Abstract
Objective
To determine whether insulin sensitizers lower androgen levels and whether androgen suppression improves insulin resistance in non-diabetic postmenopausal women.
Design
Randomized, double-blind, placebo-controlled study
Setting
Clinical and Translational Research Center of a university hospital
Patients
Thirty-five postmenopausal women aged 50-79 yr with insulin resistance and higher testosterone levels
Interventions
Subjects were randomized to metformin plus leuprolide placebo (LP), leuprolide plus metformin placebo (MP), or LP plus MP in a 1:1:1 fashion over a 12 week period.
Main Outcome Measures
Insulin sensitivity (M) assessed by euglycemic-hyperinsulinemic clamp and free testosterone by equilibrium dialysis.
Results
In those randomized to metformin, free testosterone decreased by 19% (p=0.02) compared to placebo, along with an expected improvement in M. Total testosterone also decreased significantly (p=0.001) whereas sex hormone binding globulin (SHBG) did not change. In those randomized to leuprolide, the percent change in M was not different from placebo (p=0.56), despite a 48% relative decrease in free testosterone levels (p<0.001).
Conclusions
These data are the first to establish a causal link between insulin resistance and testosterone in postmenopausal women. They confirm that treatment of insulin resistance decreases testosterone production in this population and demonstrate that pharmacologic lowering of testosterone does not affect insulin resistance.
Keywords: Testosterone, insulin resistance, polycystic ovary syndrome, metabolic syndrome, aging, elderly, women
Introduction
Mechanistic studies in women with PCOS largely suggest that hyperinsulinemia secondary to insulin resistance causes hyperandrogenism (1,2). However, the direction of causality in the association between insulin resistance and testosterone has not been examined in postmenopausal women, the group of women with the highest prevalence of metabolic syndrome and cardiovascular disease (3,4). We hypothesized that insulin resistance also increases androgen production in older women, and that endogenous androgens do not have a clinically meaningful impact on insulin resistance in this population. To test this hypothesis, we conducted a randomized, double-blind, placebo-controlled trial in which we evaluated the effects of metformin and leuprolide acetate in non-diabetic postmenopausal women with insulin resistance and higher testosterone levels.
Materials and Methods
Eligibility
This trial was conducted at the University of Pennsylvania Clinical and Translational Research Center (CTRC). Eligible women were aged 50-79 years, postmenopausal (no menses for ≥12 months, plus FSH > 30 mIU/mL for those aged 50-54 years) with at least 1 ovary, and had a calculated free testosterone concentration ≥ 3.0 pg/mL and a fasting insulin concentration ≥ 12 μU/mL; these cutoffs were based on the top quartile of a random sample of nondiabetic postmenopausal women from the Cardiovascular Health Study (5,6). There were no BMI cutoffs for inclusion, though women with a BMI of ≥ 25 mg/kg2 were targeted for recruitment. Women with a history of PCOS or evidence of hyperandrogenism were not specifically targeted for recruitment. Women were not eligible if they had diabetes mellitus or had taken glucose lowering medication in the past 3 months. Additional exclusions included contraindications to metformin or leuprolide acetate, use of medications known to affect free testosterone concentration (estrogen or estrogen agonists, androgens, antiandrogens, or oral corticosteroids), serious illness, or participation in an investigational drug study in the past 6 weeks. The study was approved by the Institutional Review Board. All participants gave written informed consent before enrollment.
Study Design
The study design was a three arm, parallel, randomized, double-blind, placebo-controlled clinical trial. From July 2005 until April 2008, 101 women were screened and 40 were eligible to participate. The 36 women enrolled in the study returned for a baseline euglycemic-hyperinsulinemic clamp and randomization. Interim visits were conducted at 4 weeks and 8 weeks. A final euglycemic-hyperinsulinemic clamp was performed at 12 weeks. Blood samples were collected at each visit between 7AM and 10AM after a 12 hour overnight fast.
Eligible women were randomly assigned in a 1:1:1 fashion to two simultaneous therapies: metformin plus leuprolide placebo (LP), leuprolide plus metformin placebo (MP), or MP plus LP. Blocked randomization with a block size of 12 was performed by the Investigational Drug Service (IDS) at the University of Pennsylvania using a web-based program available from Tufts University at www.randomization.com. The IDS purchased metformin and created an identical-appearing placebo. Lupron® was donated by the manufacturer, TAP Pharmaceuticals, which had no role in trial design, data accrual, data analysis, or preparation of the manuscript.
