Abstract
Objective
Fibromyalgia is a chronic pain disorder that is characterized by diffuse musculoskeletal pain and sensitivity to mechanical stimulation. In this pilot clinical trial, we tested the effectiveness of low-dose naltrexone in treating the symptoms of fibromyalgia.
Design
Participants completed a single-blind, crossover trial with the following time line: baseline (2 weeks), placebo (2 weeks), drug (8 weeks), and washout (2 weeks).
Patients
Ten women meeting criteria for fibromyalgia and not taking an opioid medication.
Interventions
Naltrexone, in addition to antagonizing opioid receptors on neurons, also inhibits microglia activity in the central nervous system. At low doses (4.5 mg), naltrexone may inhibit the activity of microglia and reverse central and peripheral inflammation.
Outcome Measures
Participants completed reports of symptom severity everyday, using a handheld computer. In addition, participants visited the lab every 2 weeks for tests of mechanical, heat, and cold pain sensitivity.
Results
Low-dose naltrexone reduced fibromyalgia symptoms in the entire cohort, with a greater than 30% reduction of symptoms over placebo. In addition, laboratory visits showed that mechanical and heat pain thresholds were improved by the drug. Side effects (including insomnia and vivid dreams) were rare, and described as minor and transient. Baseline erythrocyte sedimentation rate predicted over 80% of the variance in drug response. Individuals with higher sedimentation rates (indicating general inflammatory processes) had the greatest reduction of symptoms in response to low-dose naltrexone.
Conclusions
We conclude that low-dose naltrexone may be an effective, highly tolerable, and inexpensive treatment for fibromyalgia.
Keywords: Fibromyalgia, Chronic Pain, Naltrexone, Low-Dose, Novel Treatment, Microglia
Introduction
Fibromyalgia is a common and potentially debilitating condition that afflicts approximately 5% of women [1] and 1.6% of men [2] in the general population. The condition is characterized primarily by diffuse musculoskeletal pain and sensitivity to mechanical stimulation at softtissue tender points [3]. Other symptoms, including fatigue, sleep disturbance, and cognitive impairment, further contribute to the severity of the disorder [4,5]. The condition is often diagnosed in the age range of 34–53 years [6]; however, juvenile cases of fibromyalgia are not rare [7].
The etiology of fibromyalgia is unknown [8]. Many recent studies have focused on dysregulations in the central nervous system [9–12], although the possibility of combined peripheral and central contributions has not been excluded [13]. Some researchers have classified fibromyalgia as a “central sensitivity syndrome,” perhaps sharing pathophysiological mechanisms with conditions such as irritable bowel syndrome, temporomandibular disorders, interstitial cystitis, and chronic fatigue syndrome [14]. This central sensitivity may be mediated in part by centrally acting proinflammatory cytokine activity that can produce the hyperalgesia, fatigue, and other symptoms of fibromyalgia [15–17].
There are currently three Food and Drug Administration-approved medications for fibromyalgia. The first medication, pregabalin, operates via alpha2delta voltage-dependent calcium channels [18]. The other two medications, duloxetine and milnacipran, are serotonin and norepinephrine reuptake inhibitors [19,20]. While many fibromyalgia sufferers respond to these medications, a significant number either do not respond adequately, or experience intolerable side effects [21,22]. There is, therefore, still a need to introduce additional effective treatments to adjunct the conventional therapeutic approaches.
Naltrexone hydrochloride is a potential novel treatment for chronic pain. The drug is a competitive antagonist of opioid receptors, and has been used clinically for over 30 years to treat opioid addiction. More recently, naltrexone (and its shorter acting cousin, naloxone) has also been found to attenuate the production of proinflammatory cytokines and neurotoxic superoxides via suppressive effects on central nervous system microglia cells [23–27]. The reduction of proinflammatory cytokines can be achieved with ultra low doses, and can reduce thermal hyperalgesia in a rat model [28]. The effect is not due to opioid receptor activity, as the opioid nonactive isomers dextro-naloxone and dextro-naltrexone also exhibit neuroprotective benefits; it is instead potentially mediated by activity on toll-like receptor 4 [29,30]. Naltrexone has also been proposed to exert neuroprotective effects via modulation of mitochondrial apoptotic pathways [31].
Despite a solid base of basic science evidence suggesting a neuroprotective role for naltrexone, human studies are rare. One study found that naltrexone strongly attenuated the side effects associated with interferon-alpha treatment in cancer patients [32]. More recently, the drug has been used in dosages ranging from 3 mg to 4.5 mg per day to treat chronic pain and autoimmune disorders. Naltrexone used in this dosage range is typically referred to as low-dose naltrexone (LDN). Pilot trials for LDN in Crohn’s disease [33] and multiple sclerosis [34] have recently been conducted. Beneficial effects were reported in these trials; however, both were open label.
