Abstract
T cell factor-1 (TCF1) critically regulates T cell development. However, signals that control TCF1 function in developing and mature T cells remain unknown. TCF1 along with β-catenin activates gene transcription and in cooperation with Groucho family of proteins mediates gene repression. It has been established that the β-catenin-dependent gene expression is often downstream of the canonical Wnt signaling pathway. We have genetically manipulated the β-catenin gene and generated mutant mice that have shown an essential role for β-catenin and TCF1 during pre-T cell receptor (TCR) and TCR-dependent stages of T cell development. We have also demonstrated a function for TCF1 and β-catenin downstream of TCR signaling in the differentiation of mature CD4 T cells into T helper lineages.
Keywords: T cell development, β-Selection, Positive selection, CD4 T helper cell differentiation, T cell factor-1, β-Catenin
TCF1 and β-catenin signaling
T cell factor-1 (TCF1), encoded by the Tcf7 gene, was originally cloned from T cells based on its affinity for the AACAAAG motif in the CD3ε enhancer [1]. In adult mice, TCF1 is expressed exclusively in the T cell lineage. When it was discovered that TCF1 acts directly downstream of the canonical Wnt–β-catenin signaling pathway, the role of this cascade in T cell development became an area of intensive investigation.
In the absence of a canonical Wnt signal, the adenomatous polyposis coli (APC)–axin–glycogen synthase kinase-3- β (GSK3β) degradation complex sequesters cytosolic β-catenin. In this complex, GSK3β kinase phosphorylates β-catenin in the N-terminus and marks it for ubiquitylation and proteosomal degradation. The binding of Wnt, a secreted glycoprotein, to the frizzled receptors and the lipoprotein receptor-related protein (LRP) coreceptors on the cell surface, leads to inactivation of GSK3β mediated phosphorylation and degradation of β-catenin. Non-phosphorylated β-catenin accumulates in the cytoplasm, translocates to the nucleus and together with TCF1, induces expression of Wnt target genes. Thus, β-catenin modulates gene expression mediated by the Wnt–β-catenin–TCF1 pathway [2, 3]. A naturally occurring 9 kDa protein, called inhibitor of β-catenin and TCF (ICAT), binds β-catenin and prevents its interaction with TCF1 [4, 5] and thereby inhibits TCF1 and β-catenin-dependent gene expression [6]. TCF1 also interacts with the groucho-related gene (GRG) co-repressor protein to repress gene transcription [7].
In addition to mediating Wnt-dependent signals, β-catenin also participates in androgen signaling pathway and is stabilized by prostaglandins. We have shown that β-catenin is also stabilized by pre-T cell receptor (TCR) and TCR signals [8, 9]. Thus, in addition to Wnt signaling, β-catenin functions downstream of other signaling pathways.
T cell development in the thymus: DN to DP transition
T cell progenitors migrate to the thymus and undertake a highly ordered developmental program, which is regulated by stage-specific signals derived from the thymic microenvironment. The order of maturation is precisely described in terms of expression of specific cell surface markers. Overall stages are defined in terms of cell surface expression of CD4 and/or CD8 (Fig. 1). The most immature thymocytes express neither CD4 nor CD8 and are termed ‘double negative (DN)’, intermediate cells express both CD4 and CD8 and are called ‘double positive (DP)’ and the most mature thymocytes express either CD4 or CD8 and are called ‘single positive (SP)’. The DN subset is further divided into developmental stages (DN1–DN4), which bear a precursor product relationship, based on the expression of CD44 and/or CD25: DN1–CD44+ CD25−; DN2–CD44+ CD25+; DN3–CD44− CD25+ and DN4 (also called pre-DP)–CD44− CD25− [10]. At the DN3 stage, rearrangement and expression of a competent TCR β-chain and a non-rearranging pre-Tα chain results in the expression of pre-TCR and differentiation into DN4/pre-DP thymocytes. Pre-TCR also provides signals that regulate proliferation and differentiation of pre-DP thymocytes to the DP stage [11]. This is the first of two checkpoints encountered by developing thymocytes, called the β-selection checkpoint, which ensures the expression of a functional TCRβ chain and the subsequent proliferation provides the requisite numbers of thymocytes that express a productive TCRβ chain.
