Abstract
Aims
The nature of the relationship between adolescent smoking and depression is unclear and the mechanisms that account for the comorbidity have received little investigation. The present study sought to clarify the temporal precedence for smoking and depression and to determine whether these variables are linked indirectly through peer smoking.
Participants
The sample was composed of 1,093 adolescents participating in a longitudinal study of the behavioral predictors of smoking adoption.
Design & Measurements
In this prospective cohort study, smoking, depression, peer smoking and other covariates were measured annually from mid adolescence (9th grade; age 14) to late adolescence (12th grade, age 18).
Findings
Parallel Processes Latent Growth Curve Models supported a bi-directional relationship between adolescent smoking and depression, where higher depression symptoms in mid adolescence (age 14) predicted adolescent smoking progression from mid to late adolescence (ages 14-18 years old). A significant indirect effect indicated that higher depression symptoms across time predicted an increase in the number of smoking peers, which in turn predicted smoking progression from mid adolescence to late adolescence. In addition, smoking progression predicted a deceleration of depression symptoms from mid to late adolescence. A significant indirect effect indicated that greater smoking at baseline predicted a deceleration in the number of smoking peers across time, which predicted a deceleration in depression symptoms from mid adolescence to late adolescence.
Conclusions
The current study provides the first evidence of bi-directional self-medication processes in the relationship between adolescent smoking and depression and highlights peer smoking as one explanation for the comorbidity.
Keywords: adolescence, depression, smoking, longitudinal
INTRODUCTION
Depression and smoking show high rates of co-morbidity, with both typically beginning in adolescence and serving as leading causes of psychosocial, economic, and medical morbidity and premature mortality (1-5). However, the temporal relationship between adolescent smoking and depression is unclear. Research has shown that major depression and depression symptoms (subthreshold depression) predict smoking initiation, progression to regular smoking, and increases in average daily smoking rates (6-12). Conversely, other studies indicate that smoking predicts the development of depression (13-16), depression and smoking reciprocally influence each other (17-19), and smoking and depression do not influence each other in a bidirectional manner (7, 10, 16). It is plausible that the relationship between smoking and depression may not be causal, but rather due to a common set of genetic or environmental factors that contribute to both depression and smoking. The available support for common genetic influences is growing, but remains limited (20-22) and research indicates that controlling for potential confounds is insufficient to explain the association between depression and smoking (7, 23-26).
Methodological variability may help to account for some of the disparate findings (e.g., different definitions of smoking or stage of smoking, lack of repeated assessments of smoking and depression, lack of control for potential confounding variables, unidirectional assessments, and the removal of respondents who have already smoked any cigarettes or have a higher level of depression at baseline) (19, 27-29). In addition, the relationship between depression and smoking may not best represented as a direct effect, but rather an indirect or mediated effect through another variable (29).
Although the notion that depressed adolescents may more readily acquire a smoking habit in an effort to self-medicate mood (attenuate negative affect and/or increase positive affect) compared to adolescents with no or low depressive symptomatology, the self-medication hypothesis has received little prospective evaluation (30, 31). The expectation that smoking manages negative affect has been shown to discriminate smokers from nonsmokers, to predict belonging to a regular smoking trajectory among adolescents, and to predict smoking progression (32, 33). A recent lab-based study found that smoking a cigarette reduced negative affect among adolescent smokers who held strong beliefs that smoking alleviates negative affect (34). Although these studies did not evaluate depression, this research suggests that depression may promote adolescent smoking acquisition and that smoking uptake may reduce adolescent depression. However, these conclusions, in part, seem inconsistent with epidemiologic research showing that adolescent smoking contributes to the development of depression.
The relationship between depression and smoking might be better explained through more complex models that consider direct effects as well as indirect or mediated effects (29). The mechanisms that account for the comorbidity between depression and smoking have received very little investigation and research has yielded no answers (35). Peer smoking may be an important mechanism to consider. Peer smoking has been linked to adolescent smoking progression (36, 37), and has been shown to accentuate the effects of depression on adolescent smoking uptake (9). Cross-sectional data suggest that depression may make an adolescent more vulnerable to peer smoking influences, which in turn promotes smoking uptake (38). This may be due to affectively vulnerable adolescents' choice of peers who smoke, or a greater need to gain peer approval, a compromised ability to refuse cigarette offers, or the perception that cigarette smoking provides benefits to one's peers (38-41). Likewise, adolescents who smoke may select peers with similar smoking behaviors, which are often accompanied by other nonconventional behaviors (42-44). Peer substance use is linked to the development of adolescent depression, possibly because of the quality of the peer relationship or via engagement in similar nonconventional behaviors (45, 46). We are not aware of any research to date that has evaluated mechanisms that may account for smoking contributing to the development of adolescent depression. As such, peer smoking is a plausible candidate to consider.
We propose to evaluate the temporal precedence for adolescent smoking and depression and whether these variables are linked indirectly through peer smoking within a prospective cohort design. In contrast to previous research, depression, smoking, and peer smoking were repeatedly measured across time and modeled longitudinally. We anticipated that depression and smoking will be characterized by a bi-directional relationship and that peer smoking will help account for this relationship from mid adolescence to late adolescence.
METHODS
Participants and procedures
Participants were high school students (53% female and 65% White) taking part in a longitudinal study of the social, psychological, and genetic determinants of adolescent smoking adoption. Participants were enrolled in one of five public high schools in northern Virginia. This cohort was drawn from the 2,393 students identified through class rosters at the beginning of 9th grade. Students were ineligible to participate in this study if they had a special classroom placement (e.g., severe learning disability). Based on the selection criteria, a total of 2,120 (89%) students were eligible to participate, and of these, 1,533 (72%) parents provided a response regarding their teen's participation. Of the 1,533 parents who provided a response 1,151 (75%) consented to their teen's participation. Analysis of differences between students whose parents did and did not consent revealed that the likelihood of consent was greater for white parents with more than a high school education than for white parents with a high school education or less (89% vs.77%) (47).
The majority of adolescents with parental consent provided their assent (99%, N=1136). The adolescent cohort was formed in the 9th grade and was surveyed each spring until the end of 12th grade. Thus, four annual spring data waves were used in these analyses. Data were collected on-site during compulsory classes. The surveys took approximately 30 minutes to complete. Less than 10% of adolescents were lost to follow-up across the 4 years. Participants in the present study were adolescents (N=1093) with complete data on the 9th grade covariates. University Institutional Review Board approval of the study protocol was obtained.
Measures
Depression symptoms
Depression symptoms were assessed at each wave with the Center for Epidemiological Studies Depression (CES-D) inventory. The CES-D is a valid and reliable 20-item self-report measure designed to assess depression symptoms in the general population including adolescents (48-51). Whereas a CES-D score of 16 is indicative of a clinically significant level of depression symptoms in adults, cutoffs for adolescents are higher (i.e., > 22) (52). The 20-items were linearly summated and then log (base e) transformed to correct for univariate non-normality, and to form a single log depression symptoms score at each wave.
Smoking
Smoking was assessed at each wave with 13 standard epidemiological questions, such as “Have you tried or experimented with cigarette smoking, even a few puffs?” and “When was the last time you smoked a cigarette?” (53). In the present study, our dependent variable was the number of cigarettes smoked in the past 30 days. This item was log (base e) transformed for each wave for an observed continuous measure of past 30 day smoking at each wave to correct for univariate non-normality.
Peer smoking
Peer smoking was measured at each wave by summating responses to three items asking whether the adolescents' best friend smokes and how many of his or her other four best male and four best female friends smoke (range 0 to 9 friends smoking) (36, 37). Peer smoking was log (base e) transformed each wave to correct for univariate non-normality.
Covariates
Demographic variables included gender (1= “male,” 2= “female”) and race (0= “white,” 1= “non-white”). Household smoking was assessed with a binary variable (0=nobody living in the household smokes, 1=at least household member smokes). Marijuana use was assessed with one item asking “During the past 30 days, how many times have you used marijuana? Alcohol use was assessed with one item asking, “During the past 30 days, on how many days did you have at least one drink (not just a sip) of alcohol?” Response choices ranged from 0 to all 30 days for alcohol use and 0 to 40 or more times for marijuana use (53). Individual physical activity was assessed with three items tapping intensity, duration, and frequency of physical activity per week. Scores on physical activity ranged from 0 to 7 days of physical activity per week. These three physical activity items were summed and averaged to represent the average days of individual physical activity per week. Team sport participation was assessed with a single item that requested the number of sports teams on which the adolescent played during the past 12-months (1=0 teams to 4= 3 or more teams). All covariates were measured in the 9th grade. We controlled for these covariates in the model as potential confounding variables given their associations with smoking, peer smoking, and depression symptoms (7, 37, 54-66).
