Abstract
Cryopreservation of human gonadal tissue and oocytes has brought about new and exciting research in reproductive medicine, as well as new cryopreservation techniques that are dramatically improving post-thaw viability and freezing damage. The work done on gonadal tissues is aimed at improving the quality of life for infertile patients and for prepubertal patients undergoing gonadotoxic chemotherapy, patients for whom hormonal stimulation /IVF is not an option, and women without partners. Cryopreservation of mature oocytes is the best model for timing IVF. Vitrification is likely to benefit the field, and since 2005, implantation and pregnancy rates from vitrified oocytes have matched or eclipsed results from conventional methods, due to new cell-specific methods and formulas. Cryopreservation of immature oocytes leads to a new direction of egg banking in future. Preserving ovarian tissue for autografting is still promising and has resulted in folliculogenesis, resumed hormone production and live births in limited cases. The use of small cortical blocks, or mechanical/chemical digestion of ovarian tissue for isolation of follicles is a new direction for ovary preservation for reasons of cryoprotectant permeation and graft revascularization. Maturation of follicles in vitro has become more feasible with the use of alginate microencapsulation. Testicular tissue preservation has taken a sharp turn towards preservation of gonadal stem cells. Research into the mechanism for spermatogenesis points to the ability for male germ cells to resume spermatogenesis. The cryopreservation of minced testicular tissue for isolation of germ cells via chemical digestion has produced viable cells, however structural damage that may be avoided by vitrification has been noted to the surrounding cell junctions and supporting cells.
Keywords: Vitrification, Oocyte, Immature Oocyte, Ovarian Tissue, Alginate Encapsulation, Male Germ Cells
Introduction
Cryopreservation for fertility restoration has been an ever increasing field since Polge, Smith and Parkes in 1949, produced the first chicks from cryopreserved sperm.1 In the decades since their breakthrough accomplishment, assisted fertility clinics and commercial animal breeders have been using cryopreserved sperm successfully for artificial insemination. Since the development of in vitro fertilization leading to the first human birth in 1978 from a method originally developed in 19532, methods of fertility restoration have expanded from sperm cryopreservation to multiple routes for reproductive options involving not just sperm, but human embryos and oocytes also.
While this technology has dramatically impacted the lives of couples and single men and women, the applications for assisted reproductive technology have not reached their full potential, with the needs of many patients still unmet. The focus of assisted reproduction research in the late 20th century turned towards banking oocytes modeled after sperm banking and preserving human reproductive tissue, both testicular and ovarian. The simplicity and resiliency of sperm make them ideal candidates for cryopreservation. Chromosomal abnormalities, zona pellucida hardening, and parthogenesis of oocytes when cryopreserving more complex gonadal tissue have all made it clear at the beginning of the 21st century that the mechanisms of human gonadal function, as well as advanced techniques in cryopreservation must be explored in order for these goals to be met.
This paper will address individually each of these cryopreservation goals: oocytes, ovarian tissue and testicular tissue. There are some common obstacles that apply to these tissues: surface area, reproducing external signaling factors that occur in the native environment, and maintaining the structural morphology of these cells during and after cryopreservation so as to reduce time for revascularization and resumed function.3,4 Among these specializeed systems are thecal and granulosa cells of the ovary, and Sertoli and Leydig cells of the testis. Since neither prepubertal males nor prepubertal females are capable of producing mature gametes from their undeveloped sex organs, cryopreserving gonadal tissue for in vitro or in vivo maturation is necessary.5-10
Each section herein will also evaluate vitrification as a future technology when applied to cryopreserving these systems. While vitrification has been used for cryopreserving tissue and oocytes since the 1980's when Rall and Fahy published results using mouse embryos11, the normal trend in publications is still slow freezing using propandediol and glycerol or commercially available medium. Depending on the set of variables being tested, differing groups report an advantage to either technique. Toxicity, osmolality and permeability must all be taken into account when choosing cryoprotectant solutions for each system. Vitrification using alginate encapsulation of 3D cell systems in beads has produced strong results in maintenance of tissue structure and function, and represents a future direction for follicle and oocyte cryopreservation.12,13
Immature Oocyte Cryopreservation as a new direction for assisted reproduction
After proven success of embryo cryopreservation, there seems to be more focus placed on oocyte cryopreservation since the first recorded pregnancy brought to term from vitrified oocytes by Kuleshova et al. in 1999.14 Without the ability to cryopreserve oocytes, women without partners are left with the option of immediate in vitro fertilization and embryo cryopreservation using donor sperm. While it is possible to freeze oocytes and achieve fertilization, the success rates are lower than the success rate associated with frozen sperm, and the technique has many variables to consider, not the least of which is the smaller number of gametes available.
