Abstract
Viral encephalomyelitis is caused by virus infections of neurons in the brain and spinal cord. Recovery is dependent on immune-mediated control and clearance of virus from these terminally differentiated essential cells. Preservation of neuronal function is essential for prevention of neurologic sequelae such as paralysis, seizures and cognitive deficits. Using the model system of Sindbis virus-induced encephalomyelitis in mice, we have shown that immune-mediated clearance of infectious virus from neurons is a noncytolytic process. The major effectors are antibody to the E2 surface glycoprotein produced by B cells, and interferon-γ produced by T cells. These effectors work in synergy, but neuronal populations differ in their responses to each. Virus is least likely to be cleared from brain neurons and most likely to be cleared from motor neurons in the cervical and thoracic regions of the spinal cord. Because the infected neurons are not eliminated, viral RNA persists and long-term control is needed to prevent virus reactivation. Virus-specific antibody-secreting cells residing in the nervous system after recovery from infection are likely to be important for long-term control.
Keywords: Noncytolytic virus clearance, Interferon-γ, Antiviral antibody, Neuronal virus infection, Alphavirus encephalitis
Introduction
Viral encephalitis is an important cause of morbidity and mortality worldwide. During encephalomyelitis, the cells in the nervous system targeted for infection are neurons of the brain and spinal cord. Several types of viruses can infect neurons. Important human pathogens include herpes simplex virus, a DNA virus, and several RNA viruses in the enterovirus (e.g. poliovirus, enterovirus 71), flavivirus (e.g. West Nile, Japanese encephalitis, Murray Valley and tick-borne encephalitis viruses) and alphavirus (e.g. eastern equine, Venezuelan equine and western equine encephalitis viruses) families. Many of these viruses are emerging as increasing problems worldwide due to changes in virulence and spread to new geographic regions.
Particularly important are the arthropod-borne flaviviruses and alphaviruses that have caused explosive regional outbreaks of disease as demonstrated by Chikungunya in the Indian Ocean and by the spread of West Nile virus to North America. Some alphaviruses cause encephalitis, while others cause rash and arthritis. The encephalitic alphaviruses are particularly associated with the outbreaks of encephalomyelitis in the Americas [1]. Eastern equine encephalitis (EEE) is endemic on the Gulf and Atlantic coasts and has a high mortality in all age groups [2]. Numbers of cases are increasing, and in 2005 the most cases of EEE occurred in the US since 1964 [3]. Western equine encephalitis (WEE) is endemic in the western portions of North America and in South America, but has not been recently associated with human disease. In 1995, Venezuelan equine encephalitis (VEE) re-emerged in South America causing an epidemic of 75–100,000 cases [4]. As with other viral causes of encephalitis, encephalitic alphaviruses infect neurons, terminally differentiated, irreplaceable cells essential to function of the nervous system. Because mature neurons have limited capacity for regeneration, recovery that does not result in neuronal damage requires noncytolytic, rather than cytolytic, immune mechanisms for virus clearance.
The Sindbis virus model of acute alphavirus encephalomyelitis
Alphaviruses are enveloped, plus-strand, mosquito-borne RNA viruses in the Togaviridae family. The genome is approximately 11,700 nucleotides long, capped and polyadenylated. The structural proteins (C, PE2, 6 K and E1) are translated from a subgenomic RNA as a large polyprotein [5]. C is autoproteolytically cleaved from the developing nascent chain and rapidly assembled with genomic RNA into nucleocapsids. Precursor of E2 (PE2) and E1 are transported with 6 K as a noncovalently associated heterooligomeric complex through the cell secretory pathway to the plasma membrane. Later in the pathway, PE2 is processed to E2 and a small glycopeptide, E3, which is shed from the cell surface. At the plasma membrane, the specific association of E2 tails with nucleocapsids initiates a budding process that leads to the release of mature virions [6, 7]. E1 and E2 heterodimers trimerize to form the spikes on the virion surface [8]. E2 protrudes from the virion surface and is involved in attachment, while E1 forms a relatively flat skirt-like structure and is important for fusion of the virus and cell membranes to initiate infection [9].
Sindbis virus (SINV), the prototype alphavirus, is the most widespread of the alpha-viruses and causes summertime outbreaks of arthritis and rash in Northern Europe (e.g. Ockelbo, Pogosta, Karelian fevers) and southern Africa [10–12]. SINV is closely related to WEE virus [13] and Mayaro virus, an emerging cause of rash and arthritis in South America [14]. SINV causes neuronal infection in mice and serves as a model system for the study of the pathogenesis of alphavirus-induced encephalomyelitis and the mechanisms and consequences of virus clearance from neurons [15, 16].
