Abstract
Background
Microalbuminuria portends an increased risk for renal and cardiovascular diseases in diabetes. In this pilot study, we determined the effect of weight loss induced by different types of bariatric surgery on albuminuria in severely obese type 2 diabetic (T2DM) subjects.
Methods
Fifteen consecutive T2DM patients (9M/6F, 51 ± 14 years, body mass index (BMI) 49±9 kg/m2, HbA1c 7.2±1.1%) undergoing either Roux-en-Y gastric bypass (RYGB; N=9) or other types of bariatric surgery (N=6) underwent determination of urine albumin/creatinine ratio (UACR) and adipokine and insulin sensitivity during a mixed meal tolerance test performed 2 weeks prior to and 6 months following surgery.
Results
Following RYGB, there was a significant decrease in BMI (−4.74±−5.05 kg/m2), fasting glucose, cholesterol, and leptin levels. Insulin sensitivity (Matsuda index [12.05± 3.81, p=0.003]) and high molecular weight (HMW) adiponectin increased significantly along with a significant reduction in UACR (median, 36 mg/g [7–94] vs. 27 mg/g [5.5–42.5], p=0.01). The reduction in UACR following RYGB was inversely correlated with the Matsuda index (r=−0.74, p=0.02) and HMW adiponectin (r=−0.67, p=0.04). In contrast, despite reduction in BMI (−4.11±−4.10 kg/m2) following other types of bariatric surgery (n=6), there was no significant improvement in insulin sensitivity (0.88±2.40, p=0.63), UACR, or HMW adiponectin levels.
Conclusions
RYGB in severely obese DM subjects is associated with a reduction in albuminuria that correlates to the improvement in insulin sensitivity and HMW adiponectin. The data point to a need for larger studies to confirm these findings and evaluate the micro–macro-vascular benefits including renal parenchymal benefits of different types of bariatric surgery in T2DM.
Keywords: Albuminuria, Insulin sensitivity, Adiponectin, Adipokines, Obesity, Type 2 diabetes, Gastric bypass surgery, Bariatric surgery
Introduction
Obesity is an independent predictor of diabetes, hypertension, and cardiovascular disease [1]. Patients with obesity develop microalbuminuria and overt proteinuria independent of underlying comorbidities that predict the development of CKD and progression to end-stage renal disease similar to diabetics [2]. Urinary protein excretion worsens, and the kidney disease progresses rapidly as the severity of obesity increases. The pathogenesis of kidney disease in obesity is often multifactorial and could be mediated by adipogenic tissue inflammation or endothelial dysfunction giving rise to microalbuminuria [3]. Diabetes is an independent risk factor for development of microalbuminuria, and its presence reflects widespread vascular injury, a risk factor for cardiovascular disease in obese diabetic patients [4]. Apart from the traditional risk factors associated with obesity, pro-inflammatory adipokines such as low levels of total adiponectin have been linked to the increased urinary protein excretion in patients with obesity [5].
While studies have established the above-mentioned associations, the impact of weight loss on urinary protein excretion and kidney disease progression in obese and diabetic adults has not been well-studied. Few observational studies have shown that weight reduction through diet and/or exercise results in reduction of urinary protein excretion, both microalbuminuria and overt proteinuria [6, 7]. However, weight loss achieved through diet and exercise has limited efficacy and durability, and hence, their treatment effects on albuminuria may not be sustained. Surgical approaches to weight loss (bariatric surgery) are growing in popularity and have been shown to have marked effects on diabetes, dyslipidemia and hypertension [8, 9]. However, both the effects of various bariatric procedures (gastric restrictive vs. malabsorptive) on macrovascular complications associated with type 2 diabetes (T2DM) and the mechanisms of action of these procedures on T2DM improvement are unclear.
Large prospective studies and their meta-analyses have established the long-term benefits of bariatric surgery on diabetes, cardiovascular risk factors, and mortality [10]. Bariatric surgical procedures have shown a decline in urinary protein excretion and an improvement in glomerular hyperfiltration in both the general population and in patients with kidney disease [11, 12]. Bariatric surgery has potent effects on weight loss, improvement of insulin sensitivity, and glucose regulation that may contribute to the reduction in urinary protein excretion. Recent reports have highlighted the added benefits of Roux-en-Y gastric bypass (RYGB) surgery over other types of bariatric procedures for diabetes and related outcomes, but superiority in terms of renal outcomes is unclear [13].
