Abstract
Enterococcus faecalis has emerged as a prominent healthcare-associated pathogen frequently encountered in bacteremia, endocarditis, urinary tract infection, and as a leading cause of antibiotic-resistant infections. We recently demonstrated a capacity for high-level biofilm formation by a clinical E. faecalis isolate, E99. This high biofilm forming phenotype was attributable to a novel locus, designated bee, specifying a pilus at the bacterial cell surface and localized to a large ~80 kb conjugative plasmid. To better understand the origin of the bee locus, as well as to potentially identify additional factors important to the biology and pathogenesis of strain E99, we sequenced the entire plasmid. The nucleotide sequence of the plasmid, designated pBEE99, revealed large regions of identity to the previously characterized conjugative plasmid pCF10. In addition to the bee locus, pBEE99 possesses an open reading frame potentially encoding aggregation substance, as well as open reading frames putatively encoding polypeptides with 60 to 99 % identity at the amino acid level to proteins involved in regulation of the pheromone response and conjugal transfer of pCF10. However, strain E99 did not respond to the cCF10 pheromone in clumping assays. While pBEE99 was found to be devoid of any readily recognizable antibiotic resistance determinants, it carries two non-identical impB/mucB/samB-type genes, as well as genes potentially encoding a two-component bacteriocin similar to that encoded on pYI14. Although no bacteriocin activity was detected from an OG1RF transconjugant carrying pBEE99 against strain FA2-2, it was approximately an order of magnitude more resistant to ultraviolet radiation. Moreover, curing strain E99 of this plasmid significantly reduced its ability to survive UV exposure. Therefore, pBEE99 represents a novel conjugative plasmid that confers biofilm-forming and enhanced UV resistance traits that might potentially impact the virulence and/or fitness of E. faecalis.
Keywords: Enterococcus faecalis, bee locus, pBEE99, bacteriocin, ultraviolet radiation resistance, conjugation
1. Introduction
Enterococcus faecalis is a Gram-positive bacterium that commonly occupies a commensal niche in the gastrointestinal tract. However, E. faecalis is notorious for acquiring new traits through horizontal gene transfer that allow the organism to become an opportunistic pathogen capable of causing infections of the bloodstream, urinary tract, and endocardium (Coburn et al., 2007; Gilmore et al., 2002; Tendolkar et al., 2006). In fact, E. faecalis is the third leading cause of healthcare-associated infections (Hidron et al., 2008) which are commonly recalcitrant to standard treatments and have a high morbidity rate (Gilmore et al., 2002). E. faecalis is particularly adept at acquiring and disseminating genes via bacterial conjugation, a complex process whereby large portions of genetic material are transferred from a donor bacterium to a recipient. The sex-pheromone conjugative plasmid system employed by E. faecalis permits the rapid transfer of genes, including genes that encode resistance to antibiotics and genes that enhance the ability to cause disease (Clewell, 1974; Clewell and Dunny, 2002).
Recently, we identified a novel operon, designated bee (biofilm enhancer in enterococcus), by Tn917 insertional mutagenesis of the urinary tract E. faecalis isolate E99. Tn917 insertion into this locus severely impaired the ability of this strain to form a biofilm. In filter mating experiments, the bee locus was shown to transfer by conjugation and enhance biofilm formation by transconjugants. Southern hybridization analysis revealed that the bee locus was carried on an approximately 80 kb conjugative plasmid (Tendolkar et al., 2006). The discovery of a conjugative plasmid that enhances the ability of E. faecalis to form a biofilm represents another mechanism for enterococcal strains to disseminate the genes necessary to persist in the hospital setting or in susceptible patients. In the current work, we sequenced the bee-encoding conjugative plasmid, designated pBEE99, to potentially identify other factors that might enhance the pathogenesis or fitness of E. faecalis.