Age is an important determinant of outcome from SINV infection. Neonatal mice die within the first few days after infection, while older mice clear SINV from the central nervous system (CNS) within 6–8 days without signs of paralysis or neurological damage [17, 18]. Age-dependent susceptibility is not associated with the maturation of the immune response, but rather with the changing intrinsic susceptibility of immature and mature neurons to infection [15]. Maturity of the infected neuron determines the level of virus replication and the susceptibility to SINV-induced cell death independent of the immune response [19–22]. Immature neurons replicate SINV to higher titers and are susceptible to virus-induced apoptosis, while mature neurons are intrinsically more resistant to SINV replication and survive virus infection [21, 23, 24]. Recovery from infection in mature mice requires immune-mediated clearance of virus from these surviving infected neurons.
Because uncontrolled CNS inflammation can be damaging or even fatal, immune responses in the nervous system are highly regulated [25, 26]. The immunologically quiescent state of the uninfected CNS is maintained by ongoing interactions between neurons and glial cells, the presence of the blood brain barrier (BBB) and constitutive production of regulatory factors such as gangliosides, transforming growth factor (TGF)-β and interleukin (IL)-10 [25, 27–29]. Microglial cells, the resident macrophage lineage cells in the CNS, are closely apposed to neurons that express surface molecules such as CD200, CD47, HMGB1 and fractalkine/CX3CL1 that inhibit microglial cell activation [26]. Astrocytes maintain the endothelial tight junctions that form the BBB. Activated T cells routinely cross the BBB as part of normal immunologic surveillance of the CNS but leave or die if antigen is not encountered [15, 30–34].
When neurons become infected, changes in the CNS quickly occur to initiate a protective response [25]. The signaling pathway by which neurons sense most virus infections is not known, but neurons possess toll-like receptors (TLRs) and intracellular RNA helicases likely to be involved in alerting the CNS to virus infection [35, 36]. Neurons can respond to infection by producing IFN-β, IFN-γ, IL-6 and chemokines, such as CXCL10/IP-10, CCL21/SLC and CX3CL1 [37–41]. Macrophages and glial cells become activated in early phases of the response and rapidly produce an array of cytokines (e.g. IL-1, tumor necrosis factor (TNF)-α, IL-6) and chemokines (e.g. CXCL10, CCL2, CCL5) [29, 42] that up regulate major histocompatibility complex (MHC) molecules on microglial cells and increase adhesion molecule expression on cerebral capillary endothelial cells (e.g. inter-cellular adhesion molecule [ICAM]-1, vascular cell adhesion molecule [VCAM]-1) to enhance the entry of activated cells into the CNS [43].
Virus clearance
Clearance of acute virus infections requires elimination of cell-free virus to prevent continued infection of new cells and elimination of virus-infected cells to prevent continued production of virus. Only antibody can recognize the native antigen on virions. Neutralizing antibody that is generally directed to a viral surface protein and can prevent virus infection of new cells is important for preventing continued spread of infection. For viruses that bud from the plasma membrane, antibodies may also be able to interact with viral proteins on the surface of infected cells.
Elimination of virus-infected cells from tissue requires elimination of all cells in which the virus is replicating. This can occur by either virus-induced or immune-mediated cytolysis. T cells are ideal for this purpose because they recognize viral antigen as processed antigen only in the context of MHC class I (CD8+ T cells) or class II (CD4+ T cells) expressed on the cell surface and CD8+ T cells can possess cytotoxic properties. If the immune clearance mechanism is damaging to the infected neuron, then the function of that neuron will be lost, and the outcome for the host will be the same as if the virus infection had caused neuronal death. If infected cells are allowed to survive, the clearance of virus must include mechanisms for inhibiting intracellular synthesis of virus nucleic acid and protein, and for removing virus genomes from cells or preventing their replacement after degradation. If the clearance process is not complete, then mechanisms for preventing resumption of virus replication must be in place to avoid progressive or relapsing disease [44–46].
Clearance of virus from cells in the brain parenchyma and recovery from infection is a multistep process. First, there is inhibition of virus spread to new cells, then clearance of cell-free infectious virus. Subsequently, virus-infected cells must be eliminated or intra-cellular virus replication must be permanently suppressed. The most straightforward measurement of virus clearance is the amount of infectious virus that is present in CNS tissue. However, as antibody specific for the virus is produced, the ability to detect infectious virus by standard assays is compromised by the presence of neutralizing antibody in the tissue homogenates. Therefore, quantitative measurement of virus nucleic acid provides the best indicator of whether the virus has truly been eliminated.