Given: 1) naltrexone’s demonstrated suppressing effect on centrally produced proinflammatory cytokine activity; and 2) the overlap in symptoms between fibromyalgia and cytokine-induced sickness behaviors, we hypothesized that LDN would successfully reduce the symptoms of fibromyalgia. We predicted a clinical response (30% improvement) in self-reported fibromyalgia symptom severity over placebo. We also predicted that LDN would relieve a number of specific symptoms (e.g., pain, fatigue, and sleep difficulty), and decrease pain sensitivity in quantitative sensory testing of mechanical, thermal, and cold pain thresholds. To test these hypotheses, we conducted a pilot clinical trial of LDN for the treatment of fibromyalgia, using a placebo-controlled, single-blind, crossover design.
Methods
Patient Selection
All individuals were required to meet the American College of Rheumatology’s 1990 [35] criteria for fibromyalgia. Current or recent use of opioids was exclusionary, but participants were allowed to continue other medications during their participation in the study. Participants must have held their drug dosages steady for at least the previous 2 months, and were asked not to modify their pain treatment regimen without notifying the study personnel. Participants were also screened out if they exhibited joint pain/inflammation or had ever been diagnosed with an autoimmune or rheumatologic condition. Exclusionary blood test results included rheumatoid factor (RF) over 20 IU/mL, antinuclear antibody over 1:80, and erythrocyte sedimentation rate (ESR) over 60 mm/h. Initial eligibility for study participants was determined over the phone, with further screening occurring at a laboratory visit.
Study Design
This study was a placebo-controlled, single-blind, crossover, pilot investigation of LDN for reducing symptoms of fibromyalgia. A crossover design was used to minimize the statistical demand for large sample sizes. A single-blind approach was used over the more typical open-label approach for an initial, signal-detecting study. Each participant received both LDN and placebo, thereby acting as their own control. Participants were not told when they would receive the placebo capsules. All participants provided informed consent, and all procedures were approved by the Institutional Review Board at Stanford University School of Medicine.
Each study participant followed the same schedule: baseline (2 weeks), placebo (2 weeks), LDN (8 weeks), and washout (2 weeks). In the baseline phase, daily self-reports of symptom severity were obtained, but no capsules were administered. In the placebo and LDN phases, capsules were consumed by mouth daily, and daily symptom measures were collected. In the washout phase, no capsules were administered, and participants continued to complete all study measures. Total time in the study for each participant was 14 weeks. Participants attended laboratory sessions every 2 weeks, for a total of eight visits.
Treatment
LDN capsules (4.5 mg naltrexone hydrochloride) were compounded by Preuss Pharmacy (Menlo Park, CA) using standard gelatin capsules and a microcrystalline cellulose filler (Avicel, FMC BioPolymer, Rockland, ME). A noncaloric sweetener was added to all capsules. Quality assurance was provided by Front Range Laboratories (Loveland). Participants took LDN capsules once per day for a total period of 8 weeks. Capsules were taken approximately 1 hour before bedtime. At each laboratory visit, participants were given enough capsules to cover a 2-week period, plus four extra capsules in case of a delayed appointment. Participants returned bottles and extra capsules at each laboratory visit for drug accountability.
Assessments
One primary outcome measure and 13 secondary measures were assessed. The primary end point was daily, self-reported fibromyalgia symptom severity. Participants provided severity reports via a Palm Z22 handheld computer (Palm Inc., Sunnyvale, CA) and free Experiential Sampling Program (http://www.experience-sampling.org/). Participants responded on a 0–100 visual analog scale to the question, “Overall, how severe have your fibromyalgia symptoms been today?” Single-item, global outcome measures can demonstrate good reliability, validity, and responsiveness, especially when presented on a 101-point scale [36]. The system recorded the exact time and date of each response, thereby minimizing the impact of backfilling. The self-reported symptom severity measure was completed every night while participants were enrolled in the study.
A number of secondary end points were also collected via the handheld computer. These measures included: average daily pain, highest pain, fatigue, sadness, stress, sleep quality, ability to think and remember, gastrointestinal symptoms, and headaches. Additional questions were asked each night as a quality control measure, and were not tested as end points. These questions assessed if participants took their capsule each night, if any breakthrough pain medications were needed, if any highly stressful events occurred each day, the participants’ ability to tolerate the medication, and the severity of side effects.
