Abstract
Epigenetic modifications, such as DNA methylation, can occur in response to environmental influences to alter the functional expression of genes in an enduring and potentially, intergenerationally transmissible manner. As such, they may explain inter-individual variation, as well as the long-lasting effects of trauma exposure. While there are currently no findings that suggest epigenetic modifications that are specific to PTSD or PTSD risk, many recent observations are compatible with epigenetic explanations. These include recent findings of stress-related gene expression, in utero contributions to infant biology, the association of PTSD risk with maternal PTSD, and the relevance of childhood adversity to the development of PTSD. The relevance of epigenetic mechanisms to formulations of PTSD for the DSM-V is described.
Keywords: PTSD, epigenetics, development, prior trauma, gene x environment interactions, stress, risk factors
The extent to which individuals are defined by their experiences and memories is at the heart of many of the controversies that have surrounded the diagnosis of PTSD. Prior to this diagnosis, the prevailing view – as exemplified by the DSM-II diagnosis of ‘transient situational disturbance’ – was that symptoms emerging in the aftermath of an adverse event would pass. Consistent with stress theory, the expectation was that once the threat associated with an event was no longer present, the survivor would eventually recover from any lingering effects, and return to normal (pre-traumatic) functioning (American Psychiatric Association, 1968). Long-lasting effects were attributed to pre-existing constitutional problems, and not to the environmental exposure (Yehuda & McFarlane, 1995). The PTSD diagnosis explicitly recognized that the consequences of a cataclysmic exposure could be enduring, and provided validation of the subjective perception of trauma survivors that undergoing a watershed experience may result in an existential transformation. Indeed, many trauma survivors anecdotally describe their post-traumatic selves as fundamentally changed from their pre-traumatic selves. Even recovery from PTSD is not viewed by survivors as a return to one’s pre-trauma state, but rather as a resolution of symptoms in the context of the transformative nature of the traumatic experience.
In the years following establishment of the PTSD diagnosis (American Psychiatric Association, 1980), it became clear that there are marked individual differences with respect to the type of symptoms experienced after trauma as well as in the duration and course of these symptoms. First, not all persons who are exposed to trauma develop PTSD (Perkonigg, Kessler, Storz, & Wittchen, 2000). Second, many persons who do develop the disorder show spontaneous recovery (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Even so, remitted trauma survivors are at high risk for a recurrence of symptoms, if not relapse into full-blown PTSD (Macleod, 1994; Solomon & Mikulincer, 2006), suggesting that there are aspects of the response to trauma that are long-lasting, despite waning symptoms. Current bio-behavioral models of PTSD fall short of explaining these aspects of PTSD phenomenology. In preparation for the next formulation of PTSD in the DSM-V, it is important to consider biological mechanisms that provide a way of understanding effects of an environmental exposure in a manner that integrates both pre-existing risk factors and post-traumatic biological adaptations so as to account for the range of individual responses to focal events of similar intensity.
This paper considers the relevance of epigenetic mechanisms to PTSD and PTSD risk. An epigenetic modification refers to a change in the DNA produced by an environmental perturbation that alters the function, but not the structure, of a gene. Epigenetic changes are stable and long-lasting, and can, in some cases, be transmitted intergenerationally (Meaney & Szyf, 2005). Epigenetic modifications that alter gene expression explain how environmental exposures produce transformational change. When this change occurs during a critical developmental window, it may serve to recalibrate biological systems to influence the response to a subsequent traumatic exposure.
The preeminent animal models of PTSD (e.g., Cohen, Matar, Richter-Levin, & Zohar, 2006; Kesner et al., 2009; Siegmund & Wotjak, 2006; Zoladz, Conrad, Fleschner, & Diamond, 2008) have relied on stress theory, stress sensitization, and fear conditioning to explain biological mechanisms relevant to PTSD. Epigenetic models complement these approaches by addressing the persistence of the response to a stressor, which is not well explained by classic models of stress and fear or the neural architecture of fear. Indeed, the classic trajectory following the provocation of fear and stress in animals is recovery, and, upon removal of the stressor, restitution of biological responses towards homeostasis. Fear conditioning addresses the question of how neutral triggers come to be associated with fear responses – which is of high relevance to the syndrome of PTSD – but does not account well for individual variability in fear acquisition and extinction (Reviewed in Stam 2007 and Ursano et al., 2008; Yehuda & LeDoux, 2007).