2. Methods
2.1 Bacterial strains, plasmids, and growth conditions
All E. faecalis strains were cultivated in Brain Heart Infusion (BHI) medium supplemented with the appropriate antibiotics. E. faecalis strain E99 is a clinical isolate obtained from the urine of a patient at the Veterans Administration Hospital in Little Rock, AR. Selection for strain E99 consisted of 25 μg/ml kanamycin. E99 was grown in BHI medium containing kanamycin 25 μg/ml at 45°C for 10 consecutive days (subculturing was done every consecutive day) in order to cure the strain of pBEE99. After 10 days, the culture was plated on BHI containing kanamycin 25 μg/ml and random colonies were picked and screened for the absence of the bee locus and the prgK (Table 1) gene by PCR and Southern hybridization. The absence of pBEE99 from a single clone that was negative by both PCR and Southern hybridization for the bee locus and prgK was further verified in biofilm assays. This clone, designated KDS01, was significantly attenuated in biofilm formation, indicating the absence of the bee locus.
Table 1.
pBEE99 open reading frames. Asterisks indicate possible pseudogenes.
| # | Start | End | Length | Strand | Match | Affiliation | E_value | %Identity |
|---|---|---|---|---|---|---|---|---|
| 1 | 246 | 1259 | 1012 | + | gb|AAA25553.1| PrgW | E. faecalis | 3.00E-164 | 59% |
| 2 | 1390 | 2058 | 667 | + | gi|22536356|ref|NP_687207.1| Protease, putative | S. agalactiae | 1.00E-20 | 38% |
| 3 | 2100 | 3728 | 1627 | + | gi|58616130|ref|YP_195766.1| PrgZ | E. faecalis | 2.00E-185 | 59% |
| 4 | 3731 | 4714 | 982 | − | ref|YP_003329053.1| PrgX | E. faecalis | 5.00E-63 | 99% |
| 5 | 5534 | 5914 | 379 | + | gi|29377901|ref|NP_817027.1| PrgR | E. faecalis V583 | 3.00E-66 | 96% |
| 6 | 5965 | 6186 | 220 | + | pir||C56272 PrgS | E. faecalis plasmid pCF10 | 5.00E-35 | 97% |
| 7 | 6539 | 6829 | 289 | + | gi|69246067|ref|ZP_00603794.1| Transposase IS3/IS911 | E. faecium DO | 3.00E-47 | 100% |
| 8 | 6865 | 7701 | 835 | + | gi|69246262|ref|ZP_00603875.1| Integrase, catalytic region | E. faecium DO | 5.00E-140 | 89% |
| 9 | 8127 | 12044 | 3916 | + | gi|29377905|ref|NP_817031.1| PrgB | E. faecalis V583 | 0 | 89% |
| 10* | 12142 | 12470 | 311 | + | gb|AAW51309.1| PrgU | E. faecalis | 9.00E-15 | 100% |
| 11 | 12498 | 13355 | 856 | + | gi|29377906|ref|NP_817032.1| PrgC | E. faecalis V583 | 3.00E-86 | 68% |
| 12 | 13397 | 14296 | 898 | + | gi|58616088|ref|YP_195777.1| PrgD | E. faecalis | 5.00E-120 | 97% |
| 13 | 14316 | 14750 | 433 | + | gi|29377908|ref|NP_817034.1| hypothetical protein EF_B0014 | E. faecalis V583 | 6.00E-67 | 88% |
| 14 | 14797 | 15024 | 226 | + | ref|YP_195779.1| PrgF | E. faecalis | 4.00E-32 | 100% |
| 15 | 15038 | 15334 | 295 | + | gi|29377910|ref|NP_817036.1| hypothetical protein EF_B0016 | E. faecalis V583 | 2.00E-48 | 96% |
