Abstract
The study of song learning and the neural song system has provided an important comparative model system for the study of speech and language acquisition. We describe some recent advances in the bird song system, focusing on the role of offline processing including sleep in processing sensory information and in guiding developmental song learning. These observations motivate a new model of the organization and role of the sensory memories in vocal learning.
Keywords: birdsong, vocal learning, template, sensory memory, neuromodulator, speech, language
1. Introduction
Acoustic communication is broadly observed in all orders of vertebrates, including fishes, amphibians, reptiles, birds, and mammals. Aspects of vocal control in the vertebrates may share a common ancestry, such as basic spinal cord and brainstem circuits for call production that may have first evolved some 400 million years ago (Bass et al. 2008). In contrast, forebrain regulation and the emergence of learned vocalizations evolved independently multiple times in birds and mammals (Nottebohm 1972; Gahr 2000; Rendell and Whitehead 2001; Poole et al. 2005). Behavioral similarities between bird and human vocal development such as critical periods and use of auditory feedback for acquisition of vocal memories are well known (Marler 1970b). The modern reinterpretation of homologies between avian forebrain and mammalian neocortex (Karten 1991; Karten 1997; Reiner et al. 2004) further helps to draw parallels between the various vocal learning systems. For example, the identification of a striatal pathway in the songbird forebrain that is important for song learning and is homologous to elements of striatal pathways in mammals helps to interpret the avian research in a mammalian context.
The rich interplay between birdsong and speech and language research that was envisioned almost 40 years ago has emerged but only slowly. Initially, progress was impeded by a historical tendency to view human cognitive skills, especially speech and language, as beyond the purview of a biological perspective. This artificial divide is now rapidly disappearing, as progress in psychology and biology both using behavioral and neurobiological approaches identify commonalities between humans and other animals, and helps characterize quantitative, experimentally tractable differences between humans and other animals. For vocal learning, a series of behavioral studies demonstrated animal behaviors such as categorical perception or perceptual constancy, refuting early claims that these behaviors define unique features of human speech (Kuhl and Miller 1975; Kuhl and Miller 1978; Kuhl 1979; Kluender et al. 1987). More recently, a behavioral study demonstrated that starlings are capable of processing the syntactic structures of sequences of motifs (units of starling songs) defined by a simple context-free grammar (Gentner et al. 2006). Context-free grammars are defined through recursion, hence the starling behavior is a counterexample to the notion that recursive processing defines the heart of a uniquely human computational capability for language processing (Hauser et al. 2002). Given the weight of these animal examples, an alternative hypothesis is that human prowess in speech and language may emerge in part for reasons of heightened capacity, such as memory capacity associated with semantic recall (Nusbaum and Margoliash in press). The modern perspective recognizes that there are many attributes shared between animals and humans that contribute to vocal learning (Doupe and Kuhl 1999; Margoliash 2002). This perspective motivates anew the search for mechanisms of vocal processing that are common between animals and humans.
Vocal learning requires formation of memories of sounds, sequences, and higher order representations. How these memories are represented and how they influence vocal learning has been a principle focus of animal vocal learning (Konishi 1965; Konishi 1978; Konishi 2004), and could provide a focus for integrating research in animal and human systems. One feature of memory formation that has been extensively explored is the role of sleep. The prevailing hypothesis is that sleep influences learning through offline consolidation of recently acquired, labile memory traces. Sleep has been implicated in enhancing acquisition of simple rote motor sequences and visual discriminations (Karni et al. 1994; Stickgold et al. 2000), although there is some controversy as to whether sleep enhances learning in these low-level tasks (Rickard et al. 2008). Sleep also interacts with learning in a broad range of higher-level complex tasks that are characterized by generalization (Fenn et al. 2003), multimodal integration (Brawn et al. 2008), and cognitive processes (Wagner et al. 2004).
Does sleep interact with vocal learning? In this chapter we describe the role of sleep in developmental birdsong learning and in the maintenance of songs in adult birds. We describe state-dependent physiological activity in the song system, and the role of neuromodulators in state-dependent processing. We end with a brief discussion of a new model for the role of sleep in learning, and consider the future directions of animal studies in relation to speech and language.
2. Vocal Learning
Songbirds are endowed with a remarkable capacity for learning songs that have a high degree of acoustic and temporal complexity (Catchpole and Slater 1995). Many of these birds will learn their song as juveniles from their father or other males and will retain this song for the remainder of their lives. Such birds are generally referred to as closed-ended learners. Other species might learn one or more songs as juveniles but retain the ability to modify their existing song or learn new songs as adults. These birds are generally referred to as open-ended learners. In this chapter, we will focus our discussion on zebra finches, a close-ended learner, because of the wealth of behavioral and neural data that has been collected in this species. Despite the stereotyped nature of the song that these birds sing as adults, they nevertheless show a significant degree of vocal plasticity following perturbations that affect auditory feedback. Because sleep influences vocal plasticity in juveniles and adults, we review here the major features of song learning and adult vocal plasticity in this species.
a. Juvenile song learning
In zebra finches, as in many other species, song learning can generally be divided into three stages: subsong, plastic song and crystallized song. Subsong occurs at the earliest stage of singing and might develop out of the bird’s initial calling behavior, which starts soon after birds hatch. Both males and females will call, but as males mature, several of the calls they produce start to acquire more complex acoustic patterns and birds start producing these in a somewhat sequential manner (Liu et al. 2004). The production of these sequences of elements is defined as subsong and is not that different from babbling that is observed in human infants (Doupe and Kuhl 1999). Following the subsong stage, birds transition to a plastic song where acoustic elements start to resemble those in the tutor song but are still produced in a sequence that is variable. Song is defined as crystallized when syllable acoustic features and sequence are relatively stable and stereotyped. In zebra finches, the crystallized song is the song they will sing for the remainder of their lives (Immelmann 1969; Deregnaucourt et al. 2004). The exact timing of these different transitions in song development is somewhat variable and is influenced by numerous conditions in which juvenile birds are raised. These include circannual timing of birth, social factors, hormonal state, and nutrition (Kroodsma and Pickert 1980; Baptista and Petrinovich 1986; Nowicki et al. 2002). In normally laboratory raised zebra finches subsong typically begins around 25-30 days post hatch, plastic song around 50 days and song crystallization between 90 and 120 days (Immelmann 1969; Tchernichovski et al. 2004; Roper and Zann 2006).
Exposure to a song model, either from a live tutor (e.g. father or other male) or song-playback, typically results in the production of a good imitation of the tutor song. For learning to occur, birds only need to hear the song template during a short critical period in their life suggesting that auditory encoding of the song template and the process of vocal imitation are two separate events (Marler 1970a; Slater et al. 1988). This critical period for song template acquisition typically extends, in zebra finches, from about 25 to 65 days after hatching. As with recent work in other systems (Ostrovsky et al. 2006), this critical period is not predetermined and can be stretched or compressed depending on social and other environment factors (Slater et al. 1988). When juvenile birds are raised in the absence of a song model, they will eventually develop a song with some species-typical characteristics, but these “isolate songs” bear little superficial resemblance to the normal songs of their conspecifics (Immelmann 1969). Aspects of isolate songs such as overall duration, song tempo, and rhythm share similarities with the normal song implying that certain temporal features might be innately encoded (Rose et al. 2004).
In addition to the obvious necessity of hearing the song template, birds also need to hear themselves sing (Konishi 1965; Price 1979). Birds deafened as juveniles, even if this occurs after exposure to the song template, produce songs that are highly abnormal and bear little to no superficial resemblance to the song of their conspecifics or the song template, although they can retain species-typical characteristics (Marler and Sherman 1983). In fact such “songs” are much more abnormal than those produced by birds raised in isolation. Once birds have crystallized their song, in more species than was previously appreciated, auditory feedback is still necessary for song maintenance, even in critical-period learners such as zebra finches (Nordeen and Nordeen 1992). The dependency of song maintenance on auditory feedback appears to be age-dependent because birds with recently crystallized songs are much more affected by deafening than older more mature birds (Lombardino and Nottebohm 2000). The variation across songbird species in the reliance on auditory feedback for adult song maintenance (Konishi 1965; Fernando Nottebohm 1968) may be a fruitful avenue to explore the mechanistic factors associated with maintaining high levels of vocal plasticity in adults, such as in humans.
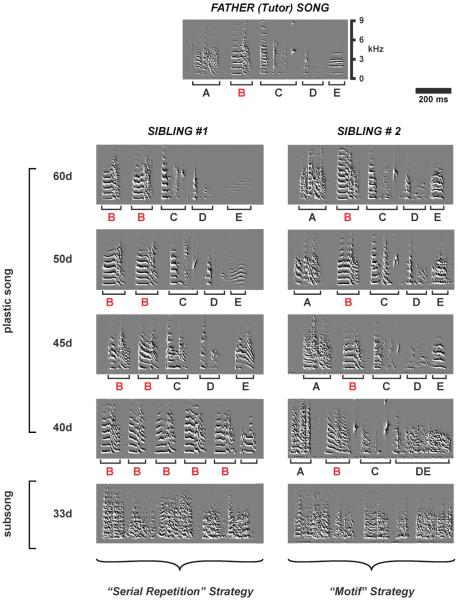
Upon exposure to the song template, birds must learn to produce accurate acoustic matches of each individual element or syllable in the song as well as learn to sing them in the correct sequence. Contrary to what might be expected, normally raised and tutored juvenile songbirds do not use a single strategy to achieve this goal (Liu et al. 2004). Even among siblings from a same clutch, some birds will choose a “serial repetition strategy” while others will opt for a “motif strategy” (Figure 1). Birds that choose the serial repetition strategy produce a single precursor syllable that they repeat in long sequences. With time, they modify the acoustic features and duration of each of these syllable precursors, eventually producing them in a sequence that matches that of the template. Birds that use the “motif strategy”, on the other hand, start very early on to produce a global imitation of the father’s song with each element already in the right place in the motif but produced in a very noisy and imprecise way. With time birds refine the acoustic properties of each element without any major modifications of the sequence. Similar differences in vocal imitation strategies appear to occur in humans as well (Macken and Ferguson 1983; Menn and Stoel-Gammon 2001).
