Abstract
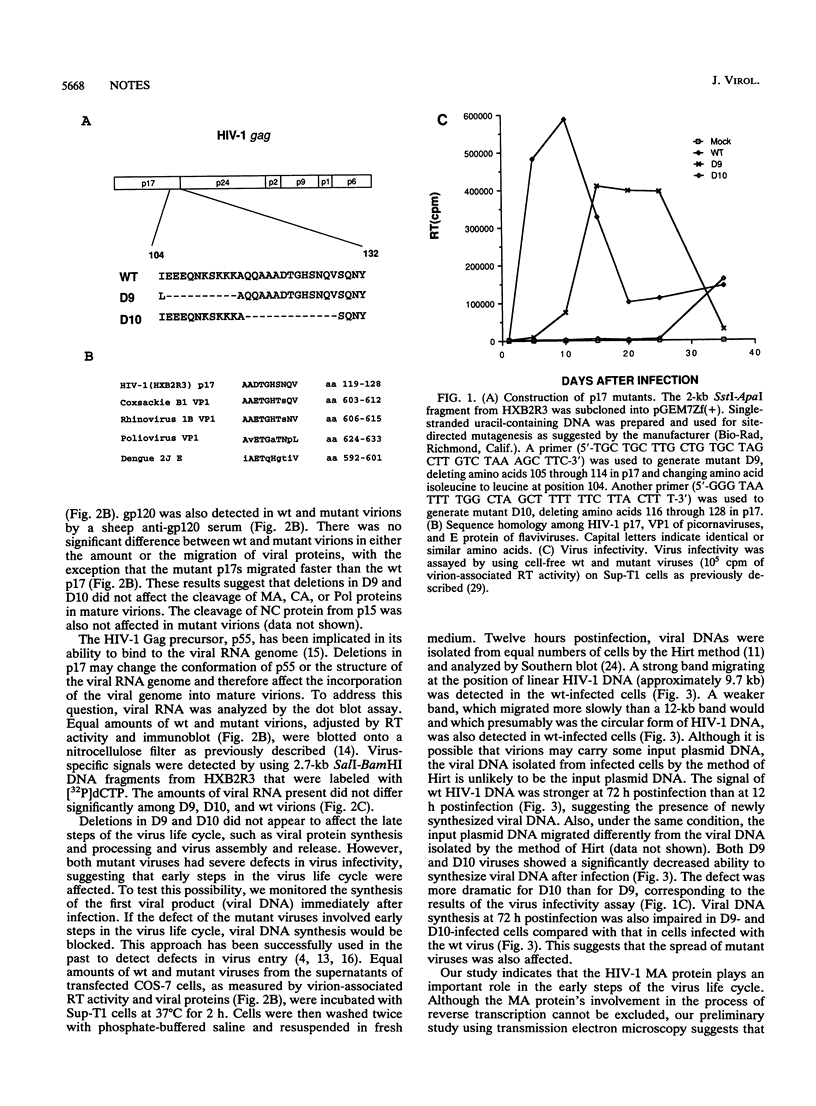
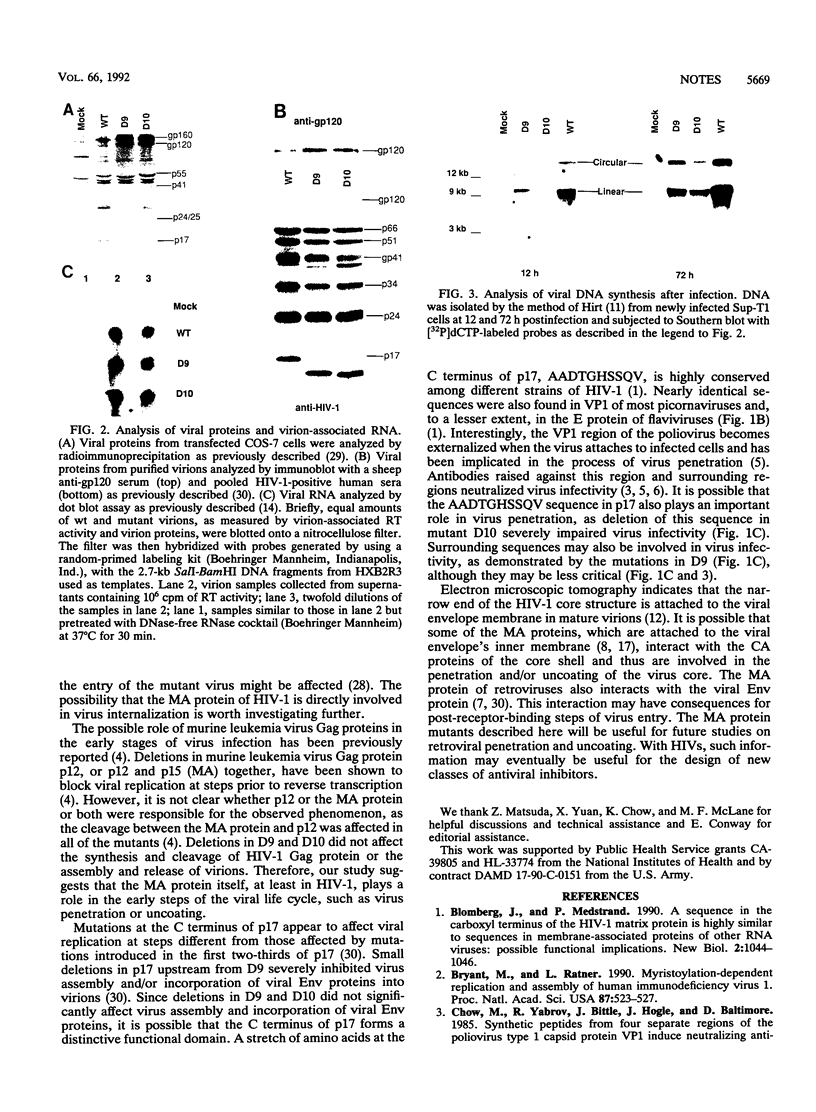
Deletion mutations at the C terminus of the matrix (MA) protein of human immunodeficiency virus type 1 (HIV-1) were generated by site-directed mutagenesis. The resultant mutant viruses had a severe defect in virus infectivity. This defect did not involve late steps of the virus life cycle, as the synthesis and processing of the Gag polyprotein and the assembly and release of mutant virions were not greatly affected. The incorporation of viral proteins and the viral RNA genome was similar for mutant and wild-type virions. In contrast, the early steps of the virus life cycle were severely affected, as the synthesis of viral DNA postinfection was dramatically reduced in mutant-virus-infected cells. One stretch of amino acids that was deleted in one of the mutants has significant homology with a region in VP1 of the picornavirus family. This region of VP1 is presumably involved in poliovirus penetration into cells. These results suggest that in addition to its functional role in virus assembly, the MA protein of HIV-1, and possibly of other retroviruses, plays an important role in virus entry.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg J., Medstrand P. A sequence in the carboxyl terminus of the HIV-1 matrix protein is highly similar to sequences in membrane-associated proteins of other RNA viruses: possible functional implications. New Biol. 1990 Nov;2(11):1044–1046. [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. Mutations in gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 1984 Mar;49(3):909–917. doi: 10.1128/jvi.49.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt A., Bosch J. V., Ziemiecki A., Friis R. R. Rous sarcoma virus p19 and gp35 can be chemically crosslinked to high molecular weight complexes. An insight into virus assembly. J Mol Biol. 1984 Apr 5;174(2):297–317. doi: 10.1016/0022-2836(84)90340-1. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Jelinek L., Whiting S., Barklis E. Transport and assembly of gag proteins into Moloney murine leukemia virus. J Virol. 1990 Nov;64(11):5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund S., Ofverstedt L. G., Nilsson A., Lundquist P., Gelderblom H., Ozel M., Skoglund U. Spatial visualization of the maturing HIV-1 core and its linkage to the envelope. AIDS Res Hum Retroviruses. 1992 Jan;8(1):1–7. doi: 10.1089/aid.1992.8.1. [DOI] [PubMed] [Google Scholar]