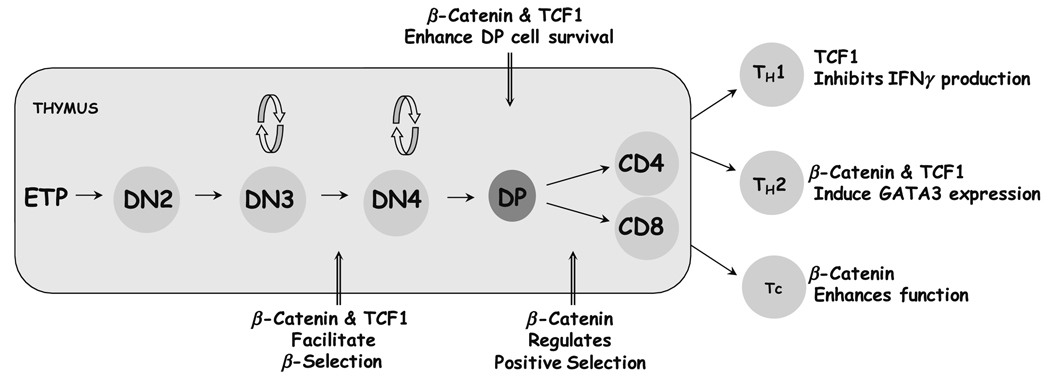
Fig. 1.
TCF1 and β-catenin regulate T cell development and function. The shaded area represents the thymus where T cells develop from bone-marrow derived precursors. Early thymic precursors (ETPs) are the most immature thymocytes that mature through well-described stages defined by the expression of cell surface markers. Mature CD4 and CD8 thymocytes migrate to the peripheral lymphoid organs where they differentiate into effectors upon encounter with antigen. Points at which TCF1 and/or β-catenin play a role during development in the thymus and mature T cell differentiation into effector subsets are indicated
Role of TCF1 and β-catenin during DN to DP transition
Clevers and colleagues showed that TCF1-deficiency impaired thymocyte maturation with multiple defects at DN1 to DN4 stages of T cell development. An incomplete block in development and proliferation results in premature thymic involution in older TCF1-deficient mice [12, 13]. Lymphocyte enhancer-binding factor-1 (LEF1)-deficient mice have normal T-cell development, but LEF1 and TCF1 double-deficient mice show a block at DN3 stage, which indicates partial redundancy between LEF1 and TCF1 during thymocyte maturation [14]. Thus, TCF1 was shown to critically regulate T cell development (Fig. 1).
First indication that loss of Wnt-dependent signaling impaired thymocyte development was obtained by using soluble frizzled receptors as decoys for Wnt proteins, in fetal thymic organ cultures (FTOCs) [15]. Subsequently, Tcf-reporter activity was directly demonstrated in all thymocyte subsets with the highest being in the DN cells [16]. Along similar lines, the secreted Wnt inhibitor Dickkopf-1 (DKK1), which binds to required LRP coreceptor, prevents Wnt signaling and inhibits thymocyte differentiation at the DN stage [16]. Finally, we provided direct evidence for the involvement of Wnts in thymocyte development. We found that Wnt1, Wnt3a and Wnt4 were predominantly expressed in the thymus and demonstrated that the thymuses of Wnt1 and Wnt4 double-deficient mice were hypocellular but maturation was unimpaired [17]. More recently, Wnt3a deficiency resulted in impaired self-renewal and reconstitution capacity of hematopoietic stem cells (HSCs) on secondary transplantation. In addition, Wnt3a deficiency also affected early stages of T-lymphoid development in the thymus [18]. These data showed a role for Wnt-signaling in T cell development and expansion.
Experiments in which ICAT expression was utilized to block interaction of β-catenin with TCF1 showed that β-catenin–TCF1 pathway was important for T cell development. Retroviral over expression of ICAT, which binds β-catenin and prevents interaction with TCF1 and blocks gene expression in DN thymocytes, resulted in decreased generation of DP thymocytes in FTOCs [19]. Decrease in the number of DP thymocytes could result from a block in development or impaired survival of newly generated DP thymocytes. We generated ICAT transgenic (ICAT-Tg) mice, which express ICAT specifically in thymocytes and T cells. These mice allowed us to identify the effects of disrupting TCF1 and β-catenin interaction during T cell development in vivo. Our analysis of ICAT-Tg mice showed that the DP thymocyte survival was impaired due to diminished expression of the pro-survival factor, BclXL [20]. Studies using transgenic or retroviral reconstitution of TCF1-deficient mice with alternately spliced forms of TCF1 also showed that the capacity in TCF1 to interact with β-catenin was required to restore T-cell development in TCF1-deficient mice [15, 21]. These data showed that TCF1 and β-catenin interaction was important for T cell development and thymocyte survival.