The recent effort into cryopreserving oocytes has shown a trend towards refinement of vitrification techniques. Groups have turned to vitrification as a possible way to permeate oocytes with cryoprotectant and replace internal water with a vitreous matrix.
In July 2006, a Cornell group published a statistical analysis of the efficiency of oocyte cryopreservation.15 They reported that pregnancy rates from transfer of vitrified oocytes increased significantly in the years compiled after 2005 (29.4% before June 2005 and 51% after June 2005). A comparison shows that the number of pregnancies and live births from vitrified oocyte transfers are close to those of control eggs, and there is no statistically significant difference between the two. In the year from June 2005 to June 2006, there were 51 clinical pregnancies and 31 live births resulting from vitrification.15
Many of the latest methods used for cryopreservation revolve around a technique that utilizes minimal volumes of cryoprotectants in order to maximize heat transfer and thus create a very rapid cooling environment. Among these are the Cryotop and Cryoloop methods. The Cryotop method, created by M. Kuwayama, consists of a thin transparent polypropylene film attached to a plastic handle with a cover straw. Oocytes can be loaded in a minimal volume of about 0.1 μL and subsequently plunged into liquid nitrogen. This method has been adopted among many promising research groups, and Oktay's group has recently evaluated its efficacy across multiple stages of oocyte development. Oktay's experiments achieved strong results in cleavage rates of embryos using intracytoplasmic sperm injection (ICSI), where in a 2008 compilation of results from this method, of 9,350 vitrified oocytes, 88% exhibited survival, of the surviving oocytes 81% fertilized and of those 44.8% matured to blastocyst stage.
Thus far, 406 babies have been delivered from 360 patients using this method.16 The Cryoloop method is another minimal volume method that uses an ultra thin loop at the end of a rod which is dipped into a cryoprotectant solution after permeation is completed. This creates a film of cryoprotectant solution upon which an oocyte can be placed. The loop is then plunged into liquid nitrogen for ultra fast cooling.
These methods, while also efficient, have been brought into question by the possibility of contamination from direct contact with liquid nitrogen. In an experiment to test liquid nitrogen as a vector for contamination, a contaminated tank was used to hold samples in both closed and open systems, showing that the open systems tested positive for contamination in 21.3% of cases17. Additionally, in open-systems used to preserve oocytes, the loss of the oocyte itself has also been a problem.17
Currently, our lab employs a system that has proven highly effective. The system uses closed vials containing enough of the final high-osmolal cryoprotectant used in the process to cover the material being vitrified, while still maintaining a volume that may be free of ice after rapid cooling. The closed system in our lab has been chosen to avoid contamination issues, as we have come to the conclusion that any method of vitrification must meet these standards regardless of effectiveness in order to move on to clinical applications. We have also not adopted the use of direct immersion into liquid nitrogen, as we rapidly cool samples to −100°C in less than 78 seconds, and then samples are left to further chill to the ambient temperature of −135°C in our ultra low temperature freezer, which occurs in the next minute. This method has been used to cryopreserve multiple cell systems, including cartilage tissue grafts, encapsulated human liver cell lines, and ovarian tissue slices. 18,19,20
As with the methods of vitrification, the conditions under which oocytes are retrieved are also being evaluated at great length. The approach towards oocyte cryopreservation has traditionally been used on mature oocytes, those with a haploid chromosome number and released from ruptured follicles after induced maturation. But this technique, if perfected, would still exclude women facing the immediate need for chemotherapy. Immature oocyte retrieval during the follicular phase is the only feasible method of allowing women to use in vitro fertilization (IVF) treatments without the use of hormonal stimulation before/during chemotherapy.
The preservation of immature oocytes for maturation before IVF is intended to counter the problem found in many studies on cryopreservation of mature oocytes, where it was suggested that disruption of the meiotic spindle of oocytes in arrested MII metaphase was a concern.17,21 New research from the past five years seems as though this point may be unfounded, though this finding still needs further support. A few groups have suggested the ability of the spindle to reform within only a few hours after thawing with incubation, with one of the earlier notations of this being made by S.U. Chen's group in Taiwan, and confirmed in multiple papers thereafter.22 In a 2004 European Journal of Reproductive Medicine paper compiling multiple data sources, Mandelbaum et al. presents a clear consensus that under correct conditions, the meiotic spindle is ultimately unharmed and the cells can divide without significant threat of aneuploidy.23 This data trend has continued throughout the late 2000's. Methods of identifying healthy spindle formation, such as the polscope system described by Rienzi et al. in 2005, suggest that selection of oocytes with healthy meiotic spindles for ICSI after re-warming may circumvent the problem entirely.23,24 The use of immature oocytes harvested without stimulation remains less studied despite the possible benefit to the patients. The key reason for this appears to be in vitro maturation (IVM) and its efficacy. Currently human case studies by Dimertas et al. and other prominent ART specialists at the McGill Reproductive Center in Montreal are providing data that will add to the figures for the retrieval of immature oocytes for IVM and cryopreservation.21 This technique is likely the future direction for the study of cryopreservation methods for immature oocyte banking.