| 16 | 15349 | 16149 | 799 | + | gi|58616084|ref|YP_195781.1| PrgH | E. faecalis | 4.00E-127 | 87% |
| 17 | 16151 | 16504 | 352 | + | gi|58616092|ref|YP_195782.1| PrgI | E. faecalis | 1.00E-47 | 82% |
| 18 | 16458 | 18848 | 2389 | + | gi|58616093|ref|YP_195783.1| PrgJ | E. faecalis | 0 | 97% |
| 19 | 18860 | 21373 | 2512 | + | gi|58616094|ref|YP_195784.1| PrgK | E. faecalis | 0 | 89% |
| 20 | 21509 | 21838 | 328 | − | No Hits Found | |||
| 21 | 22097 | 24031 | 1933 | + | gi|21693271|gb|AAM75218.1| Group II intron reverse transcriptase | E. faecalis | 2.00E-250 | 66% |
| 22 | 24304 | 24930 | 625 | + | gi|58616095|ref|YP_195785.1| PrgL | E. faecalis | 3.00E-84 | 78% |
| 23 | 24908 | 25159 | 250 | + | ref|YP_195786.1| PrgM | E. faecalis | 6.00E-35 | 97% |
| 24 | 25146 | 25754 | 607 | + | gi|58616097|ref|YP_195787.1| PcfA | E. faecalis | 3.00E-93 | 87% |
| 25 | 25867 | 26622 | 754 | + | gi|90962837|ref|YP_536752.1| hypothetical protein LSL_2159 | L. salivarius | 6.00E-11 | 39% |
| 26 | 26639 | 27097 | 457 | + | gi|58616114|ref|YP_195805.1| PcfS | E. faecalis | 9.00E-50 | 67% |
| 27 | 27143 | 27298 | 154 | + | No hits found | |||
| 28 | 27364 | 27852 | 487 | + | |113706806|ref|YP_195788.2| PcfB | E. faecalis | 4.00E-85 | 99% |
| 29 | 27852 | 29681 | 1828 | + | gi|58616099|ref|YP_195789.1| PcfC | E. faecalis | 0 | 91% |
| 30 | 29732 | 31891 | 2158 | + | gi|58616100|ref|YP_195790.1| PcfD | E. faecalis | 0 | 96% |
| 31 | 31924 | 32196 | 271 | + | ref|YP_195791.1| PcfE | E. faecalis | 2.00E-43 | 98% |
| 32 | 32442 | 32798 | 355 | + | gi|58616102|ref|YP_195792.1| PcfF | E. faecalis | 4.00E-56 | 100% |
| 33 | 32799 | 34484 | 1684 | + | gi|29377923|ref|NP_817049.1| PcfG | E. faecalis V583 | 2.00E-248 | 76% |
| 34 | 34517 | 34867 | 349 | + | gi|58616104|ref|YP_195794.1| PcfH | E. faecalis | 4.00E-49 | 77% |
| 35 | 35387 | 36727 | 1339 | − | gi|58616106|ref|YP_195796.1| PcfJ | E. faecalis | 7.00E-239 | 90% |
| 36 | 36727 | 37500 | 772 | − | gi|29377927|ref|NP_817053.1| hypothetical protein EF_B0034 | E. faecalis V583 | 5.00E-103 | 71% |
| 37 | 37605 | 38195 | 589 | − | gi|29377928|ref|NP_817054.1| hypothetical protein EF_B0035 | E. faecalis V583 | 3.00E-108 | 100% |
| 38 | 38219 | 38410 | 190 | − | gb|AAW51348.1| PcfM | E. faecalis | 8.00E-28 | 98% |
| 39 | 38395 | 38580 | 184 | − | ref|YP_195800.1| PcfN | E. faecalis | 3.00E-27 | 100% |
| 40 | 38876 | 39076 | 199 | + | ref|YP_195802.1| PcfP | E. faecalis | 5.00E-31 | 100% |
| 41 | 39178 | 39408 | 229 | − | ref|YP_195803.1| PcfQ | E. faecalis | 2.00E-37 | 100% |
| 42 | 39541 | 39957 | 415 | + | gi|58616113|ref|YP_195804.1| PcfR | E. faecalis | 6.00E-58 | 84% |
| 43* | 40023 | 40475 | 449 | + | gi|29377935|ref|NP_817061.1| PcfS | E. faecalis V583 | 2.00E-64 | 100% |
| 44 | 40489 | 40641 | 151 | + | dbj|BAH02365.1| hypothetical protein pMG2200_57 | E. faecalis | 2.00E-20 | 100% |
