Abstract
Background
Despite the increased risk of depression and conduct problems in children of depressed parents, the mechanism by which parental depression affects their children’s behavioral and emotional functioning is not well understood. The present study was undertaken to determine whether parental depression represents a genuine environmental risk factor in children’s psychopathology, or whether children’s depression/conduct can be explained as a secondary consequence of the genetic liability transmitted from parents to their offspring.
Methods
Children of Twins (COT) data collected on 2,674 adult female and male twins, their spouses, and 2,940 of their children were used to address whether genetic and/or family environmental factors best account for the association between depression in parents and depression and conduct problems in their children. Data collected on juvenile twins from the Virginia Twin Study of Adolescent Behavioral Development (VTSABD) were also included to estimate child-specific genetic and environmental influences apart from those effects arising from the transmission of the parental depression itself. The fit of alternative Children of Twin models were evaluated using the statistical program Mx.
Results
The most compelling model for the association between parental and juvenile depression was a model of direct environmental risk. Both family environmental and genetic factors accounted for the association between parental depression and child conduct disturbance.
Conclusions
These findings illustrate how a genetically mediated behavior such as parental depression can have both an environmental and genetic impact on children’s behavior. We find developmentally specific genetic factors underlying risk to juvenile and adult depression. A shared genetic liability influence both parental depression and juvenile conduct disturbance, implicating child CD as an early indicator of genetic risk for depression in adulthood. In summary, our analyses demonstrate differences in the impact of parental depression on different forms of child psychopathology, and at various stages of development.
Keywords: children of twins, parental depression, juvenile depression, conduct disturbance, genetic risk, family environment
Introduction
It has been well established that a family history of depression is an important predictor of emotional and behavioral problems in children (Merikangas et al., 1988; Beardslee, 1996) (Harrington, 1996; Kim-Cohen et al., 2006; Pilowsky et al., 2008a). Despite convincing evidence for parent to offspring transmission, the processes by which parental depression increases children’s risk are not yet fully understood. Psychosocial theories postulate that impaired parenting best accounts for the inter-generational link between mother’s depression and children’s psychopathology (Johnson et al., 2001; Elgar et al., 2007). Depressed mothers have been described as more inconsistent, insensitive, inattentive, and less psychologically available to their children (Cox et al., 1987; Goodman & Brumley, 1990) (Burt et al., 2005). A depressogenic parenting style characterized by hostility, irritability, and enmeshment (Parker et al., 1979) has been shown to be a significant risk factor for children (Weissman & Paykel, 1974; Radke-Yarrow, 1998; Hammen et al., 1987) and lack of parental warmth and over-protectiveness is predicted by a history of depression (Kendler, 1996). Given the pervasive difficulties experienced by depressed parents in providing an optimal rearing environment, it is not clear whether the problems exhibited in the children are a unique consequence of the parent’s depression or a consequence of the myriad parenting factors associated with it. Moreover, despite psychosocial theories suggesting the risk to children is environmental, it has yet to be shown unequivocally whether children’s behavioral and emotional problems arise from the direct environmental impact of parental treatment or through some form of genetic mediation. It is possible that certain aspects of the so-called parental environment are not truly environmental, but indicators of genetic risk that is transmitted from parents to their offspring. Studies showing that recurrence and severity of maternal depression, and not necessarily its timing, are most predictive of children’s depression coincide with a genetic model of risk (Halligan et al., 2007); (Hammen et al., 1991). On the other hand, the effect of negative maternal behavior is most pronounced when it is associated with a current depressive episode (Lovejoy et al., 2000). A recent series of intervention (Pilowsky et al., 2008b) and adoption studies (Tully et al., 2008) do suggest the risk is environmental.
To directly test whether parental depression represents a genetic and/or family environmental risk factor for children’s depression and conduct problems we analyzed data from a large population sample of children of adult MZ and DZ twins. The study of the Children of Twins (COT) is a powerful strategy for disaggregating the nature of transmissible effects between parents and their children (Heath et al., 1985; Truett et al., 1994; Silberg & Eaves, 2004). Specifically, we sought to determine whether the impact of parental depression and children’s depression and conduct disorder are due to the sharing of genes, the provision of a high-risk environment, or both.
The logic of the children of twins design is as follows: a child is as alike genetically to the MZ cotwin as to the biological parent, with a 50% correlation for their additive genetic effects, but is provided a direct rearing environment only by the biological parents. This has three implications:
The difference in association between the child and the MZ twin parent and the child and his/her aunt/uncle provides a direct estimate of the non-genetic interaction between parental behavior and risk to childhood behavioral and emotional disorders;
The difference in association between children and their parents’ MZ co-twin versus children and their DZ aunt/uncle is a simple test of the inter-generational transmission of hereditary factors.
The co-twins of the MZ parent as compared to DZ co-twins represent indices of the long term behavioral consequences of genes whose effects may initially be expressed in the nieces and nephews in childhood.
Methods
Ascertainment
Informed consent for the study was obtained from the Institutional Review Board at Virginia Commonwealth University (IRB# 2127). After screening for eligibility for the Children of Twins study, 7407 twins from the Mid-Atlantic Twin Registry (MATR) were selected for possible inclusion in the study. Of those twins we were able to locate, 4887 had a child or niece/nephew between the ages of 9 and 17. Of the 3343 twins that were eligible and we were able to contact, data was obtained data on 83% of the individual twin families (n=2774). The resulting sample was comprised of 1043 complete twin pair families having at least one child. Of these, 354 were monozygotic female twin pairs, 144 monozygotic male, 225 dizygotic female, 98 dizygotic male, and 222 opposite sex twin pairs. Data from incomplete families were used for estimating the association across generations and between spouses, critical parameters in the Children of Twins model (see Figure 1). In addition to the nearly 2700 male and female twins that agreed to participate, data has been collected on 1639 spouses/partners of the twins, and 2940 of their children (1608 of the children provided self ratings). The total number of parent-child and avuncular-child dyads in MZ and DZ twins is shown in Table 2.
Figure 1.