Antibody is produced, and T cells begin to infiltrate the CNS 3–4 days after SINV infection and virus clearance begins shortly thereafter [34, 47, 48]. Type I interferon (IFN) is essential for initial control of virus replication [49–52], and both humoral [21, 49, 53] and cellular [23, 54] arms of the adaptive immune response play important roles in clearance. Mice deficient in all components of adaptive immunity (SCID or Rag-/-) develop persistent nonfatal infection [55] and have been used as a test system to determine the contribution of different components of the immune response to virus clearance.
Role of antibody
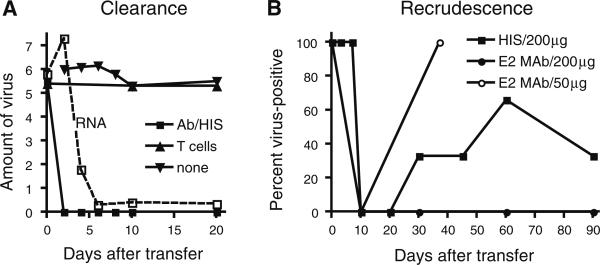
Passive transfer of hyperimmune serum (HIS) containing polyclonal SINV antibody to persistently infected SCID mice results in clearance of infectious virus from the CNS (Fig. 1a). Clearance of SINV from the brain and spinal cord occurs within 48 h without apparent neurologic damage indicating an important role for antibody in noncytolytic clearance [55]. Analysis of the specificity of the effective antibody using a panel of monoclonal antibodies to the E1 and E2 glycoproteins has shown that clearance is dependent on the amount of antibody transferred and that antibody to the E2 glycoprotein is most potent [55]. In addition to clearance of infectious virus, viral RNA is decreased after passive transfer of antibody indicating inhibition of virus replication, not just neutralization of virus by the passively transferred antibody [21, 55]. However, RNA persists at a low level and virus replication resumes when antibody has decayed (Fig. 1b), indicating that replication competent viral RNA persisted [21, 56]. Investigation of virus clearance in immunologically normal mice has shown that viral RNA can be detected by RT–PCR in these mice for at least 12 months after infection. Therefore, failure to eliminate the infected neurons results in preservation of neuronal function, but persistence of viral RNA with the potential for recrudescent virus replication.
Fig. 1.
Effect of passive transfer of antibody or T cells to SCID mice persistently infected with SINV. a Passive transfer of SINV-specific hyperimmune serum (HIS) on infectious virus in the brain (pfu/gram) and on levels of viral RNA (in situ hybridization/RT–PCR). Transfer of T cells had no effect. b Recrudescence of infectious virus in the brain after passive transfer of hyperimmune serum (200 μg IgG) or anti-E2 monoclonal antibody at two different doses (50 μg and 200 μg). Data from (21, 53, 56)
The mechanism by which antibody to a surface glycoprotein suppresses intracellular virus replication has been intensively studied using a variety of in vitro systems but is not yet completely understood. The E2 glycoprotein is expressed on the surface of virus-infected cells, and anti-E2 antibody binds to the cell surface. These studies have shown that clearance requires bivalent antibody but does not require complement or leukocytes and is independent of IgG isotype [21, 57]. Antibody treatment of infected cells blocks virus budding, restores Na+K+ATPase function, membrane potential, host protein synthesis and the ability of the cells to respond to IFN-α/β [58, 59]. These data imply that E2 cross-linking on the surface results in membrane alteration and transmission of a signal from the surface of the infected cell to intracellular sites of virus replication.
Because this process does not eliminate viral RNA, a mechanism for long-term immunologic control of virus replication is needed to prevent reactivation. Antibody is likely to participate in control, as well as initial clearance, and there are two mechanisms for maintaining antibody in the CNS: passage of immunoglobulin from the blood across the BBB into the brain parenchyma or local production of antibody by antibody-secreting cells that are resident in the CNS. Although the BBB normally restricts the entry of proteins from the blood into the CNS, this function is compromised by the inflammatory response during the acute phase of infection and plasma antibody enters the CNS in larger amounts than during recovery when the barrier has healed [60–62]. With a normally functioning BBB, interstitial brain levels of antibody are 1:100–1:200 of plasma levels, a level that may be inadequate for long-term control. Therefore, locally produced antibody may be needed to maintain levels in the CNS sufficient for continued suppression of virus replication.