Figure 1. Learning the same song using different developmental strategies.
Song development is shown for two juvenile zebra finch siblings (sibling #1 and sibling #2) from the same clutch. Both birds mastered the same 1-second long song motif they heard from their father. The highly stereotyped song motif of the father (labeled “Father (Tutor) Song”) is shown at the top and consists of five different syllables, identified by letters A through E. Sibling #1 utilizes a “serial repetition” strategy where he transitions from subsong to a song that consists of repetitions of the same syllable (shown in red). He then modifies each syllable so that they eventually match the different syllables produced in the father’s song. Sibling #2 on the other hand, transitions from subsong by producing a global imitation of the father’s song with each element already in the right place in the motif but produced in a very noisy and imprecise way. With time he refines the acoustic properties of each element without any major modifications of the sequence. The vertical axis corresponds to frequency, in kilohertz, and the horizontal axis corresponds to time, in msec. (Modified from Figure 3 in Liu and Nottebohm (2004) PNAS 101: 18177 - 18182)
Much understanding of the acoustic refinement strategies in songbirds has been obtained from Tchernichovski and colleagues who have used a highly quantitative approach to study the vocal imitation process (Tchernichovski et al. 2000). Using a simplified song-tutoring paradigm where they expose naïve male juveniles in the subsong stage to limited repetitions of a tutor song (as little as 20 times/day), they are able to track and quantify many of the vocalizations the bird produces. This technique allows them to “go back in time” from each crystallized song syllable all the way near to the onset of song tutoring and the first syllable precursor, and provide a much more complete description of the developmental trajectory of each acoustic element than had previously been achieved. Interestingly, birds tutored with this paradigm use primarily the “serial repetition strategy” because they very rapidly (within 24 hours from tutoring onset) transition from producing subsong, which has little acoustic or temporal structure, to producing a single syllable precursor that they repeat in long sequences. Within just 3-4 days of tutoring onset, however, out of this single precursor, the bird develops 3 to 5 acoustically unique elements, which will then eventually become the specific syllable in the crystallized song. Much of what has been learned of this massive and rapid acoustic transformation is that changes often occur in a highly non-linear manner (Tchernichovski et al. 2001).
b. Adult vocal plasticity
Many species of birds show a significant amount of vocal plasticity as adults. The so-called “open learner” birds, such as the canary, are able to learn new songs each breeding season with songs becoming richer and the repertoires more complex with each year. Many of the “closed learners” such as the zebra finch, were assumed initially to not show any vocal plasticity once they crystallized their song (Konishi and Nottebohm 1969; Slater et al. 1988). A number of recent studies have shown that as a generalization, this is incorrect. While adult zebra finches can no longer learn new songs, they certainly show a high degree of acoustic plasticity, both spontaneously (Kao and Brainard 2006) as well as under experimental conditions when auditory feedback is modified (Nordeen and Nordeen 1992; Williams and McKibben 1992; Leonardo and Konishi 1999; Tumer and Brainard 2007).
Male zebra finches produce a single song as adults but they produce them under two different behavioral contexts (Sossinka and Boehner 1980). When singing directly to females, they deliver the song very rapidly and sing a song that is highly stereotyped. Males will also engage in singing bouts when they are alone or in the presence of other males. These “undirected songs” are delivered with a slightly slower tempo and show a greater deal of acoustic variability than “directed songs”. Recent work in Bengalese finches (Tumer and Brainard 2007), a closely related species to the zebra finch, suggests that the observed variability in fundamental frequency (FF) of individual syllables during “undirected song” might be part of vocal exploration strategy that can lead to long-term changes in the acoustic features of the song. Of significance in the context of adult vocal plasticity, the authors of this study can cause an adaptive shift of the FF by altering the auditory feedback the bird hears while singing. This auditory feedback-dependent adaptive change underlines the degree of vocal plasticity that is possible in adult birds. It is also consistent with a number of studies showing that long term manipulations of auditory feedback, either by deafening (Nordeen and Nordeen 1992) or perturbation of auditory feedback (Williams and McKibben 1992; Leonardo and Konishi 1999; Hough and Volman 2002), can lead to dramatic re-arrangements of the bird’s song. These changes include acoustic changes of individual syllables but also more profound temporal rearrangements of the song that include syllable insertions, syllable repetitions and deletion of existing syllables. These changes are likely to be adaptive because they can, for nearly each manipulation described above, be prevented by the ablation of dedicated striatal pathways in the song system (Brainard and Doupe 2000).
3. Sleep and vocal learning
a. Sleep-dependent circadian singing and song tutoring
Serial repetition and motif strategies are large-scale patterns that are expressed over the 60-day duration of vocal development. Systematic variation in song patterns is also observed over much shorter time scales, and analysis of circadian patterns of singing has given additional insight into the mechanisms of song learning (Deregnaucourt et al. 2005). Exposure to tutor songs initiates a process that is characterized by two principle features. First, birds start to imitate aspects of those songs. This can be quantitatively characterized as a long-term developmental trajectory from less complex songs early in development to more complex adult songs (Tchernichovski et al., 2001). Second, birds develop circadian aspects in their song patterns, producing songs with relatively low complexity in the morning when compared to the songs they produce in the afternoon. This pattern repeats each day, so that songs in the morning are less complex than songs of the preceding or following afternoon. Birds who are never exposed to tutor songs (“isolate” birds) fail to develop this circadian pattern of singing. Birds whose exposure to tutor songs is artificially delayed until later in life will continue to develop isolate-like songs without exhibiting the circadian patterns, but once they are exposed to tutor songs then the imitation process begins and the circadian patterns emerge. To date, these circadian patterns have been observed only for juvenile zebra finches that develop song via the serial-repetition strategy. Whether this pattern also obtains for birds that adopt the motif strategy, and for other species of birds, remains to be determined.
The daily variations in singing behavior are not dependent on time of day but are driven by sleep-specific processes (Deregnaucourt et al. 2005). For example, birds that are prevented from singing in the morning will exhibit the deterioration and recovery when they are permitted to sing later in the day, and the phenomenon is also observed after birds awake from ectopic periods of sleep in the afternoon that are artificially induced by drugs. Furthermore, the magnitude of the daily variation in song quality is positively correlated with the final quality of the copied song - birds that exhibit the greatest change in song structure each morning are the birds that will ultimately achieve the best song copying (Figure 2). This observation establishes that these sleep-dependent processes are adaptive and help to shape song learning. Of course, it is important to note that these trends are assessed across populations of birds, with individual birds showing considerable variation around these trends.
Figure 2. Developmental manifestation of a circadian singing pattern following tutor exposure.
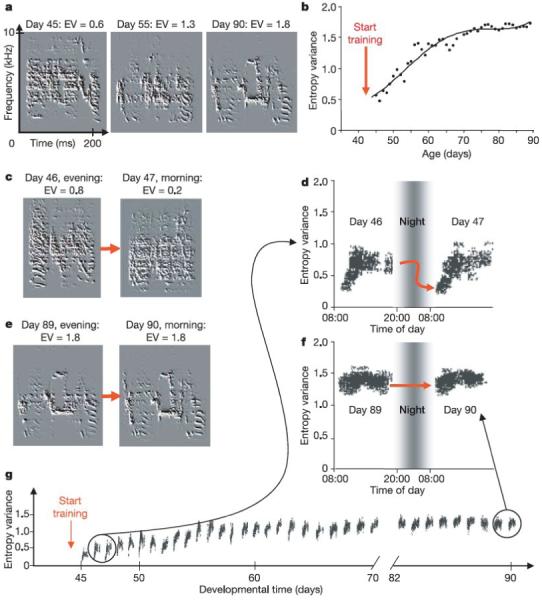
(a, b) Song acoustic structure increases incrementally following tutor song exposure. Acoustic structure is quantified using a measure known as Wiener entropy variance (EV) where high values represent more structure and low values less structure. Changes in EV throughout development are shown in (b). Examples of the same syllable with their corresponding EV values are shown in (a). (c - g) Using the same measure, it is possible to quantify the changes in syllable acoustic structure that occur within a given day. In juvenile birds, there is a dramatic drop in EV that is observed between syllables produced in the evening and those produced the following morning (c, d). This drop in acoustic structure is not observed in adult birds that sing a stable song (e, f). In figures d and f, each point represents the EV value measured for each rendition of this sample syllable. In addition to the marked drop in EV that occurs from one day to the next, there is also a steady increase in EV throughout the day. EV values measured at the end of the day show a steady increase throughout development (especially between days 45 and 60) (g). The degree of circadian change in EV is directly correlated with the quality of the vocal imitation (not shown). (Modified from Figure 3 in Deregnaucourt et al. (2005) Nature 433: 710-716.)
b. Ontogeny of circadian singing
To investigate how soon after the onset of tutoring the circadian variation in singing appears, one can use the same tutoring paradigm but with an analysis procedure that permits songs to be analyzed earlier in song development, even on days prior to tutor song exposure. To test the hypothesis that birds might begin to alter some aspects of their singing on the actual day of tutor song exposure, different subsets of songs sung on the first day are directly compared to those sung the day after tutor presentation. These subsets consist of songs sung immediately after each of the 20 presentations of the tutor song, the top 5% most complex songs sung that day, and finally a subset of songs sung late in the day after all 20 tutor songs are presented. For all three measures, there is no evidence that birds begin to change their songs on the day of tutor song exposure, and yet there is clear change in singing behavior starting the following day (Shank and Margoliash 2009). This result further focuses attention on the hypothesis that during sleep, a memory trace of the tutor song drives changes in vocal-motor networks that are expressed as changes in singing behavior the following morning.