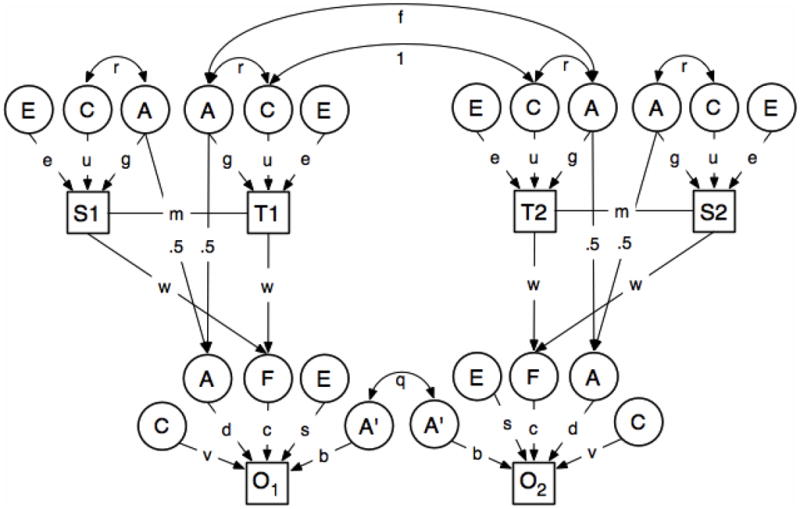
Children of Twins Model (COT)
All latent and measured variables are assumed to be standardized to unit variance to simplify the derivation of expected correlations between relatives. Note that there is assumed to be no residual variance on the parentally-derived shared environment (F) because differences are assumed to be explained entirely by regression on parental phenotype. The effects of Mendelian segregation, however, contribute residual variance in life-course persistent genetic effects (A) which explains a proportion 1− ½ (1+g2m) of the total variance in A.
Key to symbols: T1=Twin 1
T2=Twin 2
S1=Spouse of Twin 1
S2=Spouse of Twin 2
O1=Offspring of Twin 1
O2=Offspring of Twin 2
A= additive genetic effects expressed in both adults and children (“life course persistent”)
A′ = residual additive genetic effects specific to children (“juvenile limited”)
C = shared environmental effects adults
C′= shared environmental effects on children explained by parental phenotype
C″= residual, juvenile specific, shared environmental effects in twins and siblings.
E=adult unique environmental effect
Table 2.
Twin, parent - child, avuncular – offspring, and cousin correlations in MZ and DZ twins.
| Twin correlations | Depression* | Conduct Disturbance** |
|---|---|---|
| MZ adult1 | .32 (n=498) | |
| DZ adult1 | .12 (n=545) | |
| MZ child2 | .34 (n=692) | .73 (n=684) |
| DZ child2 | .17 (n=645) | .34 (n=627) |
| Adult - Child correlations3 | ||
| MZ parent | .18 (n=753) | .21 (n=1347) |
| DZ parent | .20 (n=845) | .23 (n=1508) |
| MZ avuncular | .07 (n=661) | .11 (n=1141) |
| DZ avuncular | .01 (n=654) | .06 (n=1129) |
| Cousin Correlations | ||
| MZ twin pair families | .01 (n=261) | .15 (n=526) |
| DZ twin pair families | .02 (n=185) | .15 (n=441) |
Adult twin correlations - Children of Twins Study (COT)
Juvenile twin correlations - Virginia Twin Study of Adolescent Behavioral Development (VTSABD)
Complete and incomplete twin pair families
Child ratings of depression
Parental ratings of conduct
Zygosity Determination
Zygosity was assigned to each pair based on questionnaire responses using an algorithm developed for an intersecting sample who had also been genotyped for multiple microsatellite markers (Kendler & Prescott, 2006).
The Virginia Twin Study of Adolescent Behavioral Development (VTSABD)
The Virginia Twin Study of Adolescent Behavioral Development (“VTSABD”) is a population-based, multi-wave, prospective study of 1412 male and female juvenile twin pairs between the ages of 8 and 17. The study was originally designed to elucidate the relative influence of genetic and environmental factors on the most common forms of childhood psychopathology. Details concerning the ascertainment, participation rates, and assessment protocol are available in numerous publications (Hewitt et al., 1997; Eaves et al., 1997; Simonoff et al., 1997). The twin data from the VTSABD were included in the present study to estimate any child-specific genetic and environmental effects on depression and conduct disturbance. For the purpose of elucidating the causes of depressive and conduct disturbance symptoms in the twin children most comparable in age to the children in the COT study, we used data from the first wave of the VTSABD study.
Assessment of Parental and Child Depression and Child Conduct Disturbance
As part of a telephone interview, twins and their spouses were asked to report on depressive symptoms experienced during the past 3 months using a shortened version of the Mood and Feelings Questionnaire (MFQ) (Angold et al., 1985). The children were also asked to rate depressive symptoms presenting in the past 3 months, using the same protocol used for evaluating depression in their parents. The ratings of conduct problems were obtained via maternal report using the Rutter ‘A’ scale (Rutter et al., 1970). These identical instruments were used to generate the twin correlations for depression and conduct disturbance and the data for model fitting for the VTSABD sample.
Data Analysis
Familial Correlations
The causes underlying the transmission of risk from parent’s depression to their children’s behavior (whether genetic and/or environmental) is based upon the pattern of association between the phenotypes of MZ and DZ twin parents, their cotwins, spouses, and children. To establish a baseline for decomposing the intergenerational association between parental depression and depression and conduct problems in the children, the correlations both within and across generations for each behavior were estimated for MZ and DZ twins using SAS (SAS Institute, 2000). These included the MZ and DZ parent – offspring correlations between parental depression and juvenile depression and conduct disturbance, and the correlations between children’s depression and conduct behavior and depression in their parents’ cotwin - the child’s aunt or uncle. To elucidate the causes of adult depression and depression and conduct in childhood, the twin correlations for MZ and DZ COT twins and MZ and DZ juvenile twins from the VTSABD were also estimated.
Structural Equation Modeling
We adopt a more rigorous approach for disaggregating the effect of the shared family environment from any genetic liability transmitted from parent to child using the Children of Twins model shown in Figure 1. The model is based upon the familial association between depression in MZ and DZ twin pairs, their spouses, and their children’s depression and CD, fitted using the statistical program Mx (Neale et al., 2003). The model includes twins of an adult pair, T1 and T2, their spouses, S1 and S2, and a child from each twin family, offspring 1 (O1) and offspring 2 (O2) (up to 3 children were included in the model). To take account of the possible effect of age, gender, and sample origin these variables were regressed out and the models fitted to the residual raw data.