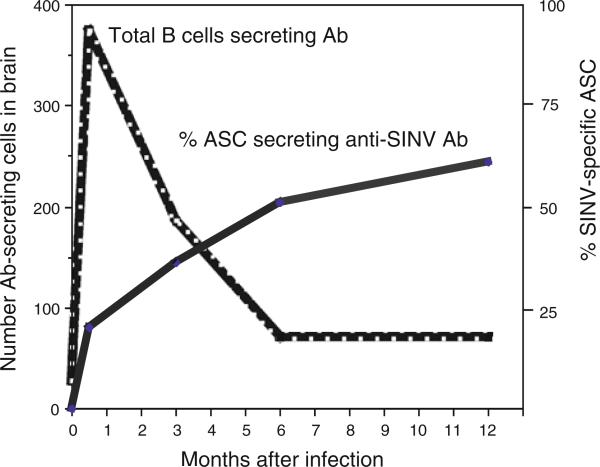
Immunohistochemical studies have shown that B cells infiltrate the CNS as a prominent part of the inflammatory response to SINV infection [63, 64]. Small numbers of perivascular IgM-secreting cells are detected 3 days after infection, followed by IgG- and IgA-secreting cells beginning at 5 days after infection [53, 61, 63]. By day 10-14, 80% of the B cells in the CNS express either IgG or IgA. Antibody-secreting cells are maintained in the CNS for at least a year after recovery with continuous enrichment for cells secreting antibody to SINV (Fig. 2) [53]. The relative roles of plasma antibody and resident B cells in the antibody-mediated clearance process and in suppression of reactivation have not been defined.
Fig. 2.
Numbers of total B cells secreting immunoglobulin in the CNS after intracerebral infection of BALB/c mice with SINV and percent of those antibody-secreting cells (ASC) producing antibody specific for SINV (53)
Role of T cells in immune-mediated clearance
No mice deficient in mature B cells are able to clear virus from the brain or prevent persistent virus replication. However, antibody-deficient mice can clear infectious virus from the brain stem and spinal cord without apparent neuronal damage, indicating a role for T cells in noncytolytic clearance of SINV from some, but not all types of neurons [23, 52]. Lymphocyte depletion studies showed that both CD4+ and CD8+ T cells are involved in this clearance process suggesting a soluble factor. The potential role of different T cell cytokines was investigated by expression from recombinant SINV to provide a local source. These studies demonstrated that IFN-γ is the main effector of T cell-mediated virus clearance [23].
During SINV-induced encephalomyelitis, IFN-γ is produced as a part of the adaptive immune response that begins 3–4 days after infection [29]. Therefore, IFN-γ must induce an antiviral response in neurons that are already infected, rather than protect neurons from becoming infected. The IFN-γ receptor is expressed on many cells, including neurons, and has two subunits [65].
The cytoplasmic domains of both chains of the receptor are necessary for signal transduction and are constitutively associated with Janus tyrosine kinases (Jaks) 1 and 2 that phosphorylate signal transducer and activators of transcription (Stats) [65]. Stat-1 phosphorylated by Jak1 at Tyr701, forms homodimers that are translocated to the nucleus where they bind gamma-activated site (GAS) elements to initiate expression of antiviral genes. Transcription is dependent on a C-terminal transcriptional activation domain in Stat-1α and is regulated in a cell-type-dependent way by Ser727 phosphorylation and recruitment of other co-regulators [66–71]. GAS regulatory elements have been identified in over 200 genes, suggesting the broad array of responses that can be initiated by IFN-γ [65, 72].
To identify the mechanism of IFN-γ-mediated clearance, we have studied CSM14.1 neuronal cells that can be differentiated in vitro. These cells become persistently infected with SINV, and treatment with IFN-γ results in virus clearance and improved cell survival [24, 73]. IFN-γ induces prolonged phosphorylation of Stat-1 at both Tyr701 and Ser727 and transient phosphorylation of Stat-5. This is accompanied by a transient increase in synthesis of viral RNA and protein followed by cessation of viral protein and RNA synthesis and restoration of host cell protein synthesis [24]. Prolonged activation of Stat-1 may be important for the shut down of virus replication that progresses over several days [24]. The importance of Stat-5 activation is unclear. Inhibition of the Jak/Stat pathway with Jak inhibitor 1 blocked the beneficial effects of IFN-γ treatment on cell viability and virus clearance indicating an essential role of Jak/Stat signal transduction in the IFN-γ-mediated noncytolytic control of SINV replication in neurons [74].