Given the potent effects of RYGB on insulin sensitivity and biochemical resolution of diabetes, we hypothesized that gastric bypass surgery would reduce urinary albumin excretion associated with T2DM independent of weight loss associated with other types of bariatric surgery.
Study Design and Methods
Subjects
This pilot study included 15 severely obese adults (nine male and six female Caucasians) with T2DM who met the National Institutes of Health eligibility criteria for bariatric surgery [14]. Table 1 depicts the baseline demographic and clinical characteristics. No active smokers were included; no women were taking hormonal therapy, and no subjects used steroid medications. Nine subjects underwent RYGB, and six underwent other types of bariatric surgery with four subjects undergoing laparascopic sleeve gastrectomy, and two subjects receiving laparascopic adjustable gastric banding. The Cleveland Clinic’s Institutional Review Board approved the protocol, and all participants provided written informed consent.
Table 1.
Baseline characteristics of study participants
| Characteristic | Number=15 (mean±SD or percent) |
|---|---|
| Demographics | |
| Age | 51.2±14.3 |
| Gender (percent female) | 40% |
| Weight (kg) | 147.6±28.0 |
| Body mass index (kg/m2) | 48.8±9.4 |
| Presence of hypertension | 53% |
| Use of RAS blockers | 20% |
| Type of surgery | |
| RYGB | 60% |
| Restrictive procedures | 40% |
| Laboratory parameters | |
| Fasting glucose (mg/dl) | 140.4±41.5 |
| Total cholesterol (mg/dl) | 165.6±47.3 |
| LDL cholesterol (mg/dl) | 96.5±43.5 |
| HDL cholesterol (mg/dl) | 37.6±6.5 |
| Triglycerides (mg/dl) | 153.9±92.8 |
| HbA1C (%) | 7.2±1.0 |
| Serum creatinine (mg/dl) | 0.78±0.2 |
| Urinary albumin/creatinine ratio (mg/g)a | 17.0 (5–126) |
RAS Renin–angiotensin system blockers, RYGB Roux-en-Y gastric bypass
Reported as median and interquartile range (25th percentile to 75th percentile)
Study Design
Patients were studied 1–2 weeks prior to surgery and retested at 6 months after surgery. During the baseline testing, all subjects were on a liquid meal diet consisting of approximately 800 cal. Medications for diabetes, hypertension, and hypercholesterolemia were discontinued 24 h prior to testing. Blood samples for fasting blood sugar, HbA1c, lipid profile, adipokines (total and high molecular weight [HMW] adiponectin, resistin, and leptin), TNF-α, serum creatinine, and cystatin C were drawn before and after surgery. A mixed meal tolerance test (MMTT) was performed prior to and 6 month post-surgery for the determination of insulin sensitivity [15].
Surgical Procedures
The techniques we use to perform laparoscopic sleeve gastrectomy, laparoscopic adjustable gastric banding, and laparoscopic RYGB have been previously described [16, 17].
Metabolic Testing
MMTT-Ensure plus (4 oz; calories, 175 kcal; protein, 6.5 g; fat, 5.5 g; carbohydrate, 25 g) was given after a 12–14-h overnight fast. Fasting blood samples were obtained for lipids, HbA1c, complete metabolic panel, thyroid stimulating hormone, glucose, c-peptide, and insulin. Blood was drawn every 30 min for 120 min during the MMTT. Levels of glucose, insulin, and c-peptide were determined at each time point.
Analytic Determinations
Blood glucose was measured immediately using the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH). Blood samples for insulin measurements were centrifuged at 4°C, and the plasma was stored at −80 C for subsequent analysis by a double antibody radioimmunoassay (RIA, Linco Research, St. Charles, MO). Total adiponectin and resistin were run on a commercial human serum adipokine LINCOplex panel (Linco, St. Charles, MO). Both HMW adiponectin and serum leptin were assayed via commercial ELISA (Millipore, Billerica, MA and Linco, St. Charles, MO, respectively). Plasma TNF-α was assayed via an ultra-sensitive ELISA (Biosource International, Camarillo, CA). Cystatin C was measured using an ELISA assay (Quantikine Human Cystatin C, R &D systems, Minneapolis, MN). To correct for inter-assay variability, all pre-and 6-month measurements for each individual subject were run on the same plate for each kit.