The basic unreduced model incorporates a number of potentially important genetic and environmental sources of variation. In many respects, it is an extension of the well-known “ACE” model for the resemblance between twins (see e.g. Neale and Cardon, 1994; Eaves et al., 1997) which recognizes that differences in a trait may be due the additive genetic effects, A, shared or “common” environmental effects, C, and individual-specific within-pair environmental influences, E. However, this basic model is extended in several directions (see Figure 1) to take into account developmental differences between adults and juveniles, and to represent alternative theories about the influence of parents on their children. (Note that there are many equivalent parameterizations of the same basic model. Some alternatives are being explored in anticipation of revisions of the Mx platform).
Firstly, we recognize that different genes may affect the phenotype in adults (adult twins and their spouses) and juveniles (the children of twins). The model allows for genes, represented by the latent variables “A” in Figure 1 that have effects that persist over the life course. Although their effects may persist, through development, the phenotype may be different in adults and children. This difference is captured in the model by specifying separate parameters, g and d, for the path from life-course persistent genetic effects, A, to adult and juvenile measures respectively. Genetic theory (see e.g. Neale and Cardon, 1994) predicts that the regression of the additive genetic effects on juveniles on those of their parents is ½. Not all genetic effects are life-course persistent. Some, represented by A′ in Figure 1 are only expressed in juveniles and do not contribute to adult variation. The effect of these juvenile-limited genetic effects is denoted by the path b in the figure and the accompanying code. Since these juvenile-genetic specific effects do not contribute to the adult phenotype, their correlation between relatives (q in the Figure) is 1 in juvenile MZ twins, ½ in juvenile DZ twins and siblings, ¼ in the (juvenile) offspring of MZ twins (biologically half-siblings) and 1/8 in the offspring of DZ twins (first cousins).
Secondly, we include parameters for the individual, unique, environmental effects, E, on adults and juveniles. These effects are shown for adults in the figure, but omitted in the diagram for juveniles for simplicity.
Finally, the model involves parameters to account for the shared environment, C, of adult and juvenile twins and siblings. We assume that some of these effects persist over the life-course and affect the phenotype of both adults (C) and juveniles (C′). Some, C′, are assumed to be juvenile-specific. A critical feature of the current model and data set is its ability to identify some aspects of the non-genetic effect of parents on the environment of their offspring and to separate these direct environmental effects of parents from any secondary correlation due to the genetic correlation between generations. In this case, we assume that all the life-course persistent effects of the juvenile shared environment may be traced to the parental phenotype. The regression of juvenile C′ on the measured phenotype of mothers and fathers is represented by the path coefficient w in Figure 1. The regression of the adult phenotype on life-course persistent shared environment is u. The corresponding regression on the life-course persistent shared environment in juveniles, C′, is c in the figure. The path from juvenile-specific shared environmental effects, C″, to juvenile phenotype is v. C″ is assumed to be completely correlated in juvenile MZ and DZ twins and siblings, and uncorrelated between the children of adult MZ and DZ twins. The direct path from parental phenotype to juvenile phenotype (i.e. the environmental effect of each parent on his/her child with the correlated effects of genes and the other parent partialled out) is given by the product wc.
Mating is seldom completely random for adult behavioral disorders, such as depression and antisocial behavior (Merikangas, 1982; Maes et al., 1998). Assortative mating increases the correlation between relatives for transmissible genetic and environmental influences that affect the trait on which assortment is based. There are many possible mechanisms for assortative mating, some of which are reviewed systematically and modeled (Heath & Eaves, 1985) and, more recently (Medland & Keller, 2009). In the current application we assume that assortment is based on the measured phenotype for the trait. Typically, the traits that are of primary interest here show statistically significant but small correlations between mates and tend to have only moderate heritability, so their overall effect on the resemblance between relatives is likely to be modest. The correlation between mates is represented by m in the figure and the genetic correlation between adult twins by f. In MZ twins, f−1. In DZ twins and siblings, f= ½ (1+a2m), where a=(g+ru) and r is the so-called “passive” genotype-environment correlation, between the life-course persistent shared environment, C, and life-course persistent additive genetic effects, A. r may be expressed in terms of other model parameters (see below). Under random mating (m=0), f reduces to ½ (see e.g. Neale and Cardon, 1994). The model for the shared environment makes the arbitrary identifying assumption that there are no adult-specific shared environmental effects on adult-twins. Thus, all shared environmental effects in parents are assumed to be adult expressions of the life-course persistent environmental impact of their parents. Among other things, this assumption makes it possible to equate the passive genotype-environment correlation in parents and offspring.
The full model for the correlations between relatives involves 7 free parameters: three “genetic parameters”, g, d and b; and three “shared environmental” parameters, u,c, and v and the correlation between spouses, m.
Other parameters may be expressed as functions of other parameters of the model and may be obtained by difference or by imposing constraints on the parameter values. Thus, since all variation in C′ of children is explained by the regression of C′ on parental phenotype, the standardized path coefficient, w, must satisfy the constraint 1−2w2(1+m)=0. Further, since parents are assumed to influence the children genetically, through genes showing life-course persistent genetic effects, and environmentally through their effects of their phenotypes on the shared environment of their children, we expect a passive genotype-environment correlation, r in Figure 1, between the life-course persistent genetic effects, A, and the life-course persistent environmental effects C. Assuming that there are no secular changes in the contributions of genes, environment and mate selection, we anticipate that r will approach an equilibrium value after relatively few generations implied by the constraint that r−w(g+ru)(1+m)=0.
The free and derived parameters of the model are summarized for convenience in Table 1. In addition to the tabulated parameters of the structural model, the model includes parameters for the means of adult and juvenile male and female subjects, and for the standard deviations of adults and juveniles. Models are fitted to the raw square root transformed data regressing out the effects of age, gender, and sample origin.
Table 1.
