SUMMARY
Autophagy is a homeostatic, carefully regulated, and dynamic process for intracellular recycling of bulk proteins, aging organelles, and lipids. Autophagy occurs in all tissues and cell types, including the brain and neurons. Alteration in the dynamics of autophagy has been observed in many diseases of the central nervous system. Disruption of autophagy for an extended period of time results in accumulation of unwanted proteins and neurodegeneration. However, the role of enhanced autophagy after acute brain injury remains undefined. Established mouse models of brain injury will be valuable in clarifying the role of autophagy after brain injury, and are the topic of discussion in this review.
Keywords: Autophagosome, Controlled cortical impact, Hypoxia-ischemia, Mitophagy, Traumatic brain injury
1. Introduction
Autophagy is a homeostatic, carefully regulated, and dynamic process for intracellular recycling of bulk proteins and aging organelles [1, 2], and very recently discovered, lipids [3]. Alteration in the dynamics of autophagy has been observed in many diseases of the central nervous system (CNS). Increased autophagy has now been reported in experimental models of traumatic brain injury, stroke, and excitotoxicity [4–7], and in patients with Alzheimer’s Disease and critical illness [8, 9]. Mice with genetic disruption of autophagy develop accumulation of unwanted proteins and neurodegeneration, mimicking human conditions like Huntington’s, Parkinson’s, and Alzheimer’s disease [10]. However, while lifetime disruption of autophagy is clearly deleterious, it remains unclear whether increased autophagy after critical injury or extended periods of cell stress is beneficial—or actually detrimental. Several studies using transgenic mice and/or established mouse models of brain injury have shed some light onto the role of autophagy after brain injury. However, the role of autophagy, particularly in the brain, remains controversial and is the topic of this review.
2. Autophagy
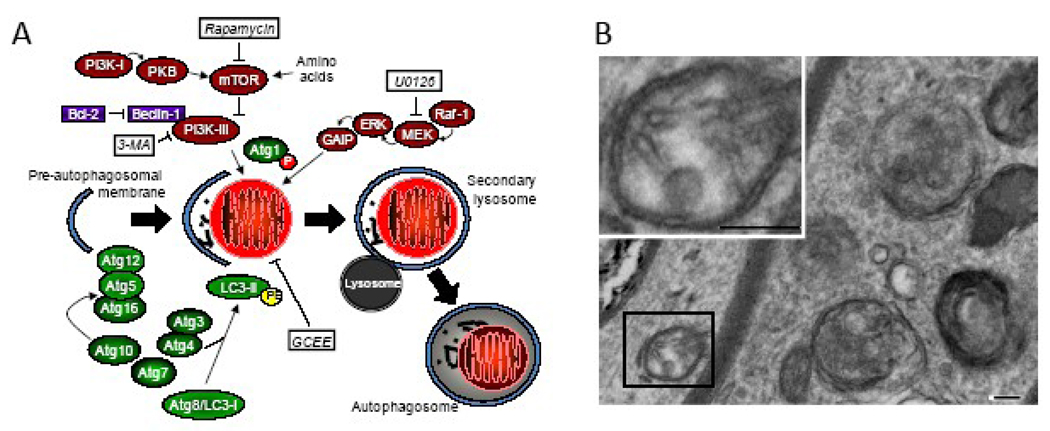
Autophagy occurs in all tissues and cell types, including the brain and neurons. Autophagy is an important cellular response to environmental stress. The classic stimulus for autophagy is starvation. Starvation-induced autophagy is initiated by extended nutrient deprivation, the hormone glucagon, and the second-messenger cAMP [11, 12]. Autophagy is a highly regulated process requiring the ubiquitin E1-like enzyme Atg7 (autophagy-associated proteins abbreviated “Atg”), phosphorylation of pre-autophagosomal membranes, formation of Atg12-Atg5 complexes, and processing of Atg8/microtubule-associated protein light chain-3 (LC3, is the mammalian homologue of the yeast Atg8), and covalent attachment of phosphatidyl-ethanolamine to LC3-I resulting in LC3-II (Figure 1). LC3-II is a relatively specific biochemical footprint of autophagy, referred to as an “LC3 shift” or “LC3 lipidation” [13]. These processes culminate in the formation of autophagosomes containing cytoplasmic material and/or organelles such as mitochondria. Autophagosomes fuse with lysosomes, facilitating enzymatic disassembly of large proteins and organelles leading to recycling of amino acids and other nutrients [14]. Starvation-induced autophagy is regulated by class III phosphatidylinositol 3-kinase (PI3-K) and the Bcl-2 interacting partner, Beclin-1 [15]. In contrast, regulation of autophagy may differ in non-starvation conditions. For example, the mitogen activated protein kinase/extracellular signal regulated protein kinases (MEK), rather than PI3-K, appear to regulate autophagy triggered by oxidative stress as the (MEK) inhibitor 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126), but not the class III PI-3K inhibitor 3-methyladenine, reduces autophagy induced by 1-methyl-4-phenylpyridinium [16]. Importantly, oxygen radicals are also essential for autophagy to proceed normally [17].