Urine measurements of albumin/creatinine ratio were performed on a first-morning-void midstream urine sample. The urine specimen was refrigerated at 2–8°C after collection and was analyzed the same day. Urine albumin was measured via the immunoturbidimetric method. Albuminuria was expressed as urine albumin/creatinine ratio (UACR) in milligrams per gram. Urine creatinine was analyzed by colorimetric analysis on an automated platform using the Jaffé method (Roche Modular Diagnostics, Indianapolis, IN).
Statistical Analysis
Analyses were conducted using STATA 10 (Stata Corporation, Texas). Normal distributions of the study variables were checked using Shapiro–Wilk test and non-normal variables (UACR) were log-transformed when necessary. All values represent the mean and SD except UACR reported as median and interquartile range (25th percentile to 75th percentile). Within surgery group differences were determined by a Student’s paired t test and Wilcoxon signed rank test where appropriate. Pearson’s correlation and Spearman correlation coefficients (where appropriate) were used to evaluate the relationship between the various metabolic parameters and the UACR at 6-month follow-up. Two-tailed p<0.05 were considered statistically significant.
Results
Clinical and Metabolic Parameters Following Bariatric Surgery
In all patients (n=15) who underwent bariatric surgery (RYGB and other types of surgery combined), there was a significant decrease in body mass index (BMI), fasting blood glucose, and total cholesterol after surgery (p<0.05). The Matsuda index increased significantly reflecting the improvement in insulin sensitivity after weight reduction surgery. Oral hypoglycemic use decreased from an average of 1.9 medications per patient to 0.4 medications per patient. Five patients discontinued insulin post-operatively. Total adiponectin and resistin levels did not alter significantly while HMW adiponectin increased and leptin levels decreased significantly. Pro-inflammatory status as measured by TNF-α was not significantly reduced after surgery. There was a marked reduction in serum creatinine after bariatric surgery (0.78 vs. 0.64 mg/dl, p<0.001) but no significant decrease in cystatin C level was noted. There was a non-significant decrease in UACR [17 mg/g (6–65) vs. 8 mg/g (14–46), p=0.09] for the entire group. No correlation was noted between the UACR and BMI, adiponectin, leptin, resistin, or insulin resistance for the entire group (Table 2).
Table 2.
Change in body mass index, metabolic characteristics, and adipokines before and after 6 months weight reduction surgery
| Variable | Baseline mean±SD | Follow-up mean±SD | p value |
|---|---|---|---|
| Body mass index (kg/m2) | |||
| All (n=15) | 48.81±9.46 | 44.31±8.9 | <0.001 |
| RYGB (n=9) | 49.69±10.88 | 44.95±10.55 | <0.001 |