Feasibility of cryopreserving encapsulated ovarian follicles
Attempts to remove an ovary for whole-organ cryopreservation have been met with success in animal models, yet a human model is lacking. Revascularization problems, permeation capabilities of cryoprotectants, and the complexities of folliculogenesis, has shifted focus from the macro to the micro. Jaques Donnez's group in Belgium reported in 2004 the first ever live birth from cryopreserved ovarian tissue in humans.25 Donnez's accomplishment was soon followed by a live birth from the group of Meirow et al., in 2005.26 Two live births have been attributed to patients who have received ovarian autotransplantation after cryopreservation.4
These two births were clearly medical milestones. Yet the very high degree of variables concerning the autograft procedure has led to mixed results when the process is repeated. Questions have been raised as to whether incidences of spontaneous resumption of ovarian function, including menstruation and follicular maturation, may be responsible for these pregnancies.4
The overwhelming evidence of extreme damage to natural hormonal function post cancer therapy must be reconciled with the variables of freezing damage to follicles, revascularization of the implanted tissue, implant-site efficacy and hormonal regimens. Typically, resumed menses after re-implantation takes place in a time that is between 8 weeks to 8 months post surgery, if at all. And, in the great majority of cases of resultant fertilization, aneuploidy is the most common result, with the pregnancy ending in miscarriage.
Vitrification of Ovarian Grafts
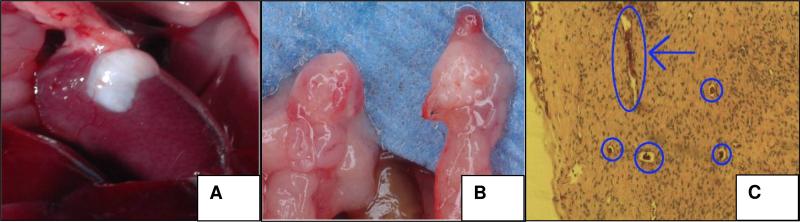
Thus far, freezing has been the method of cryopreservation for human ovarian tissue grafts. Vitrification has been explored in animal models. Our own studies on the use of implanted cortical tissue have yielded positive results in the animal model, like many other groups. 20 The relative complexity of human folliculogenesis is likely why these techniques often fail in the human model. In an attempt to move from the mouse, we conducted trials using sheep ovarian tissue xenographed into the mouse model. Fresh, vitrified or frozen sheep ovarian tissues were implanted into 20 Severe Combined Immunodeficiency (SCID) mice. The tissue was implanted beneath the renal capsule or in an orthotopic location (Fig 1a and b) and angiogenesis was demonstrated (Fig 1c). We chose the renal capsule as a grafting location due to its highly vascular nature and its ability to hold a graft in place. This graft site, while a good candidate, became problematic. Not only does the size of the kidneys limit the amount of tissue that can be transplanted, but the surgery is difficult and highly invasive for the animal. This led to a redesign of the grafting model and a new animal protocol submission that would remove variables of surgery survival and increase statistical significance.
Figure 1. The location of implantation in SCID mouse model.
A. fresh sheep ovarian grafting in kidney capsule. B. vitrified sample in orthotopic location (Right, the graft. Left, the mouse ovary). C. angiogenesis of ovarian grafts. Small blood vessels can be seen in cross section (circles) and longitudinal section (arrow). X20
Contentions over graft site efficacy concerning revascularization, chemical signaling and graft space have been present in most publications exhibiting similar work. We agreed with investigators that have used a subcutaneous graft site above the flanks in SCID mouse models.27 This site has been used to graft up to six 1-2mm3 tissue blocks on dorsal areas of the mouse, and has also been shown to allow for larger follicle growth due to its expanding nature.