Fig. 1.
A. Simplified schematic of starvation- and non-starvation-induced autophagy. Putative effectors of autophagy are highlighted in white boxes. B. Electron micrograph showing autophagosomes (t he “gold standard” for detection of autophagy) in an axons after traumatic brain injury in mice (bar = 100 nm). Abbreviations: 3-MA, 3-methyladenine; Atg, autophagy complex component; ERK, extracellular signal regulated protein kinase; GAIP, Gα-interacting protein; GCEE, γ-glutamyl cysteine-ethyl ester; LC3, light chain 3; MEK, mitogen activated protein kinase/ERK; mTOR, mammalian target of rapamycin; P, phosphate; PE, phosphatidylethanolamine; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; U0126, 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene.
3. Mouse Model to Monitor Autophagy
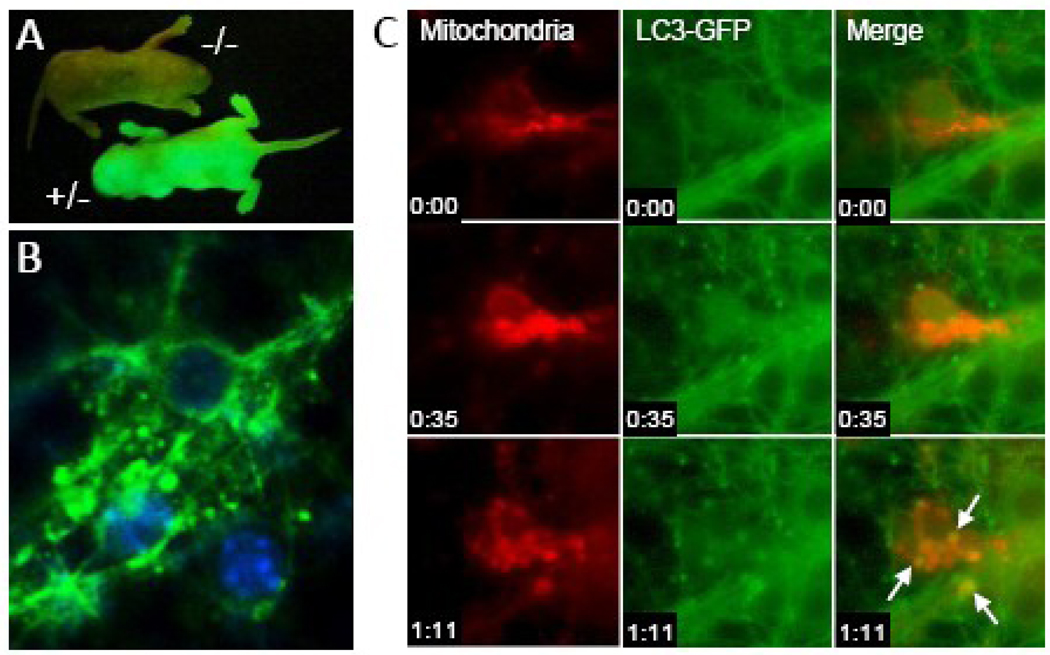
As briefly noted above, LC3-II is a reliable biomarker of autophagy. Taking advantage of this, Mizushima et al. developed a transgenic mouse where LC3 is tagged with green fluorescent protein (GFP) [18]. GFP-LC3 is concentrated in autophagosomes, and thus formation of GFP-enriched vesicles can be used to monitor autophagy [1]. Hemizygous expression of GFP-LC3 does not itself affect autophagy rates, and GFP-LC3 localizes to autophagosome membranes upon stimulation of autophagy similar to endogenous LC3 [19]. GFP fluorescent macroscopy can be used to identify transgenic embryos (Figure 2A). GFP-LC3+/− transgenic mice can be used to monitor autophagy both in vivo and in vitro. Mizushima et al. evaluated GFP-LC3+/− mice and found that the response to nutrient deprivation was tissue-specific [18]. In some tissues, such as the brain, ovaries, and testes, an increase in autophagy after 48 hours of starvation was not detectable. In others, such as the kidney, pancreas, and thymus, baseline autophagy was observed in some cell types. In most tissues, particularly the liver and muscle, 48 hours of nutrient deprivation produced a reliable and robust increase in autophagosome formation.