Summary of parameters of structural model for correlations between relatives
| Parameter | Description | Free1 |
|---|---|---|
| g | Path from persistent additive genetic effect to adult phenotype | F |
| d | Path from persistent additive genetic effect to juvenile phenotype | F |
| b | Path from juvenile limited genetic effect to juvenile phenotype | F |
| u | Path from adult shared environment to adult phenotype | F |
| c | Path from juvenile shared environment to juvenile phenotype | F |
| v | Path from juvenile-specific shared environment to phenotype | F |
| w | Path from parental phenotype to juvenile shared environment | D |
| m | Correlation between spouses | F |
| f | Correlation between additive genetic effects of siblings/twins | D |
| r | Correlation between persistent genetic and shared environmental effects | D |
| q | Correlation between juvenile-limited additive genetic effects | D |
| a | Correlation between genes of parents and phenotype of parents | D |
| wc | Partial regression of juvenile outcome on parental phenotype | D |
Note:
Parameters are designated as free (F), or derived (D) from other model parameters. Derived parameters may be expressed explicitly or implied by constraints (see text).
We do not present detailed algebraic derivation of the expected correlations between relatives. However, the annotated Mx code that implements the algebra in our chosen platform for maximum-likelihood estimation of structural models is available upon request. For convenience, parameter definitions in the code follow those in Figure 1 and Table 1.
Results
Demographics of COT families
The age range of the children was 9 through 17 with an average age of 13.53. Seventy percent of the families included one child, 25% two children, and the remainder three or more children. Seventy five percent of the children were from intact families, 20% from non-intact families through divorce or separation, and 5% of the sample of parents were widowed. Four percent of the parents had less than a high school education, 22% received a high school diploma, 39% an advanced degree, and the remainder some college education. The median income of the sample was $50–$70,000 per year.
Pattern of Association
Three sets of correlations are shown in Table 2. 1) twin correlations for adult depression, 2) twin correlations for juvenile depression and conduct disturbance, 3) parent- child correlations in MZ and DZ twin pair families, and 4) aunt/uncle – niece/nephew correlations in MZ and DZ twin families. Adult twin correlations of .32 MZ and .12 DZ implicate genetic factors (and possibly non-additive genetic effects) with little or no effect of the shared environment. The correlation of .17 in DZ twins and .34 in MZ twins for juvenile depression is also consistent with genetic mediation. Genetic factors are also most influential in liability to parental ratings of children’s conduct disturbance (.73MZ/.34DZ). The average parent offspring correlation in MZ and DZ twin families is .19 for depression and .22 for conduct. In MZ twin pairs, the parent – child correlation of .18 is more that two times the avuncular – child correlation, implicating non-genetic effects of parental depression. A similar pattern of association is observed for parental depression and juvenile conduct disturbance with an MZ correlation of .21 and an avuncular correlation of .11.
Intergenerational transmission of risk from parental depression to children’s depression
The model fitting results and associated parameter estimates under the best fitting model for child depression are presented in Table 3.
Table 3.
Summary of Model-Fitting Results for Adult and Juvenile Depression Data in Children of Twins
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 |
|---|---|---|---|---|---|---|---|---|
| IFAIL | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| m | 0.1771 | 0.1775 | 0.1786 | 0.1761 | 0.1760 | 0.1760 | 0.1761 | .1786 |
| g | 0.6033 | 0.0000! | 0.5463 | 0.5410 | 0.5410 | 0.5410 | 0.5411 | .5463 |
| d | −0.0362 | 0.0000! | 0.4263 | 0.0000! | 0.0000! | −0.0305 | 0.0000! | .4263 |
| b | 0.4839 | 0.0000! | 0.3953 | 0.5339 | 0.4854 | 0.5329 | 0.5339 | .3953 |
| u | −0.2138 | 0.4514 | 0.0000! | 0.0000! | 0.0000! | 0.0000! | 0.0000 | .0000 |
| w | 0.6514 | 0.6516 | 0.6513 | 0.6520 | 0.6520 | 0.6520 | 0.6520 | .6513 |
| c | 0.2239 | 0.2156 | 0.0000! | 0.2101 | 0.2094 | 0.2248 | 0.2101 | 0.0000! |
| v | 0.2010 | 0.4442 | 0.0000! | 0.0000! | 0.2002 | 0.0000! | 0.0000! | .0000 |
| r | 0.3976 | 0.0000 | 0.0000 | 0.4149 | 0.4149 | 0.4149 | 0.4149 | .4194 |
| wc | 0.1458 | 0.1404 | 0.0000 | 0.1369 | 0.1365 | 0.1466 | 0.1369 | .0000 |
| a | 0.5183 | 0.0000 | 0.5462 | 0.5410 | 0.5410 | 0.5410 | 0.5411 | .5463 |
| F | 0.5240 | 0.5000 | 0.5000 | 0.5226 | 0.5257 | 0.5258 | 0.5258 | .5266 |
| −2lnL | 7116.502 | 7137.561 | 7127.460 | 7116.827 | 7117.007 | 7117.962 | 7118.012 | 7127461 |
| K | 7 | 4 | 4 | 4 | 3 | 3 | 3 | 6 |
| Comparison | - | Model 1 | Model 1 | Model 1 | Model 4 | Model 4 | Model 4 | Model 1 |
| χ2 | - | 21.059 | 10.958 | 0.325 | 0.505 | 1.160 | 1.510 | 10.959 |
| d.f. | - | 3 | 3 | 3 | 1 | 1 | 1 | 1 |
| P | - | <10−4 | 0.0119 | 0.9552 | 0.4773 | 0.2269 | 0.2207 | 0.0009 |
Notes: K= # of free (unconstrained) parameters in the model.
=parameter fixed at zero ex hypothesi; “Comparison” denotes model with which reduced model is compared; χ2=log-likelihood ratio chi-square for model comparison; d.f. = degrees of freedom for χ2; “IFAIL” indicates level of concern about precision of numerical estimation – “0” implies all convergence criteria met; “1” implies not all criteria met but solution is probably satisfactory. Estimates for the “best” model are highlighted in bold type.