The recognition of individual differences in the response to trauma has led to the search for genetic markers (polymorphisms) associated with PTSD risk (Broekman, Olff, & Boer, 2007). The identification of susceptibility genes and gene by environment (GxE) interactions in PTSD has been prompted not only by the limited prevalence of PTSD following exposure, but by demonstrations that PTSD runs in families (Nugent, Amstadter, & Koenen, 2008). A greater prevalence of PTSD has been reported among trauma survivors who also had a twin with PTSD (Koenen, Nugent, & Amstadter, 2008) and among first-degree relatives of persons with PTSD, including children of trauma survivors with PTSD (Yehuda, Bell, Bierer, & Schmeidler, 2008). Even after controlling for the familial clustering that contributes to risk for exposure, genetic factors continue to account for approximately one-third of the variance in PTSD (True et al., 1993).
To date, only few genes of interest have been identified in association with PTSD (see Koenan, Amstadter, & Nugent, this volume) and even fewer studies have investigated potentially relevant GxE interactions in PTSD (Table 1, Koenan et al., this volume). Recent GxE findings associate early (but not adult) adverse exposures with PTSD in response to adult trauma (Binder et al., 2008; Bradley et al., 2008). The results of these studies may explain some individual differences in trauma exposure and vulnerability to PTSD. Epigenetic mechanisms, however, offer an additional explanation for the impact of personal and family history on vulnerability, the cumulative effects of repeated exposures, predisposition to particular trauma types, and intergenerational influences that are reflective of experience.
The epigenetic model, further elaborated below, differs from that associated with either the identification of risk alleles or of GxE interactions in which response to an environmental insult is largely determined by the gene variant (i.e., specific polymorphism) (Koenen et al., 2008). In the epigenetic model, exemplified by glucocorticoid gene methylation, an environmental exposure alters the function of the gene, which then biases an individual’s response to a subsequent traumatic event (Meaney & Szyf, 2005). To date, it is not known whether there are relationships between genotype and specific epigenetic modifications; however, it is entirely plausible that genotypic differences may contribute to the directionality (e.g., methylation, demethylation) or specificity (e.g., methylation, acetylation, etc.) of epigenetic alterations in response to environmental influences. While epigenetic modifications may be involved in the interaction of environment with a polymorphism, the influence of epigenetics does not (necessarily) depend on the existence of a specific polymorphism. As such, it is appropriate to extend the search for genetic polymorphisms to epigenetic markers, as both could conceivably impact gene expression and vulnerability to PTSD.
Epigenetics: A Brief Description
This section will consider how epigenetic mechanisms alter the function of genes to produce stable, and possibly, intergenerationally transmissible alterations in the expression of DNA. A gene is a segment of DNA that provides the “instruction” for biological activity (i.e., protein synthesis) within the cell. The instruction consists of four bases (guanine, cytosine, adenine, and thymine) that are repeated in a unique sequence. Protein synthesis is accomplished in a series of steps, first through transcription of DNA to RNA, and then through translation of RNA to a protein. In the first step, transcription factors within the cell read and interpret the DNA instruction and recruit RNA polymerases, which facilitate the copying of DNA into its analogue, messenger RNA (mRNA). Cytosine methylation refers to a chemical reaction in which a methyl group is added to a specific location on the cytosine molecule; conversely, in cytosine demethylation, a methyl group is removed (Novik et al.. 2002). Enduring changes in gene expression are accomplished when such changes affect transcription factors that then alter gene expression, thus changing the inherited biological program (Meaney & Szyf, 2005).
Although several mechanisms of stable epigenetic regulation have been described, the best characterized in the mammalian genome is DNA methylation at the cytosine site (Novik et al., 2002). Methylation changes within specific regions of a gene can occur at any time in the life cycle. They might completely “silence” the gene, or, depending on their directionality, otherwise diminish or augment gene expression (Sutherland & Costa, 2003). There are many other types of epigenetic modifications (e.g., histone acetylation/deacetylation) in addition to methylation that can be assessed quantitatively by examining specific locations within a gene (Sutherland & Costa, 2003). Epigenetic changes are site specific and must be considered in the context of a specific tissue (e.g., brain region, peripheral tissue, blood cell). At this time, laboratory methods have been established for the assessment of epigenetic modifications within an individual gene, and even using genome-wide approaches using polymorphonuclear lymphocytes. However, interpreting the functional significance of an epigenetic mark involves obtaining additional measures to assess the impact on phenotype. Furthermore, as with gene expression studies, it will be critical to ascertain the relevance of epigenetic modifications observed in peripheral blood cells (lymphocytes) to central nervous system functioning.
