Abstract
Type 1 diabetes (T1DM) is associated with increased microvascular complications and is a pro-inflammatory state. The toll-like receptors (TLRs) are pattern recognition receptors on monocytes and important in atherosclerosis. We have shown increased TLR2 and TLR4 expression on monocytes of T1DM compared to controls. In this report, we tested the surface expression of TLR2 and TLR4 on monocytes of T1DM patients with microvascular complications (T1DM-MV) compared to those without (T1DM) and healthy controls (C). The study was performed at the University of California Davis. Healthy controls (n = 31), T1DM patients (n = 31) and T1DM-MV patients (n=34) were included. TLR2 and TLR4 surface expression was significantly increased in T1DM-MV monocytes compared with T1DM and controls (p<0.01). Also, nuclear factor κB, and IL-1β release was significantly increased in monocytes from T1DM-MV compared with T1DM (p<0.005). Thus, we make the novel observation that TLR2 and TLR4 expression and signaling are increased in T1DM-MV compared to T1DM and may contribute to the accentuated pro-inflammatory state and complications of T1DM.
Introduction
Type 1 diabetes (T1DM) is associated with an increased risk of vascular complications, and type 1 diabetic patients with proteinuria and/or retinopathy have a significantly increased risk of fatal coronary artery disease [1]. Inflammation plays a pivotal role in all stages of atherosclerosis. The monocyte-macrophage, a crucial cell in atherogenesis, is readily accessible for study. We and others have demonstrated that patients with T1DM exhibit increased inflammation as evidenced by increased plasma CRP levels and increased monocyte activity, and these are more pronounced in T1DM with microvascular complications [2-5].
Members of the toll-like receptor (TLR) family play a critical role in the inflammatory components of atherosclerosis. TLRs are a family of pattern recognition receptors that are important in the regulation of immune function and inflammation [6-8]. Their activation by various ligands triggers a signaling cascade leading to cytokine production and initiation of an adaptive immune response [6-8]. TLR2 and TLR4 expression is up-regulated in atherosclerotic plaque macrophages and in animal models of atherosclerosis [6-8]. Knockout of TLR4 is associated with reduction in lesion size, lipid content, and macrophage infiltration in hypercholesterolemic apolipoprotein E –/– mice [9]. In addition, TLR2/low-density lipoprotein receptor-deficient –/–, and in a recent paper, TLR2/apolipoprotein E –/–, mice are protected from the development of atherosclerosis [10,11].
We have previously demonstrated that TLR2 and TLR 4 are upregulated in monocytes of patients with T1DM [12]. This was associated with an increase in cytokines, chemokines, NFκB activity, MyD88 and Trif. However, although T1DM with microvascular complications (T1DM-MV) exhibit accentuated inflammation compared to T1DM [5], there is a paucity of data examining TLR2 and TLR4 surface expression in T1DM-MV compared to T1DM and healthy controls and this was the aim of the present report.
Research Design and Methods
T1DM patients (n = 31) and T1DM-MV patients (n=34), (onset < 20 yr and on insulin therapy since diagnosis; present age 18 yr or older with duration of diabetes 1 yr or more) were recruited from the Diabetes and Pediatric Clinics at University of California Davis Medical Center and advertisements in the local newspaper. Microvascular complications included retinopathy and nephropathy. None of the patients were on Glucophage (Bristol-Myers Squibb Co., Princeton, NJ), and/or the thiazolidinediones, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or statins as described previously [5,12]. Other exclusion criteria were: mean HbA1c over the last year >10% , inflammatory disorders e.g. rheumatoid arthritis; macrovascular complications such as strokes, myocardial infarction etc., abnormal liver, renal or thyroid function; malabsorption; steroid therapy, smoking, abnormal complete blood count and alcohol consumption > 1 oz/day; consumption of N-3 PUFA capsules (>1g/day) and chronic high intensity exercisers. Microvascular complications were defined as retinopathy, nephropathy and neuropathy and determined in T1DM patients.
Healthy controls (n = 31), age older than 18 yr, were included if they had normal complete blood count, no family history of diabetes or other chronic diseases, normal kidney, liver, thyroid function, and fasting plasma glucose less than 100 mg/dL. Healthy controls and T1DM and T1DM-MV patients were matched for age (within 10 yr), gender, and race. Exclusion criteria were as described previously [5,12]. Informed consent was obtained from participants in the study, which was approved by the institutional review board at University of California Davis. After history and physical examination, fasting blood (30 ml) was obtained.