Similar to juvenile birds learning to sing, adult zebra finches rely on auditory feedback to maintain their songs. This motivates the hypothesis that sleep in adult birds might play similar roles as in juveniles, under the assumption that similar plastic mechanisms are engaged in juvenile and adult birds. One prediction is that adult birds exhibit circadian variation in song structure. The magnitude of the circadian variation in song structure, however, decreases throughout song development to the point that it cannot be detected at the time of song crystallization and in adulthood. It remains unknown if this implies that the phenomenon is extinguished or simply diminished in adulthood. Small-magnitude ultradian patterns of song production (systematic variation in duration and timing) have been detected in adult birds, but these have not been directly tied to auditory feedback mechanisms of adult song maintenance (Chi and Margoliash 2001; Glaze and Troyer 2006). If an adult zebra finch is deafened, however, then the change in syllable structure as song deteriorates is similar to that seen in normal circadian syllabic deterioration in juvenile birds (Deregnaucourt et al., 2005). A parsimonious explanation of these observations is that the sleep-dependent variation of song structure is also expressed in adult birds, but with too small a magnitude to be detected with current techniques. It would be interesting to search for circadian variation in vocal production in species that exhibit more profound vocal plasticity in adulthood, which might be correlated with a larger magnitude of circadian rhythm. This represents an attractive opportunity for seeking interesting new correlates between human and songbird vocal behavior (Margoliash 2003).
If first exposure to a tutor song leads to changes in singing behavior that are delayed to the following day, it implies that physiological processes that are expressed during sleep induce changes that result in adaptive responses the following day. During sleep, brain regions are reactivated in humans (Peigneux et al. 2004; Rasch et al. 2007) and specific patterns of neural activity are replayed in rats (Nadasdy et al. 1999; Ji and Wilson 2007) that resemble those observed in prior waking behavior (Hennevin et al. 2007). Reactivation/replay has been linked to memory consolidation, which may involve transferring information from one brain region to another (Buzsaki 1998). The specific information represented in replay, and how plastic changes at night and during the day interact, remains unknown. To investigate these questions requires a behavioral-physiological approach, as described below for zebra finches.
c. A possible role of napping and song learning
Songbirds, like many small diurnal animals, frequently engage in daytime naps and frequently briefly awake throughout the night. Songbirds, especially juveniles, therefore transition relatively frequently from periods of active engagement, such as when they listen to their tutor or when they vocalize, to off-line periods where they no longer engage the outside world. Switching to these offline sleep states completely changes the physiology of the brain circuits involved in vocal production (see below). During the height of the song-learning process, a typical juvenile bird will therefore transition many times a day into these very different brain states. In humans, napping is sufficient to promote sleep-dependent learning (Mednick et al. 2003), and in juvenile songbirds, an artificially imposed period of daytime sleep released an additional bout of daytime song degradation/recovery (Deregnaucourt et al. 2005). Thus, the high frequency of wake to sleep transitions during the day is therefore likely to contribute significantly, in concert with nighttime sleep, to the process of vocal learning.
The question of how sleep might contribute to offline learning is likely of fundamental importance to our understanding of vocal learning in both songbirds and humans. Of similar importance might be to understand how the sensory experiences (such as tutor song exposure) that drive vocal learning might also drive the transition into the sleeping states that might be crucial for such learning. If juvenile birds have relatively immature auditory systems, then any auditory memory that results from tutor song exposure may be relatively poorly formed and labile, so that a bout of sleep immediately after song exposure might be particularly beneficial for consolidating that memory.
This is an area that remains poorly investigated but preliminary findings in songbirds suggest that such a link may exist (T. Lints and O. Tchernichovski, unpublished results). Specifically, when juvenile birds are tutored with song that they have never heard before, there is a tendency for juveniles to transition from a highly alert wakeful state to one that resembles a sleep-like state of stupor just minutes after exposure to that new song. This sleep-like state lasts several minutes, after which the birds wake up and become fully alert again. The reliability and behavioral significance of this phenomenon remains to be established, and may in any case be exaggerated by the isolate rearing conditions of the birds, so that the tutor song represents a supranormal stimulus (Tinbergen 1951). Nevertheless, these results suggest a novel flow of information: perception of an ultra-salient signal (here a conspecific song that the bird will use as its template) can cause behavioral state changes in service of memory consolidation.
d. Sleep staging in birds
Mammalian studies have benefited from extensive knowledge regarding the organization of sleep in mammals. The structure of sleep has been examined in multiple species of birds (Campbell and Tobler 1984; Rattenborg et al. 2002), but the surprisingly few studies in songbirds suggest a pattern of sleep that is different from what is seen in other orders of birds (Low et al. 2008). The complex structure of songbird sleep was recently confirmed with an extensive analysis of electroencephalograms in sleeping zebra finches that identified three stages of sleep including slow wave sleep, intermediate sleep, and rapid eye movement sleep. These mammalian-like features of sleep in zebra finches also extend to ultradian variation in these stages across the night (Low et al. 2008). These data pose the interesting question as to how such complex sleep patterns could have evolved in passerines and mammals, that are distantly related (Rattenborg 2006).
4. The avian song system
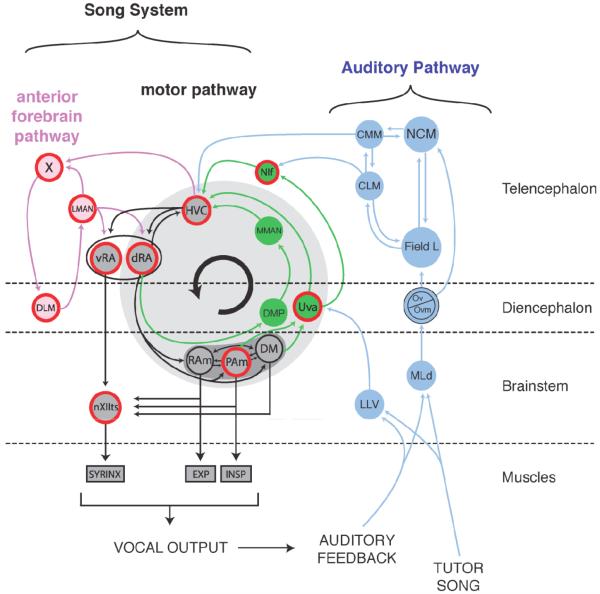
Song learning requires an auditory circuit and a motor circuit, and these should be functionally linked (Figure 3). In the avian forebrain, the ascending auditory pathway includes the thalamic nucleus ovoidalis and its projection to the telencephalic Field L complex. Field L comprises multiple subdivisions, which are often reciprocally innervated with each other and with a number of secondary forebrain auditory nuclei (Fortune and Margoliash 1992; Wild et al. 1993; Vates et al. 1996). Molecular and physiological studies have established that memory traces of songs learned as juveniles and as adults likely are stored in these secondary nuclei (Terpstra et al. 2004; Bolhuis and Gahr 2006; London and Clayton 2008). These sensory representations have interesting dynamics associated with learning (Chew et al. 1995; Phan et al. 2006) but the specific representation of the song template acquired during juvenile song learning remains unknown. One possible form of the template would be a specific locus with a high concentration of cells with tutor-song selective response properties. This has yet to be described, and it remains possible that cells in multiple nuclei are modified during sensory acquisition, leading to a template representation that is distributed over multiple nuclei.
Figure 3. Schematic representation of the avian song system and its auditory inputs.
The avian song system can be divided into three main divisions. The descending motor pathway (shown in black) includes telencephalic areas HVC and RA as well as brainstem nuclei that drive the muscles of the syrinx (nXIIts) or the respiratory system (RAm and PAm). These later two structures form part of a vocal respiratory network that also includes DM. The second division, sometimes called the ventral motor pathway, consists of projections from the diencephalon and brainstem back to HVC (shown in green). The third major division of the song system consists of the anterior pathway (shown in light red), which is made up of Area X, DLM, and LMAN. The song system receives processed auditory information from an ascending auditory pathway (shown in blue). Areas where BOS-selective responses have been recorded are outlined in red. Anatomical names: DLM, medial part of the dorsolateral thalamic nucleus; LMAN, lateral magnocellular nucleus of the anterior nidopallium; Field L is the primary auditory forebrain structure in birds; Area X, Area X of the medial striatum; NIf, nucleus interfacialis of the nidopallium; RAm, nucleus retroambigualis; PAm, nucleus paraambigualus; DM, dorsomedial nucleus of the intercollicular complex; CMM, caudal medial mesopallium; CLM, caudal lateral mesopallium; Field L, auditory forebrain areas consisting of Field L1, L2, L2a, L2b and L3; Ov/Ovm, nucleus ovoidalis; MLd, dorsal lateral nucleus of the mesencephalon; NCM, caudal medial nidopallium; LLV, ventral nucleus of the lateral lemniscus.
A series of pathways distinct from the auditory pathways, but receiving input from the auditory pathways, are collectively known as the song system. One simple starting point that can help orient the reader is to consider nucleus RA (see Figure 3 for terminology). RA represents the sole forebrain output of the song system, giving rise to a series of descending projections to midbrain and brainstem nuclei involved in motor control of the syrinx and in respiratory control. RA receives inputs from HVC (and a subset of RA cells project back to HVC; (Roberts et al. 2008)), and both RA and HVC participate in moment-to-moment control of singing in adult birds. HVC in turn receives from the nuclei NIf and Uva in the forebrain, and Uva receives from some of the brainstem structures that RA projects to, forming a loop whose functional significance is under active investigation (Schmidt and Ashmore 2008). This pathway is sometimes referred to as the ventral motor pathway (VMP). HVC also receives additional inputs, from the auditory pathways (Fortune and Margoliash 1995; Bauer et al. 2008).
In addition to RA, HVC also projects to the striatal component of the song system, Area X, part of a three nuclei pathway that projects to RA via it’s output, LMAN. This anterior forebrain pathway (AFP) is functionally distinct from the VMP. The AFP has been implicated as important in juvenile song learning, early song production, and adult song maintenance, but not in large-scale moment-to-moment regulation of vocal output in adults (Bottjer et al. 1984; Brainard and Doupe 2000; Aronov et al. 2008). This pathway shares many similarities with the basal ganglia-thalamo cortical pathways that play an important role in sensorimotor learning in mammals (Pearson et al. 2008).