| OS (n=6) | 47.50±7.59 | 43.39±6.64 | 0.001 |
| Fasting blood glucose (mg/dl) | |||
| All (n=15) | 140.41±41.57 | 104±43.28 | 0.001 |
| RYGB (n=9) | 132.57±36.85 | 85.77±12.10 | <0.001 |
| OS (n=6) | 152.16±48.85 | 131.33±59.30 | 0.16 |
| SBP (mmHg) | |||
| All (n=15) | 125±17.14 | 139.8±18.13 | 0.86 |
| RYGB (n=9) | 120.22±15.08 | 135.55±16.44 | 0.95 |
| OS (n=6) | 132.16±18.87 | 146.16±20.18 | 0.93 |
| Total adiponectin (mcg/ml) | |||
| All (n=15) | 26.80±15.44 | 26.28±15. 91 | 0.53 |
| RYGB (n=9) | 20.74±8.46 | 30.80±17.10 | 0.06 |
| OS (n=6) | 35.88±19.71 | 19.50±12.21 | 0.97 |
| Resistin (ng/ml) | |||
| All (n=15) | 38.6±4.0 | 61.120±9.8 | 0.19 |
| RYGB (n=9) | 39.7±3.9 | 38.717±3.4 | 0.33 |
| OS (n=6) | 37.7±4.4 | 94.724±15.8 | 0.79 |
| Leptin (ng/ml) | |||
| All (n=15) | 18.9±8.9 | 9.86±6.4 | <0.001 |
| RYGB (n=9) | 17.8±8.9 | 6.96±2.1 | 0.002 |
| OS (n=6) | 20.4±9.6 | 14.8±5.9 | 0.03 |
| Total cholesterol (mg/dl) | |||
| All (n=15) | 165.66±47.35 | 146.33±29.65 | 0.02 |
| RYGB (n=9) | 171±49.73 | 141.88±28.82 | 0.02 |
| OS (n=6) | 157.66±46.84 | 153±32.30 | 0.34 |
| LDL cholesterol (mg/dl) | |||
| All (n=15) | 96.46±43.51 | 82.46±26.61 | <0.001 |
| RYGB (n=9) | 101.66±43.40 | 82.44±21.55 | 0.07 |
| OS (n=6) | 88.66±46.54 | 82.5±35.21 | 0.28 |
| HDL cholesterol (mg/dl) | |||
| All (n=14) | 37.6±6.45 | 39.13±4.29 | 0.14 |
| RYGB (n=8) | 36.22±7.25 | 39.77±4.91 | 0.03 |
| OS (n=6) | 39.66±4.88 | 38.16±3.31 | 0.23 |
| Triglycerides (mg/dl) | |||
| All (n=15) | 152.93±92.79 | 117.26±63.79 | 0.06 |
| RYGB (n=9) | 159.33±102.34 | 87.33±28.54 | 0.02 |
| OS (n=6) | 145.83±84.95 | 162.16±77.83 | 0.72 |
| HbA1C (%) | |||
| All (n=15) | 7.1±1.0 | 6.0±0.7 | 0.004 |
| RYGB (n=9) | 6.8±0.9 | 5.6±0.4 | 0.005 |
| OS (n=6) | 7.5±1.0 | 6.5±0.8 | 0.02 |
| TNF-alpha | |||
| All (n=15) | 1.84±1.07 | 1.76±3.11 | 0.45 |
| Roux-en-Y (n=9) | 1.46±0.86 | 1.25±1.41 | 0.29 |
| OS/Banding (n=6) |
1.50±4.77 | 4.13±4.77 | 0.93 |
| Serum creatinine (mg/dl) | |||
| All (n=15) | 0.78±0.21 | 0.64±0.10 | <0.001 |
| RYGB (n=9) | 0.75±0.13 | 0.65±0.07 | 0.007 |
| OS (n=6) | 0.81±0.30 | 0.62±0.13 | 0.02 |
| Serum cystatin (ng/ml) | |||
| All (n=14) | 1,322.76±339.22 | 1,246.12±238.49 | 0.18 |
| RYGB (n=8) | 1,426.10±389.29 | 1,360.72±209.03 | 0.32 |
| OS (n=6) | 1,184.96±192.91 | 1,093.33±194.12 | 0.17 |
RYGB Roux-en-Y gastric bypass, OS other types of bariatric surgery
Clinical and Metabolic Parameters 6 Months Post-Surgery for RYGB vs. Other Types of Surgery
Despite similar reduction in BMI following surgery only, RYGB demonstrated a significant reduction in fasting plasma glucose, HbA1c (7.0±0.9% vs. 5.6±0.5%, p< 0.01), total cholesterol, triglyceride, and elevations in HDL levels. Serum creatinine levels reduced significantly in both groups. However, no changes in serum cystatin C levels were noted in either group (Table 2).