Cytosine Methylation in Response to Variations in Maternal Care: A Behavioral Model
A prominent paradigm demonstrating environmental influences on epigenetic modifications may have implications for PTSD (Seckl, 2008; Seckl & Meaney, 2006). In the rat, variations in maternal care produce persistent effects on physiological and behavioral responses in the offspring by programming the hypothalamic-pituitary-adrenal (HPA) axis (Liu et al., 1997; Francis & Meaney, 1999). These effects are mediated by changes in DNA methylation of the glucocorticoid receptor gene in the hippocampus (Weaver, Szyf, & Meaney, 2002).
The HPA axis is one of the major endocrine systems involved in coordinating short-term responses to stress. Alterations in HPA function have been observed in association with early life events and PTSD, and also with major depressive disorder (MDD) (Pariante & Lightman 2008; Yehuda, 2002). The observation that differences in early rearing can recalibrate the HPA axis in an animal model (Francis & Meaney, 1999) has provided an important paradigm for how environmental exposures can produce enduring modifications in human HPA axis activity. However, there appears to be a developmental window for these effects, in that similar alterations in maternal behavior conferred in older rat pups do not produce the same level of modification of the HPA axis (Champagne & Meaney, 2001).
Initial studies focused on producing phenotypic differences in maternal behavior by exposing mothers to handling (i.e., removing mothers who had given birth to rat pups approximately two weeks earlier from their home cage, having them held by an investigator for a 15 minute period over several days, and then returning them to their pups) (Liu et al., 1997). Investigators found that such a manipulation resulted in increased licking and grooming of the pups compared to mothers who had not received any manipulation. Rat pups of mothers expressing greater licking and grooming had lower cortisol levels in adulthood compared to pups of (non-handled) mothers displaying lower licking and grooming (Liu et al., 1997). The responsiveness of glucocorticoid receptor was also greater in adulthood in the offspring of the handled dams, as demonstrated by a greater suppression of cortisol in response to low dose dexamethasone administration (Cook, 1999).
Brain studies demonstrated a greater number of hippocampal glucocorticoid receptor (Francis, Diorio, Liu, & Meaney, 1999), and a greater expression of the glucocorticoid receptor gene (Nr3c1) in offspring of high licking and grooming mothers. Hypomethylation within the promoter region of the hippocampal glucocorticoid receptor gene was identified as responsible for its increased expression; in contrast, rat pups receiving low maternal licking and grooming showed greater cytosine methylation at the same promoter site (Weaver et al., 2002). The observed biological effects and maternal behaviors were transmitted from female offspring to the next (third) generation (Francis et al., 1999; Meaney & Szyf, 2005). This elegant series of studies provides a clear molecular link between an early environment influence (in this case maternal behavior) and gene expression, producing functional biological correlates in endocrine and behavioral measures related to stress reactivity (Weaver, 2007). As such, these studies offer proof of concept for intergenerational transmission of vulnerability to stress related consequences and detail a plausible mechanism for explaining how childhood adversity increases risk for the development of PTSD following traumatic events experienced in adulthood (Seckl, 2008; Seckl & Meaney, 2006; Yehuda & LeDoux, 2007).
It has been striking to observe that the neuroendocrine findings associated with high licking and grooming mothers parallel those associated with PTSD and PTSD risk (Yehuda & Bierer, 2008). PTSD and PTSD risk have been associated with relatively lower basal cortisol levels, a greater number of glucocorticoid receptor (on lymphocytes), and greater cortisol suppression in response to dexamethasone, a synthetic glucocorticoid administered orally to test the responsivity of the peripheral (pituitary) glucocorticoid receptor (Yehuda, 2002). Adult rats that were exposed to higher licking and grooming mothers also showed an attenuated cortisol response to stress, which has been largely interpreted as protective (Barha, Pawluski, & Galea, 2007). In that low basal cortisol levels in the acute aftermath of trauma have been linked with the subsequent development of PTSD (Yehuda, McFarlane, & Shalev, 1998), this may reflect an altered endocrine set-point resulting from hypomethylation. Lower cortisol levels at the time of a traumatic exposure would lead to sustained elevations of stress-induced catecholamine levels, facilitating consolidation of traumatic memories (Yehuda 2002).