Fig. 2.
Dynamic tracking of autophagy in primary cortical neurons in vitro. A. Discrimination of GFP-LC3+/− mice using fluorescent macroscopy. B. Identification of GFP-LC3-enriched vesicles suggestive of autophagosomes in primary cortical neurons from GFP-LC3+/− mice after 24 hours of nutrient deprivation using fluorescent microscopy. C. Dynamic tracking of mitophagy in primary cortical neurons from GFP-LC3+/− mice using time-lapsed microscopy. Autophagosomes (green) fusing with mitochondria (red; MitoTracker Red, Invitrogen, Carlsbad, CA) are highlighted by arrows. Time stamp is hours:minutes.
The value of these mice in the dynamic assessment of autophagy in neurons, was first demonstrated in an in vitro model of starvation. Depriving neurons of D-glucose, sodium pyruvate, L-glutamate, L-glutamine, L-aspartate, and supplements containing protein and lipid, leads to formation of GFP-LC3 enriched vacuoles (consistent with autophagosomes) within an hour (Figure 2) [20]. Cultures from these mice can be used to track fusion of autophagosomes with lysosomes [20] and mitochondria (Figure 2C). In contrast to fibroblasts, inhibition of autophagy using 3-methyladenine—a classic but non-specific inhibitor of autophagy, or siRNA targeting Atg7, increased neuronal survival, suggesting that in non-dividing cells such as neurons, increased autophagy is deleterious. Another interesting finding in this study was that during starvation, induction of autophagy was sex-dependent [20]. Neurons from male mice show a robust increase in autophagosomes versus their female counterparts during starvation. Both resistance to starvation and decreased autophagy in neurons was associated with the capacity of neurons from females to mobilize lipid sources and form lipid droplets. Very recently, a critical link between LC3 lipidation and autophagy of lipid droplets was reported, a process coined “lipophagy” [3].
Because 48 hours of food-deprivation does not produce detectable autophagy in brains from mice [18], the in vivo relevance of this in vitro starvation model, can be questioned. However, studies in humans with prolonged periods of profound starvation—those undergoing voluntary hunger strikes and patients with anorexia nervosa, show radiographic evidence of brain tissue loss, consistent with some degree of autophagy and/or lipophagy in brain [21–23]. Since it would be difficult to justify subjecting animals to extended periods of starvation, this question may never be answered. Nevertheless, what is clear is that neurons retain the capacity to undergo autophagy, the role of autophagy in response to stress may be different in neurons compared to other tissues—particularly those that also serve as nutrient depots such as adipose, liver, and skeletal muscle, and that the autophagic response to stress is sex-dependent.
4. Mouse Models of Disrupted Autophagy
4.1. Atg7 deficient transgenic mice
In 2005, Komatsu et al. reported generation of conditional knockout mice deficient in Atg7 [24]. They found that mice with completely lacking Atg7 die within 1 day after birth. The following year, this group reported generation of mice with tissue-specific Atg7 knockdown in the CNS [10]. These mice had growth retardation, motor and behavioral deficits, and extensive neuronal loss, dying within 28 weeks of birth. Additionally, age-dependent increases in ubiquitin-containing inclusion bodies were present in neurons reflecting disrupted autophagy, and correlating with neurodegeneration and neurological dysfunction.