Insulin Sensitivity and Adipokines Following RYGB vs. Other Types of Surgery
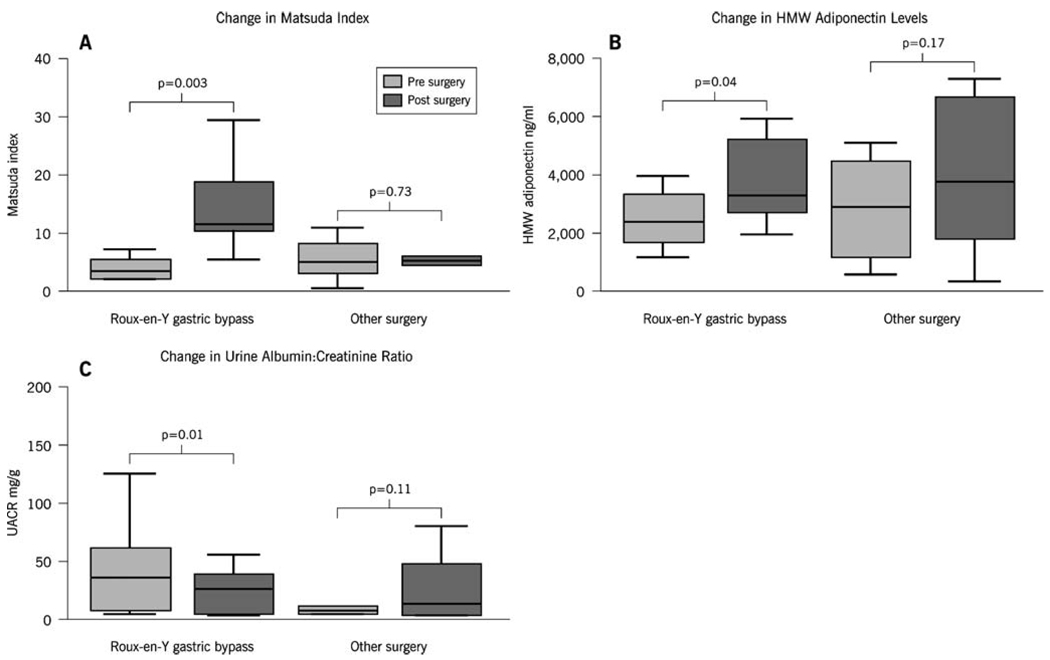
Insulin sensitivity determined by the Matsuda index improved more than 4-fold following RYGB (3.85±1.90 vs. 18.54±9.63) and did not change following other types of surgery despite weight loss (Fig. 1). Leptin levels reduced with both RYGB and other types of surgery, and a non-significant increase in total adiponectin occurred with RYGB but not with other surgical procedures (Table 2). HMW adiponectin increased significantly after RYGB but not with other procedures (Fig. 1).
Fig. 1.
Changes in a insulin sensitivity, b HMW adiponectin, and c urine albumin–creatinine ratio before and after Roux-en-Y gastric bypass and other types of surgery
Urinary Albumin Excretion Following RYGB vs. Other Types of Surgery
There was a significant decrease in UACR (36 mg/g [7–94] vs. 27 mg/g [5.5–42.5], p=0.01) following RYGB. This effect was not observed following other surgical procedures (8 mg/g [5.7–25.2] vs. 13.5 mg/g [4.7–56.2], p=0.11). In patients with preexisting microalbuminuria (n=7 who underwent RYGB or other surgery), there was a significant decrease in UACR (65 mg/g [61–126] vs. 39 mg/g [27–56], p=0.04) with 4/7 patients regressing from microalbuminuria to normoalbuminuria. In patients with normoalbuminuria (n=8), there was no significant change in UACR noted after surgery (6.5 mg/g [5–11.25] vs. 5.5 mg/g [4.25–12.25], p=0.77; Fig. 1).
Correlation Analysis
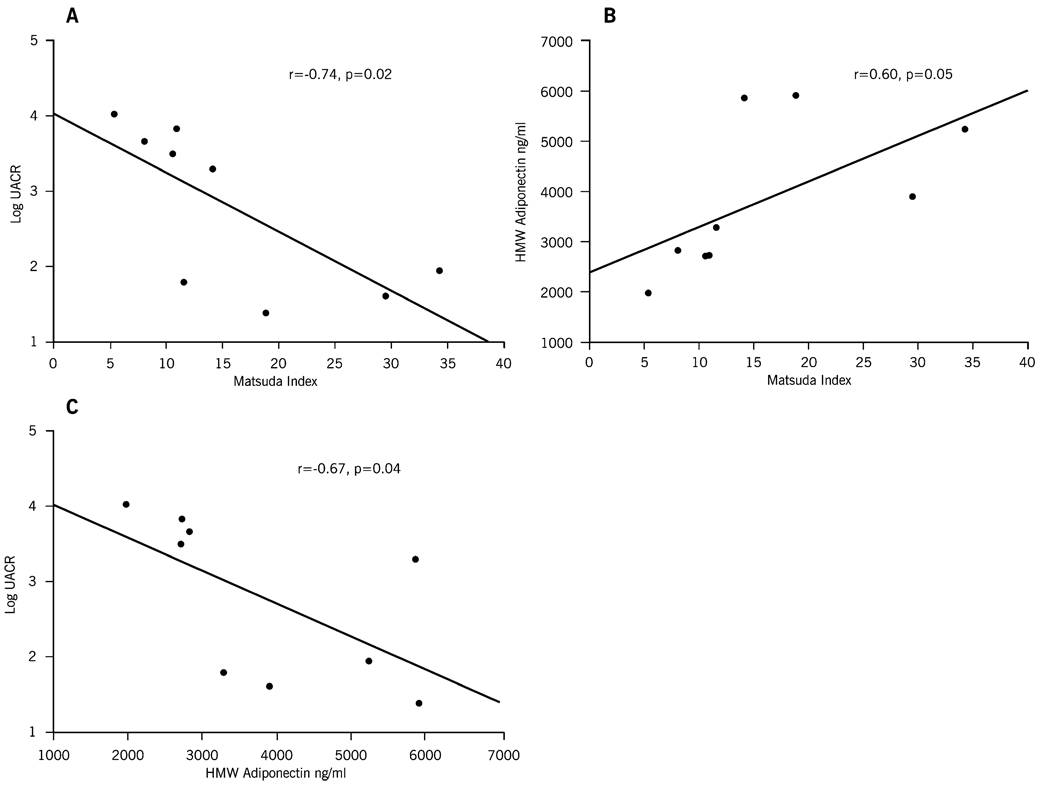
Urinary albumin excretion at 6 months following RYGB correlated strongly with insulin sensitivity and HMW adiponectin levels. An inverse correlation was noted between UACR and the Matsuda index (r=−0.62, p=0.02) and between UACR and HMW adiponectin levels (r=−0.74, p=0.02) at 6 months after RYGB surgery. A positive correlation existed between Matsuda index and HMW adiponectin levels (Fig. 2) at 6 months after RYGB surgery. No correlation was found between the post-operative levels of UACR and BMI, total cholesterol, blood pressure, leptin, or resistin after RYGB surgery (Fig. 2).
Fig. 2.
Correlation between a log-transformed values of urine albumin–creatinine ratio and Matsuda index. b Log-transformed values of urine albumin–creatinine ratio and HMW adiponectin. c HMW adiponectin and Matsuda index