In contrast to the findings associated with PTSD, in MDD, the glucocorticoid receptor are less responsive (Pariante & Lightman, 2008), similar to pups that receive low licking and grooming, alterations that may also reflect glucocorticoid programming (Oberlander et al., 2008). With increased methylation of the glucocorticoid receptor gene promoter, reduced expression would diminish glucocorticoid receptor responsiveness, increasing cortisol levels. Increased methylation, in a homologous region of the glucocorticoid receptor gene in which such changes were observed in rats exposed to variations in maternal care as pups, was recently observed in post-mortem hippocampal tissue of suicide victims (most with MDD) in association with history of childhood trauma exposure (McGowan et al., 2009). A study of cord blood from infants born to depressed mothers similarly found increased methylation that predicted subsequent infant cortisol levels and cortisol responses to stress (Oberlander et al., 2008). In that study, levels of methylation were linearly associated with cortisol responses in the infants.
Relevance of Epigenetics to Familial (Maternal) Transmission of PTSD
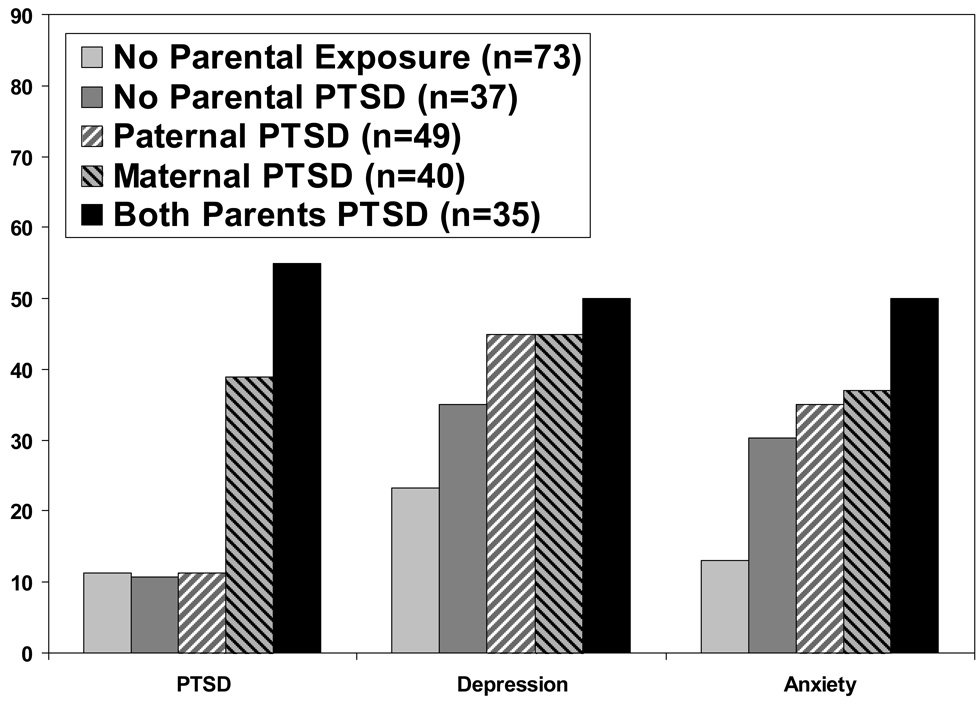
There have been no empirical demonstrations of epigenetic modifications per se in association with PTSD or PTSD risk. However, we recently observed that while maternal and paternal PTSD were equally associated with increased prevalence of depression, only maternal PTSD was associated with PTSD in Holocaust offspring (Yehuda et al., 2008). These findings are presented in Figure 1. The increased prevalence of PTSD by maternal PTSD complements the observation of low cortisol levels in association with maternal PTSD (Yehuda et al., 2007).
Fig. 1.
Psychiatric diagnoses in offspring of Holocaust survivors and comparison subjects ('No Parental Exposure' group) according to parental PTSD diagnosis. Data are redrawn from means presented in Yehuda et al. (2008). Effects of having maternal PTSD, paternal PTSD and both parents with PTSD were tested by χ2, controlling for age, gender and PTSD in the other parent. Results demonstrated significant effects of maternal but not paternal PTSD on the occurrence of offspring PTSD: paternal PTSD, χ2 (1, N = 234) = 1.18, ns; maternal PTSD (χ2 (1, N = 234) = 6.49, p < 0.05; and the maternal PTSD x paternal PTSD interaction, χ2 (1, N = 234) = 5.51, p < 0.05.