Koike et al. evaluated the influence of CNS Atg7 deficiency in a mouse model of neonatal hypoxia-ischemia [25]. This adaptation of the Levine preparation [26] is often referred to as the Rice-Vannucci model when used in immature rodents [27], and involves unilateral carotid artery ligation followed by a period of hypoxia. This insult can be done in rodents of very young age, including mice, and produces a unilateral brain infarction. Neonatal Atg7 deficient mice had less hippocampal neuronal death and autophagy compared with their wild-type counterparts, implying that autophagy contributes to neurodegeneration after acute cerebral ischemia. Importantly, the protective effect of Atg7 deletion is not seen in older mice, suggesting that any advantage Atg7 mice may have in response to ischemia is counterbalanced by accumulation of proteins with increasing age related to disrupted autophagy.
4.2. Atg5 deficient transgenic mice
In 2004, Kuma et al. reported generation of homozygous mice deficient in Atg5−/− [28]. These mice appeared normal at birth, yet most died within 1 day of life. Atg5 and Atg12-Atg5 conjugates were undetectable in these mice. LC3-II was significantly reduced or absent, and LC3-I was increased. In neural-cell-specific Atg5−/− mice, development of progressive motor and behavioral deficits and degenerative changes in neurons is observed, again illustrating the necessity of autophagy in cellular homeostasis.
5. Mouse Models of Enhanced Autophagy
Both transgenic and non-transgenic mouse models have been used to provide valuable insight into the pathobiology and pathophysiology of acute brain injury [29]. Recently, contemporary mouse models of traumatic and ischemic brain injury have shown increased autophagy, and have begun to shed some light—or perhaps a cloud of controversy, as to the role of autophagy in the mature and developing mammalian brain. Specifically, whether autophagy is beneficial and/or detrimental, or merely an epiphenomenon after acute brain injury.
5.1. Traumatic brain injury
The first report that traumatic brain injury may stimulate autophagy was by Diskin et al. in 2005 [30]. Using a mouse model of closed head injury produced by dropping a weight onto the intact skull, they assessed changes in Beclin-1, a bcl-2 interacting partner that regulates starvation-induced autophagy. They found that while Beclin-1 levels are relatively low in brain from uninjured animals, Beclin-1 increased in neurons and activated astrocytes at the site of injury [30]. Thus, providing indirect evidence that autophagy is increased after traumatic brain injury. Investigators from this laboratory later evaluated the effects of treatment with rapamycin in this closed head injury model [31]. Rapamycin triggers autophagy via inhibition of mammalian target of rapamycin (mTOR) and consequential disinhibition and activation of PI3-K (Figure 1A). Rapamycin was developed as an anti-fungal agent, and also has anti-inflammatory effects and the ability to cause cell cycle arrest and inhibit cell proliferation [32]. Erlich et al. [31] found that intraperitoneal injection of rapamycin administered 4 hours after injury resulted in improved neurobehavioral function as determined by a neurological severity score and increased neuronal survival in the injured region compared to vehicle treated mice. Additionally, western blot showed increased Beclin-1 at 5 hours in with rapamycin treatment, consistent with an augmented autophagic response. Collectively, these data support rapamycin-enhanced autophagy producing beneficial neurological effects after traumatic brain injury. Although, non-autophagic salutatory effects of rapamycin cannot be discounted.