Analytical Approach
Univariate statistics were generated to describe the study population in terms of demographics, smoking practices, and depression symptoms. Univariate estimates were generated with SAS 9.2 software, and can be found in Table 1.
Table 1.
Non-standardized regression coefficients (β), standard errors, z statistics, and p-values for the dependent factors in the primary model (depression symptoms to smoking) and the alternative model (smoking to depression symptoms).
| Primary Model (Depression Symptoms to Smoking) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Smoking Level | Smoking Trend | |||||||
| β | SE | z | p-value | β | SE | z | p-value | |
| Depression symptoms level | -0.07 | 0.08 | -0.95 | 0.34 | 0.16 | 0.06 | 2.44 | 0.02 |
| Depression symptoms trend | - | - | - | - | -0.47 | 0.26 | -1.76 | 0.08 |
| Peer smoking level | 0.90 | 0.09 | 9.79 | <0.0001 | 0.22 | 0.06 | 3.80 | <0.0001 |
| Peer smoking trend | - | - | - | - | 1.45 | 0.22 | 6.52 | <0.0001 |
| Non-White | -0.19 | 0.07 | -2.75 | 0.01 | -0.06 | 0.05 | -1.23 | 0.22 |
| Female | -0.09 | 0.06 | -1.55 | 0.12 | -0.06 | 0.04 | -1.36 | 0.18 |
| Household smoking | -0.01 | 0.02 | -0.49 | 0.62 | 0.00 | 0.02 | 0.24 | 0.81 |
| Alcohol use (past 30 days) | 0.00 | 0.02 | -0.22 | 0.82 | -0.04 | 0.02 | -2.01 | 0.05 |
| Marijuana use (past 30 days) | -0.07 | 0.03 | -2.69 | 0.01 | 0.01 | 0.02 | 0.42 | 0.68 |
| Individual physical activity | -0.04 | 0.02 | -1.82 | 0.07 | 0.03 | 0.02 | 1.67 | 0.10 |
| Team sport participation | 0.05 | 0.02 | 2.07 | 0.04 | 0.00 | 0.02 | -0.22 | 0.83 |
| Depression Symptoms Level | Depression Symptoms Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | z | p-value | β | SE | z | p-value | |
| Smoking level | - | - | - | - | 0.003 | 0.03 | 0.13 | 0.90 |
| Peer smoking level | - | - | - | - | -0.09 | 0.04 | -2.61 | 0.01 |
| Non-White | 0.05 | 0.04 | 1.24 | 0.22 | 0.05 | 0.03 | 2.02 | 0.04 |
| Female | 0.16 | 0.04 | 4.04 | <0.0001 | 0.01 | 0.02 | 0.57 | 0.57 |
| Household smoking | -0.01 | 0.02 | -0.82 | 0.41 | 0.00 | 0.01 | 0.30 | 0.76 |
| Alcohol use (past 30 days) | -0.02 | 0.01 | -1.36 | 0.17 | -0.02 | 0.01 | -2.50 | 0.01 |
| Marijuana use (past 30 days) | -0.03 | 0.02 | -1.32 | 0.19 | 0.02 | 0.01 | 1.30 | 0.19 |
| Individual physical activity | -0.01 | 0.01 | -0.77 | 0.44 | 0.02 | 0.01 | 1.82 | 0.07 |
| Team sport participation | -0.02 | 0.02 | -1.01 | 0.31 | 0.00 | 0.01 | -0.06 | 0.95 |
| Peer Smoking Level | Peer Smoking Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | z | p-value | β | SE | z | p-value | |
| Smoking level | - | - | - | - | -0.05 | 0.02 | -2.13 | 0.03 |
| Depression symptoms level | 0.37 | 0.06 | 5.99 | <0.0001 | -0.06 | 0.03 | -1.64 | 0.10 |
| Depression symptoms trend | - | - | - | - | 0.52 | 0.14 | 3.85 | <0.0001 |
| Non-White | 0.24 | 0.05 | 4.86 | <0.0001 | -0.08 | 0.03 | -2.68 | 0.01 |
| Female | -0.03 | 0.05 | -0.70 | 0.48 | 0.00 | 0.03 | -0.11 | 0.91 |
| Household smoking | 0.01 | 0.02 | 0.77 | 0.44 | -0.01 | 0.01 | -0.89 | 0.37 |
| Alcohol use (past 30 days) | -0.03 | 0.02 | -1.92 | 0.05 | 0.02 | 0.01 | 1.56 | 0.12 |
| Marijuana use (past 30 days) | -0.02 | 0.02 | -0.80 | 0.42 | 0.01 | 0.01 | 0.74 | 0.46 |
| Individual physical activity | 0.06 | 0.02 | 3.88 | <0.0001 | -0.02 | 0.01 | -1.74 | 0.08 |
| Team sport participation | -0.01 | 0.02 | -0.71 | 0.48 | 0.01 | 0.01 | 0.78 | 0.44 |
| Alternative Model (Smoking to Depression Symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Depression Symptoms Level | Depression Symptoms Trend | |||||||
| β | SE | z | p-value | β | SE | z | p-value | |
| Smoking level | -0.07 | 0.05 | -1.37 | 0.17 | 0.07 | 0.04 | 1.71 | 0.09 |
| Smoking trend | - | - | - | - | -.15 | 0.07 | -2.14 | 0.03 |
| Peer smoking level | 0.31 | 0.07 | 4.30 | <0.0001 | -0.07 | 0.04 | -1.84 | 0.07 |
| Peer smoking trend | - | - | - | - | 0.60 | 0.19 | 3.21 | 0.001 |
| Non-White | -0.03 | 0.05 | -0.56 | 0.58 | 0.07 | 0.03 | 2.25 | 0.02 |
| Female | 0.15 | 0.04 | 3.65 | <0.0001 | 0.02 | 0.03 | 0.61 | 0.55 |
| Household smoking | -0.02 | 0.02 | -1.04 | 0.30 | 0.01 | 0.01 | 0.60 | 0.55 |
| Alcohol use (past 30 days) | -0.01 | 0.01 | -0.63 | 0.53 | -0.03 | 0.01 | -2.68 | 0.01 |
| Marijuana use (past 30 days) | -0.02 | 0.02 | -1.19 | 0.24 | 0.01 | 0.01 | 0.79 | 0.43 |
| Individual physical activity | -0.03 | 0.02 | -1.94 | 0.05 | 0.02 | 0.01 | 2.30 | 0.02 |
| Team sport participation | -0.01 | 0.02 | -0.44 | 0.66 | -0.01 | 0.01 | -0.71 | 0.48 |
| Smoking Level | Smoking Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | z | p-value | β | SE | z | p-value | |
| Depression symptoms level | - | - | - | - | 0.09 | 0.06 | 1.63 | 0.10 |
| Peer smoking level | - | - | - | - | 0.12 | 0.06 | 1.82 | 0.07 |
| Non-White | 0.05 | 0.06 | 0.70 | 0.48 | -0.14 | 0.04 | -3.38 | 0.001 |
| Female | -0.07 | 0.06 | -1.29 | 0.20 | -0.05 | 0.04 | -1.38 | 0.17 |
| Household smoking | -0.002 | 0.03 | -0.10 | 0.92 | -0.01 | 0.02 | -0.45 | 0.65 |
| Alcohol use (past 30 days) | -0.04 | 0.02 | -1.73 | 0.08 | -0.02 | 0.02 | -1.13 | 0.26 |
| Marijuana use (past 30 days) | -0.09 | 0.03 | -3.17 | 0.002 | 0.03 | 0.02 | 1.64 | 0.10 |
| Individual physical activity | 0.01 | 0.02 | 0.62 | 0.54 | 0.01 | 0.02 | 0.89 | 0.37 |
| Team sport participation | 0.04 | 0.03 | 1.30 | 0.20 | 0.003 | 0.02 | 0.20 | 0.84 |
| Peer Smoking Level | Peer Smoking Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | z | p-value | β | SE | z | p-value | |
| Depression symptoms level | - | - | - | - | -0.09 | 0.03 | -3.02 | 0.003 |
| Smoking level | 0.50 | 0.05 | 10.18 | <0.0001 | -0.11 | 0.03 | -4.18 | <0.0001 |
| Smoking trend | - | - | - | - | 0.30 | 0.03 | 9.53 | <0.0001 |
| Non-White | 0.24 | 0.05 | 5.24 | <0.0001 | -0.02 | 0.03 | -0.91 | 0.36 |
| Female | 0.07 | 0.04 | 1.61 | 0.11 | 0.01 | 0.03 | 0.47 | 0.64 |
| Household smoking | 0.01 | 0.02 | 0.61 | 0.54 | -0.01 | 0.01 | -0.67 | 0.50 |
| Alcohol use (past 30 days) | -0.02 | 0.02 | -1.32 | 0.19 | 0.01 | 0.01 | 1.29 | 0.20 |
| Marijuana use (past 30 days) | 0.02 | 0.02 | 0.94 | 0.35 | 0.01 | 0.01 | 0.40 | 0.69 |
| Individual physical activity | 0.05 | 0.02 | 3.52 | <0.0001 | -0.02 | 0.01 | -1.84 | 0.07 |
| Team sport participation | -0.04 | 0.02 | -2.29 | 0.02 | 0.01 | 0.01 | 1.04 | 0.30 |
|
|
||||||||
Note. Level represents the baseline level for a given growth process; trend represents the rate of change in a given process for a unit change in time; Female (1=Male, 2=Female); Non-white (0=white, 1=non-white); 30-day alcohol use (30-day alcohol use, 1=0 days to 7=20 or more days); 30-day marijuana use (30-day marijuana use, 1=0 times to 6=40 or more times); Household smoking (1=at least one household member smokes, 0=nobody living at home smokes).