Although previous studies have implicated both maternal and paternal trauma exposure as significant predictors of PTSD in offspring (e.g., Dijanić Plasć et al., 2007; Meijer, 1985), few studies to date have been able to examine the impact of having two parents exposed to the same event. Had maternal or paternal PTSD resulted in a comparable effect on offspring, this would be consistent with contemporary models of GxE interactions (in which two parents could equally contribute to the “genetic” component or collectively influence the “environmental” aspects). Although genetics cannot be ruled out as an explanation of the increased risk for PTSD in offspring with maternal compared to paternal PTSD, epigenetic mechanisms would be sufficient to explain the preferential contribution of maternal PTSD to offspring PTSD risk (Maurel & Kanellopoulos-Langevin, 2008).
One explanation for the increased risk conferred by maternal PTSD may involve variations in maternal care. Disrupted post-natal maternal attachment with consequent deficits in the development of appropriate emotional regulation in the infant has been noted in association with increased risk for PTSD (Charuvastra & Cloitre, 2008). In the animal model linking variations of maternal care to glucocorticoid programming, increased licking and grooming has been interpreted as beneficial (Barha et al., 2007). However, the attribution of a positive valence to enhanced licking and grooming should not discourage translation of this important animal model to PTSD. The anthropomorphic notions that more infant contact means better parenting in rats has often been supported by observation of lower cortisol responses to perturbations or stressors in pups of high licking mothers than is evidenced by pups of low licking and grooming mothers. However, this interpretation fails to consider that mounting a cortisol response to a stressor is an adaptive response (Yehuda & McEwen, 2004).
The primary reason to link the early handling phenomenon in rats with transgenerational vulnerability is that it offers a mechanism through which environmental exposures can result in persisting alterations in glucocorticoid receptor expression that underlie individual differences in endocrine function that strongly resembles those observed in PTSD and PTSD risk. Maternal PTSD may confer risk for PTSD in offspring by modifying biological substrates that will influence how the offspring will respond to a future environmental challenge. What gets programmed may simply be the set point of cortisol secretion and an enhanced capacity for responsiveness of the HPA axis.
Furthermore, what may seem a protective maternal behavior in an animal may be different in the context of human behavior. Offspring of Holocaust survivor mothers with PTSD rated them as more overprotective than did offspring of Holocaust survivor mothers without PTSD, and cortisol levels in Holocaust offspring were inversely related to ratings of maternal overprotection (Yehuda & Bierer, 2008). A frequent clinical complaint of Holocaust survivor offspring is that increased attention reflected a greater reluctance on their mothers' part to undergo voluntary separation, resulting in less secure attachments (Yehuda, Blair, Labinsky, & Bierer, 2007; Yehuda & Bierer, 2008). Thus, Holocaust survivor offspring report childhood histories that are not incompatible with having had mothers who excessively “licked and groomed,” but unlike rodents, this cohort can articulate the subjective emotional consequences of such behaviors.
Epigenetic Mechanisms in Utero: Relevance to PTSD
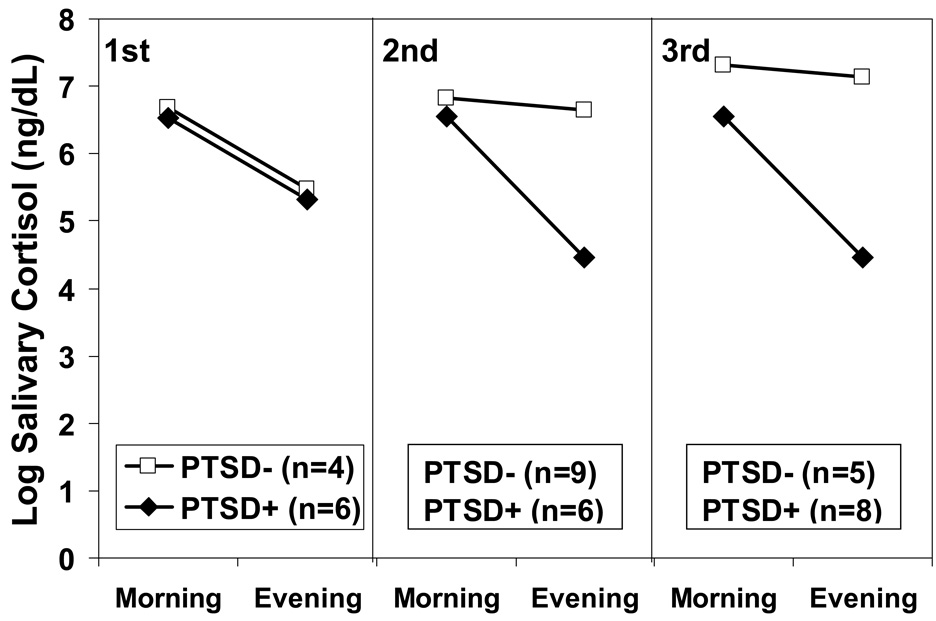
The finding of lower salivary cortisol levels in infants of mothers who developed PTSD and low cortisol as a result of their exposure while pregnant to the 9/11/01 attacks on the World Trade Center in comparison to infants of similarly exposed mothers who did not develop PTSD (Yehuda et al., 2005) may also reflect glucocorticoid programming (Seckl & Meaney, 2006). That in utero epigenetic mechanisms are involved is implied by a trimester effect on cortisol levels in offspring (Figure 2). Exposure to stress in utero results in developmental programming of tissue structure and function associated with cortisol and cortisol metabolism in the fetus, and later, adult offspring. Exposing animals and humans to glucocorticoids during pregnancy (particularly in the second and third trimesters) reduces offspring birth weight (Drake, Walker, & Seckl, 2005). Low birth weight is associated with the subsequent development of cardiometabolic disorders, such as hypertension, type 2 diabetes, and cardiovascular disease (Kajantie, 2006; Seckl, 1994). Low birth weight following maternal stress has also been linked with the development of behavioral and psychiatric problems (Susser et al., 1996). Indeed, infants of mothers exposed to the attacks of 9/11 were born small for their size (Berkowitz et al., 2003).