In addition to the daily symptom sampling, participants attended brief (45-minute) visits to the laboratory every 2 weeks. In these lab visits, quantitative sensory testing protocols were performed to obtain mechanical, heat, and cold pain thresholds. For the mechanical pain tests, pressure was applied to each of the 18 tender points [35] at a rate of 1 kg/cm2/s until the participants indicated the first sensation of pain. Pressure was applied and measured using a JTech Commander Algometer (JTech Medical, Salt Lake City, UT). The kg/cm2 pressure was recorded to the nearest tenth place and averaged for all 18 points to provide an overall score of mechanical pain threshold. Thermal pain was presented via a 3 cm × 3 cm Peltier-type, fast-ramping thermode driven by a Medoc Thermal Sensory Analyzer II (Medoc, Durham, NC) and placed on the palm (thenar eminence) of the right hand. From a baseline temperature of 32°C, heat was increased at a rate of 0.3°C/sec until the participant stopped the temperature increase at the first sensation of pain. The procedure was repeated three times, and all three resulting threshold temperatures averaged for a single estimate. Cold pain followed a similar protocol. From a baseline of 20°C, and placed on the left palm, the temperature was reduced at a rate of 0.3°C/sec until the participant stopped the decrease at the first sensation of pain. The procedure was repeated three times and all results averaged. The heat and cold pain tests were alternated, switching hands for each test, to allow adequate recovery time between stimulus repetitions. The fibromyalgia impact questionnaire (FIQ) [37] was also administrated at each visit as a separate end point.
Finally, basic individual responder analyses were performed to determine if baseline participant characteristics could predict a positive response to LDN. Drug response was defined as the reduction of severity symptoms in the drug condition, minus the reduction of symptoms in the placebo condition. This drug response variable was correlated with three predictors: ESR, fibromyalgia severity at baseline (measured with the FIQ), and duration of illness.
Statistical Analysis
All clinical outcome analyses were conducted with SPSS v17 (SPSS Inc., Chicago, IL), utilizing linear mixed models. Linear mixed models are superior over repeated-measures ANOVAs for estimating treatment effects when outcome measures are assessed frequently, when autocorrelation may be present, and when individual differences in treatment response are expected. For all outcomes tests (daily diary and laboratory), the subject identification number was entered as a random effect, so that results could be generalized to the larger population. Study condition (baseline, placebo, drug, washout) was entered as the primary predictor. For daily symptom tests, there was strong evidence of a lag-1 autocorrelation. A first-order autoregressive covariance type was confirmed to best fit the data using the Akaike’s information criteria (AIC) and Schwarz’s Bayesian criteria (BIC), and was used for all daily symptom tests. For tests conducted during laboratory visits, the AIC and BIC indicated the use of a compound symmetry covariance type. Individual responder analyses were conducted using Pearson’s correlations. All statistical tests were two-tailed. A total of 17 primary, secondary, and individual responder analyses were performed. All tests were controlled for multiple comparisons, using a method of false discovery rate modified for dependent outcomes (B-Y method) [38,39]. For all 17 tests, the adjusted statistical significance P value threshold of 0.014 was used.
For the primary outcome measure of daily fibromyalgia symptom severity, the clinical significance threshold for a positive drug effect was set at 30% reduction of symptoms over placebo. This degree of symptom reduction corresponds with “much improved” or “very much improved” on a patient global impression of change scale [40]. The crossover design, together with the high number of repeated observations, provided a high degree of statistical power for detecting drug effects. Power was estimated using G*Power 3.0 [41]. Assuming an adjusted P value of 0.014, moderate correlation between repeated measures, and four conditions, projected power for finding a 30% reduction of symptoms was calculated to be over 0.99.
Results
Participants and Demographics
Twelve women were recruited, with an average age of 44.0 years (standard deviation [SD] = 10.3, range = 22–55). Participant demographics are presented in Table 1. The mean fibromyalgia severity at baseline, as scored by the FIQ, was 67.2 (SD = 15.0), indicating moderately severe symptoms. The average duration of illness was 9.6 years (SD = 6.5). All participants returned negative values for RF and normal values of ANA. Several participants returned slightly elevated or elevated ESR levels, but were not excluded from participation.
Table 1.
Baseline patient characteristics of 10 completing protocol
| ID | Age | Duration Illness (years) |
BMI | FIQ | ESR (mm/h) |
Concomitant Drug List |
|---|---|---|---|---|---|---|
| 1 | 22 | 11 | 21.8 | 39.4 | 0 | None |
| 2 | 54 | 10 | 28.4 | 76.7 | 45 | None |
| 3 | 37 | 15 | 19.8 | 45.0 | 2 | None |
| 4 | 47 | 14 | 22.1 | 70.1 | 8 | Pregabalin, duloxetine, gabapentin |
| 5 | 52 | 2 | 24.0 | 70.1 | 28 | Fluoxetine, nortriptyline |
| 6 | 55 | 5 | 40.7 | 60.8 | 50 | None |
| 7 | 46 | 15 | 29.4 | 85.8 | 27 | None |
| 8 | 51 | 20 | 18.8 | 70.0 | 20 | Cyclobenzaprine |
| 9 | 38 | 3 | 32.0 | 83.3 | 28 | Cyclobenzaprine |
| 10 | 38 | 1 | 36.6 | 70.7 | 31 | None |
BMI = body mass index; ESR = erythrocyte sedimentation rate; FIQ = fibromyalgia impact questionnaire.