The song system is also a target of an array of neuromodulatory pathways that arise from brainstem, basal forebrain, and hypothalamic nuclei, and release a diversity of transmitters (e.g. Li and Sakaguchi 1997; Appeltants et al. 2000; Ball et al. 2003). The histochemical properties, anatomical organization, and patterns of connectivity of these systems are highly conserved among vertebrates, and are homologous in birds and mammals (Reiner et al. 2004; Jarvis et al. 2005). Relatively few studies have characterized the electrophysiological properties of these pathways in avian species in vivo, however where examined, behavioral pharmacological experiments are generally consistent with broadly shared functional properties with mammals (e.g. da Silva et al. 2008; Gibbs 2008). These pathways regulate behavioral state including sleep, and are poised to profoundly influence song-related neurophysiology and behavior.
Many techniques have been applied to investigate the functional organization of the song system, but perhaps the most powerful has been the use of single cell electrophysiology in behaving animals in the context of controlled behavioral conditions. This has been applied in the context of auditory processing and singing (Yu and Margoliash 1996; Dave et al. 1998; Dave and Margoliash 2000; Hahnloser et al. 2002; Leonardo and Fee 2005; Kozhevnikov and Fee 2007; Keller and Hahnloser 2009; Shank and Margoliash 2009). As we describe below, it also gives powerful insights when applied to sleeping birds.
5. Vocal replay during sleep
During singing neural activity in many of the forebrain song control nuclei is precisely time locked to specific acoustic features of individual syllables in the song. In fact, in HVC this precision is so extreme that individual neurons that project to RA produce only a single burst of 3-4 action potentials at the exact same time in each motif (Hahnloser et al. 2002; Kozhevnikov and Fee 2007) (see Fee chapter for more detail). The precision of this song related activity is maintained in nucleus RA where neurons produce several precisely timed bursts during each motif (Yu and Margoliash 1996; Chi and Margoliash 2001; Leonardo and Fee 2005). The temporal precision of the neural output in both HVC and RA is absolutely remarkable. Typically, bursts in these structures are time-locked to syllable-specific acoustic features of the song with an overall timing jitter across song rendition that is less than 1 ms (Chi and Margoliash 2001; Leonardo and Fee 2005; Kozhevnikov and Fee 2007). The source of this timing is likely to arise within HVC (Long and Fee 2008).
a. Replay of song-like motor activity during sleep
The precise and stereotyped nature of song-related neural activity offers a tremendous advantage to probe for replay of vocal motor activity during off-line states like sleep. Neural activity in RA in particular is ideally suited because the bursting pattern recorded during the production of a song motif provides a “neural fingerprint” to probe for spontaneous activity patterns during sleep that resemble premotor activity (Dave and Margoliash 2000). When a bird falls asleep, RA activity, which is remarkably tonic and regular in nature in awake birds during quiescent non-singing periods, begins to express transient patterns of phasic activity. Taking advantage of the ability to maintain well-isolated recordings from single neurons, it has been possible to record the unique song-related neural pattern from a single neuron in a singing bird and then probe for the same patterns in that same neuron when the bird is asleep (Dave and Margoliash 2000). Using this approach spontaneous activity patterns can be identified in sleeping birds that match with high statistical significance portions of the unique stereotyped neural patterns recorded during singing (Figure 4a). These experiments are technically challenging, and to date direct comparison of the activity of the same neurons recording during singing and during sleep has only been reported for RA neurons in zebra finches. Nevertheless, the coordinated bursting activity observed during ongoing discharge and in response to song playback in anesthetized birds (e.g. Margoliash 1986; Williams and Vicario 1993; Sutter and Margoliash 1994; Hahnloser and Fee 2007; Ashmore et al. 2008; Hahnloser et al. 2008), and comparisons of ongoing discharge and auditory responses in sleeping birds (Chi et al. 2003), suggest the possibility that the entire song motor circuit replays song-related activity during sleep. Judging from the limited RA data, any one neuron only experiences partial snippets of an entire song during a replay bout. This suggests that there is a complex temporal dynamics of song replay at the population level that has yet to be described.
Figure 4. Comparing ongoing neuronal activity during sleep and during singing in an adult zebra finch.
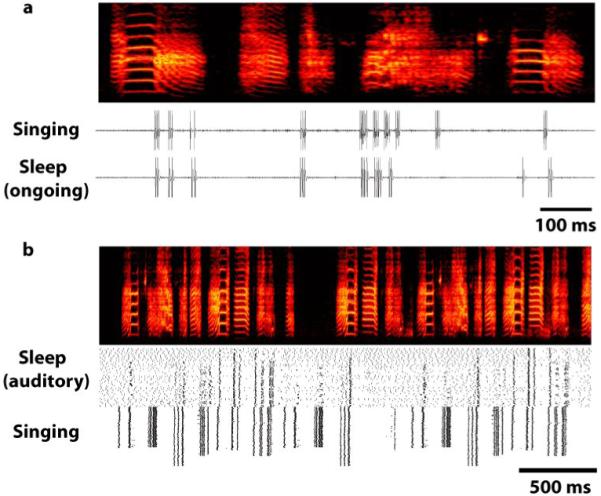
a) Neuronal trace of an RA neuron emitting 10 distinct bursts of 2 - 7 spikes per burst (“Singing”). The bursts are precisely timed to when the bird sang a song whose motif consisted of a sequence of five syllables (see spectrograph, frequency vs. time representation; top). For each song bout, the sequence of syllables and the structure of each spike burst (timing of spikes and numbers of spikes) was highly reliable. b) The recording was maintained for several hours until the bird was deeply asleep. During sleep, occasionally the neuron emitted one or more bursts. Individual bursts, and sequences of bursts, had far greater variability than during singing, but nevertheless sleep bursts matched bursts emitted during singing. In the example shown (“Sleep, ongoing”), the neuron spontaneously emits nine distinct bursts of 1 - 4 spikes per burst. The structure and timing of many of the bursts are remarkably similar but distinct from the equivalent bursts during singing. (Modified from Dave & Margoliash 2000)
b. Auditory representation of song in sleeping birds
In addition to song-related motor activity, neurons in HVC and RA also respond to auditory stimuli. In sleeping, as well as in anesthetized and sedated zebra finches, the majority of auditory responses in HVC respond selectively to the passive presentation of the bird’s own song (BOS) (Margoliash 1983; Theunissen and Doupe 1998). These neurons therefore typically respond very little to other auditory stimuli such as tones, white noise stimuli, conspecific song or BOS played in reverse order. This is in contrast to neural responsiveness in awake zebra finches where HVC auditory responses are more variable and sensitive to the level of arousal, BOS does not always elicit the strongest (or even strong) responses, and neurons typically show much broader auditory tuning (Cardin and Schmidt 2003; Rauske et al. 2003). This decrease in selectivity during wakefulness might not be a universal feature because BOS-preferring responses have been observed during wakefulness in other songbird species (Margoliash 1986; Prather and Mooney 2008). In juvenile zebra finches where song changes from day to day, and even in adult birds where song shows slow changes over time, sleep responses in HVC are strongest to the presentation of song variants that are produced immediately before the time of the neural recording (Volman 1993; Nick and Konishi 2005a). This shift in auditory tuning suggests that song production shapes the selectivity of neural responses recorded in sleeping birds (Nick and Konishi 2005b).
Using the same strategy used to compare spontaneous activity during sleep with that recorded during singing, it is possible to compare the song-related motor response in the singing bird to that elicited in the sleeping bird by passive presentation of the song the bird produces just hours earlier. Such a comparison reveals that the neural response patterns recorded during the presentation of BOS in the sleeping bird (“BOS-sleep”) are strikingly similar to those recorded in the singing bird (Figure 4b). There are some differences however. A given neuron will be recruited for more syllables during singing than during BOS-sleep, and for each burst singing tends to elicit more spikes than does BOS-sleep (Dave and Margoliash 2000). A simple interpretation is that BOS playback does not drive the system as vigorously as does singing.
Surprisingly, however, the timing relationship of individual bursts, as measured by a cross-correlation time lag between singing and BOS-sleep, is much shorter than one would expect between a sensory response (where the response lags the stimulus) and pre-motor response (where activity precedes the song being produced). Assuming a conservative auditory latency of 20 ms (the shortest auditory latency to HVC in white-crowned sparrows is 18 ms; Margoliash 1983) and a time lag in RA of 15-20 ms between pre-motor activity and sound production (Yu and Margoliash 1996; Ashmore et al. 2005), one would expect a latency of approximately 35 - 45 ms between the pre-motor and the auditory response. The observed time lag, however, is between 10 and 15 ms and is much shorter than expected. This short time lag suggests that neural responses to BOS during sleep should not be thought of as simple auditory responses but rather as auditory-driven responses that also engage the song motor network. This manifests in a pattern that can be thought of as predictive. The onsets of RA bursts elicited by BOS-sleep often precede the onsets of the “target” syllable they are associated with. This reflects a non-linear temporal integration over multiple syllables prior to the “target” syllable (Dave and Margoliash 2000). In this fashion, the timing of pre-motor bursting during singing (which leads the “target” syllables) can more closely match the timing of the same bursting during BOS-sleep. The identity of “target” syllables is confirmed in cases where the bird fails to sing the syllable; in all those cases the leading premotor bursting is also missing (Dave and Margoliash 2000).
c. Sleep responses to BOS might represent an entrainment of the motor system by a “sensory” stimulus
The finding that spontaneous activity in HVC and RA during sleep matches premotor patterns recorded during singing suggests that auditory-driven responses in HVC and RA might be able to engage spontaneous fluctuations of the underlying vocal motor network. Presentation of the BOS to a sleeping bird could therefore, in principle, be able to entrain the song motor system. A prediction of such entrainment is that auditory-driven responses during sleep should become progressively stronger and more motor-like as the duration of the stimulus progresses. Close inspection of the time course of BOS responses in sleeping birds reveals that the neural pattern that most closely resembles premotor activity often occurs towards the middle and end of the motif as if such entrainment takes time to ramp up. This is also consistent with the observation that the timing of auditory responses match premotor activity patterns, with some spike bursts immediately preceding the syllable, and that this requires temporal integration (Figure 5). Direct evidence for temporal integration of auditory responses in the song system derives from experiments showing that the auditory response at a given syllable is sensitive to removal of many of the preceding syllables (Margoliash 1983, Margoliash and Fortune 1992, Dave and Margoliash 2000).