Fig. 2.
Awakening and bedtime salivary cortisol levels of infants born to mothers exposed to the World Trade Center disaster of 9/11/2001 divided on the basis of subsequent development of maternal PTSD are presented in Yehuda et al. (2005). Examination of maternal PTSD effects on infant salivary cortisol in each trimester separately revealed a significant effect only in the third trimester, F(1, 12) =10.86, p < .05, controlling for maternal age.
The trimester effect observed above for salivary cortisol of infant offspring of mothers with PTSD may be explained by direct effects of the trauma in utero or by related factors such as the temporal proximity of the birth to the trauma, potentially affecting maternal behavior, and resulting in greater postnatal disruptions in domains of attachment or other aspects of maternal care. Similarly, that increased PTSD risk and decreased cortisol associate with maternal PTSD in Holocaust survivor offspring might be explained by in utero changes. Regardless, the biological findings from these two disparate samples of offspring provide clinical support to the considerable theoretical rational for examining the involvement of epigenetic mechanisms in PTSD vulnerability.
Interaction of Genetics and Epigenetics
As stated above, it is not known whether epigenetic modifications are more likely to occur in specific gene variants. However, epigenetic modification may explain why similar “risk alleles” demonstrate opposite levels of gene expression. For example, the FKBP5 gene codes for a protein that is a co-chaperone of the glucocorticoid receptor, influencing its responsiveness. The same polymorphism of this gene has been associated with depression (Binder et al., 2004), and interacts with childhood abuse to increase risk for PTSD in traumatized adults (Binder et al., 2008; Yehuda et al., 2009). In depression, however, increased FKBP5 gene expression was observed, and was positively associated with increased cortisol levels (Binder et al., 2004). In PTSD, decreased FKBP5 gene expression was observed in association with low cortisol levels (Yehuda et al., 2009). That the risk polymorphism may lead to functionally different consequences demonstrates the importance of obtaining information about molecular mechanisms regulating the activity of specific genes. Ultimately, it will be important to evaluate how genotype and epigenetic modifications of many genes interact. Indeed, this will undoubtedly explain what may to some seem like paradoxical observations (e.g., that childhood abuse is a risk factor for both PTSD and depression are underpinned by glucocorticoid programming alterations in opposite directions.
Implications for DSM-V
If epigenetic contributions influence the prevalence and phenomenology of PTSD, this would have implications with respect to two major issues currently under debate in the context of the DSM-V. The first concerns the definition of Criterion A and the second concerns whether PTSD should be considered an anxiety disorder vs. a stress disorder.
Much discussion has been focused on attempting to determine the type of events that precipitate PTSD. Comparatively little attention, however, has been afforded to understanding focal traumatic events (i.e., the exposures that precipitate PTSD expression) in the context of prior experience. Furthermore, the effects of single events might differ significantly from those associated with multiple exposures. These concepts have not been well-represented in previous iterations of Criterion A. The idea of vulnerability also implies that the threshold of trauma severity to an adult event may be influenced by prior exposures, explaining marked individual variation in prevalence rates by trauma types (Kessler et al., 1995).