Two participants who began the study protocol were excluded from all analyses. One participant reported taking opioid medications during the study period. The participant also had a poor response rate on the handheld computer, including large periods of time (weeks) with no responses. Sampling was especially insufficient in the placebo period, preventing any type of meaningful comparison to the drug condition. The second excluded participant had a major physical accident that caused severe and prolonged pain, and required a major change in medication status.
Daily Symptom Reporting
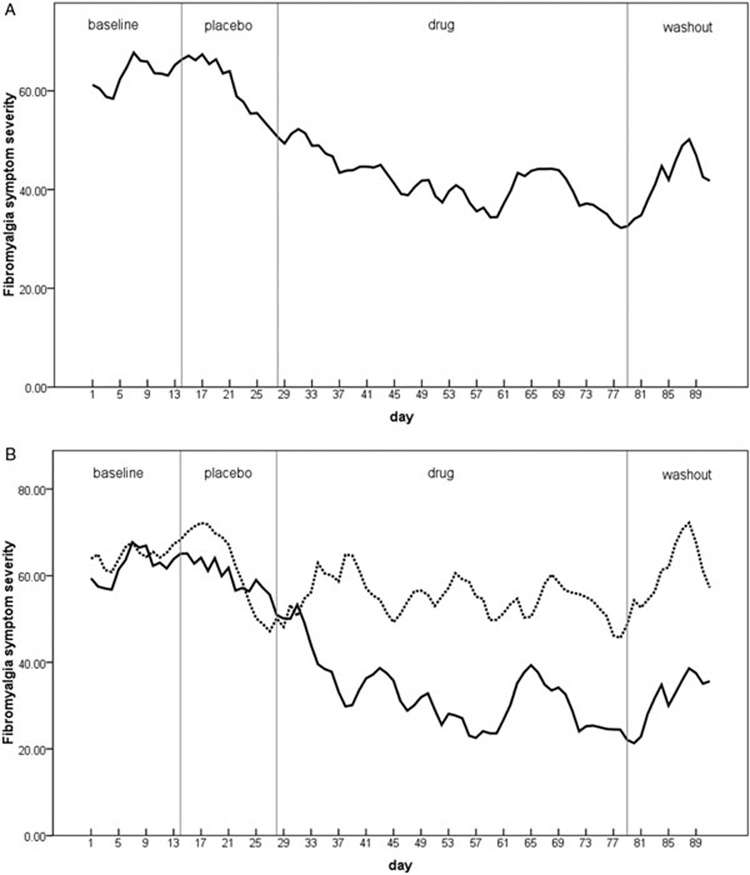
Entries were successfully completed on 92% of all study days. The primary outcome measure of daily, overall fibromyalgia symptom severity was first tested. The drug condition had a significant impact on fibromyalgia symptom severity (F[3, 254] = 8.67, P < 0.0005). During placebo, symptoms were reduced by 2.3% in the entire cohort from baseline. In the drug condition, symptoms were reduced by 32.5% (Figure 1A). Post hoc pairwise comparisons (least squares differences) found that fibromyalgia symptoms were significantly lower during drug than both baseline (P < 0.0005) and placebo (P = 0.003) conditions. No difference was found between the drug and washout conditions (P = 0.891). Six individuals were classified as drug responders, given a 30% greater reduction of symptoms during LDN compared with placebo. Figure 1B shows daily symptoms over the study period for drug responding (N = 6) and nonresponding (N = 4) groups.
Figure 1.
Overall, self-reported, daily fibromyalgia symptoms (scale 0–100, with 100 being most severe) as a function of placebo and low-dose naltrexone administration. Sections are: baseline, placebo, drug, and washout. A 3-day smoothing has been applied. (A) Data from all participants (N = 10). (B) Data separated by drug responders (30% or greater reduction of symptoms over placebo; solid line, N = 6) and nonresponders (broken line, N = 4).
The effects of naltrexone were then tested on the daily, self-reported secondary outcome measures. Drug condition had a significant impact on: daily pain (F[3, 287] = 5.6, P = 0.001), highest pain (F[3, 277] = 4.41, P = 0.005), fatigue (F[3, 224] = 4.05, P = 0.008), and stress (F[3, 236] = 4.67, P = 0.003). Statistical significance (using the adjusted P = 0.014 threshold) was not reached for sleep quality (F[3, 230] = 3.28, P = 0.022), gastrointestinal problems (F[3, 302] = 2.991, P = 0.031), headaches (F[3, 279] = 2.538, P = 0.057), thinking and concentration (F[3, 226] = 1.338, P = 0.263), or sadness (F[3, 229] = 1.016, P = 0.386). Post hoc, pairwise contrasts revealed that each of these symptoms followed the same pattern as the primary end point, with symptom severity being lower in the drug condition as contrasted with the baseline and placebo conditions.
