Abstract
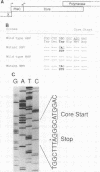
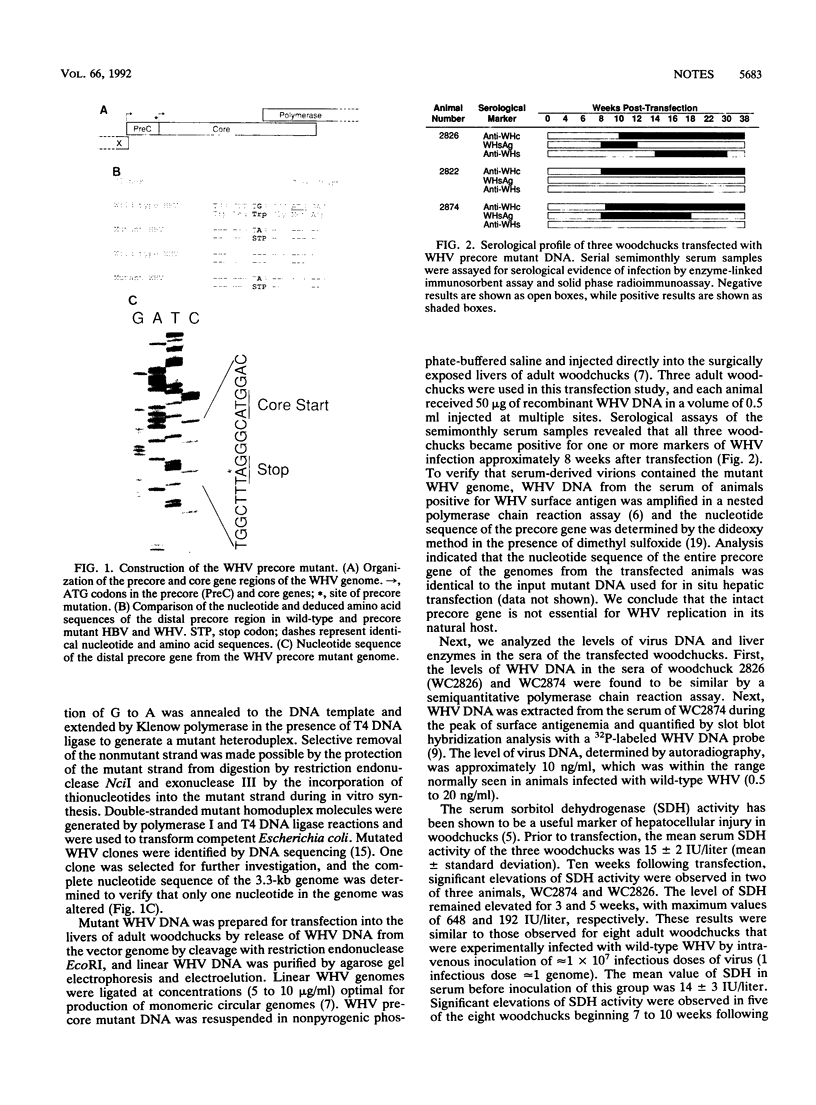
A number of naturally occurring hepatitis B virus mutants that cannot synthesize the virus precore protein have been identified. Such mutants have been associated with more severe forms of hepatitis, including fulminant hepatitis. The most common mutation observed is a substitution of G to A in the distal precore gene that converts a codon specifying Trp (TGG) to a termination codon (TAG). Using oligonucleotide-directed mutagenesis, we have produced the same point mutation in the precore gene of an infectious clone of woodchuck hepatitis virus (WHV). Transfection of mutant WHV DNA into the livers of adult woodchucks resulted in replication of the mutant in three of three susceptible animals. Levels of virus replication and transient elevations in liver enzymes in serum were similar to those of adult animals infected with wild-type WHV. Virions, found to possess mutant precore genes by polymerase chain reaction amplification and DNA sequencing, were recovered from the serum of one of the animals and inoculated subcutaneously into neonatal woodchucks. They produced infection in all five animals studied. The level of virus replication in neonatal animals infected with this mutant virus was comparable to that found in neonatal woodchucks infected with wild-type WHV, but none of five woodchucks infected with the precore mutant virus as neonates became chronic virus carriers. It was concluded that the precore gene of the WHV genome is not essential for virus replication in the natural host but may be important for chronic infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunetto M. R., Giarin M. M., Oliveri F., Chiaberge E., Baldi M., Alfarano A., Serra A., Saracco G., Verme G., Will H. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4186–4190. doi: 10.1073/pnas.88.10.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Enders G., Sprengel R., Peters N., Varmus H. E., Ganem D. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J Virol. 1987 Oct;61(10):3322–3325. doi: 10.1128/jvi.61.10.3322-3325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin J. L., Cote P. J., Korba B. E., Tennant B. C. Hepadnavirus-induced liver cancer in woodchucks. Cancer Detect Prev. 1989;14(2):227–229. [PubMed] [Google Scholar]
- Girones R., Cote P. J., Hornbuckle W. E., Tennant B. C., Gerin J. L., Purcell R. H., Miller R. H. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1846–1849. doi: 10.1073/pnas.86.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbuckle W. E., Graham E. S., Roth L., Baldwin B. H., Wickenden C., Tennant B. C. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax). Lab Anim Sci. 1985 Aug;35(4):376–381. [PubMed] [Google Scholar]