Women randomized to metformin (or MP) took 500 mg (1 capsule) daily for the first week, and via weekly follow-up phone calls were advised to uptitrate by 500 mg (1 capsule) each week until they reached the maximum recommended dose of 1000 mg (2 capsules) twice a day. All women achieved the maximum recommended dose with the exception of one woman taking MP. Women randomized to leuprolide (or LP) were administered a leuprolide acetate (or saline) IM injection at a dose of 3.75 mg at baseline and interim visits for a total of 3 injections. All women received all of the leuprolide injections with the exception of one woman taking LP, who refused her second and third injections.
Women were reminded not to change their diet and physical activity for duration of the study. Pill compliance was assessed by pill count at each visit and measurement of metformin levels after study completion.
Euglycemic-hyperinsulinemic clamp and glucose disposal (M)
All study participants underwent a euglycemic-hyperinsulinemic clamp at baseline and final visits. After a 12 hour overnight fast, a continuous infusion of regular insulin was administered at a rate of 1 mU/kg·min for 120 minutes (7). A variable infusion of 20% glucose was delivered to maintain a target plasma glucose concentration of 85-90 mg/dL. Plasma glucose was measured every 5 minutes at the bedside with a YSI glucose analyzer to adjust the glucose infusion rate and achieve the desired plasma glucose concentration. Additional plasma samples were taken every 15 minutes for glucose and insulin. Insulin sensitivity (M) was estimated from the glucose infusion rate required to maintain euglycemia, determined between 90-120 minutes of the clamp (8).
Bioassays
Free testosterone was measured by equilibrium dialysis (Esoterix, Inc). This assay had a detection limit of 0.03 pg/mL and CV of 6.6%. Total T was measured by liquid chromatography with mass spectrometry after nonpolar solvent extraction (Esoterix, Inc). The detection limit was 3 ng/dL and CV was 2.6%. SHBG was measured by immunoradiometric assay (IRMA, Esoterix, Inc) with a CV of 2.7%. Free testosterone was also estimated by the law of mass action (9). Insulin levels were measured in duplicate by radioimmunoassay (Millipore, Billerica, MA) and glucose levels were measured on a Hitachi 912 analyzer. Serum measurements of fasting insulin and glucose were used to calculate the homeostasis model assessment of insulin resistance (HOMA-IR) (10). LH and FSH were measured by IRMA (Seimens Medical Diagnostics, Los Angeles, CA) and each had detection limits of 1 mIU/ml and CVs of 2.5 and 4.5%, respectively. Estradiol and DHEAS were measured in duplicate by radioimmunoassay (Seimens Medical Diagnostics, Los Angeles, CA), with detection limits of 5 μg/dL and 5 pg/mL and CVs of 5.4 and 11.6%, respectively.
Blood concentrations of metformin were measured at the final visit by HPLC (NMS Labs, Willow Grove, PA).
Statistical analysis
The first part of our hypothesis was that improving insulin sensitivity would lower free testosterone levels. Assuming an effect size of 1.25 (similar that detected in studies of PCOS) and a significance level (alpha) of 0.05 using a two-sided two-sample t-test, we anticipated 80% power with group sizes of 12. This estimate was conservative because power was increased by the longitudinal data structure. The second part of our hypothesis was that lowering testosterone levels would not affect insulin sensitivity (M). Proof of equivalence would have required 1749 subjects per arm. Therefore, we calculated the detectable difference in M with 12 subjects per arm. Using an SD of 0.4 mg/kg·min (obtained from PCOS studies of the effects of androgen suppression on insulin sensitivity), the effect size of 1.25 corresponded to a detectable difference in M of 0.5 mg/kg·min relative to placebo, suggesting power to detect a clinically significant difference. One study subject who had a baseline fasting glucose of 123 mg/dL took metformin outside of the study randomization, against study protocol, and was subsequently excluded from the study, yielding a final sample of 35 women.
Differences in baseline characteristics between the groups were analyzed based on the chi-square test for categorical variables, ANOVA for normally distributed continuous variables, and the Kruskall-Wallis test for non-normally distributed continuous variables.