Laboratory Visits
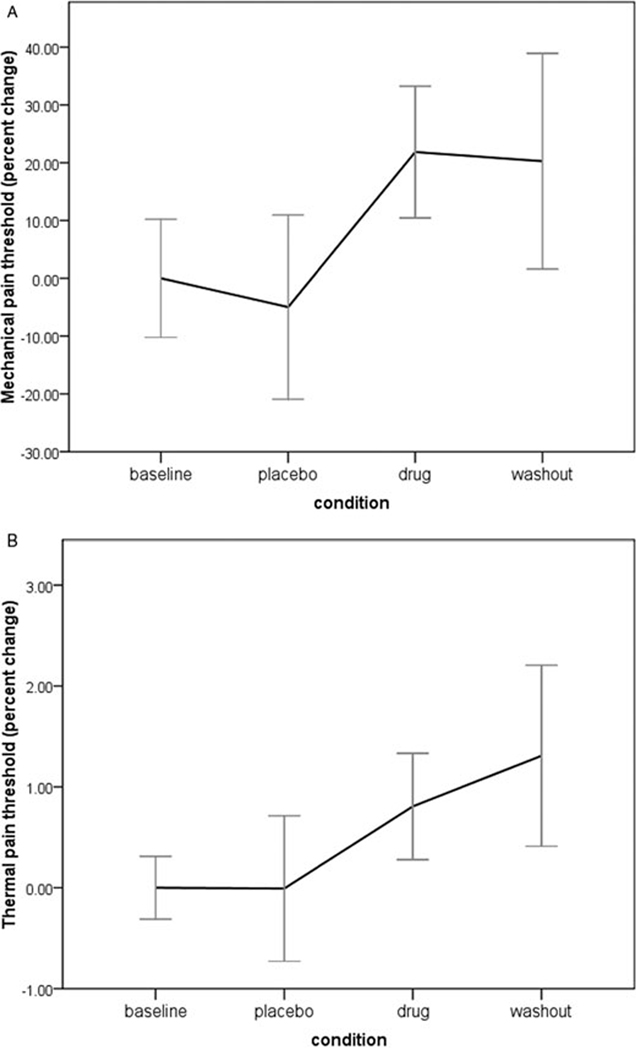
During baseline, participants had an average mechanical pain threshold of 1.02 kg/cm2 at the fibromyalgia tender sites. Drug condition significantly predicted the secondary outcome of mechanical pain threshold (F[1, 67] = 5.88, P = 0.001). Pain thresholds were reduced by 0.07 kg in the placebo condition, and raised by 0.22 kg in the drug condition (Figure 2A). Post hoc, pairwise comparisons showed that mechanical threshold was significantly improved in the drug condition compared with both baseline (P = 0.012) and placebo (P = 0.010), but was not different than washout (P = 0.829). Drug condition was then tested on the lab measures of thermal and cold pain threshold. At baseline, participants first experienced thermal pain at 37.9°C. The drug significantly impacted heat pain threshold (F[1, 67] = 3.858, P = 0.013). During placebo, heat threshold was unchanged. During the drug condition, thermal pain thresholds were increased by 0.9°C (Figure 2B). For cold pain threshold, participants experienced the first sensation of pain at 17.7°C. The drug condition had no impact on cold pain thresholds (F[1, 67] = 1.49, P = 0.224).
Figure 2.
Changes in pain threshold at laboratory sessions during baseline (visits 1 and 2), placebo (visit 3), drug (visits 4–7), and washout (visit 8) phases. (A) Mechanical pain threshold; baseline is 1.02 kg/cm2. (B) Thermal pain threshold; baseline is 37.9°C.
The final secondary outcome variable tested was FIQ-rated symptom severity. FIQ scores were significantly affected by drug condition (F[1, 66] = 8.96, P < 0.0005). FIQ scores were reduced by 16.7% in the placebo condition and 31.7% in the drug condition. Post hoc contrasts showed that the drug condition was significantly different from baseline (P < 0.0005) and placebo (P = 0.042), but not washout (P = 0.160).
Side Effects
Average daily tolerability during the drug condition was 96.3% (compared with 89.7% during placebo).Two individuals in the study reported more vivid dreams. One individual reported transient nausea and insomnia for the first few nights of capsules. No other symptoms were reported. All side effects were reported as mild, and no change in dosage or dosing schedule was required.
Individual Responder Analysis
Three variables were used to predict positive drug responders: ESR, severity of fibromyalgia during baseline, and duration of illness. Baseline ESR values predicted over 82% of the variance in LDN response. The correlation between response and ESR was 0.91, P < 0.0005 (Figure 3). Those who had elevated ESR levels had the greatest positive response to LDN. Neither duration of illness (r = −0.48, P = 0.16) nor baseline symptom severity (r = 0.02, P = 0.95) predicted response to LDN.
Figure 3.
Relationship between drug response (reduction of fibromyalgia symptoms in the drug condition) and baseline erythrocyte sedimentation rate.