Ultrastructural identification of autophagosomes remains the “gold standard” method for detection of autophagy [1]. As such, the first direct evidence that autophagy is increased after traumatic brain injury was reported by Lai et al. using a controlled cortical impact model of traumatic brain injury [4]. Controlled cortical impact is a contemporary model of traumatic brain injury that produces a contusion with cortical necrosis, delayed neuronal death in brain regions such as the hippocampus and thalamus, and sustained motor and cognitive deficits [33]. An increase in autophagosomes—vacuoles, multilamellar bodies, and secondary lysosomes, was observed from 2–48 hours after traumatic brain injury. Biochemical evidence of autophagy, increased LC3-II levels in injured brain was also reported. As oxidative stress can induce autophagy [17] and oxygen radicals are essential for autophagy to proceed normally [16], the effect of the cysteine-donor antioxidant γ-glutamylcysteinyl ethyl ester (GCEE) was evaluated. Systemic administration of GCEE after injury resulted in reduced autophagy as determined by LC3-II formation, increased antioxidant reserves, improved cognitive performance, and reduced histological damage compared with vehicle treatment. The same caveat mentioned above regarding non-autophagy related effects of rapamycin also applies to GCEE. However, there does appear to be a link between autophagy and oxidative stress after traumatic brain injury. Oxidative stress is a prominent feature of traumatic brain injury in mice [34] and humans [35].
5.2. Hypoxia-ischemia
Similar to traumatic brain injury, autophagy was also reported to be upregulated after hypoxic-ischemic injury in mice in 2005. Zhu et al. examined the effects of age and hypoxia-ischemia induced by unilateral carotid artery occlusion and hypoxemia (Rice-Vannucci model) on LC3-II levels as a biomarker of autophagy [6]. They detected levels of LC3-I and LC3-II in control brains, and found that younger (post-natal day 5; PND 5) animals had higher levels of both LC3-I and LC3-II compared with older animals (PND 60). Thus, similar to apoptosis, autophagy appears to be age-dependent and more prominent during early stages of development. After unilateral hypoxia-ischemia, relative LC3-II abundance was increased in the ipsilateral hemisphere, with the greatest increase in adult brains [6]. The following year, this group also reported that this increase in LC3-II was not sex-dependent in this model of neonatal hypoxia-ischemia [36].
Autophagic neurodegeneration is also seen after cerebral ischemia in adult mice. After transient middle cerebral artery occlusion, Degterev et al. showed an increase in autophagy that was not affected by the novel “necroptosis” inhibitor Necrostatin-1 [5]. Adhami et al. reported the effect of unilateral carotid occlusion with hypoxemia in adult mice [37]. They noted the presence of vacuole-associated damage via electron microscopy consistent with autophagy in the ischemic brain. Using GFP-LC3 transgenic mice, they also showed increased punctate GFP immunolabeling at 6 and 18 hours after hypoxia-ischemia. An increase in LC3-II was not detected after hypoxia-ischemia; however, a transient reduction in LC3-I and depletion of cytosolic LC3 was observed.
6. Reconciliation of Current Studies
To date, mouse models have been valuable in establishing that autophagy is a vital homeostatic process, and that disrupted autophagy in normal tissues—including in the brain, is detrimental [10, 28]. Contemporary mouse models have also established that the autophagic response can be triggered in brain by trauma [4], ischemia [5, 6], and excitotoxicity [7]. However, the role of autophagy—beneficial, detrimental, or an epiphenomenon, after acute brain injury remains uncertain and controversial [38, 39].
6.1. If not enough autophagy is bad, is more autophagy better?
Undoubtedly autophagy is essential for the clearance and recycling of accumulated proteins and aging or dysfunctional organelles. Disruption of autophagy, especially for the lifetime of an animal leads to accumulation of proteins. If accumulation of the particular protein or the protein itself happens to be toxic, then functional autophagy is of greater importance to cellular homeostasis. Examples of toxic protein accumulation associated with CNS pathology include mutated Tau, β-amyloid, α-synuclein, and Huntington protein [40]. As mentioned above, mice with disrupted autophagy due to deletion of Atg5 or Atg7 demonstrate neuropathology with accumulated protein aggregates and phenotypic changes mimicking human neurodegenerative disease [10, 28]. Extrapolating to diseases where increased autophagy is evident, perhaps this increase is designed to clear protein aggregates? For example, β-amyloid—sufficient to induce neuronal apoptosis [41], is known to accumulate after traumatic brain injury [42].