There has been a longstanding dialectic in stress research between the sensitizing versus inoculating effects of prior trauma (Yehuda & LeDoux, 2007). Whether or not a prior event mediates or moderates the response to a subsequent event may depend on the nature of both, in the context of other developmental experiences. For example, Holocaust offspring are more likely to develop PTSD following traumatic experiences involving interpersonal loss, but may be relatively resilient to those involving accident and disaster (Kellerman, 2001). The effects of early experience may not only depend on what occurred, but on the timing of the occurrence.
Epigenetic modifications are ideally suited to explain PTSD phenomenology in that they affirm the centrality of trauma exposure in PTSD and PTSD risk. Current deliberations pertaining to the DSM-V concern whether PTSD should be classified as a stress disorder, which would be a new category, or should remain classified as an anxiety disorder. Epigenetic mechanisms permit an understanding of how predisposing and traumatic exposures are integrated to account for individual differences in response bias, and explain the relative permanence of biological and associated psychological features that are central to PTSD. An etiology that integrates the presence of epigenetic modifications supports a conception of PTSD as a disordered stress response due, in part, to earlier or predisposing influences.
Conclusions
The major advance made by the diagnosis of PTSD in 1980 was in emphasizing the importance of trauma exposure as a major etiologic factor in the development of chronic symptoms. It is important to preserve this conception in the future diagnostic formulation of PTSD. This is in part accomplished by identifying and reinforcing the potential etiologic importance of mechanisms by which environmental exposures can modify gene expression. Epigenetic modifications are conceptually distinguished from GxE interactions, which do not place the emphasis on exposure as a principal source of variability. While genes of interest are likely to be identified in association with vulnerability to trauma exposure or even specific trauma types, there is less anticipation of a prominent role for genotype in vulnerability to PTSD. This would be reminiscent of the DSM-II where prolonged responses to adversity were based on a predetermined diathesis of non-environmental origin.
The application of epigenetic methods to the field of PTSD represents an exciting frontier because of their ability to account for individual differences in response to trauma based on environmental exposures that permanently alter gene function. Integrating epigenetics into a model that permits prior experience to have a central role in determining individual differences is also consistent with a developmental perspective of PTSD vulnerability. Importantly, an appreciation of the mechanisms through which experience may alter the expression of genes regulating biological substrates critical to PTSD pathophysiology may help establish relevant biological subtypes of the disorder.
Acknowledgements
This work was supported by a VA Merit Review Grant (RY), by an NIMH Innovation Award RO1 Genetics, Endocrinology and PTSD Risk in the Population (RY), and funding from the Department of Defense (RY). The authors express gratitude to Dr. Janine Flory for carefully reading and commenting on this manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: Author; 1968. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: Author; 1980. [Google Scholar]
- Barha CK, Pawluski JL, Galea LA. Maternal care affects male and female offspring working memory and stress reactivity. Physiology and Behavior. 2007;92:939–950. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Wolff MS, Janevic TM, Holzman IR, Yehuda R, Landrigan PJ. The World Trade Center disaster and intrauterine growth restriction. Journal of the American Medical Association. 2003;290:595–596. doi: 10.1001/jama.290.5.595-b. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of PTSD symptoms in adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Liu W, Gillespie CF, et al. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman BF, Olff M, Boer F. The genetic background to PTSD. Neuroscience & Biobehavioral Review. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Charuvastra A, Cloitre M. Social bonds and PTSD. Annual Review of Psychology. 2008;59:301–328. doi: 10.1146/annurev.psych.58.110405.085650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Richter-Levin G, Zohar J. The contribution of an animal model toward uncovering biological risk factors for PTSD. Annals of the New York Academy of Science. 2006;1071:335–350. doi: 10.1196/annals.1364.026. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Patterns of weaning and adult response to stress. Physiology & Behavior. 1999;67:803–808. doi: 10.1016/s0031-9384(99)00107-9. [DOI] [PubMed] [Google Scholar]
- Dijanić Plasć I, Peraica T, Grubisić-Ilić M, Rak D, Jambrosić Sakoman A, Kozarić-Kovacić D. Psychiatric heredity and PTSD: Survey study of war veterans. Croatian Medical Journal. 2007;48:146–156. [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Kajantie E. Fetal origins of stress-related adult disease. Annals of the New York Academy of Science. 2006;1083:11–27. doi: 10.1196/annals.1367.026. [DOI] [PubMed] [Google Scholar]
- Kellerman NP. Psychopathology in children of Holocaust survivors: A review of the research literature. Israeli Journal of Psychiatry and Related Sciences. 2001;38:36–46. [PubMed] [Google Scholar]