Discussion
This pilot study is the first to examine the effectiveness of LDN in reducing the symptoms of fibromyalgia. Overall symptom severity was significantly reduced in the drug condition, as contrasted to baseline and placebo conditions. In the entire group of participants, LDN reduced fibromyalgia symptoms by 30.2% over and above placebo. Specific symptoms, including average pain, highest pain, fatigue, and stress, were also significantly impacted by the drug. The observed effects were accompanied by a very low incidence of side effects, suggesting LDN may be an effective and well-tolerated treatment option for individuals with fibromyalgia.
Six of the 10 participants were significant responders to the drug, showing a greater than 30% reduction of symptoms over and above placebo. Daily symptom ratings (Figure 1B) showed that the nonresponding group demonstrated a significant placebo effect that was not maintained during LDN treatment. In the responding group, symptoms slowly and steadily decreased during placebo, but sharply decreased after the start of LDN administration. The time to peak effect was roughly 28 days, which is consistent with reports of 4.5 mg LDN for Crohn’s disease [33]. That report also found a comparable responder rate (67%). During washout, nonresponders showed a rapid return to baseline levels of symptom severity. Drug responders showed a rapid but noncomplete return to baseline severity levels. A longer washout period (greater than 2 weeks) might show a complete return to baseline. Post hoc analyses on the outcome variables revealed no difference between drug and washout, potentially suggesting continued beneficial action following cessation of the drug, which has also been previously reported [33].
Qualitative sensory testing in the laboratory yielded interesting results. Mechanical pain thresholds rapidly increased after administration of LDN. These effects were maintained at the 2-week washout period. Similar results were found for thermal pain thresholds. These tests provide more objective support of sensory changes resulting from LDN administration. The changes in threshold levels, while small in absolute terms, represent significant improvements.
Individual responder analyses showed that baseline levels of ESR was strongly correlated with drug response, and predicted over 80% of the variance in response to LDN. These results, which suggest the presence of inflammatory processes in some fibromyalgia patients, must be viewed with caution because of low sample size. We note, however, that the LDN trial for Crohn’s disease found a significant reduction of ESR due to the drug [33]. It is unlikely that ESR can serve as a biomarker for fibromyalgia, given that our study and previous studies have found ESR levels to be normal or only mildly elevated in these patients [42–44]. Furthermore, ESR is found to be elevated in a number of conditions, such as rheumatoid arthritis, systemic lupus erythematosus, and acute infection. For these reasons, the ESR test may be most useful in defining one important subgroup of fibromyalgia patients who are experiencing lowlevel, systemic inflammation and are responsive to LDN. It is possible that the term fibromyalgia describes a discrete number of conditions with similar symptoms, but nonoverlapping pathophysiological mechanisms [8,45]. To our knowledge, there are no reports of ESR being used to predict treatment responders. By further attempting to identify treatment responders using physiological and psychological predictors, we may be able to define important subgroups and better understand the underlying pathophysiological mechanisms.
FM is a costly condition, both in terms of lost productivity and the cost of available treatments [46,47]. LDN is an inexpensive drug, with total costs usually running under $40 per month. There are several additional advantages to LDN. The drug is easily dosed, with a once-a-day schedule. Side effects are infrequent and mild. And, while safety of long-term administration for the low dose has not been assessed, the drug has a long history of safe use at much higher dosages. Liver functioning may need to be monitored, as elevated liver transaminases have been reported with 50 mg and greater dosages in opioid-addicted and alcohol-addicted patients [48]. Changes in these markers have not been found for the 4.5 mg dosage [32]. Also, because of the proposed mechanism of action (i.e., attenuation of microglia activity), there is a theoretical increased risk of infection. While no anecdotal reports suggest increased frequency of infections under naltrexone, this risk should continue to be monitored.
There are a few methodological limitations in this study that are associated with the exploratory nature of the project. First, as an initial signal-detecting study, a single-blind design was used. While this approach provides more scientific control than an open-label design, the results will need to be confirmed in a double-blind study. Second, placebo was administered before LDN in all participants, which could have led to confounding order effects. Third, while a rich dataset was obtained on participants, the subject size was small, and generalization to a larger population needs to be established. Fourth, the pooled data from responders and nonresponders show a slow decline of symptom reporting over the placebo period and continuing into the drug period. The slope of the line (Figure 1A) could suggest that the lower symptom reports given during the drug period were just a continuation of the placebo effect. Future studies may distinguish drug effects from placebo with longer conditions, and by utilizing crossover or parallel group research designs. Fifth, the lack of a difference between washout and the drug condition suggests that effects of the drug were sustained after cessation of capsules. The complete clinical picture of LDN for fibromyalgia will therefore be made clearer by utilizing a longer washout or follow-up period. Sixth, subsequent trials may use a validated scale for the primary outcome measure, rather than the single-item fibromyalgia severity measure employed in this study. Seventh, more exploration of dose-response relationships are needed. Future studies employing stricter, double-blind designs could easily address all of these methodological concerns.