For outcomes only obtained at baseline and the final study visit, absolute and percent change analyses were performed comparing metformin and placebo groups and/or leuprolide and placebo groups. A standard t-test was used to compare M values, and the Wilcoxon Rank Sum test was used for non-normal distributions (DHEAS and lipids). For all other outcomes, longitudinal analyses examined data from baseline through final visits and compared metformin and placebo groups and/or leuprolide and placebo groups by fitting a non-linear mixed effects model at 4 timepoints. Log transformations were used where appropriate. For these outcomes, percent change from baseline to the final visit is reported in the text along with the p-value for the adjusted longitudinal analysis. Because of an imbalance in baseline weight among study groups, all analyses were adjusted for weight except for those examining M, since M is weight-corrected. We did not adjust for multiple comparisons, as we had defined specific hypotheses a priori for each analysis. For the reproductive hormones, the primary comparison was metformin to placebo, and for insulin sensitivity, it was leuprolide to placebo. All analyses were performed using Stata version 9.
Results
The mean age of study subjects was 58 years and mean BMI was 36.6 kg/m2. Forty percent of women had PCOS, defined as a history of irregular menses, excess facial or body hair growth, or difficulty achieving pregnancy, and 69% met NCEP criteria for the metabolic syndrome (11,12). Baseline median free testosterone levels by dialysis were lower than expected due to differences between this assay and estimation of free testosterone by the law of mass action (9) at the screening visit. Weight and estradiol concentrations were higher in the leuprolide group, but there were otherwise no statistically significant differences in baseline characteristics among the three groups (Table 1).
Table 1.
Baseline Characteristics by Treatment Group
| Characteristics |
Leuprolide Group (n=12) |
Metformin Group (n=12) |
Placebo Group (n=11) |
Total (n=35) |
|---|---|---|---|---|
| Age, yr |
58 (7) |
56 (4) |
62 (7) |
58 (7) |
| Race, no. (%) | ||||
| White | 6 (50) | 6 (50) | 8 (73) | 20 (57) |
| African-American | 5 (42) | 6 (50) | 3 (27) | 14 (40) |
| Asian |
1 (8) |
0 |
0 |
1 (3) |
| Current smoking, no. (%) |
3 (25) |
3 (25) |
1 (9) |
7 (20) |
| Alcohol intake, no. (%) | ||||
| 0-1 drinks/day | 12 (100) | 11 (92) | 11 (91) | 33 (94) |
| 2 drinks/day | 0 (0) | 1 (8) | 1 (9) | 2 (6) |
| Weight, kg |
108.7 (33.7) |
96.5 (19.6) |
93.3 (11.1) |
99.7(23.9)a |
| BMI, kg/m2 |
41.2 (12.1) |
34.4 (6.1) |
34.0 (3.6) |
36.6 (8.6) |
| Waist circumference, cm |
109.9 (19.3) |
95.9 (13.0) |
98.0 (6.4) |
101.4 (15.1) |
| PCOS, no. (%) |
5 (42) |
5 (42) |
4 (36) |