“Model 1” constitutes the baseline in the principal genetic and environmental parameters which are estimated without constraint. The results for other reduced models are also summarized. Model 2 excludes all genetic effects from the model (i.e. g=d=b=0) and Model 3 attempts to remove all shared environmental effects (u=c=v=0). Both reduced models show a significantly poorer fit yielding χ2(3)=21.06 for the joint effects of genes and χ2(3)= 10.96 for those of the shared environment, respectively indicating that a complete understanding of family resemblance for depression requires both genetic and shared environmental effects. Models 4–8 attempt other post-hoc reductions of the model in the attempt to identify the most salient effects. Model 4 removes shared environmental effects for adult depression (u=0), and excludes both juvenile-specific shared environmental effects (v=0) and any life-course persistence of juvenile genetic effects (d=0) while retaining a direct effect of parental depression on the shared environment of their offspring (c unconstrained). This reduced model does not fit significantly worse than the full model 1 which implies that these three effects jointly might reasonably be excluded. This model implies that, after allowing for modest assortative mating, twin resemblance in adult depression is explained entirely by the additive effects of genes (g), without any effect of the shared environment (u). Models 5–7 explore the effects of allowing each of u,v and d to take their own values in turn in comparison with Model 4. These model-comparison statistics confirm that each of the three parameters may be omitted individually without worsening the fit significantly or producing marked changes in the other model parameters.
Model 8 provides a critical test of the environmental impact of parental depression on depression in the juvenile offspring. All the model parameters are free (c.f. Model 1) except for c which is fixed at zero. The change in likelihood is highly significant (χ2(1)=10.96, P<0.0001) indicating strong support for the environmental effect of parental depression.
The final, “best-fitting”, model implies that, after allowing for modest assortative mating, twin resemblance in adult depression is explained entirely by the additive effects of genes (g) and the individual specific environment, without any effect of the shared environment (u). Twin resemblance in juveniles can be attributed both to the effects of genes (b) and the shared environment (c) but the genetic effects are specific to adolescence and do not exercise long-term effects over the life course (d=0). By contrast, the effects of the shared environment on juveniles appear to be due entirely to the environmental impact of their parents. In summary, the best-fitting model implies that a proportion g2 = 29% of the total variation in adult depression can be attributed to the cumulative additive effects of genetic differences. The remaining 71% is attributable to the unique environmental effects that are uncorrelated between adult twins. Juvenile specific genetic effects explain a proportion b2=29% of variation in depression among juveniles, with a small but significant contribution of c2=4% due to the environmental impact of parental depression on that of their children. The remaining 68% of the variation is assigned to unique environmental influences. These estimates are summarized in Table 5.
Table 5.
Proportions of variance (%) in juvenile outcome explained by sources in final “best’ model.
| Genes | Shared Environment | Passive rGE | Unique Environment | |||
|---|---|---|---|---|---|---|
| Parental | Juvenile | Parental | Juvenile | |||
| Depression (Model 4) | 0.00 | 28.50 | 4.41 | 0.00 | 0.00 | 67.09 |
| Conduct disorder (Model 10) | 15.19 | 45.90 | 1.70 | 0.00 | 4.28 | 32.89 |
Note: “parental” genetic and environmental effects refer to contribution of parental depression to the juvenile outcome (depression or conduct disorder).
Intergenerational transmission of risk from parental depression to children’s conduct disturbance
The model fitting results and parameter estimates for parental depression and children’s conduct disturbance are shown in Table 4.
Table 4.
Summary of Model-Fitting Results for Effects of Adult Depression on Juvenile CD Data in Children of Twins
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | Model 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| IFAIL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | 0.2073 | 0.2076 | 0.2136 | 0.2019 | 0.2019 | 0.2087 | 0.2064 | 0.2141 | 0.2073 | 0.2064 |
| G | 0.6054 | 0.0000! | 0.5476 | 0.0000! | 0.6094 | 0.2293 | 0.5426 | 0.5941 | 0.6054 | 0.5426 |
| D | 0.4401 | 0.0000! | 0.6544 | 0.3852 | 0.0000! | 0.7886 | 0.3898 | 0.7610 | 0.4401 | 0.3898 |
| B | 0.6486 | 0.0000! | 0.4996 | 0.6620 | 0.7657 | 0.0000! | 0.6675 | 0.3142 | 0.6486 | 0.6775 |
| U | −0.2434 | 0.4596 | 0.0000! | 0.4573 | −0.2206 | 0.3593 | 0.0000! | −0.3135 | −0.2434 | 0.0000! |
| W | 0.6435 | 0.6435 | 0.6419 | 0.6450 | 0.6450 | 0.6432 | 0.6438 | 0.6417 | 0.6435 | 0.6438 |
| C | 0.1209 | 0.3202 | 0.0000! | 0.2858 | 0.2862 | 0.1007 | 0.1304 | 0.0000! | 0.1209 | 0.1304 |
| V | −0.0000 | 0.5742 | 0.0000! | 0.0000 | −0.0000 | 0.0000 | −0.0000 | −0.0000 | 0.0000! | 0.0000! |
| R | 0.3956 | 0.0000 | 0.0000 | 0.0000 | 0.4034 | 0.2474 | 0.4215 | 0.3720 | 0.3956 | 0.4215 |
| Wc | 0.0835 | 0.2060 | 0.0000 | 0.1843 | 0.1846 | 0.0648 | 0.0840 | 0.0000! | 0.0835 | 0.0839 |
| A | 0.5091 | 0.0000 | 0.5476 | 0.0000 | 0.5204 | 0.3182 | 0.5426 | 0.4775 | 0.5091 | 0.5246 |
| F | 0.5269 | 0.5000 | 0.5320 | 0.5000 | 0.5273 | 0.5016 | 0.5304 | 0.5244 | 0.5269 | 0.5304 |
| −2lnL | 3914.939 | 4082.851 | 3921.923 | 3936.857 | 3923.365 | 3925.784 | 3916.157 | 3919.328 | 3914.939 | 3916.157 |
| K | 7 | 4 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 5 |
| Comparison | - | Model 1 | Model 1 | Model 1 | Model 1 | Model 1 | Model 1 | Model 1 | Model 1 | Model 1 |
| χ2 | - | 167.392 | 6.984 | 21.918 | 8.426 | 10.845 | 1.218 | 4.389 | 0.000 | 1.218 |
| d.f. | - | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| P | - | <10−4 | 0.0724 | <10−4 | 0.0036 | 0.0009 | 0.2697 | 0.0361 | >0.9999 | 0.5438 |
Notes: See notes to Table 3.