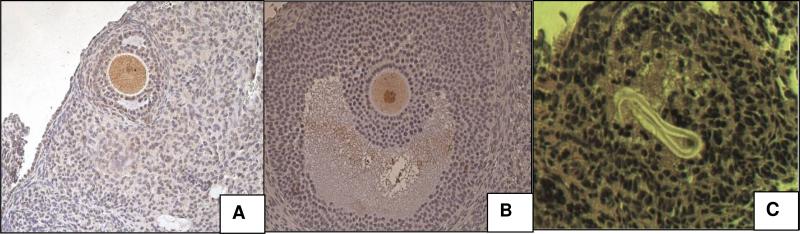
We have detected follicle growth in both conventionally cryopreserved and vitrified ovarian tissues without follicle stimulation. However, because of the difficultly in determining whether a follicle is progressively maturing or decaying using only H & E staining techniques, we used Proliferating Cell Nuclear Antigen (PCNA) and apoptosis (caspase-3 and CD-95) analysis to supplement our evaluation. PCNA is actively expressed by cells in G1/S-phase and can be used as a growth marker for developing follicles.28 Although there was follicle growth in frozen tissues, we found apoptotic oocytes of the explanted tissues (Fig 2 a and b). Follicle degeneration was found in frozen samples, which showed oocyte loss and presence of a “glassy membrane”(Fig 2c). The study indicated vitrification could be a feasible method for banking ovarian tissues, but we have come to find that conventional cryopreservation with commercially available media and slow freezing shows no statistical difference compared to vitrification in many cell cultures. Based on experience and the ever increasing number of successes in vitrification of complex tissue systems and oocytes, we do still believe that vitrification, when applied to complex tissue structures, does hold significant advantages over freezing.
Figure 2. Apoptosis assay in frozen sheep ovarian explants.
(A) Caspase-3 (B) CD-95 showed apoptotic oocytes in brown. There was no apoptosis in other cells. (C) Oocyte loss and “glassy membrane” presence can be seen in frozen samples, but not in the vitrified grafts. A&B, x200; C, X20.
Alginate Bead Encapsulation for Stabilizing Tissue Structure
In January 2009, articles by Min Xu et al. at Northwestern University (mouse model), 13 and the Belgian group of Amorim et al. (human model) reported the most recent work of a technique of alginate hydrogel encapsulation.29 Their work was preceded by that of Torrance, Telfer and Gosden who were studying the use of preantral mouse follicles in a collagen gel as early as the late 1980's.30 In 2005, Heise et al. showed across the board growth of preantral rat follicles (rat model) in calcium alginate beads using growth medium containing FSH.12 This ability is especially relevant in proving the efficacy of alginate encapsulation after Campbell et al. published in 2000 that despite different roles of FSH in animal models, FSH does modulate early folliculogenesis.31
In the study done by Amorim's group, human ovarian tissue was frozen in strips according to the slow-freeze method published by Gosden in 1994. For thawing, tissue was then transferred from cryovials to Petri dishes coated with MEM-Glutamax for in vitro culture. Tissue was enzymatically digested with collagenase and agitated, and preantral follicles were isolated under the microscope for encapsulation. They found using the human model and preantral follicles, and growth after seven days, follicles averaging 56.73±13.10 μm.
The Northwestern University study, used mechanical isolation of secondary follicles before cryopreservation. Mouse follicles were seeded at −9°C before being plunged into liquid nitrogen. Thawing was carried out by a stepwise osmolar gradient to remove cryoprotectants. Assessment of mouse follicles after 16 hours culture for presence of a polar body, indicated metaphase II oocytes. Follicles were grown to the secondary stage, producing follicles on day 12 averaging 324.2±60.2μm.
These studies showed that the alginate encapsulation maintained the integrity of granulosa layer cells as well as a thin layer of theca cells.
New Directions of Study Involving Alginate Encapsulation
In each experimental group differing cryopreservation methods, encapsulation periods and models determined growth rate and survival similar to fresh controls. Important to future research, the Northwestern study also concluded that follicles derived from frozen strips and individual follicles were able to maintain their structure in relatively equal proportions, and comparably to that of fresh individual follicles. This information suggests that cryopreservation of ovarian tissue is still useful for fertility assistance. It is clear from each paper that fine tuning the freezing process to human tissue, as well as understanding chemical factors involved in culture and maturation, will be necessary to further develop new therapies. Most current literature suggests that vitrification may be an advantageous technique, once refinements are made to protocol concerning exposure time, concentration and components of the solution.