- Kaneko S., Feinstone S. M., Miller R. H. Rapid and sensitive method for the detection of serum hepatitis B virus DNA using the polymerase chain reaction technique. J Clin Microbiol. 1989 Sep;27(9):1930–1933. doi: 10.1128/jcm.27.9.1930-1933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Girones R., Cote P. J., Hornbuckle W. E., Chestnut T., Baldwin B. H., Korba B. E., Tennant B. C., Gerin J. L., Purcell R. H. Evidence against a requisite role for defective virus in the establishment of persistent hepadnavirus infections. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9329–9332. doi: 10.1073/pnas.87.23.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Kaneko S., Chung C. T., Girones R., Purcell R. H. Compact organization of the hepatitis B virus genome. Hepatology. 1989 Feb;9(2):322–327. doi: 10.1002/hep.1840090226. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Lee S. C., Liaw Y. F., Robinson W. S. Hepatitis B viral DNA in infected human liver and in hepatocellular carcinoma. J Infect Dis. 1985 Jun;151(6):1081–1092. doi: 10.1093/infdis/151.6.1081. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Yotsumoto S., Akahane Y., Yamanaka T., Miyazaki Y., Sugai Y., Tsuda F., Tanaka T., Miyakawa Y., Mayumi M. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990 Mar;64(3):1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata M., Ehata T., Yokosuka O., Hosoda K., Ohto M. Mutations in the precore region of hepatitis B virus DNA in patients with fulminant and severe hepatitis. N Engl J Med. 1991 Jun 13;324(24):1699–1704. doi: 10.1056/NEJM199106133242404. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper H., Roth L., Purcell R. H., Tennant B. C., Gerin J. L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1987 Feb;84(3):866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Salfeld J., Schaller H. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J Virol. 1987 Dec;61(12):3701–3709. doi: 10.1128/jvi.61.12.3701-3709.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. P., Brotman B., Li J. S., Vitvitski L., Pascal D., Prince A. M., Trépo C. In vitro and in vivo replication capacity of the precore region defective hepatitis B virus variants. J Hepatol. 1991;13 (Suppl 4):S68–S73. doi: 10.1016/0168-8278(91)90028-a. [DOI] [PubMed] [Google Scholar]
- Tong S. P., Diot C., Gripon P., Li J., Vitvitski L., Trépo C., Guguen-Guillouzo C. In vitro replication competence of a cloned hepatitis B virus variant with a nonsense mutation in the distal pre-C region. Virology. 1991 Apr;181(2):733–737. doi: 10.1016/0042-6822(91)90908-t. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]