On the other hand, since baseline autophagy in neurons is low (indeed rarely detected) and protein synthesis in general is inhibited after acute brain injury, increased autophagy may not be necessary for clearance of protein. In non-human primates after global brain ischemia, protein synthesis in all brain regions is inhibited within hours after ischemia [43]. In some regions, such as the cerebellum and cortex, protein synthesis recovered within 24 hours. In others, such as the hippocampus, thalamus, and arterial border zones of the cortex, protein synthesis was inhibited by as much as fifty percent compared with normal controls. Additionally, chaperone proteins are not subject to this general reduction in protein synthesis after ischemia [44]. Signals for autophagic degradation of proteins also include sequence specific regions (KFERQ) that interact with chaperone proteins [45]. Chaperone-assisted degradation of accumulated proteins, versus autophagosome-lysosome mediated degradation of organelles and bulk proteins, may play different roles after acute brain injury.
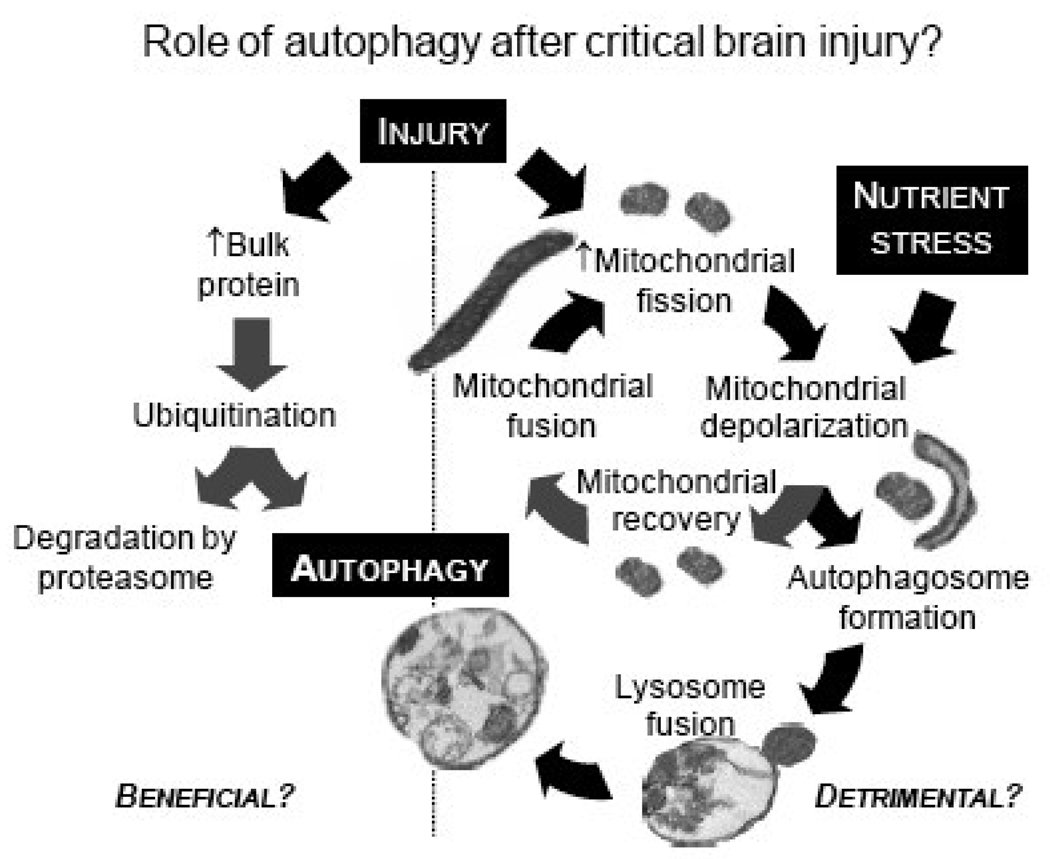
Another unanswered question is whether increased autophagy participates in the clearance of reversibly or irreversibly damaged organelles, or whether its consumption of organelles such as mitochondria in cells with low baseline autophagy is indiscriminate? Mitochondrial fission, which likely occurs before engulfment into autophagosomes (Figure 3), is not necessarily irreversible after injury [46]. Enhanced autophagy may contribute to consumption of potentially viable or uninjured organelles such as mitochondria after fission, and therefore, may exacerbate energy failure and worsen injury. Mitochondrial dysfunction [47, 48] and energy failure [49] are prominent features of both traumatic and ischemic brain injury.