We propose here a microglia mechanism of action for naltrexone’s beneficial impact on fibromyalgia symptoms. While recent in vitro and in vivo work has highlighted the importance of those cells [23–31], there is no direct evidence available that links microglia activity to fibromyalgia symptoms. We are not aware of any method by which central microglia state can be assessed in peripheral blood, so investigating microglial activity in human patients is difficult. An exciting, minimally invasive method of assessing central microglia activity may involve positron emission tomography scans with the PK11195 or DAA1106 ligands [49,50].
We conclude that LDN is a drug that should be researched more thoroughly for the treatment of fibromyalgia, and perhaps more generally for conditions associated with elevated ESR.
Acknowledgments
We would like to thank Jim and Connie Binns for their generous gift in support of this trial. We would also like to thank the American Fibromyalgia Syndrome Association for their financial and logistical support, and the Oxnard Foundation for their financial support. We also wish to thank the Arthritis Foundation for their support of Dr. Younger during the conduct of this study.
References
- 1.Nuemann L, Buskila D. Epidemiology of fibromyalgia. Curr Pain Headache Rep. 2003;7:362–368. doi: 10.1007/s11916-003-0035-z. [DOI] [PubMed] [Google Scholar]
- 2.White KP, Speechley M, Harth M, et al. The London fibromyalgia study: The prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26:1570–1576. [PubMed] [Google Scholar]
- 3.Yunus MB. Towards a model of pathophysiology of fibromyalgia: Aberrant central pain mechanisms with peripheral modulation. J Rheumatol. 1992;19:846–850. [PubMed] [Google Scholar]
- 4.Waylonis GW, Heck W. Fibromyalgia syndrome. New associations. Am J Phys Med Rehabil. 1992;71:343–348. doi: 10.1097/00002060-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan KH, Goldenberg DL, Galvin-Nadeau M. The impact of a meditation-based stress reduction program on fibromyalgia. Gen Hosp Psychiatry. 1993;15:284–289. doi: 10.1016/0163-8343(93)90020-o. [DOI] [PubMed] [Google Scholar]
- 6.Boissevain MD, McCain GA. Toward an integrated understanding of fibromyalgia syndrome. I. Medical and pathophysiological aspects. Pain. 1991;45:227–238. doi: 10.1016/0304-3959(91)90047-2. [DOI] [PubMed] [Google Scholar]
- 7.Kashikar-Zuck S, Graham TB, Huenefeld MD, Powers SW. A review of biobehavioral research in juvenile primary fibromyalgia syndrome. Arthritis Care Res. 2000;13:388–397. doi: 10.1002/1529-0131(200012)13:6<388::aid-art9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Perrot S. Fibromyalgia syndrome: A relevant recent construction of an ancient condition? Curr Opin Support Palliat Care. 2008;2:122–127. doi: 10.1097/SPC.0b013e3283005479. [DOI] [PubMed] [Google Scholar]
- 9.Gur A, Oktayoglu P. Central nervous system abnormalities in fibromyalgia and chronic fatigue syndrome: New concepts in treatment. Curr Pharm Des. 2008;14:1274–1294. doi: 10.2174/138161208799316348. [DOI] [PubMed] [Google Scholar]
- 10.Cook DB, Stegner AJ, McLoughlin MJ. Imaging pain of fibromyalgia. Curr Pain Headache Rep. 2007;11:190–200. doi: 10.1007/s11916-007-0190-8. [DOI] [PubMed] [Google Scholar]
- 11.Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 12.Mease P. Fibromyalgia syndrome: Review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl. 2005;75:6–21. [PubMed] [Google Scholar]
- 13.Staud R. New insights into the pathogenesis of fibromyalgia syndrome: Important role of peripheral and central pain mechanisms. Curr Rheumatol Rev. 2007;3:113–121. [Google Scholar]
- 14.Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Thompson ME, Barkhuizen A. Fibromyalgia, hepatitis C infection, and the cytokine connection. Curr Pain Headache Rep. 2003;7:342–347. doi: 10.1007/s11916-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DJ. Is there a role for cytokine based therapies in fibromyalgia. Curr Pharm Des. 2006;12:17–22. [PubMed] [Google Scholar]
- 17.Bazzichi L, Rossi A, Massimetti G, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25:225–230. [PubMed] [Google Scholar]
- 18.Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: Results of a randomized, double-blind, placebocontrolled trial. American College of Rheumatology. 2005;52:1264–1273. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 19.Arnold LM, Lu Y, Crofford LJ, et al. A doubleblind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 20.Gendreau RM, Thorn MD, Gendreau JF, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol. 2005;32:1975–1985. [PubMed] [Google Scholar]
- 21.Krypel LL. Fibromyalgia: A review of its patho-physiology and drug treatment. J Pharm Pract. 2009;22:6–16. [Google Scholar]