- Kesner Y, Zohar J, Merenlender A, Gispan I, Shalit F, Yadid G. WFS1 gene as a putative biomarker for development of post-traumatic syndrome in an animal model. Molecular Psychiatry. 2009;14:86–94. doi: 10.1038/sj.mp.4002109. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. PTSD in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Nugent NR, Amstadter AB. Gene-environment interaction in PTSD: Review, strategy and new directions for future research. European Archives on Psychiatry Clinical Neuroscience. 2008;258(2):82–96. doi: 10.1007/s00406-007-0787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Macleod AD. The reactivation of PTSD in later life. Australian and New Zealand Journal of Psychiatry. 1994;28:625–634. doi: 10.1080/00048679409080786. [DOI] [PubMed] [Google Scholar]
- Maurel MC, Kanellopoulos-Langevin C. Heredity -- Venturing beyond genetics. Biology of Reproduction. 2008;79:2–8. doi: 10.1095/biolreprod.107.065607. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Szyf M, Turecki G, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. Child psychiatric sequelae of maternal war stress. Acta Psychiatrica Scandinavica. 1985;72:505–511. doi: 10.1111/j.1600-0447.1985.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Novik KL, Nimmrich I, Genc B, Maier S, Piepenbrock C, Olek A, et al. Epigenomics: Genome-wide study of methylation phenomena. Current Issues in Molecular Biology. 2002;4:111–128. [PubMed] [Google Scholar]
- Nugent NR, Amstadter AB, Koenen KC. Genetics of PTSD: Informing clinical conceptualizations and promoting future research. American Journal of Medical Genetics Part C: Seminar in Medical Genetics. 2008;148:127–132. doi: 10.1002/ajmg.c.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devline AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen HU. Traumatic events and posttraumatic stress disorder in the community: Prevalence, risk factors, and comorbidity. Acta Psychiatrica Scandinavica. 2000;101:46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- Solomon Z, Mikulincer M. Trajectories of PTSD: A 20-year longitudinal study. American Journal of Psychiatry. 2006;163:659–666. doi: 10.1176/ajp.2006.163.4.659. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids and small babies. Quarterly Journal of Medicine. 1994;87:259–262. [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid "programming" and PTSD risk. Annals of the New York Academy of Science. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental “programming,” and the risk of affective dysfunction. Progress in Brain Research. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Toward an animal model of PTSD. Annals of the New York Academy of Science. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitization: A tale of brain and body Part 2: animal models. Neuroscience & Biobehavioral Reviews. 2007;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine. Further evidence. Archives of General Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Sutherland JE, Costa M. Epigenetics and the environment. Annals of the New York Academy of Science. 2003;983:151–160. doi: 10.1111/j.1749-6632.2003.tb05970.x. [DOI] [PubMed] [Google Scholar]
- True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Ursano RJ, Li H, Zhang L, Hough CJ, Fullerton CS, Benedek DM, et al. Models of PTSD and traumatic stress: the importance of research "from bedside to bench to bedside.". Progress in Brain Research. 2008;167:203–215. doi: 10.1016/S0079-6123(07)67014-9. Review. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Szyf M, Meaney MJ. From maternal care to gene expression: DNA methylation and the maternal programming of stress responses. Endocrine Research. 2002;28:699. doi: 10.1081/erc-120016989. [DOI] [PubMed] [Google Scholar]
- Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: Let's call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. American Journal of Psychiatry. 1995;152:1705–1713. doi: 10.1176/ajp.152.12.1705. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of PTSD from the acute response to a traumatic event. Biological Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Posttraumatic stress disorder. New England Journal of Medicine. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McEwen BS. Protective and damaging effects of the biobehavioral stress response: Cognitive, systemic, and clinical aspects. Psychoneuroendocrinology. 2004;29:1212–1222. doi: 10.1016/j.psyneuen.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of PTSD in babies of mothers exposed to the World Trade Center attacks during pregnancy. Journal of Clinical Endocrinology & Metabolism. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Blair W, Labinsky E, Bierer LM. Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. American Journal of Psychiatry. 2007;164:163–166. doi: 10.1176/ajp.2007.164.1.163. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM. Parental PTSD as a vulnerability factor for low cortisol trait in offspring of Holocaust survivors. Archives of General Psychiatry. 2007;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of Psychiatric Research. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Progress in Brain Research. 2008;167:121–135. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, et al. Gene expression patterns associated with PTSD following exposure to the World Trade Center attacks. Biological Psychiatry. 2009 April 24; doi: 10.1016/j.biopsych.2009.02.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Conrad CD, Fleschner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]