Fig. 3.
Hypothetical schematic illustrating potential dual-roles of autophagy after critical brain injury.
Addressing this question directly has been hampered by the fact that studies in autophagy-related transgenic mice are confounded by progressive neurodegeneration as the mouse ages, and the lack of specific pharmacological agents targeting autophagy. In the study by Koike et al. using Atg7 deficient mice, cerebral ischemia was imposed on PND 7, and brains were evaluated 3 days later [25], long before neuropathology is detectable in these mice [10]. The studies by Erlich et al. [31] and Lai et al. [4] testing pharmacological therapies that increase and inhibit autophagy, respectively, in mouse models of traumatic brain injury were conflicting. Rapamycin, which induces autophagy via inhibition of mTOR improved neurological outcome [31]; whereas the antioxidant GCEE reduced autophagy but also improved neurological outcome [4]. As noted above, both of these studies tested drugs with multiple effects in addition to those related specifically to autophagy. A more recent study in a rat model of transient focal cerebral ischemia in PND 12 rats [50], tested what was regarded as a more selective inhibitor of autophagy, the class III PI3-K inhibitor 3-methyladenine [12]. Post-treatment with 3-methyladenine administered intracerebroventricularly reduced infarction size compared with vehicle treatment and caspase inhibitors, which had no effect [50]. Similar to rapamycin and GCEE, however, 3-methyladenine also has other effects including inhibition of non-class III PI3-kinases and promotion of glycogen breakdown in hepatocytes [51]. Taken together, these pharmacological studies tend to cloud, rather than clarify, the role of autophagy after acute brain injury.
6.2. Is the role for autophagy cell-type dependent?
Another important question is whether the role of autophagy in neurons and/or brain is different from other cell-types and tissues? Autophagy at baseline and during starvation is clearly tissue-dependent [18]. It is interesting to note that tissues that also serve as nutrient depots, protein in muscle and glycogen in liver, tend to have robust autophagic responses that are probably beneficial to the organism as a whole. While curiously evidence for GFP-enriched vesicles were not routinely observed in fat [18], recent evidence suggests that regulation of lipid during starvation may be via an autophagic process [3]. In contrast, typically non-dividing cells such as neurons, spermatocytes, and oocytes have minimally detectable autophagy at baseline, and do not have an autophagic response to 48 hours of starvation [18]. Inhibition of autophagy during nutrient deprivation reduces cell death in neurons, but increases cell death in fibroblasts [20], suggesting that not only is the autophagic response tissue-dependent, but the role of autophagy is as well.
7. Conclusions
While mouse models have helped establish the presence of autophagy in brain after acute injury, they have not yet helped clarify the role of autophagy. However, it seems clear that either too little, or too much, autophagy is detrimental to normal homeostasis and the response to injury, and that this is tissue- and cell-type dependent. In this regard, autophagy is quite like apoptosis. Indeed, many other similarities and cross-talk occur between autophagy and apoptosis [52]. Important unanswered questions remain, that will require development and testing of specific interventions that either increase or inhibit autophagy in injured brain, before these questions can be answered.