Latent Growth Curve Modeling (LGCM)
LGCM was conducted to assess the direct effects depression symptoms on adolescent smoking and the indirect effects through peer smoking. All LGCM were conducted using Mplus version 5.2 software (67). LGCM is a multivariate structural equation modeling method that models repeated measures of an observed variable (e.g., smoking, depression symptoms) on factors (latent/unobserved variables) representing random effects (ηis). The random effects are free to vary by individual, thus capturing individual variability. Latent Growth Curve Models have an intercept factor representing baseline level, and a slope (trend) factor representing growth (e.g., linear, quadratic), or rate of change across time. The shape of the estimated growth curve is determined by defined factor loadings (λs), which are the path coefficients from the trend factor to the observed indicator variable. For instance, if a linear growth curve is believed to be the best representation of the average growth curve, factor loadings from the trend factor to the observed measures would be λ0 (trend factor to initial observed measure) =0, λ1(trend factor to 2nd observed measure) =1, λ2(trend factor to 3rd observed measure) =2, and λ3(trend factor to 4th observed measure) =3; there is a one unit increase in the dependent process (e.g., smoking) for a one unit (e.g., one year) increase in time. The factor loadings from the intercept factor to the observed measures are fixed at 1.0 as intercept does not change with time. Thus, LGCM models individual growth curves over time, with individual (i) specific baseline level and rate of change, and permits estimation of average growth with time.
In the present analysis, we conducted associated processes LGCM. Associated processes LGCM is an extension of single process LGCM that allows testing paths among the random effects (i.e., levels [η0] and trends [η1,2…]) of two or more LGCMs (68). For instance, we tested paths from depression symptoms trend to smoking trend, which allowed us to assess whether on average the rate of change in depression symptoms over time increased (positive effect) or decreased (negative effect) the rate of change in smoking over time. Three associated processes were modeled in the present study, one each for the repeated observed measures of depression symptoms (our exogenous process), adolescent smoking (our endogenous process), and peers smoking (mediating mechanism). We were interested in whether depression symptoms (baseline level or trend) affected smoking progression (trend) directly and indirectly through changes in peer smoking across time (trend). We were also interested in the reverse directional path which would evaluate whether smoking (baseline level or trend) affected changes in depression symptoms (trend) directly across time and indirectly across time through changes in peer smoking (trend). In both the primary and alternate LGCMs we also assessed direct and indirect effects at baseline (level to level paths).
Evaluating model fit
Model fit was evaluated with model chi-square (X2), Comparative Fit Index (CFI), Root Mean Square Error of Approximation (RMSEA), and Standardized Root Mean Residual (SRMR). Suggested fit criteria are non-significant X2, CFI > .95, RMSEA < .05-.08 (69-71). An RMSEA value zero represents exact model fit (69). We used maximum likelihood robust parameter estimation to correct for multivariate non-normality.
Missing data
To account for missing data, multivariate modeling used all available data. Mplus allows modeling with missing data using maximum likelihood estimation of the mean, variance, and covariance parameters, when requested, employing the Expectation Maximization (EM) algorithm, assuming data are missing at random (72). We only accounted for missing data on the repeated measure of smoking, depression symptoms, and peer smoking. Cases with missing data on the covariates were not included in the analysis. Final analyses were based on 1093 adolescents.
RESULTS
Descriptive Statistics
Depression symptoms fluctuated with the non-log transformed mean at 13.90 (SD=9.16) at 9th grade, increasing to 14.54 (SD=10.30) in 10th grade, and dropping to 13.00 (SD=8.85) at 12th grade. These average scores suggest a moderate level of depression symptoms in this sample. The average number of peers smoking increased slowly from 1.79 (SD=2.41) at 9th grade to 2.16 (SD=2.50) at 12th grade. At 9th grade, ~ 10% of the sample smoked at least one cigarette in the past 30 days (non-log transformed mean = 5.59 cigarettes a month, SD = 44.73). This increased to 20% of the sample smoking at least one cigarette in the past 30 days (non-log transformed mean = 20.07 cigarettes a month, SD = 81.03).
Model fit
The three process LGCM (Figure 1) fit the data reasonably well, χ2(93, 1093) =204.70, p < .0001, MLR scaling correction factor=1.125, CFI=.98, RMSEA=.03 (90% CI= .03,.04), probability RMSEA ≤ .05 = 1, SRMR=.02. For smoking, peer smoking, and depression symptoms, we allowed the last factor loading from the trend factor to the 12th grade observed measure to be freely estimated, improving model fit to the observed data. The estimated factor loadings (λ4) for smoking (ηS), depression symptoms (ηDS), and peer smoking (ηPS) were λ4ηS=2.15, λ4 ηDS=1.70, and λ4 ηPS=1.76, respectively.
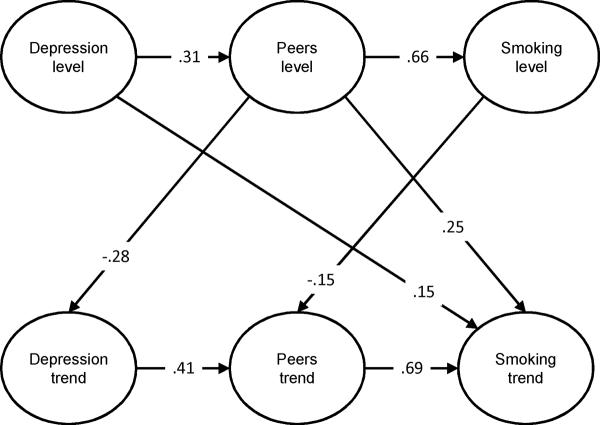
Figure 1.
The Direct and Indirect Effects of Depression on Adolescent Smoking Progression
Note: The model only depicts significant paths among latent variables and presents standardized values.
Direct Effects of Depression on Smoking
The non-standardized regression coefficients (β), standard errors (SE), z-statistics (β/SE), and associated p-values for all model effects are presented in Table 1. Standardized regression coefficients for significant model pathways are presented in Figures 1 and 2.
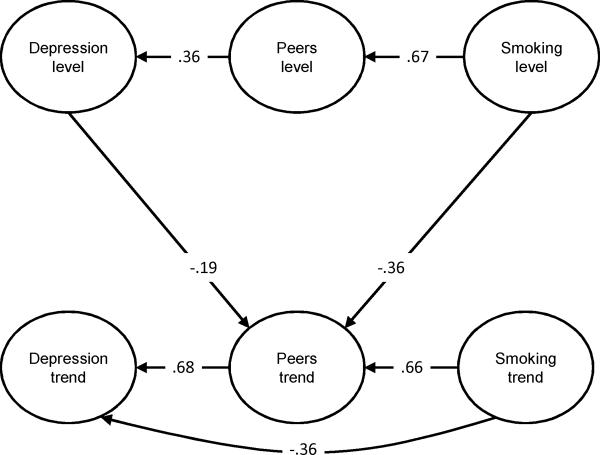
Figure 2.