| 45 | 40653 | 41243 | 589 | + | ref|YP_195806.1| PcfT | E. faecalis | 9.00E-84 | 82% |
| 46 | 41249 | 41569 | 319 | + | gi|58616116|ref|YP_195807.1| PcfU | E. faecalis | 8.00E-53 | 94% |
| 47 | 41596 | 41664 | 67 | + | ref|NP_815781.1| hypothetical protein EF2118 | E. faecalis V583 | 2.00E-09 | 86% |
| 48 | 41677 | 41922 | 244 | + | dbj|BAH02368.1| hypothetical protein pMG2200_60 | E. faecalis | 5.00E-40 | 100% |
| 49 | 41976 | 42158 | 181 | + | dbj|BAG12397.1| hypothetical protein pMG2200_61 | E. faecalis | 1.00E-24 | 100% |
| 50 | 42353 | 43069 | 715 | + | gi|169635863|dbj|BAG12398.1| hypothetical proteinpMG2200_62 | E. faecalis | 3.00E-104 | 82% |
| 51* | 43207 | 44994 | 1784 | + | gi|169635864|dbj|BAG12399.1| BacL1 | E. faecalis | 7.00E-289 | 95% |
| 52 | 45180 | 45815 | 634 | + | gi|169635865|dbj|BAG12400.1| BacL2 | E. faecalis | 2.00E-105 | 100% |
| 53 | 45838 | 46269 | 430 | + | gi|169635866|dbj|BAG12401.1| putative holin | E. faecalis | 7.00E-49 | 79% |
| 54 | 46272 | 46799 | 526 | + | gi|169635867|dbj|BAG12402.1| hypothetical protein pMG2200_66 | E. faecalis | 7.00E-84 | 90% |
| 55 | 46842 | 49022 | 2179 | + | gi|169635868|dbj|BAG12403.1| BacA | E. faecalis | 0 | 100% |
| 56 | 49130 | 49672 | 541 | + | gi|169635869|dbj|BAG12404.1| BacI | E. faecalis | 8.00E-70 | 77% |
| 57 | 49739 | 50314 | 574 | + | gi|169635870|dbj|BAG12405.1| hypothetical protein | E. faecalis | 2.00E-88 | 87% |
| 58 | 50457 | 50789 | 331 | + | gi|29377949|ref|NP_817075.1| hypothetical protein EF_B0057 | E. faecalis V583 | 5.00E-57 | 100% |
| 59 | 50953 | 51555 | 601 | + | gi|29377950|ref|NP_817076.1| site-specific recombinase | E. faecalis V583 | 3.00E-84 | 83% |
| 60 | 51622 | 52416 | 793 | + | No Hits Found | |||
| 61 | 52956 | 53126 | 169 | − | No Hits Found | |||
| 62 | 53406 | 54218 | 811 | + | No Hits Found | |||
| 63 | 54379 | 55695 | 1315 | + | gi|29377952|ref|NP_817078.1| UvrA | E. faecalis V583 | 3.00E-237 | 97% |
| 64 | 55700 | 56206 | 505 | + | gi|29377891|ref|NP_817018.1| hypothetical proteinEF_C0016 | E. faecalis V583 | 2.00E-85 | 90% |
| 65 | 56154 | 56384 | 229 | + | ref|NP_817080.1| UvrC | E. faecalis | 1.00E-17 | 68% |
| 66 | 56462 | 56875 | 412 | − | gi|29377892|ref|NP_817019.1| hypothetical proteinEF_C0017 | E. faecalis V583 | 3.00E-59 | 85% |
| 67 | 56882 | 57121 | 238 | − | ref|NP_817020.1| RepB | E. faecalis V583 | 9.00E-36 | 100% |
| 68 | 57159 | 57845 | 685 | − | gi|29377809|ref|NP_816937.1| Transposase IS1216 | E. faecalis V583 | 3.00E-126 | 97% |
| 69 | 58530 | 61781 | 3250 | + | gi|72388797|gb|AAZ68037.1| Bee1 | E. faecalis | 0 | 100% |
| 70 | 61783 | 62514 | 730 | + | gi|72388798|gb|AAZ68038.1| Bee2 | E. faecalis | 5.00E-113 | 100% |
| 71 | 62579 | 64066 | 1486 | + | gi|72388799|gb|AAZ68039.1| Bee3 | E. faecalis | 2.00E-283 | 100% |
| 72 | 64152 | 65348 | 1195 | + | gi|72388800|gb|AAZ68040.1| Srt1 | E. faecalis | 6.00E-229 | 100% |