Discussion
This study demonstrates the beneficial effects of RYGB in reducing urinary albumin excretion in severely obese patients with T2DM despite remaining anthropometrically obese following surgery. The decrease in albuminuria correlated with the improvement in insulin sensitivity and HMW adiponectin in the RYGB group, but not with BMI and other adipokines. Although the BMI decreased significantly with other surgical procedures, there were no significant changes noted with insulin sensitivity, adipokines, and UACR. Furthermore, in the entire group, the reduction in albumin excretion was higher in patients with preexisting microalbuminuria. Serum creatinine decreased after bariatric surgery while levels of cystatin C did not decrease significantly.
Although all surgery types resulted in similar reductions in BMI at 6 months follow-up, only RYGB was associated with marked improvements of fasting glucose, triglyceride, HDL levels, and insulin sensitivity. Since insulin resistance is a central feature of T2DM linked to the cluster of glucose, lipid, and vascular abnormalities, our data point to robust effects of intestinal bypass associated with RYGB to enhance insulin sensitivity and reduce metabolic syndrome features in T2DM subjects that remain anthropometrically obese following surgery. Long-term prospective studies, and their meta-analyses have suggested that the improvement of T2DM following bariatric surgery is related to the amount of weight loss with gastric restriction (GR) procedures (i.e., lap banding) resulting in lower rates of remission as compared with malabsorptive procedures like the RYGB and biliopancreatic diversion that result in 83% and 98% remission, respectively [9, 10]. However, rodent models of diabetes suggest direct anti-diabetic effects of duodenal bypass independent of gastric restriction and weight loss [18]. Our data support that intestinal bypass of nutrients associated with RYGB has unique effects that improve insulin sensitivity aside from surgical GR that induces early satiety, enforced caloric restriction, and weight loss. Further larger studies are being developed to explain the mechanisms of intestinal bypass on insulin sensitivity.