Figure 5. Comparing auditory responses during sleep and spike bursting during singing in an adult zebra finch.
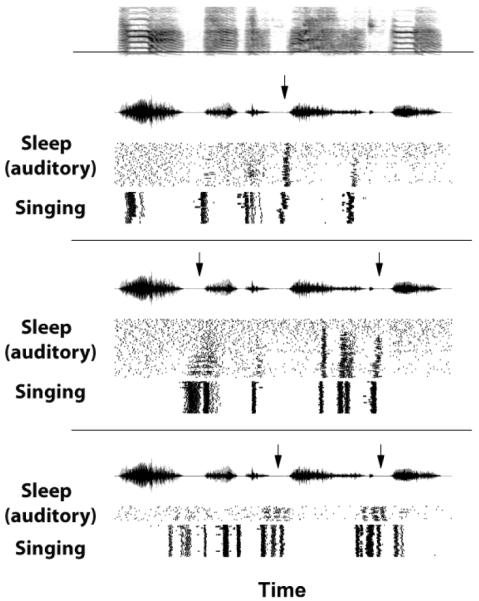
The spectrograph (top) of a representative motif of the bird’s song (same bird as in Figure 4). This song was played back while the bird slept during recordings from three distinct RA neurons. The responses of the neurons are shown in the three panels below the spectrograph, with each panel composed of three displays. Within each panel, the top display is an oscillograph of the song, and the bottom two displays are rasters of spikes. Within the rasters, one horizontal trace represents one repetition of the song, with the tick marks at the locations when the neuron spiked. The middle rasters (“Sleep, auditory”) show the neuron’s response to song playback during sleep; the bottom rasters (“Singing”) show the singing-related activity recorded during daytime singing. Note that there is more and better structured activity in response to song playback towards the ends of the motif, and that some spike bursts during song playback precede the syllables (arrows). (Modified from Dave 2001)
Additional support for the concept that BOS responses in HVC and RA in sleeping (or sedated) birds are linked to song motor activity is the location where BOS-selective responses are observed in the avian song system in anesthetized birds. In addition to HVC and RA, BOS responses are observed in areas traditionally considered exclusively motor in nature (see Figure 3). These include efferent targets of RA in the brainstem that project directly to the muscles of the vocal organ (nXIIts) (Williams and Nottebohm 1985; Sturdy et al. 2003) or down the bulbospinal tract to respiratory motoneurons in the spinal cord (PAm and RAm) (Ashmore et al. 2008). BOS-selective responses have in fact been observed in nearly all of the structures associated with song production. Many of these structures (e.g. PAm, Nif, Uva) also show spontaneous activity patterns during sleep that are correlated in time with spontaneous premotor-like activity in HVC or RA (Hahnloser and Fee 2007; Ashmore et al. 2008; Hahnloser et al. 2008). Although the relationship between these spontaneous activity patterns and song premotor activity has not been explicitly tested in any of these structures, it is conceivable many of these areas are engaging in song-like motor patterns during sleep and that presentation of the BOS helps entrain this premotor activity.
It is also noteworthy that the similarities and differences that are observed when comparing auditory and premotor activity patterns - such as burst timing and burst structure - are also observed when comparing spontaneous activity and premotor activity patterns (Dave and Margoliash 2000). In fact, spontaneous activity is more similar to BOS-sleep bursts than to bursts during singing (see Figure 4). This suggests the possibility that the natural condition, spontaneous bursting in the song system during sleep, carries sensory information. As described below, recent results relating incipient bursting during early juvenile learning gives further support for this hypothesis.
6. Song-related sleep activity and its relationship to vocal learning
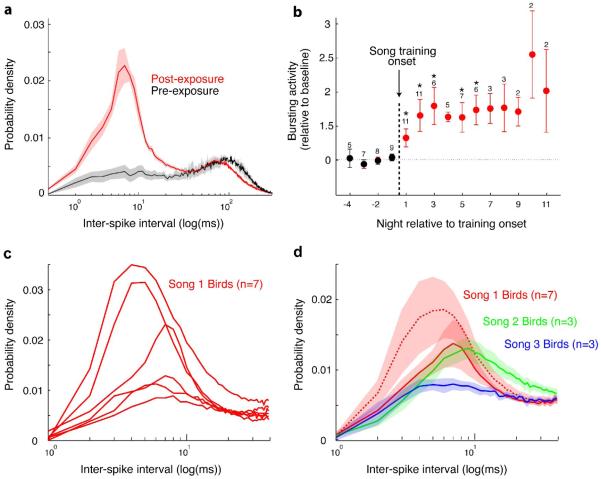
A strong connection between vocal plasticity and nighttime neuronal activity likely exists for juvenile zebra finches who are at the onset of vocal development following initial presentation of the tutor song. Using the same tutoring paradigm described above, neural activity can be recorded in naive juvenile zebra finches raised in isolation of adult male song during the day and nights prior to and following tutor song presentation. As expected, exposure to the tutor song results in the appearance of a circadian pattern of singing starting the day following the onset of tutoring (Shank and Margoliash, 2008). Neural recording of spontaneous activity patterns in RA neurons during sleep show relatively little high-frequency activity on nights prior to the tutor onset. Starting on the first night after tutor song exposure, however, RA neurons express a dramatic increase in the amount of high-frequency activity they spontaneously emit (Figure 6a). This activity is organized into prototypic bursts (measured as increases in high-frequency spiking activity), albeit without the highly structured pattern observed in RA in sleeping adults. As in adult birds (Dave et al. 1998), RA bursting during sleep in these young birds is distinct from a more tonic regular firing pattern otherwise observed during sleep and while birds are awake. Thus, inter-spike interval (ISI) histograms of RA activity collected during sleep show bimodal distributions. Examination of the changes in ISI distributions following the onset of tutoring reveals a substantial increase in ISI the very first night after the bird experiences tutoring earlier that day. By the second night the amount of bursting reaches a plateau that does not change thereafter (Shank and Margoliash, 2009) (Figure 6b).
Figure 6. Changes in RA neuronal activity during sleep and the onset of juvenile song learning.
a) Interspike interval histogram (ISI) distributions for all 19 RA neurons recorded on three nights prior to the onset of tutor song exposure (black curve) and for all 59 cells on 12 following nights (red curve). Cells recorded in the period when the bird is learning to sing have much greater high frequency bursting. b) Data from all 13 birds recorded on nights prior to (black points) and following (red points) the onset of tutor song exposure. Bursting activity is quantified as the area under the ISIs from 1 - 40 ms, relative to the baseline area defined as the average of the four nights preceding the first post-tutoring night. Each point is the average (± s.e.m.) across all birds contributing to that night; the number above each point is the number of birds contributing to that point. For each bird the average was calculated across all cells recorded that night. Note that the amount of spike bursting increases on the first night following tutor song exposure and reaches a plateau by the second night. c) ISI distributions, one per bird averaged across all cells for that bird, from birds that learned one particular tutor song (“Song 1”). The magnitude of the curves varies but the shape (location and width of peak) is generally conserved. d) Comparison of means (± s.e.m.) of ISI distributions across three groups of birds, that learned either “Song 1” (red dashed curve), “Song 2” (green curve), or “Song 3” (blue curve). The ISI distributions vary depending on which tutor song the birds were exposed to. Eliminating the two “Song 1” birds with the greatest high-frequency bursting (see c) (red solid curve) preserves differences between “Song 1” and the other two tutor song types. (Modified from Shank & Margoliash 2009)
As suggested by the behavior described above, the exposure of a naive bird to a tutor song may engage powerful attentional mechanisms. But beyond any non-specific increases in neuronal activity attendant to the first exposures to song, RA activity seems to be shaped by the song the bird is exposed to. In fact, the shape of the ISI distributions, that is the location and width of the short ISIs are more similar within groups of birds that are exposed to the same tutor song than they are across groups (Figure 6c, d) (Shank and Margoliash, 2009). These results suggest that the acoustic nature of the tutor shapes sleep bursting patterns in RA. It is also particularly salient because tutor song-specific changes in ISI distributions are observed on the night after tutor song exposure, but before changes in singing behavior are detected the following morning.
RA bursting is also dependent on a second signal, auditory feedback (Shank and Margoliash, 2009). Surgically muting birds (by making holes in the trachea and bronchial tubes), or raising them in the presence of a continuous loud masking noise (the masking noise is briefly eliminated to allow birds to hear the tutor song when it is broadcast) causes nighttime RA bursting to become suppressed even prior to tutor song exposure. Bursting then remains suppressed even following tutor song exposure and only recovers once singing recovers. In the case of the muted birds, this occurs when the masking noise is withdrawn, or in the case of the muted birds when the holes in the vocal tract heal (Shank and Margoliash, 2009). These observations support the hypothesis that during sleep the premotor network for singing in RA carries sensory cues. This presumably arises by information carried by nuclei afferent to RA, which implies that the major song system forebrain pathways carry sensory information in sleeping juvenile birds.
7. The transition to offline processing: A possible role for neuromodulator systems
We have identified behavioral and single cell correlates of offline processing associated with song learning. What candidate systems would be recruited to transition between the various online states (singing, non-singing aroused) and the various offline states (napping, sleeping)? Such candidate systems would likely be strongly activated by sensory stimuli that have high salience, possess the ability of causing long-term modification of the neural circuits that convert the sensory stimulus into a vocal-motor representation, and be intimately linked to arousal systems that control the transition from wakefulness to sleep. In the avian song system, several neuromodulator systems, notably the noradrenergic and the cholinergic system, fit all three of these criteria and seemingly are well placed for playing an important role in linking behavioral state changes and song learning.
Neuromodulator systems tend to be localized in a few small densely packed brain nuclei, but despite their relative small size their projections fan out throughout most of the central nervous system including the forebrain, brainstem and many areas of the spinal cord. These structures are therefore capable of influencing vast areas of the brain at once (Foote et al. 1983). There is evidence however, that the functional influence of some of these neuromodulators can be localized to specific circuits in the brain (Marrocco et al. 1987), including good evidence for this in the song system (Shea and Margoliash 2003a; Cardin and Schmidt 2004). This implies that neuromodulator systems can have both global and local influences on song system function.