Mononuclear cells were isolated from fasting heparinized blood by Ficoll Hypaque centrifugation, followed by magnetic separation of monocytes using the depletion technique (Miltenyi Biotech, Auburn, CA), as described previously [5,12]. Briefly, non-monocytes, such as T cells, NK cells, B cells, dendritic cells, and basophils, are indirectly magnetically labeled using a cocktail of biotin-conjugated antibodies against CD3, CD7, CD16, CD19, CD56, CD123, and CD235a (Glycophorin A), as well as Anti-Biotin MicroBeads. Highly pure unlabeled monocytes are obtained by depletion of the magnetically labeled cells.
Monocytes from control and T1DM were incubated with antihuman TLR2 and TLR4 antibodies (InvivoGen) or isotype controls, and surface expression of TLR2 and TLR4 was analyzed using BD FACSArray (Franklin Lakes, NJ) [12]. Results were expressed as mean fluorescence intensity of 10,000 cells. NFκB activity was examined as readout of TLR signaling as described previously [12] and expressed as nanograms of NFκB p65 per milligram cell protein. The release of IL-1β in the supernates of monocytes in resting and LPS activated cells was also determined as a readout of up-regulated TLR expression, and expressed as picograms per milligram of cell protein as described previously [5,12].
Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC). Data are expressed as mean ± SD for parametric data, and as median and interquartile range for nonparametric data. Following ANOVA, Parametric data were analyzed using paired, two-tailed t tests and nonparametric data using Wilcoxon signed rank tests. Level of significance was set at P < 0.05. Spearman's rank correlation was computed to assess association between variables.
Results
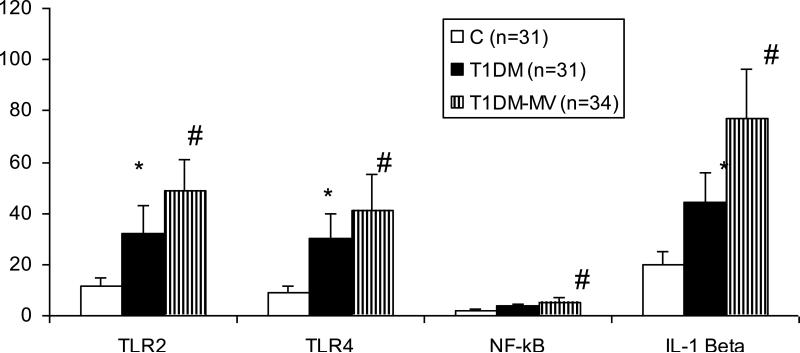
Baseline subject characteristics have been provided previously [5,12]. There were no significant differences in age, body mass index, and male to female ratio between control, T1DM and T1DM-MV groups. In addition, there were no significant differences in the lipid profile. As expected, levels of glucose, HbA1c, and free fatty acids were significantly higher in T1DM and T1DM-MV compared with controls [5]. Also, levels of hsCRP and circulating IL-1β were significantly increased in T1DM and T1DM-MV compared to controls and this was more pronounced in the T1DM-MV group (Table 1) Monocyte surface expression of TLR2 and TLR4 was significantly up-regulated in T1DM-MV compared to T1DM and controls (Fig. 1). Downstream signaling of TLR, i.e. NFκB activity and release of IL-1β was significantly increased in resting and activated monocytes from T1DM-MV compared to T1DM patients and controls (p < 0.005) (data for NFκB and IL-1β are shown for resting monocytes). In addition, there was a significant correlation between TLR2 expression and NFκB activity (r = 0.57; p < 0.005) and IL-1β release (r=0.67, p<0.01) , and TLR4 expression and NFκB activity (r = 0.7; P < 0.005) and IL-1β release (r=0.79, p<0.01), respectively.
Table 1.
Baseline Characteristics
| Controls (n=31) | T1DM (n=31) | T1DM-MV (n=34) | |
|---|---|---|---|
| Age (years) | 32 ± 13 | 32 ± 13 | 34±11 |
| BMI (kg/sq.m) | 25 ± 4 | 25 ± 4 | 26±6 |
| Male:Female Ratio | 12:19 | 12:19 | 13:21 |
| Glucose (mg/dL) | 85 ± 11 | 131 ± 68* | 153±80* |
| HbA1C(%) | 5.4 ± 0.3 | 7.8 ± 1.1* | 8.4± 1.5* |
| Free Fatty Acids (mM) | 0.28 ± 0.1 | 0.39 ± 0.23* | 0.44± 0.26* |
| Total Cholesterol (mg/dL) | 175 ± 26 | 180 ± 37 | 182±37 |
| Triglycerides (mg/dL) | 80 ± 39 | 81 ± 47 | 71 ± 57 |
| LDL Cholesterol (mg/dL) | 112 ± 20 | 111 ± 28 | 112±34 |
| HDL Cholesterol (mg/dL) | 47 ± 15 | 52 ± 17 | 51±19 |
| Hs-CRP (mg/L) | 1.1 (0.6,1.7) | 1.7 (0.9,2.1)* | 2.5 (0.7,2.1)*a |
| Interleukin-1 beta (pg/mL) | 10.3 (4.1,7.8) | 25.3 (5.3,44.2)* | 46.4 (14, 38)*a |
Data are expressed as mean ± S.D and median and interquartile range for CRP.