- 22.Lawson K. Pharmacological treatments of fibromyalgia: Do complex conditions need complex therapies? Drug Discov Today. 2008;13:333–340. doi: 10.1016/j.drudis.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Hong JS. Neuroprotective effect of naloxone in inflammation-mediated dopaminergic neurodegeneration: Dissociation from the involvement of opioid receptors. Methods Mol Med. 2002;79:43–54. doi: 10.1385/1-59259-358-5:43. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Du L, Kong L-Y, et al. Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience. 2000;97:749–756. doi: 10.1016/s0306-4522(00)00057-9. [DOI] [PubMed] [Google Scholar]
- 25.Czlonkowski A, Stein C, Harz A. Peripheral mechanisms of opioid antinociception in inflammation: Involvement of cytokines. Eur J Pharmacol. 1993;242:229–235. doi: 10.1016/0014-2999(93)90246-e. [DOI] [PubMed] [Google Scholar]
- 26.Greeneltch KM, Haudenschild CC, Keegan AD, et al. The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factoralpha production. Brain Behav Immun. 2004;18:476–484. doi: 10.1016/j.bbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–617. [PubMed] [Google Scholar]
- 28.Tsai RY, Jang FL, Tai YH, et al. Ultra-low-dose naloxone restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in PTX-treated rats. Neuropsychopharmacology. 2008;33:2772–2782. doi: 10.1038/sj.npp.1301672. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Jiang JW, Wilson BC, et al. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J Pharmacol Exp Ther. 2000;295:125–132. [PubMed] [Google Scholar]
- 30.Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: Involvement of toll-like receptor 4 (TLR4) European Journal of Neuroscience. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.San-Emeterio EP, Hurlé MA. Modulation of brain apoptosis-related proteins by the opioid antagonist naltrexone in mice. Neurosci Lett. 2006;403:276–279. doi: 10.1016/j.neulet.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 32.Valentine AD, Meyers CA, Talpaz M. Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest. 1995;13:561–566. doi: 10.3109/07357909509024923. [DOI] [PubMed] [Google Scholar]
- 33.Smith JP, Stock H, Bigaman S, et al. Low-dose naltrexone therapy improves active Crohn’s disease. Am J of Gastroenterol. 2007;102:820–828. doi: 10.1111/j.1572-0241.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 34.Gironi M, Martinelli-Boneschi F, Sacerdote P, et al. A pilot trial of low-dose naltrexone in primary progressive multiple sclerosis. Mult Scler. 2008;14:1076–1083. doi: 10.1177/1352458508095828. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 36.de Boer AGEM, van Lanschot JJB, Stalmeier PFM, et al. Is a single-item visual analogue scale as valid, reliable, and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 37.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: Development and validation. J Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 38.Bejamini Y, Yekutieli D. The control of false discovery rate under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 39.Narum SR. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conserv Genet. 2006;7:783–787. [Google Scholar]
- 40.Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 41.Faul F, Erdfelder EG, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 42.Viitanen JV, Kautiainen H, Isomäki H. Pain intensity in patients with fibromyalgia and rheumatoid arthritis. Scand J Rheumatol. 1993;22:131–135. doi: 10.3109/03009749309099257. [DOI] [PubMed] [Google Scholar]
- 43.Leeb BF, Andel I, Sautner J, et al. The DAS28 in rheumatoid arthritis and fibromyalgia patients. Rheumatology. 2004;43:1504–1507. doi: 10.1093/rheumatology/keh322. [DOI] [PubMed] [Google Scholar]
- 44.Pamuk Ö, Çakir N. The frequency of thyroid antibodies in fibromyalgia patients and their relationship with symptoms. Clin Rheumatol. 2007;26:55–59. doi: 10.1007/s10067-006-0237-y. [DOI] [PubMed] [Google Scholar]
- 45.Giesecke T, Williams DA, Harris RE. Subgrouping of fibromyalgia patients on the basis in pressurepain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 46.Robinson RL, Birnbaum HG, Morley MA, et al. Economic cost and epidemiological characteristics of patients with fibromyalgia claims. J Rheum. 2003;30:1318–1325. [PubMed] [Google Scholar]
- 47.Penrod J, Adam V, Bernatsky S, et al. Direct and indirect costs of fibromyalgia in women. J Rheum. 2004;31:1391–1398. [PubMed] [Google Scholar]
- 48.Mitchell JE. Naltrexone and hepatotoxicity. Lancet. 1986;1:1215. doi: 10.1016/s0140-6736(86)91196-7. [DOI] [PubMed] [Google Scholar]
- 49.Debruyne JC, Versijpt J, van Laere KJ, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10:257–264. doi: 10.1046/j.1468-1331.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- 50.Venneti S, Wagner AK, Wang G, et al. The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds specifically to microglia in a rat model of traumatic brain injury: Implications for PET imaging. Exp Neurol. 2007;207:118–127. doi: 10.1016/j.expneurol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]