Overall, genetic effects are highly significant (Model 2, χ2(3)=167.39) while the overall effects of the shared environment show borderline statistical significance (Model 3, χ2(3)=6.98, P=0.07). None of the genetic parameters can be omitted without substantial deterioration in fit (Models 4–6), while only the environmental impact of parental depression, c, approaches statistical significance (Model 8, χ2(1)=4.39,P<0.04). Overall, the “best” model, combining parsimony and goodness of fit appears to be Model 10 that includes all three genetic parameters but omits the shared environment for adult depression and any juvenile-specific shared environmental effects for conduct disorder (χ2(2)=1.20, P>0.54).
As expected, the estimates of genetic and environmental components of adult depression hardly differ from those reported for the previous analysis (g2 explains 29% of the total variance, as before). The pattern of results for the transmission of parental depression to child conduct disturbance is different than those for child depression. In addition to a slightly significant environmental impact of parental depression on children’s conduct disturbance (χ2(1)=4.39, P<0.04), the best fitting model shows a significant genetic association between parent depression and child conduct (χ2(1) =4.39, p<.0.04). As expected, not all genetic variation in juvenile CD can be explained by the same genes that influence adult depression since the estimate of juvenile-specific genetic effects on conduct disorder is highly significant (χ2(1)=10.84, P<0.001). The fact that the correlation between parental depression and juvenile conduct disorder has both a genetic and environmental component derived from the parents results in a modest contribution of passive genotype-environmental covariance to individual differences in conduct disorder that accounts for an estimated 2rdc=4.28% of the total variance. Genetic effects that are shared with adult depression explain d2=15.2%, juvenile-specific genetic effects account for a further b2 =45.9%, and the shared environment explained by parental depression accounts for c2=2% of the total variation in juvenile conduct disorder.
Though we found the aggregate contribution of the shared environment to be highly statistically significant, they were still relatively small because the effects of the shared environment, c, are attributed to a measured covariate (parental depression) rather than to a latent variable as in the typical “ACE” model for twin resemblance. The variance of estimates of the effects of latent shared environmental effects in twin data is much larger. The estimate of c2 is the total contribution of depression in both parents to depression in their children, after making allowance for the small effects of phenotypic assortative mating. This effect is larger than any that has currently been attributed reliably to an individual measured genetic polymorphism in models for juvenile psychopathology.
Discussion
The key goal of this study was to determine whether the impact of parental depression on child depression and conduct disturbance is truly environmental, or rather the consequence of a shared genetic liability between parents and their children. The results of model fitting show a significant environmental effect of parental depression on both children’s depression and conduct disturbance. The pattern of transmission is different for the two behaviors. Whereas juvenile depression is accounted for solely by family environmental factors, both family environmental and genetic factors are significant in the association between parental depression and childhood conduct disturbance.
The lack of a transmissible genetic effect for juvenile depression does not imply that genes are unimportant – our models clearly show significant, child-specific genetic effects, unrelated to the genes for adult depression, that have no long term continuity into adulthood. Despite the prominent role of the family environment in childhood depression, these familial effects are also limited to childhood evidenced by the absence of any shared environmental effect for depression in adulthood. These findings underscore the developmental nature of depression and are consistent with studies showing juvenile depression to be different in kind from depression occurring in adulthood (Rutter et al., 2006). Our findings also implicate a different etiologic role of parental depression depending upon the age of the child. Early in childhood, the effect of the parental depression is environmental. As children approach adulthood, the association between parental and child depression appears to be primarily genetic given.
As with depression, the family environment is a significant parental risk factor in children’s conduct disturbance. There are also a shared genetic liability between parental depression and juvenile CD, underscoring the importance of developmental factors in the expression of genetic risk underlying the two traits. Any persistence of effect is due to common genetic factors, indicative of heterotypic continuity, in which a common set of genes influences different behaviors at different phases of development. Given the shared genetic liability between juvenile CD and adult depression, children with conduct problems should be considered at increased genetic risk for depression as they enter young adulthood. The association between childhood conduct problems and adult depression, even in the absence of comorbid childhood depression, has been reported in a number of follow-up studies (Kim-Cohen et al., 2006; Rutter et al., 1998). However, the nature of this association, until now, has not been well understood.
The provision of a deleterious rearing environment provided by depressed parents for both children’s emotional and behavioral problems is consistent with the notion that adverse parenting and difficulties in the parent-child relationship are important mediators in the association between parental depression and children’s behavioral and emotional problems. The predominant transmissible effect of the shared family environment on juvenile depression is consistent with recent studies showing the greatest risk to the child to be the environmental impact of depression itself. However, this is the first demonstration of a different etiological pattern underlying the transmission of parental depression on children’s conduct disturbance.
Because there is often little evidence for shared environmental influences in behavior genetic studies, it is sometimes erroneously concluded that parents are unimportant in their children’s development (Fonagy, 2003). Consistent with psychosocial theory and the efficacy of family intervention in reducing children’s risk, this study provides strong empirical support for the critical role of the family environment in children’s behavioral and emotional functioning. Conventional twin studies are relatively weak in detecting an effect of the shared environment which may explain why potentially important parental effects on child outcomes remain undetected in such studies. In this study, the twin correlations by themselves do not show a significant shared environmental effect. The children of twins design may serve as an important alternative for identifying important aspects of the rearing environment that increase children’s liability to behavioral and emotional problems (D’Onofrio et al., 2003; Silberg & Eaves, 2004; Narusyte et al., 2008).
Since we found children’s depression to arise, in part, from family environmental factors related to parental depression, interventions should be targeted at treating the parental depression in lowering children’s risk. The significant effect of child specific genes for depression also underscores the importance of including the child in such treatments. The effectiveness of parental interventions has been elegantly demonstrated in a series of studies showing a significant ameliorative effect of treating maternal depression in minimizing children’s symptoms (Pilowsky et al., 2008b). However, a recent study (Creswell et al., 2008) showed that change in parenting behavior, and not necessarily the treatment of maternal anxiety itself was more effective in lowering children’s risk. The present study did not address the nature of association between parenting behaviors and children’s depression and CD. Given the inconsistency in the relative role of parental diagnoses versus adverse parenting in risk to children’s emotional and behavioral functioning, future work may involve the study adverse parenting, parental psychopathology, and children’s outcome within a Children of Twins framework.