To determine whether β-catenin played a role in T cell development, we generated T cell-specific conditional deletion of β-catenin gene (Catnb) using Lck-Cre. Using these mice we demonstrated that deletion of β-catenin at the DN3–DN4 stage impaired T-cell development at the β-selection checkpoint [9]. β-catenin expression was only partially lost at the DN3 stage and then more fully deleted in DN4 thymocytes. Even so pre-TCR signaling was impaired, leading to a partial block in DN3-to-DN4 transition and reduced pre-TCR-dependent proliferation, but not survival, of DN4 (pre-DP) thymocytes [9]. Along these lines, vav-Cre-dependent deletion of β-catenin in HSCs also impaired long-term growth and maintenance of HSCs following transplantation [22]. Together, these loss-of-function manipulations demonstrated a role for Wnt–β-catenin–TCF1 pathway in hematopoiesis and T cell development in the thymus. By contrast, deletion of β-catenin with inducible Mx1-Cre in HSCs was shown to reconstitute normal hematopoiesis or lymphopoiesis when transplanted in lethally irradiated mice [23]. The authors postulated that a β-catenin homolog, γ-catenin (plakoglobin), might compensate for the lack of β-catenin. However, mice with combined γ-catenin and β-catenin-deficiency also showed normal hematopoiesis and lymphopoiesis [24, 25]. The authors concluded that these proteins played no role in hematopoiesis and lymphopoiesis. Notably, their data showed that TCF-reporter activity was retained in the absence of both β- and γ-catenin [24]. Overall these data show that β-catenin deletion perturbs HSC survival, self-renewal and subsequent development into T cells. However, this regulation may be complex and requires further attention.
Enforced expression of stabilized β-catenin has further identified a role for β-catenin at multiple stages of T cell development. The first such mouse was generated by deletion of exon3 of β-catenin, which encodes the GSK3β phosphorylation domain, using Lck-Cre at the DN3 stage of thymocyte development. This manipulation led to very high levels of β-catenin expression (~a log higher than that naturally stabilized by pre-TCR signals), which blocked TCRβ chain rearrangement and expression. In this model, TCRβ negative DN thymocytes transitioned to the DP stage in the absence of pre-TCR signaling [26]. The same observation was made using another model in which β-catenin was stabilized at very high levels in thymus by Lck-Cre mediated deletion of the Apc gene [27]. These studies show that high levels of β-catenin expression in DN thymocytes impairs T cell development by blocking TCRβ rearrangement and by allowing pre-TCR-independent maturation of a small number of cells to the DP stage.
We studied normally developing thymocytes and found that pre-TCR signals induce stabilization of β-catenin in DN thymocytes [8], which is down-regulated prior to transition to the DP stage [28]. Taken together with the observation that partial deletion of β-catenin at the DN3 stage impaired pre-TCR signaling [9], these data suggest that the pre-TCR utilized β-catenin for β-selection. To study this notion further, we generated β-CAT-Tg mice with enforced expression of stabilized β-catenin. In β-CAT-Tg mice, expression of β-catenin in DN3-DN4 thymocytes was increased by approximately two- to fourfold in comparison with that stabilized by pre-TCR signals and β-selection was facilitated as judged by phenotypic and several molecular criteria [8]. Detailed analysis showed that β-selected β-CAT-Tg thymocytes fail to develop further due to persistent expression of transgenic β-catenin in post-β-selected thymocytes. Molecular analysis β-CAT-Tg thymocytes revealed that the failure to down-regulate β-catenin expression in pre-DP thymocytes induced expression of transforming growth factor β (TGFβ) and impeded expression of the orphan nuclear hormone receptor RORγt that is required for transition of DN thymocytes to the DP stage [28]. Thus, modest increase in β-catenin at the DN3–DN4 stage facilitates pre-TCR signaling and β-selection but must be turned down in β-selected thymocytes as sustained expression impairs further maturation. Thus, pre-TCR signals induce and utilize β-catenin to facilitate β-selection. Together, these studies provide an additional important insight, which is that the level of β-catenin over-expression critically controls the biological outcome.