The Direct and Indirect Effects of Adolescent Smoking on the Development of Depression.
Note: The model only depicts significant paths among latent variables and presents standardized values.
Smoking
Peer smoking level had a significant positive effect on smoking level (β=.90, z=9.79, p<.0001), suggesting that the greater the number of peers smoking at baseline, the greater the baseline level of smoking. Depression symptoms level had a significant positive effect on smoking trend (β=.16, z=2.44, p=.02), which indicated that the higher the depression symptoms at baseline, the greater the acceleration in smoking uptake over time. In addition, peer smoking level had a significant positive effect on smoking trend (β=.22, z=3.80, p<.0001), indicating that a greater number of smoking peers at baseline predicted smoking progression. Peer smoking trend also had a significant positive effect on smoking trend (β=1.45, z=6.52, p<.0001), indicating that the acceleration in the number of peers smoking over time was paralleled by an acceleration in smoking over time. There were no other significant between factor effects on smoking trend.
Peer Smoking
Baseline depression symptoms had a significant positive effect on baseline peer smoking (β=.37, z=5.99, p<.0001) indicating that the greater the baseline level of depression symptoms, the greater the number of peers who smoked at baseline. Baseline smoking had a significant negative effect on peer smoking trend (β= -.05, z= -2.13, p=.03) indicating that the greater the smoking at baseline, the slower the acceleration in the number of peers smoking over time. However, depression symptoms trend had a significant positive effect on peer smoking trend (β=.52, z=3.85, p<.0001), indicating that acceleration in depression symptoms over time was associated with acceleration in the number of peers smoking over time. There were no other significant between factor effects on peer smoking trend.
Depression Symptoms
To control for the effects of baseline smoking and peer smoking, the multivariate model also estimated the effects of baseline levels of repeated measures of these two variables on depression symptoms trend. Peer smoking baseline level had a significant negative effect on depression symptoms trend (β= -.09, z= -2.61, p=.01), indicating that the greater the peer smoking at baseline, the slower the acceleration in depression symptoms over time. There were no other significant effects on depression symptoms trend.
Indirect Effects of Depression on Smoking
We evaluated whether there were significant indirect effects of depression symptoms on smoking progression through peer smoking. As such, we tested indirect effects from depression symptoms level to smoking level through peer smoking level, depression symptoms level on smoking trend through peer smoking level or trend, and for depression symptoms trend to smoking trend through peer smoking trend, for significance with Delta method standard errors (67). The total effect, total and specific indirect effects, and direct effects are presented in Table 2, along with the 95% confidence intervals for the significant indirect effects. To assess the strength of a mediated effect on a continuum from absense of mediation to complete mediation, we estimated the ratio of the specific indirect effect to total effect, the effect proportion mediated (Bindirect / Btotal) (73). Further, the 95% confidence interval (CI), if it does not include zero, provides a measure of the strength of the indirect effect, suggesting minimum and maximum effects (73).
Table 2.
Partitioning the mediated paths: Total effects, direct effects, and indirect effects.
| Primary model: Depression symptoms predict smoking | ||||||
|---|---|---|---|---|---|---|
| 95% CIb | ||||||
| Effect | Estimate | S.E.a | Estimate/S.E. | p-value | Low | High |
| Depression symptoms level to peer smoking level to smoking level | ||||||
| Total effect | .26 | .08 | 3.13 | .002 | .10 | .42 |
| Total indirect effectc | .33 | .07 | 5.09 | <.0001 | .21 | .46 |
| Specific indirect effectd | .33 | .07 | 5.09 | <.0001 | .21 | .46 |
| Direct effect | -.07 | .08 | -.95 | .34 | -.22 | .08 |
| Depression symptoms level to peer smoking level to smoking trend | ||||||
| Total effect | .13 | .05 | 2.48 | .01 | .03 | .23 |
| Total indirect effect | -.03 | .05 | -.50 | .62 | -.13 | .08 |
| Specific indirect effect | .08 | .02 | 3.35 | .001 | .03 | .13 |
| Direct effect | .16 | .06 | 2.44 | .02 | .03 | .28 |
| Depression symptoms trend to peer smoking trend to smoking trend | ||||||
| Total effect | .29 | .22 | 1.29 | .20 | -.15 | .72 |
| Total indirect effect | .75 | .24 | 3.11 | .002 | .28 | 1.23 |
| Specific indirect effect | .75 | .24 | 3.11 | .002 | .28 | 1.23 |
| Direct Effect | -.47 | .26 | -1.76 | .08 | -.98 | .05 |
| Alternative model: Smoking predicts depression symptoms | ||||||
|---|---|---|---|---|---|---|
| 95% CI | ||||||
| Effect | Estimate | S.E. | Estimate/S.E. | p-value | Low | High |
| Smoking level to peer smoking level to depression symptoms level | ||||||
| Total effect | .09 | .03 | 3.08 | .002 | .03 | .14 |
| Total indirect | .15 | .04 | 4.08 | <.0001 | .08 | .23 |
| Specific indirect effect | .15 | .04 | 4.08 | <.0001 | .08 | .23 |
| Direct effect | -.07 | .05 | -1.37 | .17 | -.16 | .03 |
| Smoking level to peer smoking trend to depression symptoms trend | ||||||
| Total effect | -.04 | .02 | -1.73 | .08 | -.08 | .01 |
| Total indirect effect | -.10 | .04 | -2.97 | .003 | -.17 | -.04 |
| Specific indirect effect | -.06 | .02 | -2.76 | .01 | -.11 | -.02 |
| Direct effect | .07 | .04 | 1.71 | .09 | -.01 | .15 |
| Smoking trend to peer smoking trend to depression symptoms trend | ||||||
| Total effect | .04 | .03 | 1.17 | .24 | -.02 | .10 |
| Total indirect effect | .18 | .06 | 2.97 | .003 | .06 | .30 |
| Specific indirect effect | .18 | .06 | 2.97 | .003 | .06 | .30 |
| Direct effect | -.15 | .07 | -2.14 | .03 | -.28 | -.01 |
Note: Only significant indirect effects are reported
Delta Method Standard Errors
95% confidence intervals containing zero are not significant
total indirect effect represents the total of all indirect effects, including the specific indirect effect presented
the specific indirect effect is the significant indirect effect. Non-significant indirect effects are not presented in this table for parsimony
Depression symptoms level to smoking level
The total effect of depression symptoms level to smoking level, including the direct and indirect effects through peer smoking, was significant (Btotal = .26, z = 3.13, p=.002, 95% CI = .10, .42). The specific indirect effect of baseline depression symptoms on baseline smoking through baseline peer smoking was significant (Bindirect = .33, z = 5.09, p<.0001, 95% CI = .21, .46). As the direct effect of depression symptoms level on smoking level (Bdirect= -.07 z = -.95, p=.34, 95% CI = -.22, .08) was negative, the effect proportion mediated exceeded 1.00 (i.e., Bindirect = .33/ Btotal = .26 = 1.27). However, because the direct effect (Bdirect) was not significant, we capped the proportion mediated effect at a value of 1.00 (73). Thus, we can conclude that increases in baseline depression symptoms predict an increase in the number of smoking peers at baseline, which in turn, predicts baseline smoking level (complete mediation).
Depression symptoms level to smoking trend
The total effect of depression symptoms level to smoking trend, including the direct and indirect effects through peer smoking, was significant (Btotal = .13, z = 2.48, p=.01, 95% CI = .03, .23). The specific indirect effect of baseline depression symptoms on smoking trend through baseline peer smoking was significant (Bindirect = .08, z = 3.35, p=.001, 95% CI = .03, .13). The direct effect of depression symptoms level on smoking trend (Bdirect = .16 z = 2.44, p=.02, 95% CI = .03, .28) was also significant. The effect proportion mediated (i.e., Bindirect = .08/ Btotal = .13) was .61, suggesting only partial mediation. Thus, higher baseline depression symptoms predict a greater number of smoking peers at baseline, which in part, predicts smoking progression.