Here we focus on two specific neuromodulator systems because these have been shown to display in songbirds some of the characteristics that were stated above. Noradrenergic (NE) neurons are located in the brainstem in a series of small nuclei, the major one being the locus coeruleus. In songbirds, this nucleus projects to many of the primary song system nuclei in songbirds (Appeltants et al. 2000; Castelino et al. 2007). The noradrenergic system is thought to be associated with the control of arousal state and selective attention (Berridge and Waterhouse 2003) and recent work, based on its regulation by prefrontal areas and its influence on sensory processing, suggest that it plays an important role in highlighting sensory features that have saliency (Aston-Jones and Cohen 2005). Cholinergic neurons are located in the brainstem, thalamus, and basal forebrain. In the song system, certain thalamic and basal forebrain neurons are thought to project directly to song system nuclei (Li and Sakaguchi 1997; Akutagawa and Konishi 2005). Forebrain cholinergic activation is associated with plasticity and selective attention (Wenk 1997), both in sensory cortices (e.g. Kilgard and Merzenich 1998) and in motor cortices (e.g. Conner et al. 2003). Brainstem cholinergic systems also help to regulate sleep states (see McCormick and Bal 1997).
During sleep, RA neurons display spontaneous replay of song motor activity and neurons throughout the song system are highly selective to the BOS. These two properties are often viewed as synonymous because awake birds do not show spontaneous replay and many neurons that show BOS-selective responses in the sleeping bird become completely suppressed when the animal wakes up. Using a paradigm that mimics the transition from sleep to waking (sedated birds are aroused with a brief air puff), it is possible to show that arousal mediated suppression of BOS responsiveness in HVC is mediated by the noradrenergic system (Cardin and Schmidt 2004) because injection of norepinephrine receptor antagonists into nucleus Nif, which provides auditory input to HVC, can reverse this suppression. This targeted manipulation is therefore able to retain the sleep-like response properties in HVC (and presumably the rest of the song system) even though the animal is aroused. The converse can also be shown where it is possible to “activate” HVC, and place it in an awake-like state by injecting NE directly into Nif. This causes a complete suppression of auditory responses in HVC even though the rest of the animal is in a sleep-like sedated state. The tight relationship in the song system between auditory response properties and spontaneous motor replay implies that targeted changes in NE levels acting at the level of NIf might be intimately linked to localized switching between on-line and offline processing of song.
Acetylcholine is the other neuromodulator known to influence auditory activity in the song system. In anesthetized animals, injections of cholinergic agents into HVC can block auditory activity in HVC and/or RA, depending on whether muscarinic or nicotinic receptors are activated, and these effects can be blocked by receptor-type specific cholinergic antagonists. Thus, there are likely to be different cholinergic systems within HVC. Stimulation of the cholinergic basal forebrain causes profound suppression of auditory responses in HVC in sedated and sleeping birds. Interestingly, despite the broad range of targets that are activated by such stimulation, the effects on HVC and RA auditory responsiveness can be reversed by infusing ACh receptor antagonists directly into HVC (Shea and Margoliash 2003a). This suggests that the stimulation effect is due to local effects on HVC, and that auditory pathways prior to HVC are spared even while stimulating the cholinergic basal forebrain.
The existence of an extensive cholinergic system in HVC that is sensitive to muscarine has been confirmed with a brain slice preparation (Shea and Margoliash 2003b). Interestingly, HVC interneurons are strongly inhibited by muscarine, and there are both excitatory and inhibitory effects on HVC neurons projecting to RA and Area X. Thus, increased cholinergic activation may serve to decouple the premotor and AFP pathways at the level of HVC. It remains to be determined, however, whether and how different cholinergic systems in the song system are activated under different behaviors such as singing and listening, as well as during sleep.
These two examples illustrate the complex effect neuromodulators can have on the underlying circuit properties of the song system. They also show the surprisingly localized influences these broadly acting agents can have. Because different neuromodulators are able to suppress auditory responsiveness by acting on different nuclei, it is likely that under natural conditions, auditory responses are sculpted by the complex interplay of these and other neuromodulators. It is also imaginable that the neuromodulators, which are known to be associated with arousal levels and sleep, might play a role in activating auditory memories stored during the day and release them into the song system for offline processing during sleep. Complex actions of neuromodulators that sculpt neural circuitry and guide changes in behavior are well-known in invertebrate systems (Harris-Warrick and Marder 1991) but this is not well-established for vertebrate systems. The song system represents an excellent opportunity to explore such questions.
8. Conclusions
a. A new model for sleep and sensorimotor learning
In recent years, a series of experiments have provided strong support for the hypothesis that off-line processing plays a role in sensorimotor vocal learning. The greatest amount of work has focused on sleep. Behavioral results demonstrate that a circadian rhythm of singing is released the day after birds gain their first exposure to tutor songs; this rhythm depend on sleep, and veridical song copying depends on the rhythm (Deregnaucourt et al. 2005; Shank and Margoliash 2009). The role of napping in song learning is more speculative. If tutor song exposure elicits napping (T. Lints and O. Tchernichovski, unpublished results), this may help to consolidate the song “template” - the auditory memory of the tutuor song. Naps, in any case, have different consequences than does sleep, since the sleep-triggered circadiam rhythm in singing is not reset after each nap. This difference could arise simply from the magnitude (duration) of napping relative to sleep or could arise because the structure of napping is different from sleep.
The recent data describing night-time changes in RA bursting activity related to the onset of song tutoring suggest a new mechanism for vocal learning. Two signals support RA bursting in juvenile birds. The first is auditory feedback, a powerful signal whose absence fundamentally suppresses activity in the song system, independent of tutoring. This permissive effect of feedback in structuring night-time RA bursting is a physiological correlate of the fundamental organizing effect of auditory feedback on song development (Konishi 1965, 1978). The second signal is the sensory representation of the tutor song, the song “template”. Whereas feedback is necessary and permissive for structuring night-time RA bursting, it is sensory exposure that profoundly influences night-time RA physiology. Thus, feedback can be viewed as a control or “gain” signal for sensory input. When the appropriate sensory signal is experienced, it rapidly elicits a change in physiological structure. The mechanisms whereby such changes occur are unknown, but may involve sensory gating by neuromodulators and expression of immediate-early genes (Bolhuis and Gahr 2006; London and Clayton 2008).
The changes in RA are observed at night, prior to the onset the following morning of changes in objective singing behavior. This suggests an active process during sleep is an integral component of the mechanism for vocal learning, although this remains speculative since the observation is correlational. Given that RA activity reflects sensory experience, one hypothesis is that plastic changes occur at night based on spontaneous activity driven by the template (“sensory replay”). The hypothesis predicts that the circadian pattern observed during song development results from an interaction between spontaneous activity reflecting sensory activity driving plastic mechanisms at night and sensorimotor-driven plastic mechanisms during daytime singing (Shank and Margoliash, 2009). This model has some resemblance to a “wake-sleep” algorithm for unsupervised training of neural networks (Hinton et al. 1995).
b. Birdsong and language revisited
Our understanding of plasticity in birdsong learning is advancing at a rapid pace. The role of feedback and the role of sensory memories are beginning to be recast from purely behavioral to behavioral-neurophysiological explanations. Recent observations focus attention on interactions between representations at night and during the day in driving aspects of developmental song learning. Offline mechanisms of learning highlight the importance of state-dependent functional wiring, and implicate neuromodulators in mediating these processes.
These observations can also motivate re-examination of plausible candidate mechanisms in speech and language acquisition (Margoliash 2003). If these are influenced by similar offline sensory representations as has been observed for birdsong, then that influence might be particularly strong since auditory feedback has a powerful influence on spoken language. A recent study demonstrated a powerful role of sleep in a speech perceptual learning task that required generalization of phonological categories (Fenn et al. 2003). It remains to be seen if sleep also influences higher-level properties of spoken language such as learning recursive structures. A potential role of sleep mechanisms in productive speech also bears consideration. One approach would be to explore potential circadian aspects of speech production, either in adults or in the context of infant development.