| 73 | 65345 | 66466 | 1120 | + | gi|72388801|gb|AAZ68041.1| Srt2 | E. faecalis | 2.00E-188 | 100% |
| 74 | 67237 | 68196 | 958 | + | gb|AAA68982.1| Transposase IS6770 | Insertion sequence IS6770 | 2.00E-185 | 99% |
| 75 | 68193 | 68423 | 229 | + | ref|ZP_00874586.1| PadR | S. suis | 1.00E-13 | 60% |
| 76 | 68765 | 69697 | 931 | − | gi|167746666|ref|ZP_02418793.1| hypothetical protein ANACAC_01377 | A. caccae DSM 14662 | 8.00E-102 | 55% |
| 77 | 69793 | 70422 | 628 | + | gi|124485630|ref|YP_001030246.1| hypothetical protein Mlab_0808 | M. labreanum Z | 7.00E-74 | 64% |
| 78 | 70604 | 71197 | 592 | + | gi|57854758|ref|YP_187535.1| Site-specific recombinase | S. epidermidis RP62A | 1.00E-52 | 65% |
| 79 | 71169 | 72206 | 1036 | + | gi|33416281|ref|NP_878027.1| Transposase Tn552 | S. aureus | 2.00E-114 | 59% |
| 80 | 72184 | 72612 | 427 | + | gi|67077955|ref|YP_245575.1| putative transposase | B. cereus E33L | 2.00E-31 | 54% |
| 81 | 72605 | 73423 | 817 | + | gi|49483988|ref|YP_041212.1| ATP-binding protein | S. aureus | 9.00E-94 | 62% |
| 82 | 73566 | 74162 | 595 | + | gb|AAW51342.1| PcfY | E. faecalis | 5.00E-93 | 87% |
| 83 | 74152 | 74466 | 313 | + | gi|58616121|ref|YP_195812.1| PcfZ | E. faecalis | 8.00E-45 | 94% |
| 84 | 74460 | 74681 | 220 | + | ref|YP_195813.1| UvrC | E. faecalis | 3.00E-35 | 95% |
| 85 | 74745 | 74954 | 208 | + | gb|AAO83067.1| hypothetical protein EF_A0075 | E. faecalis V583 | 1.00E-29 | 94% |
| 86 | 75424 | 75546 | 121 | + | No Hits Found | |||
| 87 | 75601 | 76038 | 436 | − | No Hits Found | |||
| 88 | 76130 | 76378 | 247 | + | ref|NP_817016.1| hypothetical protein EF_C0014 | E. faecalis V583 | 9.00E-36 | 95% |
| 89 | 76497 | 77813 | 1315 | + | gi|29377952|ref|NP_817078.1| UvrA | E. faecalis V583 | 1.00E-206 | 93% |
| 90 | 77817 | 78332 | 514 | + | gi|158021643|gb|ABW08102.1| hypothetical protein EF_C0016 | E. faecalis | 2.00E-88 | 91% |
| 91 | 78280 | 78447 | 166 | + | gb|ABW08103.1| hypothetical protein ABW08103 | E. faecalis | 2.00E-06 | 65% |
| 92 | 78559 | 78645 | 85 | + | gb|ABW08104.1| hypothetical protein ABW08104 | E. faecalis | 3.00E-18 | 100% |
| 93 | 78903 | 79217 | 313 | + | gi|29377955|ref|NP_817081.1| PrgN | E. faecalis V583 | 1.00E-38 | 80% |
| 94 | 79455 | 80237 | 781 | + | gi|29377956|ref|NP_817082.1| ParA | E. faecalis V583 | 4.00E-142 | 100% |
| 95 | 80230 | 80586 | 355 | + | gi|29377957|ref|NP_817083.1| hypothetical protein EF_B0065 | E. faecalis V583 | 8.00E-60 | 97% |
The plasmid free strain OG1RF (Dunny et al., 1978) and the previously generated transconjugant IG9, that was derived from mating E99 with OG1RF (Tendolkar et al., 2006), were cultivated in the presence of 25 μg/ml rifampicin and 10 μg/ml fusidic acid. OG1RF (pCF10) (Dunny et al., 1981) possesses the 67.6 kb pheromone responsive conjugative plasmid pCF10 that encodes a response to the cCF10 pheromone (Hirt et al., 2005).