- Kim S., Ikeuchi K., Groopman J., Baltimore D. Factors affecting cellular tropism of human immunodeficiency virus. J Virol. 1990 Nov;64(11):5600–5604. doi: 10.1128/jvi.64.11.5600-5604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever A., Gottlinger H., Haseltine W., Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989 Sep;63(9):4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Goff S. P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 gag polyprotein. J Virol. 1991 Jun;65(6):3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990 Oct 5;63(1):77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 1991 Mar;10(3):535–546. doi: 10.1002/j.1460-2075.1991.tb07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Structural role of the matrix protein of type D retroviruses in gag polyprotein stability and capsid assembly. J Virol. 1990 Sep;64(9):4383–4389. doi: 10.1128/jvi.64.9.4383-4389.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Viglianti G. A., Mullins J. I. Functional comparison of transactivation by simian immunodeficiency virus from rhesus macaques and human immunodeficiency virus type 1. J Virol. 1988 Dec;62(12):4523–4532. doi: 10.1128/jvi.62.12.4523-4532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. A., Panganiban A. T. N myristoylation of the spleen necrosis virus matrix protein is required for correct association of the Gag polyprotein with intracellular membranes and for particle formation. J Virol. 1990 Aug;64(8):3995–4001. doi: 10.1128/jvi.64.8.3995-4001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Weldon R. A., Jr, Nelle T. D., Erdie C. R. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J Virol. 1991 Jul;65(7):3804–3812. doi: 10.1128/jvi.65.7.3804-3812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. F., Matsuda M., Essex M., Lee T. H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990 Nov;64(11):5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Yuan X., Matsuda Z., Lee T. H., Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992 Aug;66(8):4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]