Traditionally, the sensory aspect of language learning has been considered from the perspective of auditory plasticity (but see Houde and Jordan 1998). Yet non-vocal aspects of speech production such as gesturing are also important for language production and possibly language comprehension (McNeill 1992; Goldin-Meadow et al. 2001). Recent work has been interpreted to implicate so-called mirror neurons, which link externally observed behavior with similar behavior produced by an individual, in language processing areas of the brain (Rizzolatti and Craighero 2004; Petrides et al. 2005). Although beyond the scope of this review, such mirror neurons have also been observed in songbirds (Prather et al. 2008). These new observations further extend the breadth of interaction between the study of birdsong learning, speech and language.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akutagawa E, Konishi M. Connections of thalamic modulatory centers to the vocal control system of the zebra finch. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14086–14091. doi: 10.1073/pnas.0506774102. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. Journal of Chemical Neuroanatomy. 2000;18(3):117–133. doi: 10.1016/s0891-0618(99)00054-x. Scopus. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320(5876):630–634. doi: 10.1126/science.1155140. Scopus. [DOI] [PubMed] [Google Scholar]
- Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. Journal of Neuroscience. 2008;28(10):2613–2623. doi: 10.1523/JNEUROSCI.4547-07.2008. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. Journal of Neuroscience. 2005;25(37):8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. Scopus. [DOI] [PubMed] [Google Scholar]
- Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Annals of the New York Academy of Science. 2003;1007:211–231. doi: 10.1196/annals.1286.021. Scopus. [DOI] [PubMed] [Google Scholar]
- Baptista LF, Petrinovich L. Song development in the white-crowned sparrow: social factors and sex differences. Animal Behaviour. 1986;34:1359–1371. [Google Scholar]
- Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321(5887):417–421. doi: 10.1126/science.1157632. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. Journal of Neuroscience. 2008;28(6):1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. Scopus. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature reviews. 2006;7(5):347–357. doi: 10.1038/nrn1904. Scopus. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224(4651):901–903. doi: 10.1126/science.6719123. Scopus. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404(6779):762–766. doi: 10.1038/35008083. Scopus. [DOI] [PubMed] [Google Scholar]
- Brawn TP, Fenn KM, Nusbaum HC, Margoliash D. Consolidation of sensorimotor learning during sleep. Learning and Memory. 2008;15(11):815–819. doi: 10.1101/lm.1180908. Scopus. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. Journal of sleep research. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Campbell S, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neuroscience & Biobehavioral Reviews. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. Scopus. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol. 2003;90(5):2884–2899. doi: 10.1152/jn.00391.2003. Scopus. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. Journal of Neuroscience. 2004;24(35):7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino CB, Diekamp B, Ball GF. Noradrenergic projections to the song control nucleus area X of the medial striatum in male zebra finches (Taeniopygia guttata) Journal of Comparative Neurology. 2007;502(4):544–562. doi: 10.1002/cne.21337. Scopus. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song. Biological Themes and Variations. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proceedings of the National Academy of Science U.S.A. 1995;92(8):3406–3410. doi: 10.1073/pnas.92.8.3406. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron. 2001;32(5):899–910. doi: 10.1016/s0896-6273(01)00524-4. Scopus. [DOI] [PubMed] [Google Scholar]
- Chi Z, Rauske PL, Margoliash D. Pattern filtering for detection of neural activity, with examples from HVc activity during sleep in zebra finches. Neural Computation. 2003;15(10):2307–2337. doi: 10.1162/089976603322362374. Scopus. [DOI] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38(5):819–829. doi: 10.1016/s0896-6273(03)00288-5. Scopus. [DOI] [PubMed] [Google Scholar]
- da Silva E.S.o., dos Santos TV, Hoeller AA, dos Santos TS, Pereira GV, Meneghelli C, Pezlin AI, dos Santos MM, Faria MS, Paschoalini MA, et al. Behavioral and metabolic effects of central injections of orexins/hypocretins in pigeons (Columba livia) Regulatory Peptides. 2008;147(1-3):9–18. doi: 10.1016/j.regpep.2007.12.003. Scopus. [DOI] [PubMed] [Google Scholar]
- Dave AS. Ph.D. Thesis. University of Chicago; 2001. Mechanisms of Sensorimotor Vocal Integration. [Google Scholar]
- Dave A, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. Scopus. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290(5492):812–816. doi: 10.1126/science.290.5492.812. Scopus. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433(7027):710–716. doi: 10.1038/nature03275. Scopus. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra PP, O F, Maul K, J LT, Tchernichovski O. Song development: from moment-to-moment to lifetime. In: Marler P, Zeigler HP, editors. Behavioral Neurobiology of Birdsong. Annals of the New York Academy of Sciences; New York: 2004. [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual review of neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. Scopus. [DOI] [PubMed] [Google Scholar]
- Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425(6958):614–616. doi: 10.1038/nature01951. Scopus. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiological Reviews. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. Scopus. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in male zebra finches (Taenopygia guttata) Journal of Comparative Neurology. 1992;325(3):388–404. doi: 10.1002/cne.903250306. Scopus. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata) Journal of Comparative Neurology. 1995;360(3):413–441. doi: 10.1002/cne.903600305. Scopus. [DOI] [PubMed] [Google Scholar]
- Gahr M. Neural song control system of hummingbirds: comparison to swifts, vocal learning (Songbirds) and nonlearning (Suboscines) passerines, and vocal learning (Budgerigars) and nonlearning (Dove, owl, gull, quail, chicken) nonpasserines. Journal of Comparative Neurology. 2000;426(2):182–196. Scopus. [PubMed] [Google Scholar]
- Gentner TQ, Fenn KM, Margoliash D, Nusbaum HC. Recursive syntactic pattern learning by songbirds. Nature. 2006;440(7088):1204–1207. doi: 10.1038/nature04675. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME. Memory systems in the chick: regional and temporal control by noradrenaline. Brain Research Bulletin. 2008;76(3):170–182. doi: 10.1016/j.brainresbull.2008.02.021. Scopus. [DOI] [PubMed] [Google Scholar]
- Glaze CM, Troyer TW. Temporal structure in zebra finch song: implications for motor coding. Journal of Neuroscience. 2006;26(3):991–1005. doi: 10.1523/JNEUROSCI.3387-05.2006. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin-Meadow S, Nusbaum H, Kelly SD, Wagner S. Explaining Math: Gesturing Lightens the Load. Psychological Science. 2001;12(6):516. doi: 10.1111/1467-9280.00395. Scopus. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419(6902):65–70. doi: 10.1038/nature00974. Scopus. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Wang CZ, Nager A, Naie K. Spikes and bursts in two types of thalamic projection neurons differentially shape sleep patterns and auditory responses in a songbird. Journal of Neuroscience. 2008;28(19):5040–5052. doi: 10.1523/JNEUROSCI.5059-07.2008. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RHR, Fee MS. Sleep-related spike bursts in HVC are driven by the nucleus interface of the nidopallium. Journal of Neurophysiology. 2007;97(1):423–435. doi: 10.1152/jn.00547.2006. Scopus. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annual Review of Neuroscience. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. Scopus. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Chomsky N, Fitch WT. The faculty of language: What is it, who has it, and how did it evolve? Science. 2002;298(5598):1569–1579. doi: 10.1126/science.298.5598.1569. Scopus. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Huetz C, Edeline JM. Neural representations during sleep: from sensory processing to memory traces. Neurobiology of Learning and Memory. 2007;87(3):416–440. doi: 10.1016/j.nlm.2006.10.006. Scopus. [DOI] [PubMed] [Google Scholar]
- Hinton GE, Dayan P, Frey BJ, Neal RM. The “wake-sleep” algorithm for unsupervised neural networks. Science. 1995;268(5214):1158–1161. doi: 10.1126/science.7761831. [DOI] [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279:1213–1216. doi: 10.1126/science.279.5354.1213. Scopus. [DOI] [PubMed] [Google Scholar]
- Hough GE, Volman SF. Short-term and long-term effects of vocal distortion on song maintenance in zebra finches. Journal of Neuroscience. 2002;22:1177–1186. doi: 10.1523/JNEUROSCI.22-03-01177.2002. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge University Press; London: 1969. pp. 61–74. [Google Scholar]
- Jarvis ED, Gunturkun O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, et al. Avian brains and a new understanding of vertebrate brain evolution. Nature reviews. 2005;6(2):151–159. doi: 10.1038/nrn1606. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–107. doi: 10.1038/nn1825. Scopus. [DOI] [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. Journal of Neurophysiology. 2006;96(3):1441–1455. doi: 10.1152/jn.01138.2005. Scopus. [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265(5172):679–682. doi: 10.1126/science.8036518. Scopus. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Homology and evolutionary origins of the ‘neocortex’. Brain, Behavior and Evolution. 1991;38(4-5):264–272. doi: 10.1159/000114393. Scopus. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Evolutionary developmental biology meets the brain: the origins of mammalian cortex. Proceedings of the National Academy of Science U.S.A. 1997;94(7):2800–2804. doi: 10.1073/pnas.94.7.2800. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457(7226):187–190. doi: 10.1038/nature07467. Scopus. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. Scopus. [DOI] [PubMed] [Google Scholar]
- Kluender KR, Diehl RL, Killeen PR. Japanese quail can learn phonetic categories. Science. 1987;237(4819):1195–1197. doi: 10.1126/science.3629235. Scopus. [DOI] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift fur Tierpsychologie. 1965;22:770–783. Scopus. [PubMed] [Google Scholar]
- Konishi M. Auditory environment and vocal development in birds. In: Walk RD, Pick HLJ, editors. Perception and Experience. Plenum; New York: 1978. pp. 105–118. [Google Scholar]
- Konishi M. The role of auditory feedback in birdsong. Annals of the New York Academy of Science. 2004;1016:463–475. doi: 10.1196/annals.1298.010. Scopus. [DOI] [PubMed] [Google Scholar]
- Konishi M, Nottebohm F. Experimental studies in the ontogeny of avian vocalization. In: Hinde RA, editor. Bird Vocalizations. Cambridge University Press; London: 1969. pp. 29–38. [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. Journal of Neurophysiology. 2007;97(6):4271–4283. doi: 10.1152/jn.00952.2006. Scopus. [DOI] [PubMed] [Google Scholar]
- Kroodsma DE, Pickert R. Environmentally dependent sensitive periods for avian vocal learning. Nature. 1980;288:477–479. Scopus. [Google Scholar]
- Kuhl PK. Models and mechanisms in speech perception. Species comparisons provide further contributions. Brain, Behavior and Evolution. 1979;16(5-6):374–408. doi: 10.1159/000121877. Scopus. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Miller JD. Speech perception by the chinchilla: voiced-voiceless distinction in alveolar plosive consonants. Science. 1975;190(4209):69–72. doi: 10.1126/science.1166301. Scopus. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Miller JD. Speech perception by the chinchilla: identification function for synthetic VOT stimuli. Journal of the Acoustical Society of America. 1978;63(3):905–917. doi: 10.1121/1.381770. [DOI] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. Journal of Neuroscience. 2005;25(3):652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399(6735):466–470. doi: 10.1038/20933. Scopus. [DOI] [PubMed] [Google Scholar]