The antiviral proteins responsible for IFN-γ-mediated control of SINV replication are poorly characterized. As in other virus infections of the CNS, a large number of IFN-stimulated genes are expressed in response to infection [18, 75]. There is no evidence that the well-characterized PKR, Mx or RNAse L pathways are essential for control of SINV replication [76]. IFN-induced antiviral proteins that have been implicated include ISG-15 and zinc-finger antiviral protein, but their importance in neurons and mechanisms of action are unclear [77–80]. Future studies will be required to determine the downstream effectors of Jak/Stat-induced suppression of virus replication in neurons.
Synergism between antibody and IFN-γ
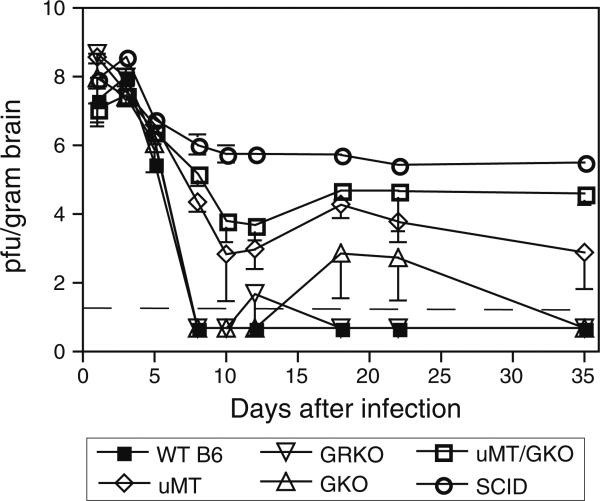
Studies in mice deficient in production of both IFN-γ (IFN-γ-/-, GKO) and antibody (μMT) indicate a synergistic role for these mediators in clearing SINV from the CNS [52]. Doubly deficient μMT/GKO mice develop persistent infection in the brain and spinal cord that is intermediate in titer between that of μMT and SCID mice (Fig. 3), suggesting that additional factors contribute to the complete clearance of SINV.
Fig. 3.
Clearance of infectious SINV from the brains of wild type (WT), IFN-β-/- (BKO), IFN-γ-/- (GKO), antibody-deficient (μMT), antibody and INF-γ-deficient (GKO/μMT) and severe combinded immunodeficiency (SCID) C57BL/6 (B6) mice. Dashed line indicates the limit of detection. Data from (52)
To investigate the roles of antibody and IFN-γ in the long-term control of SINV replication, CNS tissues were studied approximately 3 months after infection. SCID mice showed persistent virus replication at all sites. All μMT and μMT/GKO mice had persistent virus in brain, while GKO and GRKO mice did not. In the brain stem and spinal cord, virus was detectable in most μMT/GKO mice, but in only a few μMT or GKO mice. SINV is particularly likely to infect motor neurons of the anterior horn of the spinal cord [25, 81], and mechanisms of virus clearance from this site are of particular interest because of their importance for paralytic disease. To identify differences in clearance spinal cord regions of μMT/GKO, mice were analyzed separately. Peak virus replication (day 3 pi) and the initial phases of clearance (day 5) were similar in the cervical, thoracic and lumbar regions. After day 8, SINV was no longer detected in the cervical or thoracic regions of most μMT/GKO mice, while the majority had detectable virus in the lumbar spinal cord through day 81. However, levels of virus were lower than those observed in the lumbar spinal cords of SCID mice. These data indicate that IFN-γ is an important contributor to antibody-mediated control of SINV replication in the lumbar spinal cord and that virus can be cleared from cervical and thoracic motor neurons by an unidentified antibody and IFN-γ-independent mechanism [52].
These studies show that antibody and IFN-γ play synergistic roles in controlling SINV replication and preventing reactivation of infection in a site-specific manner in the CNS and that each individual arm of the immune response plays a role in virus clearance during the course of infection. Virus is least likely to be cleared from brain neurons and most likely to be cleared from motor neurons in the cervical and thoracic regions of the spinal cord. Neurons may differ either in expression of IFN-γ receptor subunits, in intracellular signaling pathways necessary for an antiviral response or in the ability to synergize with type I IFN [82–86].
Acknowledgments
This work was supported by research grant R01 NS38932 from the National Institutes of Health. The contributions of many members of the laboratory to these studies, particularly Drs. Gwen-dolyn Binder, Rebeca Burdeinick-Kerr, Philippe Despres, Beth Levine, William Tyor and Sukathida Ubol, are greatly appreciated.