14 (40) |
| Metabolic Syndrome, no. (%) | 7 (58) | 9 (75) | 8 (73) | 24 (69) |
|
Biochemical Measurements b | ||||
| Glucose (mg/dL) |
96 (90-117) |
98 (92-106) |
96 (91-110) |
97 (91-108) |
| Insulin (uIU/mL)c |
29.8 (17.8-38.5) |
21.6 (17.1-31.7) |
21.4 (10.8-22.1) |
21.9 (11.8-32.4) |
| HOMA-IR (mg/dL × uIU/mL)c |
7.06 (4.43-10.28) |
5.30 (4.46-7.86) |
4.91 (2.31-5.89) |
5.37 (2.99-8.17) |
| Total testosterone (ng/dL)c |
26 (19-35) |
19 (15-20) |
22 (17-34) |
20 (15-29) |
| SHBG (nmol/L) |
43 (32-76) |
43 (38-53) |
50 (31-54) |
43 (35-55) |
| Free testosterone (pg/mL)c |
2.8 (2.2-3.8) |
2.3 (1.8-2.7) |
3.0 (2.0-3.5) |
2.6 (2.0-3.5) |
| LH (mIU/mL)c |
28.7 (20.9-31.1) |
26.1 (17.3-29.1) |
29.8 (19.9-36.7) |
26.7 (17.9-32.6) |
| FSH (mIU/mL) |
60.9 (45.5-67.6) |
58.4 (45.9-84.7) |
80.3 (54.6-91.5) |
62.6 (49.2-80.3) |
| Estradiol (pg/mL) |
18.4 (13.5-22.1) |
10.1 (6.6-14.2) |
5.0 (5.0-18.0) |
12.4 (5.3-20.6)a |
| DHEAS (ug/dL)c |
60.4 (30.8-75.3) |
72.1 (46.3-104.9) |
39.8 (31.7-106.9) |
65.2 (37.1-89.1) |
| Total cholesterol (mg/dL) | 177 (155-194) | 209 (156-230) | 180 (152-196) | 181 (152-212) |
| LDL (mg/dL) | 97 (84-117) | 117 (85-138) | 95 (73-110) | 100 (81-132) |
| HDL (mg/dL) | 52 (47-57) | 48 (45-51) | 51 (49-61) | 51 (47-57) |
| Triglycerides (mg/dL) | 121 (84-164) | 153 (104-185) | 150 (86-173) | 132 (85-173) |
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; PCOS, polycystic ovary syndrome; HOMA-IR, homeostasis model assessment of insulin resistance; SHBG, sex hormone binding globulin
Data are presented as mean (SD) unless otherwise indicated. ANOVA was performed to assess differences in all continuous variables except for BMI, SHBG, estradiol, HDL, and triglycerides, for which the Kruskall-Wallis test was performed due to non-normal distribution.
Multiply by 0.0347 to convert total testosterone concentrations from ng/dl to nmol/l. Multiply by 3.47 to convert free testosterone concentrations from pg/ml to pmol/l. Multiply by 3.671 to convert estradiol from pg/mL to pmol/L.
p<0.05 for differences among groups
median (IQR)
log-transformed values were used in statistical comparisons
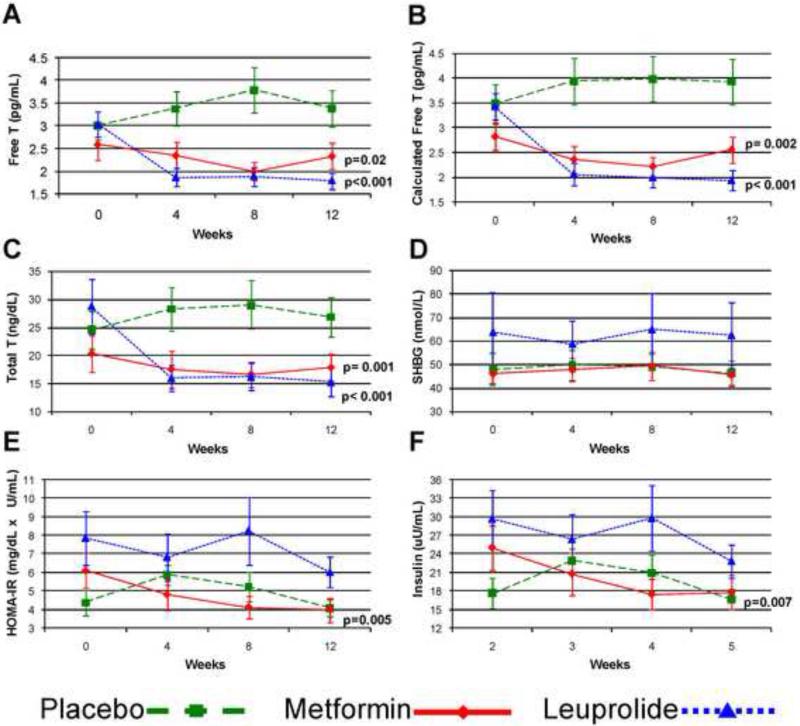
In those randomized to metformin, free testosterone by equilibrium dialysis decreased by 19% (p=0.02) over the course of the study relative to placebo (Figure 1). Calculated free testosterone similarly decreased by 22% during the study duration (p=0.002 vs placebo). Total testosterone decreased significantly (p=0.001), whereas SHBG did not change. Insulin sensitivity (M) increased by 37% (p=0.02 vs placebo), consistent with metformin's known mechanism of action (Table 2). HOMA-IR similarly improved by 36% and insulin by 31% over the course of follow-up (p=0.005 and p=0.007 vs placebo, respectively; Figure 1).