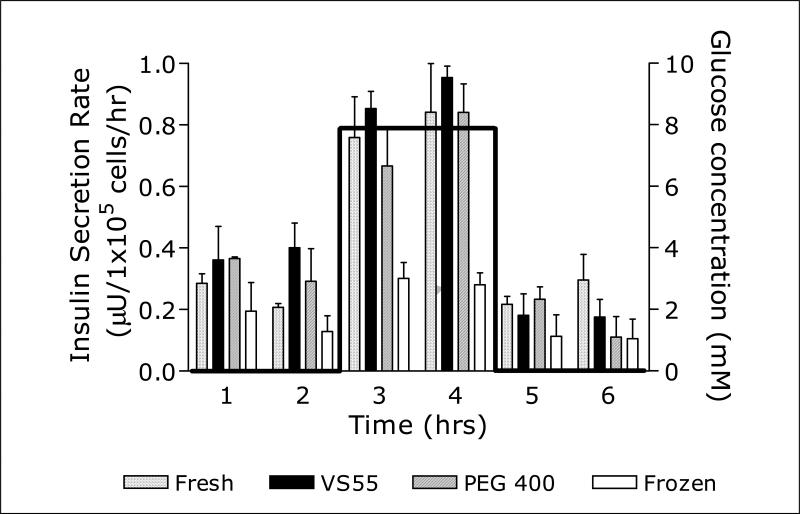
Maturation of encapsulated preantral follicles after vitrification to maintain the 3D form may be within reach. The preservation of surrounding granulosa junctions as well as theca cells may reduce damage caused by loss of intricate hormonal stimulation of the surrounding tissue, leading to fewer events of meiotic spindle malformation. Heise's work in 3D culture opens the door for further refinement, perhaps offering a method to sustain thawed follicles long enough to present mature oocytes. We have been working for some time with encapsulation of human liver cell lines using alginate hydrogel for vitrification, and the use of encapsulation to maintain the 3D structure of human follicles for maturation has great potential. When comparing this to results of vitrification of encapsulated insulin producing cells published after 2005, 19 we see that vitrification may allow mature stage follicles to survive when vitrified within alginate beads. Insulin secretion rates in liver cells vitrified and rewarmed showed significantly greater competence than frozen counterparts, and may allow mature follicles to be stored. (Fig. 3) In current studies people are preserving tissue first, then thawing and encapsulating. The future should see follicle encapsulation, cryopreservation, and then maturation to provide viable oocytes to meet the demands of a wide range of patients.
Figure 3. Insulin secretion rates from fresh, vitrified, and frozen beads containing HepG2 cells exposed to a square wave of glucose concentration.
To normalize for small variations in cell number per bead and volume of beads in each experiment, the highest secretion rate of each set of cultures studied in parallel was set equal to 1 and the other secretion rates were determined relative to that value. Bar graphs represent average secretion rates; error bars represent standard errors; n=6 for fresh, VS55, and frozen; n= 4 for PEG 400 [a polyethylene glycol (PEG)-based formulation, consisting of 5 M propanediol, 1M Me2SO and 12% PEG400]. Y Song et al., Transplantation Proceedings 37(2005)253-255.
Testicular tissue cryopreservation: ART for prepubescent males
As with the cryopreservation of human ovarian tissue, testicular tissue preservation is aimed at a sizeable yet unique cross section of the population, namely prepubertal boys who have not yet developed to the stage where spermatozoa are present in their ejaculate, with Thomson et al. reporting that 1 in 3 male childhood cancer patients fall into this category.32 Patients with low to intermediate probability of testicular involvement are good candidates for autografting procedures.33 Testis banking allows time to screen tissue samples to avoid reseeding cancer cells to the patients, and for patients with high risk of testis metastasis, testis banking and in vitro and in vivo spermatogenesis, ICSI and in vitro fertilization could be the options for fertility preservation.34,35
During the early to mid 1990's, studies by Brinster and Zimmermann discovered a process by which injected male germ cells may resume spermatogenesis. In their studies, after the injection of germ cells into the seminiferous tubules, one third of recipient mice showed colonization of spermatogenic cells.36 Since then, mapping the migration of spermatogenic stem cells during in vivo and childhood development has driven the study of male fertility restoration to seek better cell isolation methods and injection sites. There are questions that need to be addressed before this technology reaches clinical trials. Can the spermatogenic cells (pachytene primary spermatocyte and early spermatids) from cryopreserved testicular tissue proliferate at various phases of a complex differentiative processs?37 Are there sufficient type A and type B spermatogonia surviving in the basal compartment of the seminiferous epithelium after testis cryopreservation? and can surviving testis resume meiosis, fertilize, and develop to the late spermatid stage after in vivo or in vitro spermatogenesis?
Molecular signaling between spermatogenic cells and surrounding Sertoli cells is essential for the correct function and development of spermatozoa.38 Extracellular matrix environment is very important for survival of spermatogonium in long-term culture, 39 and poor survival rates (<37%) of frozen and thawed testis has been observed after in vitro maturation. The reason for the poor results of conventional cryopreservation has been linked to rupture of stroma and the cell-cell connections in the interstial tissue and inside the seminiferous tubules, mitochondria and chromosomal abnormalities, the pathogenesis of cytoplasm, and spermatogonia detachment from the basement membrane after thawing.5 Live offspring have been born to mice after transplantation of fresh, but not cryopreserved testicular cells and tissues.34 No cryopreserved and transplanted whole testis have been functional.40 Heterotopic grafting for the maturation of germ cells, of which there has been significant studies done, show the ability for spermatogenesis to occur in castrated host mice, hamsters and monkeys41 and in xenografted models. Sperm has been extracted after 27 weeks from ectopically xenografted porcine tissue placed in nude mice.35,42-44 This ability has also been shown in prepubertal cases, yielding enough sperm for possible ICSI.45 Though studies of this nature have been limited to the fresh and frozen tissue model, it is yet to be seen whether vitrification may better maintain tissue structure upon freezing, a factor that would enhance the potential of alternate site grafting.