References
- 1.Griffin DE. Alphaviruses. In: Knipe DL, Howley PM, Griffin DE, editors. Field's Virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 2.Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Disease Control Eastern equine encephalitis—New Hampshire and Massachusetts, August–September 2005. MMWR. 2006;55:697–700. [PubMed] [Google Scholar]
- 4.Weaver SC, Salas R, Rico-Hesse R, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE study group. Lancet. 1996;348:436–40. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 5.Strauss EG, Strauss JH. Structure and replication of the alphavirus genome. In: Schlesinger S, Schlesinger MJ, editors. The togaviridae and flaviviridae. Plenum Publ. Corp; New York: 1986. [Google Scholar]
- 6.Skoging U, Vihinen M, Nilsson L, Liljestrom P. Aromatic interactions define the binding of the alphavirus spike to its nucleocapsid. Structure. 1996;4:519–29. doi: 10.1016/s0969-2126(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Owen KE, Choi H–K. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure. 1996;4:531–41. doi: 10.1016/s0969-2126(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 8.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn RJ, Rossmann MG. Placement of the structural proteins in Sindbis virus. J Virol. 2002;76:11645–58. doi: 10.1128/JVI.76.22.11645-11658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurkela S, Manni T, Vaheri A, Vapalahti O. Causative agent of Pogosta disease isolated from blood and skin lesions. Emerg Infect Dis. 2004;10:889–94. doi: 10.3201/eid1005.030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malherbe H, Strickland-Cholmley M, Jackson AL. Sindbis virus infection in man: report of a case with recovery of virus from skin lesions. S Afr Med J. 1963;37:547–52. [PubMed] [Google Scholar]
- 12.Laine M, Luukkainen R, Toivanen A. sindbis viruses and other alphaviruses as cause of human arthritic disease. J Intern Med. 2004;256:457–71. doi: 10.1111/j.1365-2796.2004.01413.x. [DOI] [PubMed] [Google Scholar]
- 13.Hahn CS, Lustig S, Strauss EG, Strauss JH. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci USA. 1988;85:5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesh RB, Watts DM, Russell KL, et al. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis. 1999;28:67–73. doi: 10.1086/515070. [DOI] [PubMed] [Google Scholar]
- 15.Griffin DE. Role of the immune response in age-dependent resistance of mice to encephalitis due to Sindbis virus. J Infect Dis. 1976;133:456–64. doi: 10.1093/infdis/133.4.456. [DOI] [PubMed] [Google Scholar]
- 16.Jackson AC, Moench TR, Griffin DE. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Invest. 1987;56:418–23. [PubMed] [Google Scholar]
- 17.Johnson RT, McFarland HF, Levy SE. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972;125:257–62. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- 18.Labrada L, Liang XH, Zheng W, Johnston C, Levine B. Age-dependent resistance to lethal alphavirus encephalitis in mice: analysis of gene expression in the central nervous system and identification of a novel interferon-inducible protective gene, mouse ISG12. J Virol. 2002;76:11688–703. doi: 10.1128/JVI.76.22.11688-11703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havert MB, Schofield B, Griffin DE, Irani DN. Activation of divergent neuronal cell death pathways in different target cell populations during neuroadapted Sindbis virus infection of mice. J Virol. 2000;74:5352–6. doi: 10.1128/jvi.74.11.5352-5356.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis J, Wesselingh SL, Griffin DE, Hardwick JM. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–35. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–60. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 22.Nava VE, Rosen A, Veliuona MA, Clem RJ, Levine B, Hardwick JM. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–9. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder G, Griffin D. Interferon-c-mediated site specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–6. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- 24.Burdeinick-Kerr R, Griffin DE. Gamma interferon-dependent, noncytolytic clearance of Sindbis virus infection from neurons in vitro. J Virol. 2005;79:5374–85. doi: 10.1128/JVI.79.9.5374-5385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths M, Neal JW, Gasque P. Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int Rev Neurobiol. 2007;82:29–55. doi: 10.1016/S0074-7742(07)82002-2. [DOI] [PubMed] [Google Scholar]
- 27.Irani DN, Lin K- I, Griffin DE. Brain-derived gangliosides regulate the cytokine production and proliferation of activated T cells1. J Immunol. 1996;157:4333–40. [PubMed] [Google Scholar]
- 28.Irani DN, Lin K- I, Griffin DE. Regulation of brain-derived T cells during acute central nervous system inflammation. J Immunol. 1997;158:2318–26. [PubMed] [Google Scholar]
- 29.Wesselingh SL, Levine B, Fox RJ, Choi S, Griffin DE. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–97. [PubMed] [Google Scholar]
- 30.Irani DN, Griffin DE. Regulation of lymphocyte homing into the brain during viral encephalitis at various states of infection. J Immunol. 1996;156:3850–7. [PubMed] [Google Scholar]