Depression symptoms trend to smoking trend
The total effect of depression symptoms trend to smoking trend was not significant (Btotal = .29, z = 1.29, p=.20, 95% CI = -.15, .72). However, depression symptoms trend had a significant indirect effect on smoking trend through peer smoking trend (Bindirect = .75, z = 3.11, p=.002, 95% CI = .28, 1.23). As the direct effect of depression symptoms trend on smoking trend (Bdirect = -.47 z = 1.76, p=.08, 95% CI = -.98, .05) was negative, the effect proportion mediated exceeded 1.00 (i.e., Bindirect = .75/ Btotal = .29 = 2.59). However, because the direct effect (Bdirect) was not significant, we capped the proportion mediated effect at a value of 1.00 (73). Thus, we can conclude that increases in depression symptoms over time predicts an increase in the number of smoking peers, which in turn, predicts smoking progression (complete mediation).
Direct Effects of Smoking on Depression: The Alternative Model
We also assessed an alternative directional path whereby smoking affects the development of depression symptoms directly as well as indirectly through peer smoking and physical activity. Standardized regression coefficients for significant model pathways are presented in Figure 2.
Depression symptoms
Peer smoking level had a significant positive effect on depression symptoms level (β=.31, z=4.30, p<.0001), indicating that the greater the number of peers smoking at baseline, the higher the depression symptoms at baseline. Peer smoking trend also had a significant positive effect on rate of change in depression symptoms from 9th to 12th grade. (β = .60, z= 3.21, p=.001). These results indicated that acceleration in the number of peers smoking over time was associated with an acceleration in depression symptoms over time. Smoking trend though had a significant negative effect on depression symptoms trend (β = -.15, z= -2.14, p=.03) indicating that acceleration in smoking over time was associated with a deceleration (or slowing of acceleration) in depression symptoms over time.
Peer smoking
Baseline smoking (level) had a significant positive effect on peer smoking level (β = .50, z= 10.18, p<.0001), indicating that greater baseline smoking predicted a higher number of smoking peers at baseline. However, baseline smoking had a significant negative effect on peer smoking trend (β = -.11, z= -4.18, p<.0001). These results considered together indicate that adolescents who have higher levels of smoking at age 14 also have more peers who smoke at this age and may reach a ceiling in the number of smoking peers than those who do not smoke regularly at mid adolescence. Smoking trend had a significant positive effect on peer smoking trend (β =.30, z=9.53, p<.0001), indicating that acceleration in smoking predicted increases in the number of smoking peers across time. Finally, depression symptoms baseline level (β = -.09, z= -3.02, p=.003) had a significant negative effect on peer smoking trend suggesting that the higher the baseline level of depression symptoms the slower the acceleration in the number of peers smoking over time.
Smoking
To control for the effects of baseline depression and peer smoking, the multivariate model also estimated the effects of baseline levels of these two variables on smoking trend. However, there were no significant effects on smoking trend.
Indirect Effects of Smoking on Depression
To evaluate whether smoking affects the development of depression symptoms indirectly through peer smoking we tested several indirect pathways for significance, reversing the pathways tested in the first model (See Figure 2). First, we tested the effect of baseline smoking (level) on baseline depression symptoms (level) through baseline peer smoking level. This indirect effect was significant (Bindirect = .15, z = 4.08, p<.0001, 95% CI = .08, .23). The total effect was also significant (Btotal = .09, z = 3.08, p=.002, 95% CI = .03, .14). The effect proportion mediated exceeded 1 (Bindirect /Btotal = 1.67). However, as the direct effect was negative and non-significant (see Table 2), the effect proportion mediated was capped at 1.00, and we can conclude that the effect of baseline smoking on baseline depression symptoms was completely mediated through baseline peer smoking.
In addition to the indirect effect of baseline smoking to depression symptoms level, the indirect effect of baseline smoking level to depression symptoms trend through peer smoking trend was also significant (Bindirect = -.06, z = -2.76, p=.01, 95% CI = -.11, -.02), and there was a significant indirect effect from smoking trend to depression symptoms trend (Bindirect = .18, z = 2.97, p=.003, 95% CI = .06, .30). The total effect of baseline smoking on depression symptoms trend was not significant (Btotal = -.04, z = -1.73, p=.08, 95% CI = -.08, .01). The effect proportion mediated for this indirect effect was (Bindirect = -.06/ Btotal = -.04) = 1.50, which exceeds 1.0. This resulted from a positive direct effect from baseline smoking to depression symptoms trend (Bdirect = .07, z = 1.71, p=.09, 95% CI = -.01, .15). However, because the direct effect (Bdirect was not significant, we capped the proportion mediated effect at a value of 1.00 (73). Thus, we can conclude that the effect of baseline smoking on rate of change in depression symptoms over time, is due to in the number of peers who smoke, indicating complete mediation.
The indirect effect of smoking trend on depression symptoms trend was more complicated. The total effect was not significant (Btotal = .04, z = 1.17, p=.24, 95% CI = -.02, .10). The effect proportion mediated, though, exceeded 1.00 [i.e., (Bindirect = .18/ Btotal = .04) = 4.5]. This is because the direct effect of smoking trend on depression symptoms trend was negative (Bdirect = -.15, z = -2.14, p=.03, 95% CI = -.28, -.01). However, unlike the case of the indirect effect of baseline smoking level on depression symptoms trend in which the direct effect was not significant, this direct effect was significant, indicating effect suppression (73). In the presence of effect suppression, the calculated proportion of mediation is not meaningful and the direct effect cannot be ignored. This suggests that holding peer smoking rate of change (trend) constant, the direct effect of smoking trend on depression symptoms trend is significant and negative.
For exploratory purposes, we evaluated the moderating role of gender in the direct and indirect relationship between depression and smoking. There were no signficant gender differences in either two-group LGCM.
DISCUSSION
The present study found evidence for a bi-directional relationship between adolescent depression and smoking. Peer smoking helped account for the comorbidity. Depression symptoms measured at mid adolescence (age 14) predicted smoking progression across mid to late adolescence (ages 14-18 years old). Peer smoking mediated these developmental influences such that higher depression symptoms predicted an increase in the number of smoking peers, which in turn predicted smoking progression. The assessment of the alternative directional path indicated that smoking progression predicted a deceleration of depression symptoms from mid to late adolescence. A significant indirect effect indicated that greater smoking at baseline predicted a deceleration in the number of smoking peers across time, which predicted a deceleration in depression symptoms from mid adolescence to late adolescence.
The presence of a bi-directional relationship is partially consistent with previous research (17-19). In contrast to those studies that found smoking to be a risk factor for depression and depression to be a risk factor for smoking, the bi-directional influences observed in the present study support a self-medication hypothesis for both directional paths. That is, depression contributed to smoking uptake and smoking progression contributed to a dampening or leveling off of depression symptoms. A recent cross-sectional study of smoking associated mood variation found that adolescent smokers reported higher positive affect and lower negative affect after smoking, but these mood modulation effects diminished with high levels of smoking experience (74). Variability in negative mood or unstable affect regulation appears to be a risk factor for smoking escalation among adolescents and smoking serves to stabilize or decrease the variability in negative mood states (75). This study extends this research by showing prospectively that depression contributes to smoking uptake and that smoking modulates depression symptoms. These reciprocal relationships may reinforce and maintain smoking behavior into adulthood.
These findings also highlight the notion that there may be overlap in the neural substrates modified by smoking and anti-depressant medication (76). For example, nicotinic acetylcholine receptors are thought to be involved in modulating the release of several neurotransmitters implicated in mood, such as serotonin, norepinephrine, dopamine, GABA and glutamate (76, 77). Nicotine receptor inhibition appears to mitigate mood instability and may reduce depression (78). It is possible that long term (or even shorter term) smoking may be followed by adaptations or tolerance to nicotine, which may promote negative affective states such as depression (77, 79). The mood modulating or affective regulating functions of nicotine may decline with a greater smoking history (74, 75). As the epidemiological studies of the depression-smoking relationship extend into young adulthood, capturing longer smoking histories and higher smoking rates, findings that smoking predicts depression are consistent with this explanation (14, 24).
Our bi-directional findings may have differed, in part, from previous research (7, 10, 17-19) because we used repeated measures of depression and smoking to account for baseline levels and changes across time. This may have allowed us to better capture how these two variables impact each other from mid adolescence to late adolescence (80). We also controlled for some confounding variables (17-19, 81) and considered a more complex relationship between smoking and depression, which included an evaluation of indirect effects.