In 2008 a French group of Milazzo et al. published a comprehensive report on the cryopreservation of testicular tissue based on slow freezing and rapid freezing methods, using propanediol and Me2SO.46 Tissue was frozen for histology and enzymatic digestion, and the results tested against cultured post-thaw cells for viability, morphology, testosterone assessment and DNA fragmentation. Cell viability significantly decreased in propanediol groups when compared to fresh groups across all freezing protocols. Significant statistical difference in Me2SO freezing groups only occurred in the “controlled rapid freezing” group. Morphologically, different freezing protocols reported varying degrees of deformation. This very comprehensive study points to an overwhelming advantage towards Me2SO using controlled rate freezing protocols for the overall reduction in freezing damage. This conclusion is based on observation of Leydig cells, Sertoli cells, apoptosis assay, proliferation of germ cells and seminiferous cord morphology.
Nonhuman primate studies have the same problem faced with human ovarian tissue transplantation. Occurrences of spontaneous recovery of spermatogenesis in autotransplantation procedures make it difficult to differentiate between spermatogenesis from transplanted tissue and native tissue.41 One alternative measurement of success has been explored by a few groups, and reported in a review published by Derek McLean whereby transgenic donor mouse tissue is injected into wildtype mice. Donor cells were given 1-4 months to colonize in the testes and the recipient mice were bred, allowing the phenotype of the resultant pups to determine efficacy.47 This method is somewhat time-consuming, but there are advantages. Given the short gestation period and large average litter of mice, it is both an inexpensive and visual determinant for transplanted germ cell differentiation and resumed spermatogenesis.
Vitrification studies have shown that with some effort to modify protocols and apparatuses, vitrification has proven an effective system when applied to ovarian tissue. There is evidence that the extracellular matrix and supporting cells that feed follicles and male germ cells may be critical to germ cell survival and germ cell function.48 Vitrification may hold the answer to keeping cell systems intact for greater viability. Testicular tissue is typically frozen in small pieces and then thawed for recovery of germ cells. It is here that vitrification may hold an advantage, by preserving the surrounding Leydig and Sertoli cells. Clinical pregnancies from vitrified blastocysts have been demonstrated,49 and promising results were shown in 2004. A group was able to show that vitrification resulted in a higher degree of germ cell integrity over standard egg yolk freezing in minced testicular tissue, and in microdiced testicular tissue (P<0.05), vitrification also yielded a higher number of intact germ cells with greater viability (P<0.05).50
Conclusions
The future cryopreservation of human reproductive tissue will have to address three target groups; women without partners, prepubertal males and prepubertal females, and those that cannot delay cancer treatment for IVF hormonal stimulation. Immature and mature oocyte banking presents options for preservation of female fertility through in vitro fertilization, however there are issues to resolve: cryoprotectant toxicity, freezing techniques, and avoidance of ice damage to meiotic processes. There are many methods for oocyte cryopreservation using both open and closed systems, and closed systems may be the simplest way to ensure sterility of samples at this point.
Maturation of oocytes in vitro after cryopreservation presents the best option for fertilization, since immature oocytes are more resistant to freezing stresses. How and when meiotic spindle reformation takes place after thawing remains a concern. Since there is strong evidence that spindle reformation takes place a few days after thawing, specific standards will have to be set in this regard before cryopreserved oocytes can be used.