- Li R, Sakaguchi H. Cholinergic innervation of the song control nuclei by the ventral paleostriatum in the zebra finch: a double-labeling study with retrograde fluorescent tracers and choline acetyltransferase immunohistochemistry. Brain Research. 1997;763(2):239–246. doi: 10.1016/s0006-8993(97)00417-4. Scopus. [DOI] [PubMed] [Google Scholar]
- Liu WC, Gardner TJ, Nottebohm F. Juvenile zebra finches can use multiple strategies to learn the same song. Proceedings of the National Academy of Science U.S.A. 2004;101(52):18177–18182. doi: 10.1073/pnas.0408065101. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. Journal of Neuroscience. 2000;20(13):5054–5064. doi: 10.1523/JNEUROSCI.20-13-05054.2000. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature Neuroscience. 2008;11(5):579–586. doi: 10.1038/nn.2103. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456(7219):189–194. doi: 10.1038/nature07448. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PS, Shank SS, Sejnowski TJ, Margoliash D. Mammalian-like features of sleep structure in zebra finches. Proceedings of the National Academy of Science U.S.A. 2008;105(26):9081–9086. doi: 10.1073/pnas.0703452105. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macken MA, Ferguson CA. In: Children’s Language. Nelson KE, editor. Vol. 4. Lawence Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. Journal of Neuroscience. 1983;3(5):1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. Journal of Neuroscience. 1986;6(6):1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Evaluating theories of bird song learning: implications for future directions. Journal of Comparative Physiology A. 2002;188(11-12):851–866. doi: 10.1007/s00359-002-0351-5. Scopus. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Offline learning and the role of autogenous speech: new suggestions from birdsong research. In: B S, editor. Speech Commun. Vol. 41. 2003. pp. 165–178. Scopus. [Google Scholar]
- Margoliash D, Fortune ES. Temporal and harmonic combination-sensitive neurons in the zebra finch’s HVc. Journal of Neuroscience. 1992;12:4309–4326. doi: 10.1523/JNEUROSCI.12-11-04309.1992. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. A comparative approach to vocal learning: song development in white-crowned sparrows. Journal of Comparative and Physiological Psychology. 1970a;71((2),Part 2):1–25. Scopus. [Google Scholar]
- Marler P. Birdsong and speech development: could there be parallels? American Scientist. 1970b;58(6):669–673. Scopus. [PubMed] [Google Scholar]
- Marler P, Sherman V. Song structure without auditory feedback: emendations of the auditory template hypothesis. Journal of Neuroscience. 1983;3(3):517–531. doi: 10.1523/JNEUROSCI.03-03-00517.1983. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco RT, Lane RF, McClurkin JW, Blaha CD, Alkire MF. Release of cortical catecholamines by visual-stimulation requires activity in thalamocortical afferents of monkey and cat. Journal of Neuroscience. 1987;7(9):2756–2767. doi: 10.1523/JNEUROSCI.07-09-02756.1987. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechansims. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McNeill D. Hand and mind: What gesture reveals about thought. University of Chicago Press; Chicago: 1992. [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6(7):697–698. doi: 10.1038/nn1078. Scopus. [DOI] [PubMed] [Google Scholar]
- Menn L, Stoel-Gammon C. The Development of Language. Allyn and Bacon; Boston, MA: 2001. [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. Journal of Neuroscience. 1999;19(21):9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural auditory selectivity develops in parallel with song. Journal of Neurobiology. 2005a;62(4):469–481. doi: 10.1002/neu.20115. Scopus. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. Journal of Neurobiology. 2005b;62(2):231–242. doi: 10.1002/neu.20087. Scopus. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behavioral & Neural Biology. 1992;57(1):58–66. doi: 10.1016/0163-1047(92)90757-u. Scopus. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Auditory experience and song development in the chaffinch (Fringilla coelebs) Ibis. 1968;110(4):549–568. [Google Scholar]
- Nottebohm F. The origins of vocal learning. American Naturalist. 1972;106:116–140. [Google Scholar]
- Nowicki S, Searcy WA, Peters S. Brain development, song learning and mate choice in birds: a review and experimental test of the “nutritional stress hypothesis”. Journal of Comparative Physiology A. 2002;188(11-12):1003–1014. doi: 10.1007/s00359-002-0361-3. Scopus. [DOI] [PubMed] [Google Scholar]
- Nusbaum HC, Margoliash D. Understanding spoken language. In: Berntson GCJ, editor. Handbook of Neuroscience for the Behavioral Sciences. John Wiley & Sons; New York: in press. [Google Scholar]
- Ostrovsky Y, Andalman A, Sinha P. Vision following extended congenital blindness. Psychological Science. 2006;17(12):1009–1014. doi: 10.1111/j.1467-9280.2006.01827.x. Scopus. [DOI] [PubMed] [Google Scholar]
- Pearson AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including area X. Journal of Comparative Neurology. 2008;508(5):840–866. doi: 10.1002/cne.21699. Scopus. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–545. doi: 10.1016/j.neuron.2004.10.007. Scopus. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature. 2005;435(7046):1235–1238. doi: 10.1038/nature03628. Scopus. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proceedings of the National Academy of Science U.S.A. 2006;103(4):1088–1093. doi: 10.1073/pnas.0510136103. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JH, Tyack PL, Stoeger-Horwath AS, Watwood S. Animal behaviour: elephants are capable of vocal learning. Nature. 2005;434(7032):455–456. doi: 10.1038/434455a. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451(7176):305–310. doi: 10.1038/nature06492. Scopus. [DOI] [PubMed] [Google Scholar]
- Price PH. Developmental determinants of structure in zebra finch song. Journal of Comparative and Physiological Psychology. 1979;93:268–277. Scopus. [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. Scopus. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC. Evolution of slow-wave sleep and palliopallial connectivity in mammals and birds: a hypothesis. Brain Research Bulletin. 2006;69(1):20–29. doi: 10.1016/j.brainresbull.2005.11.002. Scopus. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Amlaner CJ, Phil D. Phylogeny of Sleep. In: Lee-Chiong TL Jr., Sateia MJ, Carskadon MA, editors. Sleep Medicine. Hanley & Belfus, Inc.; Philadelphia: 2002. pp. 7–22. [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. Journal of Neurophysiology. 2003;89(3):1688–1701. doi: 10.1152/jn.00655.2002. Scopus. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. Journal of Comparative Neurology. 2004;473(3):377–414. doi: 10.1002/cne.20118. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell L, Whitehead H. Culture in whales and dolphins. The Behavioral and Brain Sciences. 2001;24(2):309–324. doi: 10.1017/s0140525x0100396x. discussion 324-382 Scopus. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC. Sleep does not enhance motor sequence learning. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2008;34(4):834–842. doi: 10.1037/0278-7393.34.4.834. Scopus. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27(1) doi: 10.1146/annurev.neuro.27.070203.144230. 169-C-164 Scopus. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. Journal of Neuroscience. 2008;28(13):3479–3489. doi: 10.1523/JNEUROSCI.0177-08.2008. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology. 2006;112(5):458–470. Scopus. [Google Scholar]
- Rose GJ, Goller F, Gritton HJ, Plamondon SL, Baugh AT, Cooper BG. Species-typical songs in white-crowned sparrows tutored with only phrase pairs. Nature. 2004;432(7018):753–758. doi: 10.1038/nature02992. Scopus. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Ashmore RC. Integrating breathing and singing: forebrain and brainstem mechanisms. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge Univ. Press; Cambridge: 2008. pp. 285–322. [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458(7234):73–77. doi: 10.1038/nature07615. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Basal forebrain cholinergic modulation of auditory activity in the zebra finch song system. Neuron. 2003a;40(6):1213–1226. doi: 10.1016/s0896-6273(03)00723-2. Scopus. [DOI] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Basal forebrain control of HVc: cellular mechanisms and behavioral consequences. Society for Neuroscience Abstract. 2003b;29 942.912. [Google Scholar]
- Slater PJB, Eales LA, Clayton NS. Song learning in zebra finches (Taeniopygia guttata): Progress and Prospects. Advances in the Study of Behavior. 1988;18:1–33. [Google Scholar]
- Sossinka R, Boehner J. Song types in the zebra finch Poephila guttata castanotis. Zeitschrift fur Tierpsychologie. 1980;53:123–132. [Google Scholar]
- Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nature Neuroscience. 2000;3(12):1237–1238. doi: 10.1038/81756. Scopus. [DOI] [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. Journal of Neuroscience. 2003;23(3):1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter ML, Margoliash D. Global synchronous response to autogenous song in zebra finch HVc. Journal of Neurophysiology. 1994;72(5):2105–2123. doi: 10.1152/jn.1994.72.5.2105. Scopus. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Lints TJ, Deregnaucourt S, Cimenser A, Mitra PP. Behavioral Neurobiology of Birdsong. Vol. 1016. 2004. Studying the song development process rationale and methods; pp. 348–363. Scopus. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291(5513):2564–2569. doi: 10.1126/science.1058522. Scopus. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Animal Behaviour. 2000;59(6):1167–1176. doi: 10.1006/anbe.1999.1416. Scopus. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. Journal of Neuroscience. 2004;24(21):4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen F, Doupe A. Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVc of male zebra finches. Journal of Neuroscience. 1998;18:3786–3802. doi: 10.1523/JNEUROSCI.18-10-03786.1998. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N. The Study of Instinct. Oxford University Press; Oxford: 1951. [Google Scholar]
- Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450(7173):1240–1244. doi: 10.1038/nature06390. Scopus. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. Journal of Comparative Neurology. 1996;366(4):613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. Scopus. [DOI] [PubMed] [Google Scholar]
- Volman SF. Development of neural selectivity for birdsong during vocal learning. Journal of Neuroscience. 1993;13(11):4737–4747. doi: 10.1523/JNEUROSCI.13-11-04737.1993. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: One hundred years of progress. Neurobiology of Learning and Memory. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. Scopus. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) Journal of Comparative Neurology. 1993;337(1):32–62. doi: 10.1002/cne.903370103. Scopus. [DOI] [PubMed] [Google Scholar]
- Williams H, McKibben JR. Changes in stereotyped central motor patterns controlling vocalization are induced by peripheral nerve injury. Behavioral & Neural Biology. 1992;57(1):67–78. doi: 10.1016/0163-1047(92)90768-y. Scopus. [DOI] [PubMed] [Google Scholar]
- Williams H, Nottebohm F. Auditory responses in avian vocal motor neurons: a motor theory for song perception in birds. Science. 1985;229(4710):279–282. doi: 10.1126/science.4012321. Scopus. [DOI] [PubMed] [Google Scholar]
- Williams H, Vicario DS. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. Journal of Neurobiology. 1993;24(7):903–912. doi: 10.1002/neu.480240704. Scopus. [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273(5283):1871–1875. doi: 10.1126/science.273.5283.1871. Scopus. [DOI] [PubMed] [Google Scholar]