The findings of this study also indicate that depression contributes to smoking acquisition indirectly by increasing adolescent vulnerability to peers who smoke. The impact of peer smoking on adolescent smoking acquisition is well documented (43). Adolescents with higher levels of depression may be more sensitive to peer behavior, more likely to select nonconventional peers, or both (45, 46, 82). A greater number of smoking peers provides better access to cigarettes, promotes a normative perception of smoking, and may be a source of peer approval (38, 83). These issues may be especially salient for youth with higher levels of depression symptoms.
Of note, the alternative indirect path showed that smoking at mid adolescence (age 14) predicted a leveling off in the number of smoking peers across time, which in turn, contributed to a deceleration of depression symptoms. This finding is contrary to what we would have expected, although cross-sectional research has shown that adolescent smokers tend to have higher depression symptoms, if they attend a school with a lower smoking prevalence (83). This may reflect the importance of peer group belonging and perceptions of normative behavior on depression. Alternatively, this finding may simply reflect a positive relationship between greater adolescent smoking and a greater number of peers who smoke at baseline, such that fewer smoking peers are acquired across time. The leveling off in the number of peer smokers is then positively linked to the leveling off in depression symptoms. The positive trend to trend relationship between smoking progression, greater number of peers smoking, and an increase in depression symptoms across time was suppressed by a significant and negative direct effect between smoking progression and deceleration in depression symptoms (73). This may simply exemplify the complexity of the relationship between adolescent smoking and depression, reflect that the peer smoking variable measured the quantity of peers who smoke across time but did not account for the quality and types of peer relationships that may impact mood, and/or highlights the importance of other common and unique mechanisms.
Research indicates that almost 25% of adolescents are regular smokers (37, 53), about 25% of adolescents have had at least one major depressive episode by 18 years of age (2), and 20-30% of adolescents experience depressive symptoms (64, 81, 84, 85). The high rates of comorbidity between depression and smoking emphasize the importance of targeting smoking prevention efforts to this high-risk group. Interventions that have components on depression prevention and management could have an important impact on smoking uptake as well as subsequent depression. Social influence-based models of smoking prevention or intervention address peer influences to smoke (e.g., cigarette offer refusal skills). However, it may also be important to address these issues from the standpoint of adolescent depression. For example, programs that address coping and negative mood management skills, limited social networks, need for peer approval, and accessing nonsmoking peer groups may be especially beneficial for adolescents with elevated depression symptoms. Preliminary findings indicate that social learning-based approaches to smoking prevention tend to be especially helpful for depressed adolescent boys (86).
The strengths of the present study include the collection of data from a large group of adolescents, repeated measurements of smoking, depression and peer smoking across mid to late adolescence, an analytic plan that is consistent with the longitudinal nature of the data and the concept of comorbidity, inclusion of potential confounding variables, and a good retention rate. One limitation is that self-reports of depression symptoms were not confirmed by formal clinical interview. Thus, we cannot determine whether these adolescents had a history of major depression or if they met criteria for a current diagnosis. Similarly, our baseline measure of depression (depression level factor) does not encompass depression history. However, our measure of smoking at baseline (smoking level factor) does consider smoking history. Future prospective research should include a baseline variable that encompasses lifetime and present depression. Also, we did not measure co-morbid and potentially confounding psychiatric symptoms, such as anxiety (17, 85, 87). Although it may be considered a limitation that our model did not include nicotine dependence, our study shows important relationships between depression symptoms and regular smoking, which may or may not reflect nicotine dependent smoking. Many of these adolescent smokers may continue to smoke into adulthood, potentially solidifying a link between smoking, nicotine dependence, and depression. Indeed, data suggests a bi-directional relationship between smoking, nicotine dependence and major depression in young adults ages 18-31 years old (23, 24).
In addition, although our sample was 34% minority, we did not have enough adolescents in any one racial group to evaluate racial differences in the link between depression and smoking. Finally, a common limitation of protocols requiring active consent is the rate of nonresponse (88-90). Although 75% of those parents who responded provided consent and the differences between those who provided consent and those who declined were relatively small and few (47), caution is warranted in generalizing the results of this study. However, our sample was nationally and locally representative on basic demographic characteristics (91-93), and the smoking rates are fairly comparable to those found in national surveys for the geographical area of our sample (91, 94-96), and the region and the population of high school students in the county from which the sample was drawn.
In conclusion, the present study provides the first evidence of bi-directional self-medication processes in the relationship between adolescent smoking and depression and suggests that peer smoking behavior may help account for the comorbidity. Further research on mechanisms may provide novel behavioral and pharmacological intervention targets for adolescent smoking and/or depression. Based on these findings, targeting depression could have an important impact on smoking uptake as well as subsequent depression. The public health implications of further research could have a significant bearing on the psychosocial, economic, and medical morbidity and premature mortality that are associated with these conditions.
Acknowledgements
This study was supported by a Transdisciplinary Tobacco Use Research Center grant from the National Cancer Institute and the National Institute on Drug Abuse P50 84718 and National Cancer Institute RO1 CA109250. The funding agency had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data or in the preparation, review or approval of the manuscript. All authors had full access to all of the data in the study. None of the authors have a conflict of interest in the submission of this manuscript. The Principal Investigator (J.A.M.) takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.FLEMING JE, OFFORD DR. Epidemiology of childhood depressive disorders: a critical review. J Am Acad Child Adolesc Psychiatry. 1990;29:571–80. doi: 10.1097/00004583-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 2.LEWINSOHN PM, HOPS H, ROBERTS RE, SEELEY JR, ANDREWS JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSMIII-R disorders in high school students. J Abnorm Psychol. 1993;102:133–44. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- 3.MOKDAD AH, MARKS JS, STROUP DF, GERBERDING JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.MURRAY CJ, LOPEZ AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–3. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 5.MURRAY CJ, LOPEZ AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 6.ESCOBEDO LG, REDDY M, GIOVINO GA. The relationship between depressive symptoms and cigarette smoking in US adolescents. Addiction. 1998;93:433–40. doi: 10.1046/j.1360-0443.1998.93343311.x. [DOI] [PubMed] [Google Scholar]
- 7.FERGUSSON DM, GOODWIN RD, HORWOOD LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol Med. 2003;33:1357–67. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- 8.KILLEN JD, ROBINSON TN, HAYDEL KF, et al. Prospective study of risk factors for the initiation of cigarette smoking. J Consult Clin Psychol. 1997;65:1011–6. doi: 10.1037//0022-006x.65.6.1011. [DOI] [PubMed] [Google Scholar]