p<0.05 compared to Controls
p<0.05 compared to Controls and T1DM
Discussion
T1DM is a pro-inflammatory state characterized by increased levels of circulating biomarkers of inflammation and monocyte activity [3-5]. TLR2 and TLR4 play a critical role in atherosclerosis [6-10]. We have previously shown increased inflammation in T1DM-MV compared to T1DM [5,12]. The increased inflammation in T1DM-MV may be mediated in part via activation of the innate immune pathway by the TLRs. However, there are no studies examining TLR expression in T1DM-MV compared to age, gender matched T1DM and their contribution to the accentuated pro-inflammatory state of T1DM. In this report, we provide novel data on up-regulated TLR2 and TLR4 expression and signaling in monocytes of T1DM-MV compared to T1DM and Controls.
TLRs are characterized by an extracellular ligand binding domain, single transmembrane domain, and intracellular domain [6-11]. Upon ligand binding, the TLR subunits associate, leading to the formation of a complex of Toll-interacting region domain containing adaptor proteins of the MyD88 family. Subsequent downstream signal transduction events lead to the activation of NFκB and transcription of pro-inflammatory chemokines such as monocyte chemoattractant protein-1 and cytokines such as IL-1β, IL-6, and TNFα [6-11]. In addition to showing that TLR2 and TLR4 surface expression is increased on monocytes isolated from T1DM-MV compared with T1DM, we demonstrate increased NFκB DNA binding activity, as well as increased IL-1β release from monocytes of T1DM-MV compared to T1DM. There was also a significant correlation between the increased TLR2 and TLR4 expression, NFκB activity and IL-1β release, indicating a direct relationship between TLR 2 and 4 activities and increased inflammation in T1DM-MV. Furthermore, it is important to note that macronutrient intake increases while insulin suppresses TLR2 and TLR4 expression [13,14] and the latter may contribute in part to increased TLR2 and TLR4 in T1DM, an insulin deficient state.
In conclusion, this is the first demonstration of increased TLR2 and TLR4 expression and activity in T1DM-MV monocytes. Future studies will examine molecular mechanisms for increased TLR2 and TLR4 expression, and determine their contribution to microvascular complications of T1DM and examined their modulation.
Fig 1.
Increased TLR2, TLR 4 surface expression, mononuclear NFKb binding activity and IL-1β release in T1DM-MV:
Monocytes were obtained from C (n=31), T1DM (n=31) and T1DM-MV (n=34) and TLR2 and TLR4 surface expression (mfi), nuclear NFκB activity(ng/mg protein) and IL-1β release (pg/mg protein) were examined as described in Methods. *p<0.001 compared to C and #p<0.005 compared to T1DM and C.
Acknowledgement
NIH DK 69801, NIH UL1 RR024146, JDRF-2007-585, NIH K24 AT 00596 Manpreet Kaur for editorial assistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: Authors report no conflicts of interest
Approval: The protocol was approved by the University of California Davis Institutional Review Board.
References
- 1.Libby P, Nathan DM, Abraham K, et al. National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 2.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD. EURODIAB Prospective Complications Study Group-Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes–the EURODIAB Prospective Complications Study. Diabetologia. 2005;48:370–378. doi: 10.1007/s00125-004-1628-8. [DOI] [PubMed] [Google Scholar]
- 3.Schalkwijk CG, Poland DC, van Dijk W, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42:351–357. doi: 10.1007/s001250051162. [DOI] [PubMed] [Google Scholar]
- 4.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 5.Devaraj S, Cheung AT, Jialal I, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Sun B. Toll-like receptor 4 in atherosclerosis. J Cell Mol Med. 2007;11:88–95. doi: 10.1111/j.1582-4934.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 8.Mullick AE, Tobias PS, Curtiss LK. Toll-like receptors and atherosclerosis: key contributors in disease and health? Immunol Res. 2006;34:193–209. doi: 10.1385/IR:34:3:193. [DOI] [PubMed] [Google Scholar]
- 9.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Ukai T, Yumoto H, et al. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a pro-inflammatory state. J Clin Endocrinol Metab. 2008;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P. Acute modulation of toll-like receptors by insulin. Diabetes Care. 2008;31:1827–1831. doi: 10.2337/dc08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]