Our study demonstrated a significant correlation between albuminuria and insulin sensitivity as measured by the Matsuda index. Insulin resistance alters the balance between endogenous vasoactive molecules such as nitric oxide and reactive oxygen species with resultant endothelial dysfunction in the kidneys. Insulin resistance and hyperinsulinemia also stimulate angiogenesis and mesangial cell proliferation associated with the development of diabetic nephropathy through the increased expression of transforming growth factor beta, insulin-like growth factor, and downregulation of matrix metalloproteinase [19]. An improvement in insulin sensitivity was shown to impact several of these mechanistic links and might partially explain the reasons for the decrease in albuminuria noted in our study.
The reduction in urinary albumin excretion following gastric bypass inversely correlated with HMW adiponectin levels. The evidence linking adiponectin and albuminuria is emerging. Adiponectin improves insulin signaling, inhibits pro-inflammatory pathway activation by NFkappaB and has vasoprotective properties through stimulating nitric oxide generation [20]. Recently, Sharma et al. have shown that hypoadiponectinemia induces podocyte dysfunction and that albuminuria and adiponectin infusion reversed these abnormalities in rats [20]. Adiponectin circulates in three forms: tetramer, hexamer, and HMW forms. Several epidemiological studies have also shown an association between low levels of total adiponectin levels and albuminuria [5]. In the Health Professionals Follow-up Study, higher serum adiponectin concentration was associated with reduced odds of moderate renal dysfunction in men with T2DM [21]. Few studies suggested the beneficial effects of higher HMW adiponectin over total adiponectin levels for cardiovascular events and mortality [22]. However, studies linking HMW adiponectin and renal outcomes are lacking, and our findings suggest that the HMW adiponectin fraction may be more important than total adiponectin levels with respect to change in insulin sensitivity and albuminuria.
Whether the improvement in insulin sensitivity is mediated by an improvement in adiponectin levels in patients undergoing RYGB surgery cannot be ascertained from our current study. But, studies assessing the impact of exercise and diet suggested that the improvement in insulin sensitivity seen with weight loss might be due to the changes in the adiponectin oligomeric distribution [23]. Given the link between increased abdominal fat to insulin resistance and endothelial dysfunction, it is not surprising that a procedure (RYGB) that rapidly restores whole body insulin sensitivity also improves renal vascular injury. These data provide insight into the mechanistic links that mediate the improvement in urinary albumin excretion with RYGB.
Few studies have examined the impact of weight reduction surgery on renal function. Studies by Chagnac and Navarro-Diaz et al. have shown that bariatric surgery reduces both glomerular hyperfiltration and serum creatinine levels [11, 24]. We noted a significant decrease in serum creatinine which could be attributed to improvement in glomerular hyperfiltration and/or decreases in muscle mass. As an alternative, we measured cystatin C levels, but the reduction in cystatin C levels was minimal and non-significant. Cystatin C levels are higher in obese subjects in comparison to non-obese controls independent of renal function [25]. However, 6 months after surgery, our cohort had a BMI>40 which could explain the lack of significant decline in cystatin C levels.
Our study is not without limitations. This was a pilot study, and the lack of significance with other adipokines such as resistin and the lack of significance in the other surgical group might be related to the short follow-up and relatively small sample size. Renin–angiotensin inhibitors were used in three patients, which could have influenced the urinary albumin excretion; however, all three patients were on these medications prior to surgery, and the dose was not altered after surgery which reduces the possibility that these agents could have independently influenced the changes in albuminuria. Even though pro-inflammatory cytokines such as IL-6 and TNF-α are higher in obese diabetics with increased macrophage infiltration into the kidney, the TNF-α levels did not reduce significantly with weight loss. We did not measure other inflammatory markers such as IL-6 and CRP which might have helped us to better study the relationship between albumin excretion and inflammation with weight loss.
Blood pressure increased after surgery in both groups. We measured blood pressure only once during the baseline and follow-up visits, and this might be less reliable. We did not have details of duration of diabetes, hypertension, and other comorbid conditions that could influence microalbuminuria. Due to the small sample size, regression analysis was also not possible to explore if the renal parenchymal benefits would sustain even after adjusting for gender and other comorbid conditions. Furthermore, this is a prospective non-randomized study which precludes establishing causality and rather just shows an association between HMW adiponectin and albumin excretion.