Figure 1.
Longitudinal data for (A) free testosterone, (B) calculated free testosterone, (C) total testosterone, (D) SHBG, (E) HOMA-IR and (F) insulin by group. Mean values with SE bars are presented. P-values are for longitudinal comparisons to placebo of log-transformed values. Abbreviations: SHBG, sex hormone binding globulin; HOMA-IR, homeostasis model assessment of insulin resistance
Table 2.
Insulin Sensitivity
| Leuprolide Group (n=12) | Metformin Group (n=12) | Placebo Group (n=11) | |
|---|---|---|---|
| Baseline M (mg/kg−1·min−1) | 2.13 (0.85) | 1.99 (0.98) | 2.57 (0.74) |
| Final M (mg/kg−1·min−1) | 2.17 (1.07) | 2.43 (0.60) | 2.49 (0.79) |
| Absolute change in M (mg/kg−1·min−1) | 0.04 (0.81) | 0.44 (0.80) | −0.08 (0.73) |
| % change in M | 6.4 (30.9) | 36.3 (37.7)a | −1.10 (28.9) |
Data are presented as mean (SD) unless otherwise indicated. T-tests were used for comparisons.
p=0.02 compared to placebo
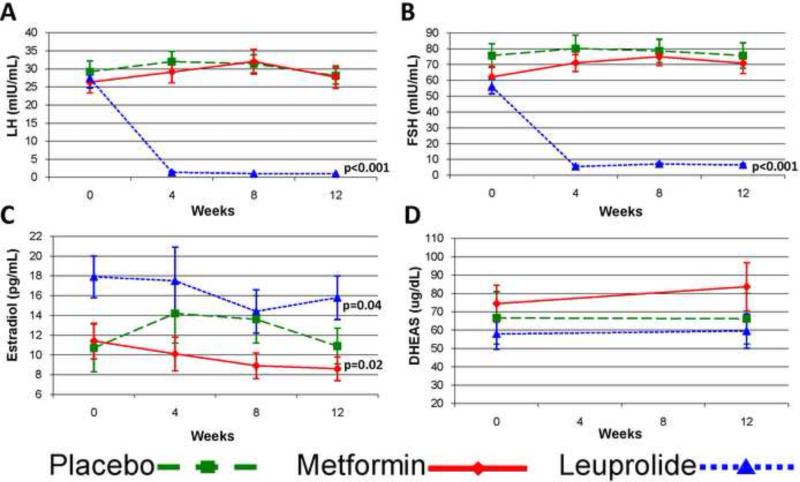
In metformin users, LH, FSH, and DHEAS did not change over the study duration, however estradiol decreased by 27% (p=0.02 vs placebo) (Figure 2). LDL decreased by 36% (p=0.007 vs placebo) and total cholesterol by 17% (p=0.02 vs placebo), while diastolic blood pressure increased by 10% (p=0.048 vs placebo). There was no significant difference in BMI (−1.3% vs. +0.3%), HDL, triglycerides, or systolic blood pressure in metformin users compared to placebo.
Figure 2.
Longitudinal data for (A) LH, (B) FSH, (C) estradiol, and (D) DHEAS by group. Mean values with SE bars are presented. P-values are for longitudinal comparisons to placebo. DHEAS values were log-transformed for analysis.
In those randomized to leuprolide, the percent change in M was not different from placebo (p=0.56, Table 2) despite a 48% relative decrease in free testosterone levels over the course of the study (p<0.001, Figure 1). Similar results were found using HOMA-IR and insulin levels (Figure 1). Total and calculated free testosterone decreased significantly during study follow-up (p<0.001), as did estradiol, whereas SHBG did not change.
In leuprolide users, LH decreased by 95% (p<0.001 vs placebo, Figure 2) and FSH decreased by 89% (p<0.001 vs placebo), consistent with leuprolide's known mechanism of action. DHEAS was not affected by leuprolide administration. Diastolic blood pressure increased by 14% (p=0.001 vs placebo) in the leuprolide users. There was no significant difference in BMI (+0.1% vs. +0.3%), cholesterol, or systolic blood pressure in leuprolide users compared to placebo.