- 9.PATTON GC, CARLIN JB, COFFEY C, et al. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health. 1998;88:1518–22. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WANG MQ, FITZHUGH EC, GREEN BL, et al. Prospective socialpsychological factors of adolescent smoking progression. J Adolesc Health. 1999;24:2–9. doi: 10.1016/s1054-139x(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 11.ROHDE P, LEWINSOHN PM, BROWN RA, GAU JM, KAHLER CW. Psychiatric disorders, familial factors and cigarette smoking: I. Associations with smoking initiation. Nicotine Tob Res. 2003;5:85–98. doi: 10.1080/1462220031000070507. [DOI] [PubMed] [Google Scholar]
- 12.ROHDE P, KAHLER CW, LEWINSOHN PM, BROWN RA. Psychiatric disorders, familial factors, and cigarette smoking: II. Associations with progression to daily smoking. Nicotine Tob Res. 2004;6:119–32. doi: 10.1080/14622200310001656948. [DOI] [PubMed] [Google Scholar]
- 13.CHOI WS, PATTEN CA, GILLIN JC, KAPLAN RM, PIERCE JP. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann Behav Med. 1997;19:42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- 14.GOODMAN E, CAPITMAN J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106:748–55. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- 15.STEUBER TL, DANNER F. Adolescent smoking and depression: which comes first? Addict Behav. 2006;31:133–6. doi: 10.1016/j.addbeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.WU LT, ANTHONY JC. Tobacco smoking and depressed mood in late childhood and early adolescence. Am J Public Health. 1999;89:1837–40. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BROWN RA, LEWINSOHN PM, SEELEY JR, WAGNER EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J Am Acad Child Adolesc Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 18.WANG MQ, FITZHUGH EC, TURNER L, FU Q, WESTERFIELD RC. Association of depressive symptoms and school adolescents' smoking: a cross-lagged analysis. Psychol Rep. 1996;79:127–30. doi: 10.2466/pr0.1996.79.1.127. [DOI] [PubMed] [Google Scholar]
- 19.WINDLE M, WINDLE RC. Depressive symptoms and cigarette smoking among middle adolescents: prospective associations and intrapersonal and interpersonal influences. J Consult Clin Psychol. 2001;69:215–26. [PubMed] [Google Scholar]
- 20.DIERKER LC, AVENEVOLI S, STOLAR M, MERIKANGAS KR. Smoking and depression: an examination of mechanisms of comorbidity. Am J Psychiatry. 2002;159:947–53. doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed] [Google Scholar]
- 21.KENDLER KS, NEALE MC, MACLEAN CJ, et al. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 22.LYONS M, HITSMAN B, XIAN H, et al. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine Tob Res. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- 23.BRESLAU N, KILBEY MM, ANDRESKI P. Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993;50:31–5. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- 24.BRESLAU N, PETERSON E, SCHULTZ L, CHILCOAT H, ANDRESKI P. Major depression and stages of smoking: A longitudinal investigation. Archives of General Psychiatry. 1998;55:165–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- 25.MARTINI S, WAGNER FA, ANTHONY JC. The association of tobacco smoking and depression in adolescence: evidence from the United States. Subst Use Misuse. 2002;37:1853–67. doi: 10.1081/ja-120014087. [DOI] [PubMed] [Google Scholar]
- 26.MUNAFO MR, HITSMAN B, RENDE R, METCALFE C, NIAURA R. Effects of progression to cigarette smoking on depressed mood in adolescents: evidence from the National Longitudinal Study of Adolescent Health. Addiction. 2008;103:162–71. doi: 10.1111/j.1360-0443.2007.02052.x. [DOI] [PubMed] [Google Scholar]
- 27.DIERKER LC, AVENEVOLI S, MERIKANGAS KR, FLAHERTY BP, STOLAR M. Association between psychiatric disorders and the progression of tobacco use behaviors. J Am Acad Child Adolesc Psychiatry. 2001;40:1159–67. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 28.JOHNSON EO, RHEE SH, CHASE GA, BRESLAU N. Comorbidity of depression with levels of smoking: an exploration of the shared familial risk hypothesis. Nicotine Tob Res. 2004;6:1029–38. doi: 10.1080/14622200412331324901. [DOI] [PubMed] [Google Scholar]
- 29.KASSEL JD, STROUD LR, PARONIS CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 30.KASSEL JD, HANKIN BL. Smoking and depression. In: Steptoe A, editor. Depression and Physical Illness. Cambridge University Press; New York, NY: 2006. pp. 321–347. [Google Scholar]
- 31.KHANTZIAN EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 32.LEWIS-ESQUERRE JM, RODRIGUE JR, KAHLER CW. Development and validation of an adolescent smoking consequences questionnaire. Nicotine Tob Res. 2005;7:81–90. doi: 10.1080/14622200412331328475. [DOI] [PubMed] [Google Scholar]
- 33.WAHL SK, TURNER LR, MERMELSTEIN RJ, FLAY BR. Adolescents' smoking expectancies: psychometric properties and prediction of behavior change. Nicotine Tob Res. 2005;7:613–23. doi: 10.1080/14622200500185579. [DOI] [PubMed] [Google Scholar]
- 34.KASSEL JD, EVATT DP, GREENSTEIN JE, et al. The Acute Effects of Nicotine on Positive and Negative Affect in Adolescent Smokers. J Abnorm Psychol. 2007 doi: 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- 35.AUDRAIN-MCGOVERN J, RODRIGUEZ D, PATEL V, et al. How do psychological factors influence adolescent smoking progression? The evidence for indirect effects through tobacco advertising receptivity. Pediatrics. 2006;117:1216–25. doi: 10.1542/peds.2005-0808. [DOI] [PubMed] [Google Scholar]
- 36.AUDRAIN-MCGOVERN J, RODRIGUEZ D, TERCYAK KP, NEUNER G, MOSS HB. The impact of self-control indices on peer smoking and adolescent smoking progression. J Pediatr Psychol. 2006;31:139–51. doi: 10.1093/jpepsy/jsi079. [DOI] [PubMed] [Google Scholar]
- 37.CHOI WS, PIERCE JP, GILPIN EA, FARKAS AJ, BERRY CC. Which adolescent experimenters progress to established smoking in the United States. American Journal of Preventive Medicine. 1997;13:385–391. [PubMed] [Google Scholar]
- 38.RITT-OLSON A, UNGER J, VALENTE T, et al. Exploring peers as a mediator of the association between depression and smoking in young adolescents. Subst Use Misuse. 2005;40:77–98. doi: 10.1081/ja-200030505. [DOI] [PubMed] [Google Scholar]
- 39.HOFFMAN BR, MONGE PR, CHOU CP, VALENTE TW. Perceived peer influence and peer selection on adolescent smoking. Addict Behav. 2007;32:1546–54. doi: 10.1016/j.addbeh.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 40.WANG MQ, EDDY JM, FITZHUGH EC. Smoking acquisition: peer influence and self-selection. Psychol Rep. 2000;86:1241–6. doi: 10.2466/pr0.2000.86.3c.1241. [DOI] [PubMed] [Google Scholar]
- 41.WILLS TA, CLEARY SD. Peer and adolescent substance use among 6th-9th graders: latent growth analyses of influence versus selection mechanisms. Health Psychology. 1999;18:453–63. doi: 10.1037//0278-6133.18.5.453. [DOI] [PubMed] [Google Scholar]
- 42.KANDEL DB. Similarity in real-life adolescent friendship pairs. Journal of Personality Social Psychology. 1978;36:306–312. [Google Scholar]
- 43.KOBUS K. Peers and adolescent smoking. Addiction. 2003;98(Suppl 1):37–55. doi: 10.1046/j.1360-0443.98.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 44.PRINSTEIN MJ, BOERGERS J, SPIRITO A. Adolescents' and their friends' health-risk behavior: factors that alter or add to peer influence. J Pediatr Psychol. 2001;26:287–98. doi: 10.1093/jpepsy/26.5.287. [DOI] [PubMed] [Google Scholar]
- 45.CONNELL AM, DISHION TJ. The contribution of peers to monthly variation in adolescent depressed mood: a short-term longitudinal study with time-varying predictors. Dev Psychopathol. 2006;18:139–54. doi: 10.1017/S0954579406060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FERGUSSON DM, WANNER B, VITARO F, HORWOOD LJ, SWAIN-CAMPBELL N. Deviant peer affiliations and depression: confounding or causation? J Abnorm Child Psychol. 2003;31:605–18. doi: 10.1023/a:1026258106540. [DOI] [PubMed] [Google Scholar]
- 47.AUDRAIN J, TERCYAK KP, GOLDMAN P, BUSH A. Recruiting adolescents into genetic studies of smoking behavior. Cancer Epidemiol Biomarkers Prev. 2002;11:249–52. [PubMed] [Google Scholar]
- 48.RADLOFF LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 49.LEWINSOHN PM, ROHDE P, SEELEY JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin Psychol Rev. 1998;18:765–94. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 50.RADLOFF LS. The use of the center for epidemiologic studies depression scale in adolescents and young adults. Journal of Youth and Adolescence. 1991;20:149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- 51.ROBERTS RE. Assessment of depression in adolescents using the center for epidemiologic studies depression scale. Psychol Assessment. 1990;2:122–128. [Google Scholar]