- 31.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–24. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 32.Wekerle H, Engelhardt B, Risau W, Meyerman R. Interaction of T lymphocytes with cerebral endothelial cells in vitro. Brain Pathol. 1991;1:107–14. doi: 10.1111/j.1750-3639.1991.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 33.Wekerle H, Linington C, Lassman H, Meyermann R. Cellular immune reactivity within the CNS. Trends in Neurosci. 1986;9:271–7. [Google Scholar]
- 34.McFarland HF, Griffin DE, Johnson RT. Specificity of the inflammatory response in viral encephalitis I. Adoptive immunization of immunosuppressed mice infected with Sindbis virus. J Exp Med. 1972;136:216–26. doi: 10.1084/jem.136.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29:185–94. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 36.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein RS, Lin E, Zhang B, et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–66. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–31. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rappert A, Biber K, Nolte C, et al. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl(-) current and chemotaxis in murine microglia. J Immunol. 2002;168:3221–6. doi: 10.4049/jimmunol.168.7.3221. [DOI] [PubMed] [Google Scholar]
- 41.Sandberg K, Eloranta ML, Campbell IL. Expression of alpha/beta interferons (IFN-alpha/beta) and their relationship to IFN-alpha/beta-induced genes in lymphocytic choriomeningitis. J Virol. 1994;68:7358–66. doi: 10.1128/jvi.68.11.7358-7366.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane TE, Asensio VC, Yu N, Paoletti AD, Campbell IL, Buchmeier MJ. Dynamic regulation of alpha-and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol. 1998;160:970–8. [PubMed] [Google Scholar]
- 43.Rempel JD, Quina LA, Blakely-Gonzales PK, Buchmeier MJ, Gruol DL. Viral induction of central nervous system innate immune responses. J Virol. 2005;79:4369–81. doi: 10.1128/JVI.79.7.4369-4381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorries R. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr Top Microbiol Immunol. 2001;253:219–45. doi: 10.1007/978-3-662-10356-2_11. [DOI] [PubMed] [Google Scholar]
- 45.Marten NW, Stohlman SA, Bergmann CC. Role of viral persistence in retaining CD8(+) T cells within the central nervous system. J Virol. 2000;74:7903–10. doi: 10.1128/jvi.74.17.7903-7910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawke S, Stevenson PG, Freeman S, Bangham CR. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–82. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moench TR, Griffin DE. Immunocytochemical identification and quantitation of mononuclear cells in cerebrospinal fluid, meninges, and brain during acute viral encephalitis. J Exp Med. 1984;159:77–88. doi: 10.1084/jem.159.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin DE, Johnson RT. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977;118:1070–5. [PubMed] [Google Scholar]
- 49.Byrnes AP, Durbin JE, Griffin DE. Control of Sindbis virus infection by antibody in interferon-deficient mice. J Virol. 2000;74:3905–8. doi: 10.1128/jvi.74.8.3905-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frolova EI, Fayzulin RZ, Cook SH, Griffin DE, Rice CM, Frolov I. Roles of nonstructural protein nsP2 and alpha/beta interferons in determining the outcome of Sindbis virus infection. J Virol. 2002;76:11254–64. doi: 10.1128/JVI.76.22.11254-11264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol. 2000;74:3366–78. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burdeinick-Kerr R, Wind J, Griffin D. The synergistic roles of antibody and interferon in noncytolytic clearance of Sindbis virus from different regions of the central nervous system. J Virol. 2007;81:5628–36. doi: 10.1128/JVI.01152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyor WR, Wesselingh S, Levine B, Griffin DE. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J Immunol. 1992;149:4016–20. [PubMed] [Google Scholar]
- 54.Kimura T, Griffin DE. The role of CD8+ T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J Virol. 2000;74:6117–25. doi: 10.1128/jvi.74.13.6117-6125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston RE, Peters CJ. Alphaviruses. In: Fields BN, Knipe DM, Howley PM, et al., editors. Virology. Lippincott–Raven Press; New York: 1996. [Google Scholar]