β-catenin is an established oncogene that causes tumors in a nmber of tissues including the colon [29, 30]. As mentioned earlier, in the thymus of mice bearing Lck-Cre-dependent deletion of exon3 of β-catenin gene, TCRβ− DP thymocytes develop in the absence of pre-TCR signals. TCRβ− DP thymocytes induce expression of c-myc, a known β-catenin target gene and develop c-myc-dependent DP lymphomas [31]. By contrast, we found that β-CAT-Tg mice fail to develop thymic lymphomas. We discovered that this was because immediately upon expression of transgenic (oncogenic) β-catenin, thymocytes undergo oncogene-induced senescence (OIS), growth arrest, DNA-damage and p53-mediated apoptosis, which prevents the development of thymic lymphoma in β-CAT-Tg mice [32]. The removal of p53 function in p53−/− β-CAT-Tg mice prevented apoptosis of thymocytes expressing oncogenic β-catenin and promoted β-catenin-dependent DN lymphoma. β-catenin-dependent DN lymphomas were c-myc and Notch independent [32] and thus were molecularly and phenotypically distinct from those observed in p53-deficient mice, which are DP in phenotype and c-myc dependent [33]. Thus, β-catenin-dependent thymic lymphomas develop in the absence of p53-function and are phenotypically and molecularly distinct from those that develop in p53-deficient mice. We believe that lymphoma formation is prevented in β-CAT-Tg (p53-sufficient) mice because intrathymic signals induce stabilization of β-catenin as a part of normal development [8]. β-catenin expression must be down-regulated prior to further development [28]; if this fails, essential ‘safety’ mechanisms are put in place to remove cells that have the lymphomagenic potential [32]. Thus, gain-of-function and loss-of-function manipulations demonstrate that Wnt-signaling, β-catenin and TCF1 regulate thymocyte proliferation and β-selection during T cell development.
We have also demonstrated that one consequence of transgenic expression of stabilized β-catenin in thymocytes in β-CAT-Tg mice is pre-mature age-dependent thymic involution. It is very likely that the failure to down-regulate β-catenin in post-β-selected thymocytes followed by OIS, growth arrest and apoptosis contribute to thymic involution [34]. We propose that these mechanisms may also contribute to normal age-dependent thymic involution.
T cell development in the thymus: DP to SP transition
Prior to expression of CD4 and CD8 and transition to the DP stage, pre-DP thymocytes undergo growth arrest, which allows the re-expression of RAG2 protein and rearrangement of the TCRα gene. Expression of TCRα and β chains along with the CD3 proteins leads to the expression of a mature αβ TCR on DP thymocytes. DP thymocytes that express a functional αβ TCR face three developmental fates depending on the avidity of their surface T cell receptor (TCR) with MHC/self-Ag complexes presented by thymic stromal cells [35, 36]. Majority of DP thymocytes do not recognize MHC/self-Ag complex, fail to receive a TCR signal and die by neglect. DP thymocytes that react with high avidity with MHC/self-Ag complexes receive strong TCR signals and are eliminated by negative selection [36, 37]. A small fraction of DP thymocytes receive just the right signal through their TCR and are selected for further differentiation into mature CD4+ SP or CD8+ SP thymocytes, by a process referred to as positive selection [36, 38, 39]. Mature SP thymocytes subsequently emigrate from thymus into peripheral lymphoid organs to contribute to the naïve CD4 and CD8 T cell pool. During positive selection, DP thymocytes undergo lineage commitment to differentiate into SP thymocytes with coordinated expression of MHC class I restricted T cell receptors (TCRs) with CD8 coreceptor (CD8SP) or MHC class II restricted TCRs with CD4 coreceptor (CD4SP) [40–42]. Positive selection is initiated and driven by signals transduced through both TCR and coreceptors. Cytokine receptor signals, particularly IL-7 receptor signals, also play an important role in the positive selection and maturation of CD8SP thymocytes [42, 43].