The mechanisms of risk from parental depression and children’s conduct problems are different than those for depression. In the case of conduct disturbance where there is also genetic transmission of risk from parent to child, a targeted intervention that includes both the child and the parents would be appropriate to reduce the child’s vulnerability. Since children with conduct problems are at increased genetic risk for depression, these children should be identified and treated early to prevent the onset of depression later in development.
Limitations
There are several limitations of the present study. Since the assessment was derived via telephone interview, we relied on a dimensional approaching for assessing psychopathology within a relatively limited temporal window. The MFQ has the advantage of allowing direct comparability between depression in children and adults and the heritability estimate for the adult MFQ in this study is nearly identical to those obtained using more intensive structured interviews (Kendler et al., 1993). The limited time frame can also be viewed as a strength for the detection of a direct causal impact of the environment in potentiating the child’s risk. The relatively modest correlation between adult and child depressive symptoms may be related to the cyclical nature of depression. The current findings require replication using more rigorous diagnostic criteria within a broader period of assessment.
Given the large sample of kinships required for resolving genetic and family environmental transmission, the present population, although relatively large, was not sufficiently large enough for separating children into pre-pubertal and pubertal groups for evaluating different mechanisms of risk. The parent offspring correlations in younger and older children of these twin families were comparable. Power was also inadequate to test for the interaction of genetic and environmental influences with gender. There is evidence to suggest that depressive syndromes in boys and girls before and after puberty are etiologically distinct (Silberg et al., 1999; Rice et al., 2002). However, regressing out the main effects of age, gender, and sample origin had a negligible effect on the components of variance within and across generations.
The genetic association between parental depression and children’s conduct disturbance could potentially arise from any genetic overlap between parental depression and parental antisocial behavior (Kim-Cohen et al., 2006). This type of analysis would require a bivariate extension of the COT model, also planned for future analyses (Maes et al., 2009). The independent effect of both parental depression and parental antisocial behavior on children’s conduct disturbance suggests that the effect may not be accounted for entirely by comorbid antisociality.
There is an extensive literature demonstrating important associations between parental depression and depression and conduct disorder in children.
Using a Children of Twins design (COT), we are able to determine whether these inter-generational associations are due to the direct of impact of the family environment, a shared genetic liability, or both. We have found that parental depression has a direct environmental impact on both children’s depression and conduct problems. Although there are juvenile specific genetic effects not associated with the genes for adult depression, the genes for childhood conduct problems appear to be early indicators of genetic risk to adult depression.
This study underscores the importance of parental depression as a family environmental risk factor on children’s risk to depression and conduct disturbance. Since we found children’s depression to arise, in part, from family environmental factors related to parental depression, interventions should be targeted at treating the parental depression in lowering children’s risk. The significant effect of child specific genes for depression also underscores the importance of including the child in such treatments. Since children with conduct problems are at increased genetic risk for depression, these children should be identified and treated early to prevent the onset of depression later in development.
Footnotes
Mx code for the version of the model used here may be obtained upon request from Dr. Lindon Eaves (eaves.lindon@gmail.com). Mx is currently undergoing extensive revision (see http://openmx.psyc.virginia.edu/). Verified code for the new platform will be uploaded as soon as it becomes available.
Declaration of interest: There are no list fees and grants from, employment by, consultancy for, shared ownership in, or any close relationship with, an organization whose interests, financial or otherwise, may be affected by the publication of the paper by Drs. Silberg, Maes, or Eaves.
Reference List
- Angold A, Costello EJ, Messer SC, Pickles A, Winder F, Silver D. The development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Methods in Psychiatric Research. 1985;5:249. [Google Scholar]
- Beardslee WR. Prediction of adolescent affective disorder: Effects of prior parental affective disorders and child psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:279–288. doi: 10.1097/00004583-199603000-00008. [DOI] [PubMed] [Google Scholar]
- Burt KB, van Dulmen MHM, Carlivati J, Egeland B, Stroufe LA, Appleyard K, et al. Mediating links between maternal depression and offspring psychopathology: The importance of independent data. Journal of Child Psychology and Psychiatry. 2005;46:490–499. doi: 10.1111/j.1469-7610.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Cox AD, Puckering C, Pound A, Mills M. The impact of maternal depression on young children. Journal of Child Psychology and Psychiatry. 1987;28:917–928. doi: 10.1111/j.1469-7610.1987.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Creswell C, Willetts L, Murray L, Singhal M, Cooper P. Treatment of child anxiety: an exploratory study of the role of maternal anxiety and behaviors in treatment outcome. Chinical Psychology and Psychotherapy. 2008;15:38–44. doi: 10.1002/cpp.559. [DOI] [PubMed] [Google Scholar]
- D’Onofrio B, Turkheimer E, Eaves L, Corey LA, Berg K, Solaas MH, et al. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff ES, Pickles A, et al. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry. 1997;38:965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Elgar FJ, Mills RSL, McGrath PJ, Waschbusch DA, Brownridge DA. Maternal and Paternal Depressive Symptoms and Child Maladjustment: The Mediating Role of Parental Behavior. Journal of Abnormal Child Psychology. 2007;35:943–955. doi: 10.1007/s10802-007-9145-0. [DOI] [PubMed] [Google Scholar]
- Fonagy P. The development of psychopathology from infancy to adulthood: the mysterious unfolding of disturbance in time. Infant Mental Health Journal. 2003;24:212–239. [Google Scholar]
- Goodman SH, Brumley HE. Schizophrenic and depressed mothers: Relational deficits in parenting. Developmental Psychology. 1990;26:31–39. [Google Scholar]