In summary, improved insulin sensitivity and HMW adiponectin levels attained through RYGB were associated with a reduction in albuminuria independent of weight loss associated with other types of surgery. Given the existing link between microalbuminuria to endothelial dysfunction and renal parenchymal disease, larger studies with longer follow-up are warranted to examine the impact of RYGB on renal parameters and other macrovascular disease in patients with diabetes and obesity.
Acknowledgements
We are grateful to the skilled assistance of the nurses and technicians in the Cleveland Clinic, Clinical Research Unit. This work was supported in part by National Institutes of Health, National Center for Research Resources [NCRR], Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264 (SRK), National Institutes of Aging Award RO1 AG12834 (JPK), National Center for Research Resources, CTSA 1UL1RR024989, Ethicon Endo-Surgery (PRS, SRK), and by Department of Nephrology and Hypertension, Cleveland Clinic (SDN).
Abbreviations
- RYGB
Roux-en-Y gastric bypass
- GR
gastric restriction
- UACR
urine albumin creatinine ratio
- T2DM
type 2 diabetes
- CKD
chronic kidney disease
Contributor Information
Sankar D. Navaneethan, Department of Nephrology and Hypertension, Glickman Urological and Kidney Institute, Cleveland Clinic, 9500 Euclid Ave, Q7, Cleveland, OH 44195, USA, navanes@ccf.org
Karen R. Kelly, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA
Firas Sabbagh, Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, OH, USA.
Philip R. Schauer, Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, OH, USA
John P. Kirwan, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA Digestive Disease Institute, Cleveland Clinic, Cleveland, OH, USA.
Sangeeta R. Kashyap, Department of Endocrinology, Cleveland Clinic, 9500 Euclid Avenue, A51, Cleveland, OH, USA, kashyas@ccf.org
References
- 1.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 3.Wahba I, Mak R. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kideny disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 4.Stevens RJ, Coleman RL, Adler AI, et al. Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care. 2004;27:201–207. doi: 10.2337/diacare.27.1.201. [DOI] [PubMed] [Google Scholar]
- 5.Yano Y, Hoshide S, Ishikawa J, et al. Differential impacts of adiponectin on low-grade albuminuria between obese and non-obese persons without diabetes. J Clin Hypertens (Greenwich) 2007;9:775–782. doi: 10.1111/j.1524-6175.2007.07321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales E, Valero MA, Leon M, et al. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41:319–327. doi: 10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 7.Saiki A, Nagayama D, Ohhira M, et al. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obesity (Lond) 2005;29:1115–1120. doi: 10.1038/sj.ijo.0803009. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparascopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–748. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 10.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 11.Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213–S217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 12.Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin Am J Soc Nephrol. 2009;4:1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodieux F, Giusti V, D’Alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 14.Burguera B, Agusti A, Arner P, et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J Endocrinol Invest. 2007;30:844–852. doi: 10.1007/BF03349226. [DOI] [PubMed] [Google Scholar]
- 15.Kashyap SR, Belfort R, Berria R, et al. Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E537–E546. doi: 10.1152/ajpendo.00541.2003. [DOI] [PubMed] [Google Scholar]
- 16.Cottam D, Qureshi FG, Mattar SG, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–863. doi: 10.1007/s00464-005-0134-5. [DOI] [PubMed] [Google Scholar]
- 17.Schauer PR, Ikramuddin S, Hamad G, et al. Laparoscopic gastric bypass surgery: current technique. J Laparoendosc Adv Surg Tech A. 2003;13:229–239. doi: 10.1089/109264203322333557. [DOI] [PubMed] [Google Scholar]
- 18.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26:232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 20.Sharma K, Ramachandrarao S, Usui QG, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Hu FB, Curhan G. Serum adiponectin and renal dysfunction in men with type 2 diabetes. Diabetes Care. 2007;30:239–244. doi: 10.2337/dc06-1296. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Kotooka N, Morooka T, et al. High molecular weight adiponectin as a predictor of long-term clinical outcome in patients with coronary artery disease. Am J Cardiol. 2007;100:569–574. doi: 10.1016/j.amjcard.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 23.O’Leary VB, Jorett AE, Marchetti CM, et al. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–E427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 24.Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 25.Naour N, Fellahi S, Renucci JF, et al. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring) 2009 Apr 9; doi: 10.1038/oby.2009.96. doi:10.1038/oby.2009.96. [DOI] [PubMed] [Google Scholar]