Adherence to study medications was high (96% for study pills and 98% for study injections) for the 12 week study duration. Additionally, there was no difference in pill adherence between those taking metformin or placebo. There were no serious adverse events. Non-serious adverse events predominantly included nausea and diarrhea in the group taking metformin.
Discussion
In this randomized trial of metformin and leuprolide acetate in non-diabetic postmenopausal women, treatment of insulin resistance significantly lowered free testosterone levels compared to controls. Conversely, pharmacologic lowering of testosterone did not significantly improve insulin resistance. These data are the first to establish a causal link between insulin resistance and testosterone in postmenopausal women.
Until now, the causal relationship between high endogenous androgen levels and insulin resistance in women was limited to premenopausal women with PCOS (13). Administration of metformin results in significant decreases in testosterone in women with PCOS (1,2). The converse effect of lowering testosterone levels, using gonadotropin-releasing hormone (GnRH) agonists, on insulin resistance has been less conclusive in PCOS; two studies have shown no effect on insulin resistance assessed via euglycemic-hyperinsulinemic clamp (14-16), whereas one showed an improvement (16). Although we retrospectively diagnosed 40 percent of our study population with PCOS, our findings were not limited to this group of women. Additional small studies examining non-obese premenopausal women without PCOS showed no effect of GnRH agonist therapy on insulin resistance (17,18).
Our data suggest that hyperinsulinemia secondary to insulin resistance may increase endogenous androgen production across a woman's lifespan. Interestingly, BMI did not change in the present study, suggesting that insulin resistance may modulate testosterone independent of changes in body weight. In PCOS, studies have suggested that the cellular mechanism for insulin resistance may involve a postreceptor defect in the insulin signal transduction pathway (19,20). The subsequent development of hyperinsulinemia may increase testosterone production by stimulation of ovarian cytochrome P450c17α (1). Whether a similar mechanism can be applied more broadly to postmenopausal women with insulin resistance is not known. Though circulating androgen levels are lower in older than in younger women, it has been shown that the ovary continues to synthesize testosterone after the menopausal transition (21), and free testosterone levels may even rise in the later decades of life (22). In our study, levels of DHEAS were not affected by an improvement in insulin sensitivity, suggesting that hyperinsulinemia may not affect adrenal androgen biosynthesis, and further implicating the postmenopausal ovary as the site where insulin acts to stimulate androgen production.
A major strength of our study, aside from its randomized and placebo controlled design, includes the application of gold standard methodology to measure our study outcomes. We performed euglycemic-hyperinsulinemic clamps to assess changes in insulin sensitivity and used testosterone assays with sufficient sensitivity to detect levels in the postmenopausal range. However, there are several study limitations. It is possible that our results would have differed if we had used an androgen receptor antagonist instead of a GnRH agonist, though our degree of testosterone suppression with leuprolide acetate was excellent. Although the prevailing mechanism by which metformin mediates improvement in hyperandrogenemia is thought to occur via reduction in hyperinsulinemia, there are data showing that supraphysiologic levels of metformin inhibit androgen production in a human thecal tumor cell line (23). Our study was only 12 weeks in duration, and it is possible that we would have seen a larger effect of metformin on testosterone levels if our study were of longer duration. We also did not have the power to prove equivalence in insulin sensitivity between leuprolide acetate and placebo; however, our data do not suggest a trend toward improvement and support no effect of leuprolide acetate on insulin sensitivity. Finally, we cannot determine from our study whether exogenous testosterone administration has any impact on insulin sensitivity in postmenopausal women.
Our study is the first to show a causal relationship between insulin resistance and testosterone production in postmenopausal women. Our data suggest that testosterone is an epiphenomenon of insulin resistance in this population and not part of the causal pathway. Several observational studies have demonstrated associations between levels of testosterone and cardiovascular disease in postmenopausal women (6,24-28). Since insulin resistance is a risk factor for atherosclerosis (29-31), it is unclear whether there are atherogenic effects of endogenous testosterone that are independent of insulin resistance. Further research is required to define the mechanism whereby hyperinsulinemia increases testosterone production and to determine any physiologic impact of endogenous testosterone in postmenopausal women.