So far, whole reproductive organ cryopreservation has shown promise in rodent models, however, due to size and complexity of human gonadal function, there have been no live births from cryopreserved whole ovary transplant in humans. Cryopreserving small sections of gonadal tissue has produced live births; however in the majority of cases the result is limited. In these techniques, the maintenance of cellular structure of and function of surrounding tissue layers is likely critical. Encapsulation of follicles in alginate beads is a leading technology for follicle growth. Cryopreservation of encapsulated follicles followed by thawing and culture while still encapsulated seems the most rational step for preserving 3D structure of follicles to produce more viable oocytes. Isolation of spermatogenic stem cells has made great strides, producing young in the animal model that are identifiable as the product of transplanted germ cells, or producing sperm from xenografted tissue. Encapsulation in alginate may also help preserve the structure of testicular tissue supporting cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polge C, Smith AY, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 2.Bunge RG, Sherman JK. Fertilizing capacity of frozen human spermatozoa. Nature. 1953;172:767–768. doi: 10.1038/172767b0. [DOI] [PubMed] [Google Scholar]
- 3.Gook DA, Osborn SM, Bourne H, Johnston WI. Fertilization of human oocytes following cryopreservation: normal karyotypes and absence of stray chromosomes. Human Reproduction. 1994;9:684–691. doi: 10.1093/oxfordjournals.humrep.a138572. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J. J, Martiniz-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Human Reproduction Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 5.Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Human Reproduction. 2005;6:1676–1687. doi: 10.1093/humrep/deh797. [DOI] [PubMed] [Google Scholar]
- 6.Bahadur G, Ralph D. Gonadal tissue cryopreservation in boys with paediatric cancers. Human Reproduction. 1999;1:11–17. doi: 10.1093/humrep/14.1.11. [DOI] [PubMed] [Google Scholar]
- 7.Kellerman J. Psychological aspects of childhood cancer. Charles C Thomas Publisher; Illinois, USA: 1980. [Google Scholar]
- 8.Nugent D, Meirow D, Brook PF, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Human Reproduction. 1997;3:267–280. doi: 10.1093/humupd/3.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM, Kramarova E, Draper GJ, Masuyer E, Michaelis J, Neglia J, Qureshi S, Stiller CA. International incidence of childhood cancer. II. International Agency for Research on Cancer; Lyon, France: 1998. IARC Scientific Publications No 144. [Google Scholar]
- 10.Spinetta JJ, Deasy-Spinetta P. Living with childhood cancer. The C. V. Mosby Company; St. Louis, Toronto, London: 1981. [Google Scholar]
- 11.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at –196°C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 12.Heise M, Koepsel R, Russell A, McGee E. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reproductive Biology and Endocrinology. 2005;3:47. doi: 10.1186/1477-7827-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Banc A, Woodruff TK, Shea LD. Secondary Follicle and Oocyte Maturation by Culture in Alginate Hydrogel Following Cryopreservation of the Ovary or Individual Follicles. Biotechnology and Engineering. 2009 Jan 6; doi: 10.1002/bit.22250. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A. Birth following vitrification of a small number of human oocytes: Case report. Human Reproduction. 1999;14:3077–3079. doi: 10.1093/humrep/14.12.3077. [DOI] [PubMed] [Google Scholar]
- 15.Oktay K, Pelin A, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertility and Sterility. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Kuwayama M, Leibo SP. Efficiency of the cryotop method to cryopreserve human oocytes; analysis of in vitro and in vivo results at eleven IVF clinics. Fertility and Sterility. 2008;90:s281–s282. [Google Scholar]
- 17.Jain JK, Paulson RJ. Oocyte Cryopreservation. Review article. Fertility and Sterility. 2006;86:1037–1046. doi: 10.1016/j.fertnstert.2006.07.1478. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee N, Li Y, Song Y, Long RC, Jr, Sambanis A. Cryoprotectant transport through articular cartilage for long-term storage: experimental and modeling studies. Osteoarthritis and Cartilage. 2008;16:1379–1386. doi: 10.1016/j.joca.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee N, Song Y, Sambanis A. Cryoprotectant delivery and removal from murine insulinomas at vitrification-relevant concentrations. Cryobiology. 2007;55:10–18. doi: 10.1016/j.cryobiol.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song YC, Chen Z, Journey C, Xie X, Emmi AM, Song RL. Vitrification of ovarian tissues. In: Tucker, Liebermann, editors. Vitrification in Assisted Reproduction. Informa Healthcare; London: 2007. [Google Scholar]
- 21.Dimertas E. Immature oocyte retrieval in the luteal phase to preserve fertility in cancer patients: Case report. Reproductive BioMedical Online. 2008;4:520–523. doi: 10.1016/s1472-6483(10)60239-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen SU, Lien YR, Chen HF, Chang LJ, Tsai YY, Yang YS. Observational clinical follow-up of oocyte cryopreservation using a slow-freezing method with 1,2-propanediol plus sucrose followed by ICSI. Human Reproduction. 2005;20:1975–1980. doi: 10.1093/humrep/deh884. [DOI] [PubMed] [Google Scholar]
