Abstract
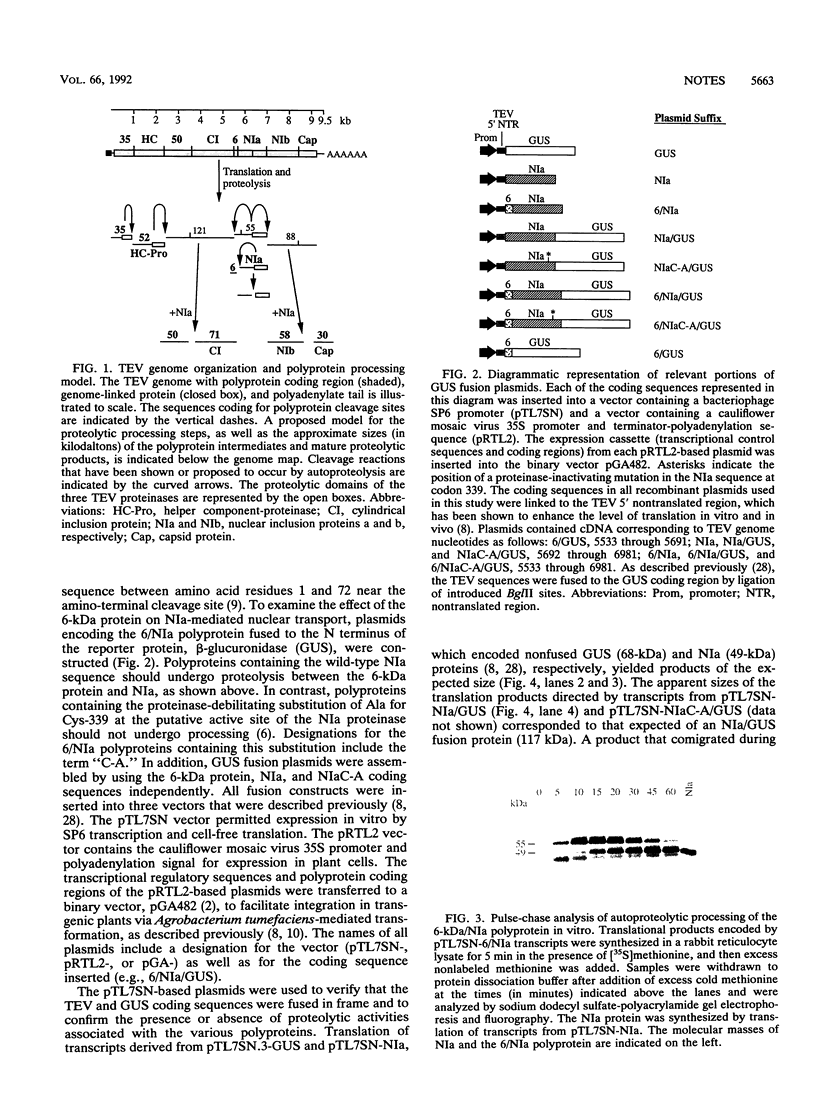
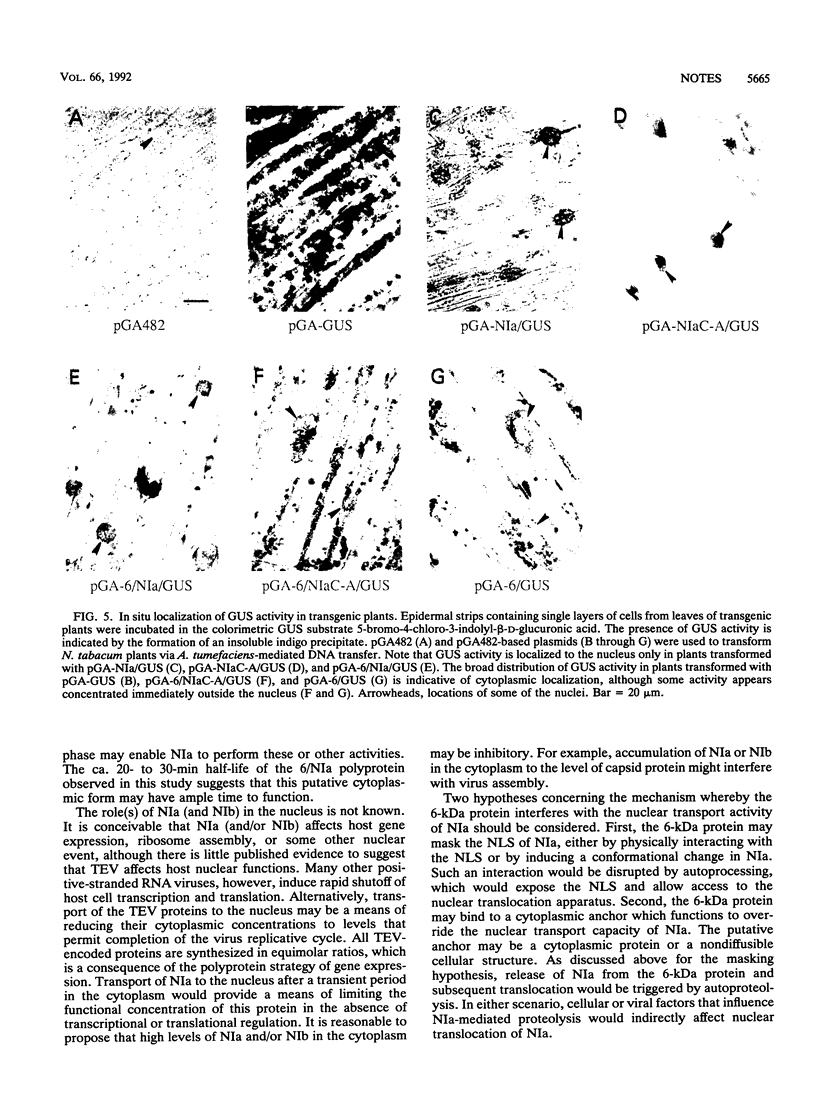
The NIa proteinase encoded by tobacco etch potyvirus catalyzes six processing events, three of which occur by an autoproteolytic mechanism. Autoproteolysis is necessary to cleave the boundaries of both NIa and the 6-kDa protein, which is located adjacent to the N terminus of NIa in the viral polyprotein. As a consequence, NIa may exist in a free form or in a transient polyprotein form containing the 6-kDa protein. While the majority of NIa molecules localize to the nuclei of infected cells, a fraction of the NIa pool is attached covalently to the 5' terminus of genomic RNA in the cytoplasm. To determine whether the presence of the 6-kDa protein affects the nuclear transport properties of NIa, we have generated transgenic plants that express genes encoding a reporter enzyme, beta-glucuronidase (GUS), fused to NIa or NIa-containing polyproteins. The NIa/GUS fusion protein was detected by histochemical analysis in the nucleus. Similarly, an NIa/GUS fusion protein that arose by autoproteolysis of a 6-kDa/NIa/GUS polyprotein was found in the nucleus. In contrast, fusion protein consisting of 6-kDa/NIa/GUS, which failed to undergo proteolysis because of the presence of a Cys-to-Ala substitution in the proteolytic domain of NIa, was detected in the cytoplasm. The inhibition of NIa-mediated nuclear transport was not due to the Cys-to-Ala substitution, since this alteration had no effect on translocation in the absence of the 6-kDa protein. These results indicate that the 6-kDa protein impedes nuclear localization of NIa and suggest that subcellular transport of NIa may be regulated by autoproteolysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Dougherty W. G. Mutational analysis of tobacco etch virus polyprotein processing: cis and trans proteolytic activities of polyproteins containing the 49-kilodalton proteinase. J Virol. 1988 Jul;62(7):2313–2320. doi: 10.1128/jvi.62.7.2313-2320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Parks T. D., Dougherty W. G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989 Feb;8(2):365–370. doi: 10.1002/j.1460-2075.1989.tb03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Dougherty W. G. Small nuclear inclusion protein encoded by a plant potyvirus genome is a protease. J Virol. 1987 Aug;61(8):2540–2548. doi: 10.1128/jvi.61.8.2540-2548.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D. Cap-independent enhancement of translation by a plant potyvirus 5' nontranslated region. J Virol. 1990 Apr;64(4):1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Leinicke A. J. Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell. 1991 Sep;3(9):953–962. doi: 10.1105/tpc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Oh C. S. Expression of potyviral polyproteins in transgenic plants reveals three proteolytic activities required for complete processing. EMBO J. 1990 May;9(5):1347–1353. doi: 10.1002/j.1460-2075.1990.tb08249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M. X., Schlesinger M. J. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. doi: 10.1016/0042-6822(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D. Molecular genetic and biochemical evidence for the involvement of the heptapeptide cleavage sequence in determining the reaction profile at two tobacco etch virus cleavage sites in cell-free assays. Virology. 1989 Sep;172(1):145–155. doi: 10.1016/0042-6822(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D. Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology. 1991 Aug;183(2):449–456. doi: 10.1016/0042-6822(91)90974-g. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J Gen Virol. 1975 Feb;26(2):183–196. doi: 10.1099/0022-1317-26-2-183. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989 Nov;63(11):4653–4664. doi: 10.1128/jvi.63.11.4653-4664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. Cytoplasmic anchoring proteins and the control of nuclear localization. Cell. 1989 Dec 22;59(6):949–951. doi: 10.1016/0092-8674(89)90747-2. [DOI] [PubMed] [Google Scholar]
- Knuhtsen H., Hiebert E., Purcifull D. E. Partial purification and some properties of tobacco etch virus induced intranuclear inclusions. Virology. 1974 Sep;61(1):200–209. doi: 10.1016/0042-6822(74)90254-2. [DOI] [PubMed] [Google Scholar]
- Martín M. T., García J. A. Plum pox potyvirus RNA replication in a crude membrane fraction from infected Nicotiana clevelandii leaves. J Gen Virol. 1991 Apr;72(Pt 4):785–790. doi: 10.1099/0022-1317-72-4-785. [DOI] [PubMed] [Google Scholar]
- Murphy J. F., Rhoads R. E., Hunt A. G., Shaw J. G. The VPg of tobacco etch virus RNA is the 49-kDa proteinase or the N-terminal 24-kDa part of the proteinase. Virology. 1990 Sep;178(1):285–288. doi: 10.1016/0042-6822(90)90405-g. [DOI] [PubMed] [Google Scholar]
- Peränen J., Rikkonen M., Liljeström P., Käriäinen L. Nuclear localization of Semliki Forest virus-specific nonstructural protein nsP2. J Virol. 1990 May;64(5):1888–1896. doi: 10.1128/jvi.64.5.1888-1896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo M. A., Freed D. D., Carrington J. C. Nuclear transport of plant potyviral proteins. Plant Cell. 1990 Oct;2(10):987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. Nuclear location signal-mediated protein transport. Biochim Biophys Acta. 1989 Aug 14;1008(3):263–280. doi: 10.1016/0167-4781(89)90016-x. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Verchot J., Koonin E. V., Carrington J. C. The 35-kDa protein from the N-terminus of the potyviral polyprotein functions as a third virus-encoded proteinase. Virology. 1991 Dec;185(2):527–535. doi: 10.1016/0042-6822(91)90522-d. [DOI] [PubMed] [Google Scholar]