Acknowledgments
This work was supported by K23 AG19161 from the National Institute on Aging, UL1RR024134 from the National Center for Research Resources, P30 DK19525 from the National Institute of Diabetes and Digestive and Kidney Diseases, the John A. Hartford Foundation, and the University of Pennsylvania Institute for Diabetes, Obesity, and Metabolism. Leuprolide acetate (Lupron®) was provided by TAP Pharmaceuticals. The views expressed are those of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Dr. Iqbal is currently employed by Bristol Myers Squibb. All of his work on this project was completed prior to his joining Bristol Myers Squibb. No other potential conflict of interest relevant to this article was reported.
Capsule: Treatment of insulin resistance in non-diabetic postmenopausal women decreases testosterone production, whereas pharmacologic lowering of testosterone does not affect insulin resistance in this population.
References
- 1.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17α activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 2.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–46. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 5.Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 2007;92:509–16. doi: 10.1210/jc.2006-1399. [DOI] [PubMed] [Google Scholar]
- 6.Patel SM, Ratcliffe SJ, Reilly MP, Weinstein R, Bhasin S, Blackman MR, et al. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2009;94:4776–4784. doi: 10.1210/jc.2009-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981;240:E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 10.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985:28412–28419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–32. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]
- 14.Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. Journal of Clinical Endocrinology Metabolism. 1990;70:699–704. doi: 10.1210/jcem-70-3-699. [DOI] [PubMed] [Google Scholar]
- 15.Lasco A, Cucinotta D, Gigante A, Denuzzo G, Pedulla M, Trifiletti A, et al. No changes in peripheral insulin resistance in polycystic ovary syndrome after long-term reduction of endogenous androgens with leuprolide. Eur J Endocrinol. 1995;133:718–722. doi: 10.1530/eje.0.1330718. [DOI] [PubMed] [Google Scholar]
- 16.Moghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–60. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- 17.Cooper BC, Sites CK, Casson PR, Toth MJ. Ovarian suppression with a gonadotropin-releasing hormone agonist does not alter insulin-stimulated glucose disposal. Fertil Steril. 2007;87:1131–8. doi: 10.1016/j.fertnstert.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth MJ, Cooper BC, Pratley RE, Mari A, Matthews DE, Casson PR. Effect of ovarian suppression with gonadotropin-releasing hormone agonist on glucose disposal and insulin secretion. Am J Physiol Endocrinol Metab. 2008;294:E1035–E1045. doi: 10.1152/ajpendo.00789.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen SS. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab. 1992;75:577–83. doi: 10.1210/jcem.75.2.1322430. [DOI] [PubMed] [Google Scholar]
- 20.Dunaif A, Wu X, Lee A, Amanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). AJP - Endocrinology and Metabolism. 2001;281:E392–E399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- 21.Adashi E. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994;62:20–7. doi: 10.1016/s0015-0282(16)56810-1. [DOI] [PubMed] [Google Scholar]
- 22.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 23.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76:517–24. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 24.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276–84. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 26.Wild RA, Grubb B, Hartz A, Van Nort JJ, Bachman W, Bartholomew M. Clinical signs of androgen excess as risk factors for coronary artery disease. Fertil Steril. 1990;54:255–259. doi: 10.1016/s0015-0282(16)53699-1. [DOI] [PubMed] [Google Scholar]
- 27.Phillips GB, Pinkernell BH, Jing TY. Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:695–701. doi: 10.1161/01.atv.17.4.695. [DOI] [PubMed] [Google Scholar]
- 28.Braunstein GD, Johnson BD, Stanczyk FZ, Bittner V, Berga SL, Shaw L, et al. Relations between endogenous androgens and estrogens in postmenopausal women with suspected ischemic heart disease. J Clin Endocrinol Metab. 2008;93:4268–75. doi: 10.1210/jc.2008-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis. Circulation. 1996;93:1809–17. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 30.Bonora E, Tessari R, Micciolo R, Zenere M, Targher G, Padovani R, et al. Intimal-medial thickness of the carotid artery in nondiabetic and NIDDM patients. Relationship with insulin resistance. Diabetes Care. 1997;20:627–31. doi: 10.2337/diacare.20.4.627. [DOI] [PubMed] [Google Scholar]
- 31.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83:2773–6. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]