- 23.Mandelbaum J, Anastasiou O, Lévy R, Guérin JF, de Laurouzière V, Antoine JM. Effects of cryopreservation on the meiotic spindle of human oocytes. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2004;113:S17–S23. doi: 10.1016/j.ejogrb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Rienzi L, Martinez F, Ubaldi F, Minasi MG, Iacobelli M, Tesarik J, Greco E. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Human Reproduction. 2004;19:655–659. doi: 10.1093/humrep/deh101. [DOI] [PubMed] [Google Scholar]
- 25.Donnez J, Domans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Van Langendonckt A. Livebirth after orthotopic transplantion of cyropreserved ovarian tissue. The Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 26.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. New England Journal of Medicine. 2005;3:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Fonseca H, Bosch P, Sirisathien S, Wininger JD, Massey JB, Brackett BG. Effect of site of transplantation on follicular development of human ovarian tissue transplanted into intact or castrated immunodeficient mice. Fertility and Sterility. 2004;81:888–892. doi: 10.1016/j.fertnstert.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulatiing hormone. Human Reprododuction. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- 29.Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Human Reproduction. 2009;24:92–99. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 30.Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87:367–374. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- 31.Campbell BK, Telfer EE, Webb R, Baird DT. Ovarian autografts in sheep as a model for studying folliculogenesis. Molecular and Cellular Endocrinology. 2000;163:131–139. doi: 10.1016/s0303-7207(00)00217-3. [DOI] [PubMed] [Google Scholar]
- 32.Thomson AB, Campbell AJ, Irvine DC, Anderson RA, Kelnar CJ, Wallace WH. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet. 2002;360:361–367. doi: 10.1016/s0140-6736(02)09606-x. [DOI] [PubMed] [Google Scholar]
- 33.Oktay K, Yih M. Preliminary experience with orthotopic and heterotopic transplantation of ovarian cortical strips. Seminars in Reproductive Medicine. 2002;20:63–74. doi: 10.1055/s-2002-23520. [DOI] [PubMed] [Google Scholar]
- 34.Hovatta Cryopreservation of testicular tissue in young cancer patients. Hum. Reprod. Update. 2001;7:378–383. doi: 10.1093/humupd/7.4.378. [DOI] [PubMed] [Google Scholar]
- 35.Orwig E. Kyle, Schlatt Stefan. Cryopreservation and Transplantation of Spermatogonia and Testicular Tissue for Preservation of Male Fertility. J Natl Cancer Inst Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- 36.Brinster L. Ralf, Zimmermann James W. Spermatogenesis following male germ-cell transplantation. Developmental Biology. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rubben H, Dhir R, Dobrinski I, Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum Reprod. 2006;21:384–389. doi: 10.1093/humrep/dei352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordhoff V, Schlatt S. The developing testis – physiology and pathophysiology. Karger publishers, series; Endocrine Development. Ed. Olle Soder, Basel, Karger: Present and future optiona for preservation of testis tissue and function; pp. 136–154, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Meistrich ML, Wilson G, Kangasniemi M, Huhtaniemi I. Mechanism of protection of rat spermatogenesis by hormonal pretreatment: stimulation of spermatogonial differentiation after irradiation. J Androl. 2000;21:464–469. [PubMed] [Google Scholar]
- 40.Yin H, Wang X, Samuel Kim S, Chen Huifang, Lin Tan Seang, Gosden Roger G. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Human Reproduction. 2003;8:1165–1172. doi: 10.1093/humrep/deg236. [DOI] [PubMed] [Google Scholar]
- 41.Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- 42.Dobrinski Ina. Germ cell transplantation and testis tissue xenografting in domestic animals. Animal Reproduction Science. 2005;1-4:137–145. doi: 10.1016/j.anireprosci.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Honaramooz A. Snedaker, Boiani M, Scholer H, Dobrinski I, Schlatt I. Sperm from neonatal mammalian testis grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 44.Nagano M, Patrizio P, Brinster R. Long-term survival of human spermatogonial stem cells in mouse testes. Fertility and Sterility. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 45.Gosden RG. Gonadal tissue cryopreservation and transplantation. Reprod. Biomed. Online. 2002;4(suppl 1):64–67. doi: 10.1016/s1472-6483(12)60014-5. [DOI] [PubMed] [Google Scholar]
- 46.Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Massé L, Mousset-Siméon N, Macé B, Rives N. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Human Reproduction. 2007;23:17–28. doi: 10.1093/humrep/dem355. [DOI] [PubMed] [Google Scholar]
- 47.Mclean J. Derek. Spermatogonial stem cell transplantation and testicular function. Cell Tissue Res. 2005;322:21–31. doi: 10.1007/s00441-005-0009-z. [DOI] [PubMed] [Google Scholar]
- 48.Goossens E, Geens M, De Block G, Tournaye H. Spermatogonial survival in long-term human prepubertal xenografts. Fertility and sterility. 2008;90:2019–2022. doi: 10.1016/j.fertnstert.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 49.Yoon TK, Chung HM, Lim JM, Han SY, Ko JJ, Cha KY. Pregnancy and delivery of healthy infants developed from vitrified oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertility and Sterility. 2000;74:180–181. doi: 10.1016/s0015-0282(00)00572-0. [DOI] [PubMed] [Google Scholar]
- 50.Neri QV, Feliciano M, Tanaka N, Palermo L, Schlegel PN, Palermo GD. Vitrification of testicular tissue is more gentle on germ cells. Fertility and Sterility. 2004;82(suppl2):S184. [Google Scholar]