Role of TCF1 and β-catenin during DP to SP transition
We found that endogenous β-catenin levels increased during transition of DP into SP thymocytes, which suggested a regulatory function for β-catenin in positive selection (Fig. 1) [44]. Accordingly, β-CAT-Tg mice showed an increase in both mature CD4 and CD8 SP thymocytes, with a preferential increase in CD8SP thymocytes [44, 45]. This observation provided an opportunity to investigate the cellular and molecular mechanisms by which β-catenin may regulate the development of DP thymocytes into mature SP thymocytes. Studies with β-CAT-Tg mice that also express transgenic TCR demonstrated that enforced expression of β-catenin enhanced positive selection of both MHC II-restricted CD4 and MHC I-restricted CD8SP thymocytes but did not affect the lineage commitment. It has been known for some time that the timing of generation of CD4 and CD8 cells from DP precursors is different; with CD4 cells developing faster compared to CD8 cells [46]. We found that β-catenin expression accelerated the rate of generation of CD8SP cells and consequently β-CAT-Tg CD4SP and CD8SP thymocytes were generated with the same kinetics [44]. This provided a cellular mechanism for the preferential effect of β-catenin on CD8SP thymocyte development. Molecular analysis showed that β-catenin expression enhanced IL-7R signals in positive selection intermediates by both up-regulating IL-7R α chain (IL-7Rα) expression and simultaneously down-regulating suppressor of cytokine signaling (SOCS)-1 expression [47]. Finally, we showed that the effect of β-catenin on CD8 development was dependent on IL-7R signals in FTOCs [47]. Together, these results indicate that β-catenin expression in DP thymocytes, presumably driven by TCR signals, promotes the positive selection and maturation of CD8SP thymocytes by enhancing IL-7R signals in developing thymocytes.
We have found that normal DP thymocytes express very low levels of β-catenin. It is likely that the increase in β-catenin levels in pre-positive selection thymocytes is an important factor in its role in positive selection. In two other models that express β-catenin at high levels in DP thymocytes, the characteristics of the DP thymocytes that are important for positive selection appear to be altered. In mice that express stabilized β-catenin from the CD4 promoter, increased level of surface CD4 and altered survival might critically affect positive selection of thymocytes [48, 49]. In mice expressing high levels of β-catenin from the deletion of exon3 (discussed above), DP thymocytes fail to express TCRβ chain and thus will not properly respond to TCR-dependent positive selection signals [26, 31]. These results also indicate that the level of β-catenin overexpression and the developmental stage where β-catenin is expressed influence the biological outcome of the action of β-catenin.
In addition to positive selection, β-catenin and TCF1 were shown to be essential mediators of TCR-induced negative selection. In a recent report, TCR signal in DP thymocytes was shown to stabilize β-catenin and induce β-catenin and TCF1-dependent gene expression [50]. Using a mouse model in which CD4-Cre mediates the deletion of exon3, the authors demonstrated that increased β-catenin enhances TCR-induced signaling pathways that promote negative selection. Thus, high levels of β-catenin expression in TCR-signaled DP thymocytes promoted apoptosis. TCF1-deficient mice showed impaired negative selection, suggesting that TCF1 and β-catenin cooperate to mediate apoptosis and negative selection of thymocytes [50]. By contrast, in β-CAT-Tg mice, where the amount of β-catenin is only modestly increased while retaining the pattern of expression of endogenous β-catenin, positive selection of thymocytes is promoted by enhancing IL-7R signaling [44, 47]. Together, these data show that high level of β-catenin expression in TCR-signaled DP thymocytes promotes negative selection whereas modest level facilitates positive selection.
Mature T cell activation and T helper cell differentiation
In the peripheral lymphoid organs, naïve CD4 and CD8 T cells survive and maintain homeostasis until they encounter antigen. Upon encountering antigen in the context of MHC presented by the antigen presenting cells (APCs), T cells become activated, expand and produce cytokines to perform effector functions (Fig. 1). CD4 T cells differentiate into specialized effector cells called T helper (TH) cells or T regulatory (Treg) cells. If antigen-activated CD4 T cells encounter APC-derived IL-12, signaling via STAT4 induces the production of IFNγ and commitment to TH1 lineage [51, 52]. Activated CD4 T cells commit to TH2 lineage upon encountering IL-4 produced by cells of the innate immune system, which induces expression of GATA-3 and IL-4 production by CD4 T cells themselves [51–54]. In addition, CD4 T cells can also utilize TCR-driven cell-intrinsic mechanisms for differentiation to the TH2 lineage [55]. If activated CD4 T cells encounter TGFβ and IL-6 or TGFβ and IL-21, signals via STAT3 lead to differentiation to the TH17 lineage and IL-17 and IL-22 production [56, 57]. Finally, TGFβ alone results in the differentiation of CD4 T cells to Treg cells that suppress the activity of activated CD4 T cells [58, 59]. Thus, CD4 T helper cell differentiation into various subsets provide the diversity required to effectively fight different types of infections.