- 56.Levine B, Griffin DE. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol. 1992;66:6429–35. doi: 10.1128/jvi.66.11.6429-6435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ubol S, Levine B, Lee S–H, Greenspan NS, Griffin DE. Roles of immunoglobulin valency and the heavy-chain constant domain in antibody-mediated downregulation of Sindbis virus replication in persistently infected neurons. J Virol. 1995;69:1990–3. doi: 10.1128/jvi.69.3.1990-1993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Despres P, Griffin JW, Griffin DE. Effects of anti-E2 monoclonal antibody on Sindbis virus replication in AT3 cells expressing bcl-2. J Virol. 1995;69:7006–14. doi: 10.1128/jvi.69.11.7006-7014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Despres P, Griffin JW, Griffin DE. Antiviral activity of alpha interferon in Sindbis virus-infected cells is restored by anti-E2 monoclonal antibody treatment. J Virol. 1995;69:7345–8. doi: 10.1128/jvi.69.11.7345-7348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin DE, Giffels J. A study of protein characteristics that influence entry into cerebrospinal fluid of normal mice and mice with encephalitis. J Clin Invest. 1982;70:289–95. doi: 10.1172/JCI110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffin DE. Immunoglobulins in the cerebrospinal fluid: changes during acute viral encephalitis in mice. J Immunol. 1981;126:27–31. [PubMed] [Google Scholar]
- 62.Knopf PM, Harling-Berg CJ, Cserr HF, et al. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J Immunol. 1998;161:692–701. [PubMed] [Google Scholar]
- 63.Tyor WR, Moench TR, Griffin DE. Characterization of the local and systemic B cell response of normal and athymic nude mice with Sindbis virus encephalitis. J Neuroimmunol. 1989;24:207–15. doi: 10.1016/0165-5728(89)90118-5. [DOI] [PubMed] [Google Scholar]
- 64.Tyor WR, Griffin DE. Virus specificity and isotype expression of intraparenchymal antibody-secreting cells during Sindbis virus encephalitis in mice. J Neuroimmunol. 1993;177:475–82. doi: 10.1016/0165-5728(93)90056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tau G, Rothman P. Biologic functions of the IFN-gamma receptors. Allergy. 1999;54:1233–51. doi: 10.1034/j.1398-9995.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–8. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 67.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 68.Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci. 2000;25:496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- 69.Zhu M, John S, Berg M, Leonard WJ. Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell. 1999;96:121–30. doi: 10.1016/s0092-8674(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 70.Ramsauer K, Farlik M, Zupkovitz G, et al. Distinct modes of action applied by transcription factors STAT1 and IRF1 to initiate transcription of the IFN-gamma-inducible gbp2 gene. Proc Natl Acad Sci USA. 2007;104:2849–54. doi: 10.1073/pnas.0610944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 72.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 73.Vernon PS, Griffin DE. Characterization of an in vitro model of alphavirus infection of immature and mature neurons. J Virol. 2005;79:3438–47. doi: 10.1128/JVI.79.6.3438-3447.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burdeinick-Kerr R, Govindarajan D, Griffin DE. Noncytolytic clearance of sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling 1. J Virol. 2009;83:3429–35. doi: 10.1128/JVI.02381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wacher C, Muller M, Hofer MJ, et al. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J Virol. 2007;81:860–71. doi: 10.1128/JVI.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryman KD, White LJ, Johnston RE, Klimstra WB. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 2002;15:53–76. doi: 10.1089/088282402317340233. [DOI] [PubMed] [Google Scholar]
- 77.Guo X, Ma J, Sun J, Gao G. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc Natl Acad Sci USA. 2007;104:151–6. doi: 10.1073/pnas.0607063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bick MJ, Carroll JW, Gao G, Goff SP, Rice CM, MacDonald MR. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J Virol. 2003;77:11555–62. doi: 10.1128/JVI.77.21.11555-11562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenschow DJ, Giannakopoulos NV, Gunn LJ, et al. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79:13974–83. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lenschow DJ, Lai C, Frias-Staheli N, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci USA. 2007;104:1371–6. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thach DC, Kimura T, Griffin DE. Differences between C57BL/6 and BALB/cBy mice in mortality and virus replication after intranasal infection with neuroadapted Sindbis virus. J Virol. 2000;74:6156–61. doi: 10.1128/jvi.74.13.6156-6161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vollstedt S, Arnold S, Schwerdel C, et al. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J Virol. 2004;78:3846–50. doi: 10.1128/JVI.78.8.3846-3850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 84.Sainz B, Jr, Mossel EC, Peters CJ, Garry RF. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology. 2004;329:11–7. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sainz B, Jr, LaMarca HL, Garry RF, Morris CA. Synergistic inhibition of human cytomegalovirus replication by interferon-alpha/beta, interferon-gamma. Virol J. 2005;2:14. doi: 10.1186/1743-422X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pierce AT, DeSalvo J, Foster TP, Kosinski A, Weller SK, Halford WP. Beta interferon and gamma interferon synergize to block viral DNA and virion synthesis in herpes simplex virus-infected cells. J Gen Virol. 2005;86:2421–32. doi: 10.1099/vir.0.80979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]