- 52.ROBERTS RE, LEWINSOHN PM, SEELEY JR. Screening for adolescent depression: a comparison of depression scales. J Am Acad Child Adolesc Psychiatry. 1991;30:58–66. doi: 10.1097/00004583-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: 2006. [Google Scholar]
- 54.AUDRAIN-MCGOVERN J, RODRIGUEZ D, MOSS HB. Smoking progression and physical activity. Cancer Epidemiol Biomarkers Prev. 2003;12:1121–9. [PubMed] [Google Scholar]
- 55.AUDRAIN-MCGOVERN J, RODRIGUEZ D, TERCYAK KP, et al. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiol Biomarkers Prev. 2004;13:2023–34. [PubMed] [Google Scholar]
- 56.BERGEN HA, MARTIN G, ROEGER L, ALLISON S. Perceived academic performance and alcohol, tobacco and marijuana use: longitudinal relationships in young community adolescents. Addict Behav. 2005;30:1563–73. doi: 10.1016/j.addbeh.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 57.ESCOBEDO LG, REDDY M, DURANT RH. Relationship between cigarette smoking and health risk and problem behaviors among US adolescents. Arch Pediatr Adolesc Med. 1997;151:66–71. doi: 10.1001/archpedi.1997.02170380070011. [DOI] [PubMed] [Google Scholar]
- 58.FERGUSSON DM, HORWOOD LJ, SWAIN-CAMPBELL N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–35. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 59.KELDER SH, MURRAY NG, ORPINAS P, et al. Depression and substance use in minority middle-school students. Am J Public Health. 2001;91:761–6. doi: 10.2105/ajph.91.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LEWINSOHN PM, BROWN RA, SEELEY JR, RAMSEY SE. Psychosocial correlates of cigarette smoking abstinence, experimentation, persistence and frequency during adolescence. Nicotine Tob Res. 2000;2:121–31. doi: 10.1080/713688129. [DOI] [PubMed] [Google Scholar]
- 61.NORRIS R, CARROLL D, COCHRANE R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J Psychosom Res. 1992;36:55–65. doi: 10.1016/0022-3999(92)90114-h. [DOI] [PubMed] [Google Scholar]
- 62.RAO U, RYAN ND, DAHL RE, et al. Factors associated with the development of substance use disorder in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:1109–17. doi: 10.1097/00004583-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 63.RODRIGUEZ D, AUDRAIN-MCGOVERN J. Physical activity, global physical self-concept, and adolescent smoking. Ann Behav Med. 2005;30:251–9. doi: 10.1207/s15324796abm3003_9. [DOI] [PubMed] [Google Scholar]
- 64.SALUJA G, IACHAN R, SCHEIDT PC, et al. Prevalence of and risk factors for depressive symptoms among young adolescents. Arch Pediatr Adolesc Med. 2004;158:760–5. doi: 10.1001/archpedi.158.8.760. [DOI] [PubMed] [Google Scholar]
- 65.SOLDZ S, CUI X. Pathways through adolescent smoking: a 7-year longitudinal grouping analysis. Health Psychol. 2002;21:495–504. [PubMed] [Google Scholar]
- 66.WILLS TA, VACCARO D, MCNAMARA G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger's theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 67.MUTHÉN LK, MUTHÉN BO. Mplus user's guide. Muthén& Muthén; Los Angeles, CA: 1998-2007. [Google Scholar]
- 68.DUNCAN TE, DUNCAN SC. An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Applications. Lawrence Erlbaum Associates; Mahwah, NJ: 1999. [Google Scholar]
- 69.CURRAN PJ, BOLLEN KA. Finite sampling properties of the point estimates and confidence intervals of the RMSEA. Sociological Methods & Research. 2003;32:208–252. [Google Scholar]
- 70.LOEHLIN JC. Latent variable models: An introduction to factor, path, and structural equation analysis. Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- 71.MUTHÉN LK, MUTHÉN BO. Mplus User's Guide. Muthén & Muthén; Los Angeles, CA: 2001. [Google Scholar]
- 72.MUTHÉN BO. Mplus technical appendices. Muthén & Muthén; Los Angeles, CA: 1998-2004. [Google Scholar]
- 73.SHROUT PE, BOLGER N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–45. [PubMed] [Google Scholar]
- 74.HEDEKER D, MERMELSTEIN RJ, BERBAUM ML, CAMPBELL RT. Modeling mood variation associated with smoking: an application of a heterogeneous mixedeffects model for analysis of ecological momentary assessment (EMA) data. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.WEINSTEIN SM, MERMELSTEIN R, SHIFFMAN S, FLAY B. Mood variability and cigarette smoking escalation among adolescents. Psychol Addict Behav. 2008;22:504–13. doi: 10.1037/0893-164X.22.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.QUATTROCKI E, BAIRD A, YURGELUN-TODD D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8:99–110. [PubMed] [Google Scholar]
- 77.PICCIOTTO MR, BRUNZELL DH, CALDARONE BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 78.SHYTLE RD, SILVER AA, LUKAS RJ, et al. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–35. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 79.BALFOUR DJ, RIDLEY DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 80.WIDIGER TA, CLARK LA. Toward DSM-V and the classification of psychopathology. Psychol Bull. 2000;126:946–63. doi: 10.1037/0033-2909.126.6.946. [DOI] [PubMed] [Google Scholar]
- 81.FERGUSSON DM, HORWOOD LJ, RIDDER EM, BEAUTRAIS AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- 82.ALLEN JP, PORTER MR, MCFARLAND FC. Leaders and followers in adolescent close friendships: susceptibility to peer influence as a predictor of risky behavior, friendship instability, and depression. Dev Psychopathol. 2006;18:155–72. doi: 10.1017/S0954579406060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.CHAITON MO, ZHANG B. Environment modifies the association between depression symptoms and smoking among adolescents. Psychol Addict Behav. 2007;21:420–4. doi: 10.1037/0893-164X.21.3.420. [DOI] [PubMed] [Google Scholar]
- 84.HORWATH E, JOHNSON J, KLERMAN GL, WEISSMAN MM. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Arch Gen Psychiatry. 1992;49:817–823. doi: 10.1001/archpsyc.1992.01820100061011. [DOI] [PubMed] [Google Scholar]
- 85.LEWINSOHN PM, SHANKMAN SA, GAU JM, KLEIN DN. The prevalence and co-morbidity of subthreshold psychiatric conditions. Psychol Med. 2004;34:613–22. doi: 10.1017/S0033291703001466. [DOI] [PubMed] [Google Scholar]
- 86.SUN P, MIYANO J, ROHRBACH LA, DENT CW, SUSSMAN S. Short-term effects of Project EX-4: a classroom-based smoking prevention and cessation intervention program. Addict Behav. 2007;32:342–50. doi: 10.1016/j.addbeh.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LEWINSOHN PM, ZINBARG R, SEELEY JR, LEWINSOHN M, SACK WH. Lifetime comorbidity among anxiety disorders and between anxiety disorders and other mental disorders in adolescents. J Anxiety Disord. 1997;11:377–94. doi: 10.1016/s0887-6185(97)00017-0. [DOI] [PubMed] [Google Scholar]
- 88.DENT CW, GALAIF J, SUSSMAN S, et al. Demographic, psychosocial and behavioral differences in samples of actively and passively consented adolescents. Addict Behav. 1993;18:51–6. doi: 10.1016/0306-4603(93)90008-w. [DOI] [PubMed] [Google Scholar]
- 89.HARRINGTON KF, BINKLEY D, REYNOLDS KD, et al. Recruitment issues in school-based research: lessons learned from the High 5 Alabama Project. J Sch Health. 1997;67:415–21. doi: 10.1111/j.1746-1561.1997.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 90.O'DONNELL LN, DURAN RH, SAN DOVAL A, et al. Obtaining written parent permission for school-based health surveys of urban young adolescents. J Adolesc Health. 1997;21:376–83. doi: 10.1016/S1054-139X(97)00108-0. [DOI] [PubMed] [Google Scholar]
- 91.Developmental Research & Programs: Fairfax County, VA Fairfax County Youth Survey Report, Communities that Care: 2001. Available at: http://www.co.fairfax.va.us/comm/demogrph/pdf/Youth20001 Accessed March 3 2002.
- 92.U.S. Census Bureau State and county quick facts: Fairfax County, Virginia 2001. Available at: http://quickfacts.census.gov/qfd/states/51/51059.html Accessed February 8 2005.
- 93.U. S. Census Bureau National Report: 2001. Available at: http://quickfacts.census.gov/qfd/states/51000.html Accessed August 29 2003.
- 94.GRUNBAUM JA, KANN L, KINCHEN SA, et al. Youth risk behavior surveillance United States, 2001. MMWR Surveill Summ. 2002;51:1–62. [PubMed] [Google Scholar]
- 95.JOHNSTON LD, O'MALLEY PM, BACHMAN JG, SCHULENBERG JE. Monitoring the Future national results on adolescent drug use: Overview of the key findings, 2005. National Institute on Drug Abuse; Bethesda, MD: 2006. (NIH Publication No. 06-5882) [Google Scholar]
- 96.JOHNSTON LD, O'MALLEY PM, BACHMAN JG, SCHULENBERG JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2003. National Institution on Drug Abuse; Bethesda, MD: 2004. [Google Scholar]