Role of TCF1 and β-catenin in T cell activation and T helper cell differentiation
Regulation of T cell activation by TCF1 and β-catenin remain poorly understood. An earlier study on the peripheral T cell function in TCF1-deficient mice showed that the total spleen cells from TCF1-deficient mice responded to ConA and alloantigen normally and certain in vivo anti-viral response was also normal in these mice [12]. However, TCR stimulation was shown to inhibit GSK3β activity in vitro but not induce TCF1-dependent reporter activity in Jurkat T cells [60]. Furthermore, it was reported that nuclear localization of LEF1 and β-catenin was not sufficient to induce target gene transcription in T cells [61]. These data supported the surprising notion that TCF1, which is exclusively expressed in T cells in adult mice, may not regulate gene expression in mature T cells.
We have found that TCF1 is highly expressed in T cells and β-catenin expression is stabilized downstream of TCR-stimulation in T cells, which results in up-regulation of TCF1-reporter activity and TCF1-dependent gene expression in T cells [62]. Likewise, as mentioned earlier, TCR signaling was shown to stabilize β-catenin in thymocytes and regulate TCR-dependent events during T cell development [44, 50].
To determine whether TCR-dependent TCF1 and β-catenin signaling regulated T cell function, we analyzed TCF1-deficient CD4 T cells in several well-defined assays. We demonstrated that TCF1 in cooperation with β-catenin regulates expression of GATA-3, the transcription factor essential for differentiation to the TH2 fate and for IL-4 production (Fig. 1) [62]. To arrive at this conclusion, we used both loss-of-function ICAT-Tg and TCF1-deficient mice and gain-of-function β-CAT-Tg mice. Using CD4 T cell from these mutant mice, we demonstrated that TCF1 and β-catenin play a critical role in the initiation of TH2 cell differentiation. GATA3 is expressed from two transcripts, Gata3-1a and Gata3-1b [63]. We showed that initial TCR-dependent GATA3 expression in activated CD4 T cells was predominantly produced from Gata3-1b transcripts and not from Gata3-1a. Gata3-1b transcript was induced at early time points after activation and was independent of IL-4R signaling via STAT-6. Using both chromatin immunoprecipitation assay and luciferase reporter assay, we found that TCF1 and β-catenin bound to the TCF1 binding site upstream of Gata3 exon-1b to directly activate Gata3-1b gene transcription. Further support for the regulation of Gata3-1b by TCF1 was found in the observation that deletion of TCF1 diminished Gata3-1b expression and IL-4 production. In contrast, enforced expression of β-catenin enhanced Gata3-1b expression and IL-4 production. Moreover, enforced expression of ICAT impaired Gata3-1b expression and IL-4 production, demonstrating a functional requirement for interaction between TCF1 and β-catenin. Importantly, TCF1 and β-catenin could induce Gata3-1b expression in the absence of STAT6, in the presence of anti-IL-4/IL-4R Abs or in the presence of dominant negative mastermind-like that blocks Notch activity. Therefore, the effect of TCF1 and β-catenin on Gata3-1b induction was independent of both IL-4R signaling and Notch signaling. These observations further support the notion that TCF1 and β-catenin promote initial TCR-dependent events during TH2 cell differentiation. By using ovalbumin-induced allergic asthma model, we demonstrated that TCF1-deficient mice develop impaired TH2 response in vivo as indicated by decreased IL-4 level in bronchoalveolar lavage, thus resulting in decreased pathogenesis of allergic airway inflammation. Finally, we showed that TCF1 deficiency resulted in increased IFNγ production in CD4 T cells, while overexpression of a mutant form of TCF1 that could not bind β-catenin led to suppression of IFNγ production [62]. These results show that TCF1 negatively regulates IFNγ production and TH1 fate, independently of its interaction with β-catenin. Thus, these data demonstrate that TCF1 plays an essential role in the initiation of TH2 lineage differentiation and it does so by a twofold mechanism: initiating GATA-3 expression, which induces IL-4 production and simultaneously inhibiting IFNγ production.
CD4 T cells differentiate into Tregs in the presence of TGFβ. Using retroviral transduction system, Ding and colleagues showed that expression of a stable form of β-catenin in Tregs promoted their survival and as a result, enhanced their ability to protect against inflammatory bowel disease in a mouse model. The same report also showed that high levels of β-catenin expressed from retroviral expression vector induced expression of anergy-associated genes such as Cbl-b, GRAIL and Itch [64]. Together, our studies and these studies show for the first time a role for TCF1 and β-catenin-dependent gene expression in the activation and function of CD4 T cells.