Long range PCR products were subcloned into pBluescript for automated sequencing. Escherichia coli XL1-Blue (Stratagene, LaJolla, CA) was used as a host for subcloned pBluescript plasmids. E. coli was cultivated in Luria Broth (LB) or 2xYT media and supplemented with 100 μg/ml ampicillin.
2.2 DNA purification
The bee locus encoding plasmid DNA was purified from strain E99 using the QIAGEN large plasmid purification kit (QIAGEN, Valencia, CA). The pBluescript plasmid clones were purified from XL1-Blue using Promega’s Wizard plasmid miniprep kit (Promega, Madison, WI).
2.3 Cloning, Long range PCR, primer walking, and DNA sequencing
We previously identified and sequenced a novel 8,320 bp locus, designated bee (Tendolkar et al., 2006). Using inverse PCR and primer walking methodologies, an additional 16,038 bp of sequence data was obtained 5′ and 3′ to the bee locus for a total of 24,358 bp. This was followed by a shotgun sequencing approach in which purified pBEE99 DNA was restricted with HindIII, fragments ligated to HindIII-restricted pBluescript and introduced into E. coli by electroporation. Clones were then selected, sequenced, and fragments were assembled into a 36,932 bp contig that interestingly did not overlap with the 24,358 bp contig, and thus represented a distinct region of the pBEE99 plasmid. The two gaps between these two regions were then closed using long range PCR and primer walking. Long range PCR was then used to confirm correct assembly of the fragments into the complete pBEE99 plasmid. Takara LA Taq was used for all PCR amplifications, and PCR products generated by long range PCR were purified using Promega’s Wizard PCR purification kit (Promega, Madison, WI). Sequencing of purified PCR products and purified pBluescript clones possessing pBEE99 fragments was performed via automated sequencing using an ABI3730 capillary sequencer at the Oklahoma Medical Research Foundation (Oklahoma City, OK). Analysis of pBEE99 sequence was performed using FGENESB from Softberry® (http://linux1.softberry.com/berry.phtml). This program predicts genes, operon organization, promoters, tRNA, rRNA, and deduce functions using BLASTP against COG and NR databases at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). A circular genome viewer java application called CGVIEW (Stothard and Wishart, 2005) was used to Visualize analyzed feature of pBEE99. Additonaly, pair-wise nucleotide analysis against related E. faecalis plasmids was performed using BLASTN algorithm. Homology searches of the sequenced DNA were performed by BLASTN analyses using National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and the FGENESB software from Softberry© (http://linux1.softberry.com/berry.phtml). The FGENESB program performs several analyses: BLASTP, tRNA and rRNA scans, promoter, terminator and operon prediction. The results were then visualized using the Circular Genome Viewer (CGVIEW) java application (Stothard and Wishart, 2005).
2.4 Clumping assay
Overnight cultures of E. faecalis strains OG1RF (pCF10) and E99 were diluted 1:100 in 5 mL Todd Hewitt Broth with or without 10 ng/mL of cCF10 peptide and incubated at 37°C. Cell aggregates appear after 2 hours of incubation.