- Halligan SL, Murray L, Martins C, Cooper PJ. Maternal depression and psychiatric outcomes in adolescent offspring: A 13-year longitudinal study. Journal of Affective Disorders. 2007;97:145–154. doi: 10.1016/j.jad.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hammen C, Burge D, Adrian C. Timing of mother and child depression in a longitudinal study of children at risk. Journal of Consulting and Clinical Psychology. 1991;59:341–345. doi: 10.1037//0022-006x.59.2.341. [DOI] [PubMed] [Google Scholar]
- Hammen C, Gordon D, Burge D, Adrian C, Jaenicke C, Hiroto D. Maternal affective disorders, illness, and stress: Risk for children’s psychopathology. American Journal of Psychiatry. 1987;144:736–741. doi: 10.1176/ajp.144.6.736. [DOI] [PubMed] [Google Scholar]
- Harrington RC. Family-genetic findings in child and adolescent depressive disorders. International Review of Psychiatry. 1996;8:355–368. [Google Scholar]
- Heath AC, Eaves LJ. Resolving the effects of phenotype and social background on mate selection. Behavior Genetics. 1985;15:15–30. doi: 10.1007/BF01071929. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: Informativeness of different relationships. Behavior Genetics. 1985;15:439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Hewitt JK, Silberg JL, Rutter M, Simonoff E, Meyer JM, Maes H, et al. Genetics and developmental psychopathology: I Phenotypic assessment in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry. 1997;38:943–963. doi: 10.1111/j.1469-7610.1997.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Smailes E, Brook JS. Association of maladaptive parental behavior with psychiatric disorder among parents and their offspring. Archives of General Psychiatry. 2001;58:453–460. doi: 10.1001/archpsyc.58.5.453. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Parenting: A genetic-epidemiologic perspective. American Journal of Psychiatry. 1996;153:11–20. doi: 10.1176/ajp.153.1.11. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The lifetime history of major depression in women: The impact of varying definitions of illness. Archives of General Psychiatry. 1993;50:863–870. doi: 10.1001/archpsyc.1993.01820230054003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott C. Genes, environment, and psychopathology: understanding the causes of psychiatric and substance use disorders. New York: Guilford Press; 2006. [Google Scholar]
- Kim-Cohen J, Caspi A, Rutter M, Tomas M, Moffit TE. The caregiving environments provided to children by depressed mothers with or without an antisocial history. American Journal of Psychiatry. 2006;163:1009–1018. doi: 10.1176/ajp.2006.163.6.1009. [DOI] [PubMed] [Google Scholar]
- Lovejoy CM, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a Meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Maes H, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, et al. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychological Medicine. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale M, Medland SE, Keller MC, Martin NG, Heath AC, et al. Flexible mx specification of various extended twin kinship designs. Twin Res Hum Genetics. 2009;12:26–34. doi: 10.1375/twin.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland S, Keller MC. Modeling Extended Twin Family Data II: Power Associated With Different Family Structures. Twin Research and Human Genetics. 2009;12:19–25. doi: 10.1375/twin.12.1.19. [DOI] [PubMed] [Google Scholar]
- Merikangas K. Assortative mating for psychiatric disorders and psychological traits. Archives of General Psychiatry. 1982;39:1173–1180. doi: 10.1001/archpsyc.1982.04290100043007. [DOI] [PubMed] [Google Scholar]
- Merikangas K, Prusoff BA, Weissman MM. Parental concordance for affective disorders: Psychopathology in offspring. Journal of Affective Disorders. 1988;15:279–290. doi: 10.1016/0165-0327(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Narusyte J, Neiderhiser J, D’Onofrio B. Testing different types of genotype-environment correlation: An extended Children of Twins model. Developmental Psychology. 2008;44:1591–1603. doi: 10.1037/a0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Department of Psychiatry, Virginia Commonwealth University; 2003. [Google Scholar]
- Parker G, Tupling H, Brown LB. A parental bonding instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Pilowsky D, Wickramaratne P, Talati A, Tang M, Hughes CW, Garber J, et al. Children of Depressed Mothers 1 Year After the Initiation of Maternal Treatment: Findings From the STAR*D-Child Study. Am J Psychiatry. 2008a;165:1136–1147. doi: 10.1176/appi.ajp.2008.07081286. [DOI] [PubMed] [Google Scholar]
- Pilowsky D, Wickramaratne P, Talati A. Children of Depressed Mothers 1 Year After the Initiation of Maternal Treatment: Findings From the STAR*D-Child Study. American Journal of Psychiatry. 2008b;165:1136–1147. doi: 10.1176/appi.ajp.2008.07081286. [DOI] [PubMed] [Google Scholar]
- Radke-Yarrow M. Children of depressed mothers: from childhood to maturity. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- Rice F, Harold G, Thapar A. Assessing the effects of age, sex, and shared environment on the genetic aetiology of depression and adolescence. Journal of Child Psychology and Psychiatry. 2002;43:1039–1051. doi: 10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- Rutter M, Tizard J, Whitmore K. Education, health and behavior. London: Longmans; 1970. [Google Scholar]
- Rutter M, Kim-Cohen J, Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry. 2006;47:276–295. doi: 10.1111/j.1469-7610.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Giller H, Hagell A. Antisocial behavior by young people. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- SAS Institute. The SAS Software for Windows for the PC: Version 8. Cary, N.C: SAS Institute, Inc; 2000. [Google Scholar]
- Silberg J, Eaves L. Analyzing contributions of genes and parent-child interaction to childhood behavioral and emotional problems: a model for the children of twins. Psychological Medicine. 2004;34:1–10. doi: 10.1017/s0033291703008948. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Pickles A, Rutter M, Hewitt JK, Simonoff E, Maes H, et al. The influence of genetic factors and life stress on depression in adolescent girls. Archives of General Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes H, Loeber R, et al. The Virginia Twin Study of Adolescent Behavioral Development: Influences of age, sex, and impairment on rates of disorder. Archives of General Psychiatry. 1997;54:801–808. doi: 10.1001/archpsyc.1997.01830210039004. [DOI] [PubMed] [Google Scholar]
- Truett KR, Eaves LJ, Walters EE, Heath AC, Hewitt JK, Meyer J, et al. A model system for the analysis of family resemblance in extended kinships of twins. Behavior Genetics. 1994;24:35–49. doi: 10.1007/BF01067927. [DOI] [PubMed] [Google Scholar]
- Tully E, Iacono W, McGue M. A adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. American Journal of Psychiatry, June. 2008;16:1–7. doi: 10.1176/appi.ajp.2008.07091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Paykel E. The depressed woman: A study of social relations. Chicago: University of Chicago Press; 1974. [Google Scholar]