Activated CD8 cytotoxic T cells release perforin and granzymes and induce infected, target cells to undergo apoptosis. Armed effector CD8 T cells also secrete cytokines such as IFNγ, TNFα and TNFβ. Activated CD8 T cells play an instrumental role in combating cytoplasmic viral, bacterial and protozoan infections. We have found that expression of β-catenin transgene induces memory-like phenotype and function in CD8 T cells (Yu, et al., manuscript in preparation). Along these lines, adoptive transfer of Wnt-treated CD8 T cells was shown to enhance anti-tumor activity in vivo [65]. Thus, Wnt, β-catenin and TCF1 have the potential to regulate CD8 T cell function. Finally, a recent report showed that Wnt proteins produced by endothelial cells induced matrix metalloproteinase (MMP) 2 and MMP9 expression in activated T cells and promoted their migration through the subendothelial basement membrane and interstitial collagen [66]. These data show that Wnt signals regulate transmigration of effector T cells. Thus, in sharp contrast to the notion that Wnts, TCF1 and β-catenin might not regulate gene expression in T cells [12, 61], these initial studies reveal a role of β-catenin and TCF1 in the activation and function of mature T cells.
Conclusion and future directions
Altogether, studies described earlier have demonstrated the ability of β-catenin and TCF1 to regulate various aspects of T cell development and CD4 and CD8 T cell function (Fig. 1). T cell development is regulated at two major checkpoints, the pre-TCR driven β-selection and the TCR-mediated positive or negative selection. Enforced expression of β-catenin or deletion of β-catenin or TCF1 affects both these checkpoints. TCR also regulates mature T cell functions including effector and memory cell differentiation, anergy and tolerance as well as transmigration. Enforced expression of β-catenin or deletion of TCF1 affects many of these functions. Together, these data suggest that TCF1 and β-catenin regulate gene transcription downstream of the pre-TCR and TCR. An important observation from these studies is that the stage of T cell development or activation when β-catenin is increased in expression affects the biological outcome. More experimental manipulations are needed to fully understand this interesting phenomenon. Furthermore, a direct connection between Wnt–β-catenin and TCF1 signaling remains to be fully established under various conditions of T cell activation and function. Finally, elucidation of the several signaling pathways that are induced upon TCR signaling will facilitate a better understanding of how TCF1, in cooperation with β-catenin or with GRG proteins, regulates T cell development, activation and function.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging at the NIH and in part by an appointment to the Oak Ridge Institute for Science and Education’s Research Associates Program at the NIH.
References
- 1.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991;10:123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macdonald BT, Semenov MV, He X. Snapshot: Wnt/beta-catenin signaling. Cell. 2007;131:1204. doi: 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 4.Graham TA, Clements WK, Kimelman D, Xu W. The crystal structure of the beta-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Mol Cell. 2002;10:563–571. doi: 10.1016/s1097-2765(02)00637-8. [DOI] [PubMed] [Google Scholar]
- 5.Daniels DL, Weis WI. ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell. 2002;10:573–584. doi: 10.1016/s1097-2765(02)00631-7. [DOI] [PubMed] [Google Scholar]
- 6.Gottardi CJ, Gumbiner BM. Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions. Am J Physiol Cell Physiol. 2004;286:C747–C756. doi: 10.1152/ajpcell.00433.2003. [DOI] [PubMed] [Google Scholar]
- 7.Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Sharma A, Wiest DL, Sen JM. Pre-TCR-induced beta-catenin facilitates traversal through beta-selection. J Immunol. 2009;182:751–758. doi: 10.4049/jimmunol.182.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 10.Ciofani M, Zuniga-Pflucker JC. A survival guide to early T cell development. Immunol Res. 2006;34:117–132. doi: 10.1385/IR:34:2:117. [DOI] [PubMed] [Google Scholar]
- 11.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–169. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 12.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 13.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 14.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 15.Staal FJ, Meeldijk J, Moerer P, Jay P, van de Weerdt BC, Vainio S, et al. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31:285–293. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR, et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci USA. 2006;103:3322–3326. doi: 10.1073/pnas.0511299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 19.Pongracz JE, Parnell SM, Jones T, Anderson G, Jenkinson EJ. Overexpression of ICAT highlights a role for catenin-mediated canonical Wnt signalling in early T cell development. Eur J Immunol. 2006;36:2376–2383. doi: 10.1002/eji.200535721. [DOI] [PubMed] [Google Scholar]