2.5 Bacteriocin assay
To test whether the pBEE99 plasmid specifies bacteriocin production, we compared strain OG1RF to a previously generated OG1RF transconjugant, designated IG9, possessing the pBEE99 plasmid (Tendolkar et al., 2006) for the ability to inhibit the growth of E. faecalis strain FA2-2. Briefly, strain FA2-2 was grown overnight in brain heart infusion medium (BHI) and 50 μl of the overnight culture added to 3 ml of molten BHI soft agar (0.75% [wt/vol]). The soft agar was then poured onto the surface of a BHI agar plate and allowed to solidify at room temperature. Three microliters of either strain OG1RF or IG9 was spotted onto the surface of the plate in triplicate, and the plates incubated at 37°C for 24 hours. Any clearing around the spots was interpreted as bacteriocin production.
2.6 Ultraviolet sensitivity assay
In order to determine whether the pBEE99 plasmid confers a UV resistant phenotype, we compared the UV sensitivity of strain OG1RF to IG9 [OG1RF (pBEE99)]. We performed a qualitative UV irradiation killing assay essentially as described by Scott et al (Scott et al., 2008). Strains OG1RF and IG9 were grown overnight in Todd-Hewitt broth (Difco) supplemented with 2% yeast extract (THY medium), diluted 1:20 in fresh, pre-warmed THY, and allowed to grow to an OD600 of 0.6. Cultures were then centrifuged at 5,000 × g for 15 minutes at 4°C, resuspended in ice-cold 0.1 M MgSO4, and incubated on ice for 10 minutes. For each strain, 5 ml of the resuspended culture was placed in a sterile glass 100 × 15 mm petri dish and exposed to a calibrated 254 nm UV lamp (300 μW/cm2). At 30, 60, 90, and 120 seconds, 1 ml aliquots were removed and serially diluted 10-fold in 0.1 M MgSO4. Two microliters of each dilution from 10−1 to 10−4 was spotted onto the surface of a THY plate and incubated in the dark at 37°C for 24 hours.
In order to precisely quantify the difference observed between strain OG1RF and IG9 and to determine whether curing pBEE99 from strain E99 results in increased UV sensitivity, we performed a quantitative UV radiation resistance assay as follows. Strains E99, KDS01, OG1RF, and IG9 were grown overnight in BHI broth supplemented with the appropriate antibiotics, diluted 1:20 in fresh, pre-warmed BHI, and allowed to grow to an OD600 of 0.6. Cultures were then centrifuged at 5,000 × g for 15 minutes at 4°C, resuspended in ice-cold 0.1 M MgSO4, and incubated on ice for 10 minutes. For each strain, 1 ml of the resuspended culture was placed in a sterile plastic 35 × 10 mm petri dish and exposed to a calibrated 254 nm UV lamp (300 μW/cm2). At 30, 60, 90, and 120 seconds, 20 μl aliquots were removed, serially diluted 10-fold in 0.1 M MgSO4, and colony forming units (CFU) per milliliter determined by the track dilution method (Jett et al., 1997). The statistical significance of the results was determined by performing pairwise comparisons at each time point using the Student’s t-test.