Acknowledgements
Supported by National Institute of Neurologic Diseases and Stroke grant NS38620 and National Institute Child Health and Human Development grant HD045968.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim. Biophys. Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Y, Hickey RW, Chen Y, Bayir H, Sullivan ML, Chu CT, Kochanek PM, Dixon CE, Jenkins LW, Graham SH, Watkins SC, Clark RS. Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J. Cereb. Blood Flow Metab. 2008;28:540–550. doi: 10.1038/sj.jcbfm.9600551. [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, Hagberg H, Blomgren K. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–176. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
- 7.Shacka JJ, Lu J, Xie ZL, Uchiyama Y, Roth KA, Zhang J. Kainic acid induces early and transient autophagic stress in mouse hippocampus. Neurosci. Lett. 2007;414:57–60. doi: 10.1016/j.neulet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark RS, Bayir H, Chu CT, Alber SM, Kochanek PM, Watkins SC. Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy. 2008;4:88–90. doi: 10.4161/auto.5173. [DOI] [PubMed] [Google Scholar]
- 9.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 11.Neely AN, Mortimore GE. Localization of products of endogenous proteolysis in lysosomes of perfused rat liver. Biochem. Biophys. Res. Commun. 1974;59:680–687. doi: 10.1016/s0006-291x(74)80033-1. [DOI] [PubMed] [Google Scholar]
- 12.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouno T, Mizuguchi M, Tanida I, Ueno T, Kanematsu T, Mori Y, Shinoda H, Hirata M, Kominami E, Kawano K. Solution structure of microtubule-associated protein light chain 3 and identification of its functional subdomains. J. Biol. Chem. 2005;280:24610–24617. doi: 10.1074/jbc.M413565200. [DOI] [PubMed] [Google Scholar]
- 14.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 15.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J. Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 16.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N. Methods for monitoring autophagy. Intl. J. Biochem. Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, Kochanek PM, Jenkins LW, Ren J, Gibson G, Chu CT, Kagan VE, Clark RS. Starving neurons show sex difference in autophagy. J. Biol. Chem. 2008 doi: 10.1074/jbc.M804396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connan F, Murphy F, Connor SE, Rich P, Murphy T, Bara-Carill N, Landau S, Krljes S, Ng V, Williams S, Morris RG, Campbell IC, Treasure J. Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Res. 2006;146:117–125. doi: 10.1016/j.pscychresns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Katzman DK, Christensen B, Young AR, Zipursky RB. Starving the brain: structural abnormalities and cognitive impairment in adolescents with anorexia nervosa. Semin. Clin. Neuropsychiatry. 2001;6:146–152. doi: 10.1053/scnp.2001.22263. [DOI] [PubMed] [Google Scholar]
- 23.Basoglu M, Yetimalar Y, Gurgor N, Buyukcatalbas S, Kurt T, Secil Y, Yeniocak A. Neurological complications of prolonged hunger strike. Eur. J. Neurol. 2006;13:1089–1097. doi: 10.1111/j.1468-1331.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am. J. Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine S. Anoxic-ischemic encephalopathy in rats. Am. J. Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 27.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxicischemic brain damage in the rat. Ann. Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 28.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 29.Statler KD, Jenkins LW, Dixon CE, Clark RS, Marion DW, Kochanek PM. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J. Neurotrauma. 2001;18:1195–1206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- 30.Diskin T, Tal-Or P, Erlich S, Mizrachy L, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. J. Neurotrauma. 2005;22:750–762. doi: 10.1089/neu.2005.22.750. [DOI] [PubMed] [Google Scholar]
- 31.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol. Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. (Tokyo) 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 33.Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- 34.Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, Ferriero DM. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann. Neurol. 1998;44:357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- 35.Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, Graham SH, Janesko K, Clark RS, Kochanek PM. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J. Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 37.Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am. J. Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 39.Chu CT. Autophagic stress in neuronal injury and disease. J. Neuropathol. Exp. Neurol. 2006;65:423–432. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Bodsch W, Barbier A, Oehmichen M, Grosse Ophoff B, Hossmann KA. Recovery of monkey brain after prolonged ischemia. II. Protein synthesis and morphological alterations. J. Cereb. Blood Flow Metab. 1986;6:22–33. doi: 10.1038/jcbfm.1986.4. [DOI] [PubMed] [Google Scholar]
- 44.Nowak TS., Jr Protein synthesis and the heart shock/stress response after ischemia. Cerebrovasc. Brain Metab. Rev. 1990;2:345–366. [PubMed] [Google Scholar]
- 45.Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol. Histopathol. 2002;17:897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- 46.Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- 48.Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- 49.Satchell MA, Zhang X, Kochanek PM, Dixon CE, Jenkins LW, Melick JA, Szabo C, Clark RS. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+ depletion and ribosylation of 14-3-3gamma. J. Neurochem. 2003;85:697–708. doi: 10.1046/j.1471-4159.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 50.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann. Neurol. 2009 doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 51.Caro LH, Plomp PJ, Wolvetang EJ, Kerkhof C, Meijer AJ. 3-Methyladenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur. J. Biochem. 1988;175:325–329. doi: 10.1111/j.1432-1033.1988.tb14200.x. [DOI] [PubMed] [Google Scholar]
- 52.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]