- 20.Hossain MZ, Yu Q, Xu M, Sen JM. ICAT expression disrupts beta-catenin-TCF interactions and impairs survival of thymocytes and activated mature T cells. Int Immunol. 2008;20:925–935. doi: 10.1093/intimm/dxn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin-TCF-1 pathway ensures CD4(+) CD8(+) thymocyte survival. Nat Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 25.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 26.Gounari F, Aifantis I, Khazaie K, Hoeflinger S, Harada N, Taketo MM, et al. Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat Immunol. 2001;2:863–869. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- 27.Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E, et al. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol. 2005;6:800–809. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M, Sharma A, Hossain MZ, Wiest DL, Sen JM. Sustained expression of pre-TCR induced beta-catenin in post-beta-selection thymocytes blocks T cell development. J Immunol. 2009;182:759–765. doi: 10.4049/jimmunol.182.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 30.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 31.Guo Z, Dose M, Kovalovsky D, Chang R, O’Neil J, Look AT, et al. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109:5463–5472. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu M, Yu Q, Subrahmanyam R, Difilippantonio MJ, Ried T, Sen JM. Beta-catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo. Mol Cell Biol. 2008;28:1713–1723. doi: 10.1128/MCB.01360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao MJ, Zhang XX, Hill R, Gao J, Qumsiyeh MB, Nichols W, et al. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol Cell Biol. 1998;18:3495–3501. doi: 10.1128/mcb.18.6.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Sen J. Beta-catenin expression in thymocytes accelerates thymic involution. Eur J Immunol. 2003;33:12–18. doi: 10.1002/immu.200390002. [DOI] [PubMed] [Google Scholar]
- 35.Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994;15:362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 36.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 37.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 38.Fowlkes BJ, Schweighoffer E. Positive selection of T cells. Curr Opin Immunol. 1995;7:188–195. doi: 10.1016/0952-7915(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 39.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 40.Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–540. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- 41.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 42.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4-versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Q, Erman B, Bhandoola A, Sharrow SO, Singer A. In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J Exp Med. 2003;197:475–487. doi: 10.1084/jem.20021765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Q, Sen JM. Beta-catenin regulates positive selection of thymocytes but not lineage commitment. J Immunol. 2007;178:5028–5034. doi: 10.4049/jimmunol.178.8.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulroy T, Xu Y, Sen JM. Beta-Catenin expression enhances generation of mature thymocytes. Int Immunol. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 46.Lucas B, Vasseur F, Penit C. Production, selection, and maturation of thymocytes with high surface density of TCR. J Immunol. 1994;153:53–62. [PubMed] [Google Scholar]
- 47.Yu Q, Xu M, Sen JM. Beta-catenin enhances IL-7 receptor signaling in thymocytes during positive selection. J Immunol. 2007;179:126–131. doi: 10.4049/jimmunol.179.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Z, Xie H, Ioannidis V, Held W, Clevers H, Sadim MS, et al. Transcriptional regulation of CD4 gene expression by T cell factor-1/beta-catenin pathway. J Immunol. 2006;176:4880–4887. doi: 10.4049/jimmunol.176.8.4880. [DOI] [PubMed] [Google Scholar]
- 49.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 50.Kovalovsky D, Yu Y, Dose M, Emmanouilidou A, Konstantinou T, Germar K, et al. Beta-catenin/Tcf determines the outcome of thymic selection in response to alphabetaTCR signaling. J Immunol. 2009;183:3873–3884. doi: 10.4049/jimmunol.0901369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 52.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 53.Corthay A. A three-cell model for activation of naive T helper cells. Scand J Immunol. 2006;64:93–96. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 54.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz J, Thiel A, Kuhn R, Rajewsky K, Muller W, Assenmacher M, et al. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J Exp Med. 1994;179:1349–1353. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 59.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Staal FJ, Burgering BM, van de Wetering M, Clevers HC. Tcf-1-mediated transcription in T lymphocytes: differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int Immunol. 1999;11:317–323. doi: 10.1093/intimm/11.3.317. [DOI] [PubMed] [Google Scholar]
- 61.Prieve MG, Waterman ML. Nuclear localization and formation of beta-catenin-lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol Cell Biol. 1999;19:4503–4515. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asnagli H, Afkarian M, Murphy KM. Cutting edge: identification of an alternative GATA-3 promoter directing tissue-specific gene expression in mouse and human. J Immunol. 2002;168:4268–4271. doi: 10.4049/jimmunol.168.9.4268. [DOI] [PubMed] [Google Scholar]
- 64.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 65.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]