3. Results and Discussion
3.1 DNA sequence and organization of plasmid pBEE99 from E. faecalis strain E99
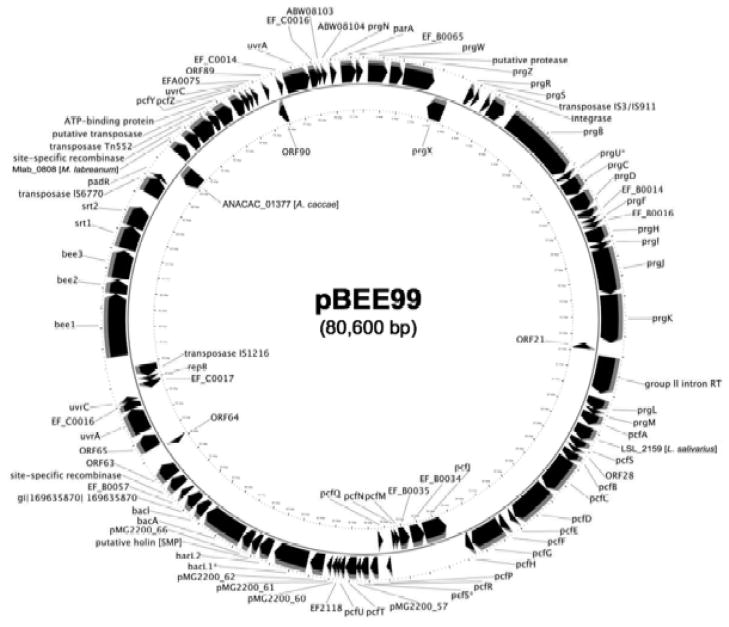
The molecular size of pBEE99 was determined to be 80,600 base pairs, and the first nucleotide was defined as a C located 245 bp 5′ to the start codon of the repA (prgW) gene. The pBEE99 plasmid possesses 95 putative ORFs identified using the FGENESB software from Softberry© (http://linux1.softberry.com/berry.phtml). Comparative BLASTN analysis of pBEE99 against other E. faecalis plasmids pCF10, pTEF1, pTEF2, pTEF3, pRE25 and pAM373, demonstrated that pBEE99 is more closely related to pTEF2 and pCF10 (Hirt et al., 2005; Dunny, 2007; Paulsen et al., 2003; Schwarz et al., 2001; De Boever et al., 2000). The putative replication initiation protein A (RepA) demonstrates 59% identity and 74% similarity to PrgW from pCF10 (Hirt et al., 2005). Within the putative RepA coding sequence is the direct repeat sequence 5′ – CGAAAGTGGGAAAAACCAAC – 3′ occurring thrice in tandem and suggestive of the direct repeats within the origin of replication (oriV) of pAD1 (Francia et al., 2001; Francia et al., 2004). While the precise location of the oriV remains to be determined experimentally, we predict that this region of pBEE99 corresponds to the oriV.
pBEE99 possesses a conjugal transfer region including an open reading frame potentially encoding aggregation substance (98% identity to PrgB from pCF10 and pTEF2) (Table 1), and open reading frames putatively encoding polypeptides with 60 to 99 % identity at the amino acid level to proteins involved in regulation of the pheromone response and conjugal transfer of pCF10 (Table 1) (Hirt et al., 2005). Interestingly, pBEE99 carries two non-identical open reading frames that show a high degree of identity and similarity to a putative ImpB/MucB/SamB family protein on pTEF2 (Table 1). The first of the 1315 bp genes begins at position 54,379 and ends at 55,695, and the second at position76,497 to 77,813 (Table 1 and Fig. 1). E. coli UmuC protein is the prototypical member of this family of genes that encode a class of DNA polymerases that are capable of replicating through UV-induced DNA lesions (Smith and Walker, 1998; Wang, 2001), and thus allow the organism to survive lethal doses of ultraviolet radiation. The E. coli MucB, the Salmonella typhimurium ImpB, and the S. typhimurium SamB proteins are all homologs of the UmuC protein; however, they are all plasmid associated (Sarov-Blat and Livneh, 1998; Koch et al., 1995; Smith and Walker, 1998). A cognate ImpA/MucA/SamA protein was not found in association with either copy of the ImpB/MucB/SamB family protein. Another pBEE99 open reading frame possibly related to UV resistance shows 95% identity and 96% similarity to a putative UvrC protein from pCF10. The pCF10 open reading frame is 95% identical and 98% similar to UvrC from pAD1, which has been demonstrated to function as a negative regulator of the uvrA gene (Ozawa et al., 1997). The pAD1 uvrA gene product increases the resistance of E. faecalis to DNA-damaging UV light (Ozawa et al., 1997). Further, pBEE99 possesses another open reading frame with 68% identity and 84% similarity to a putative UvrC family transcriptional regulator from pTEF2 (Table 1).
Fig. 1.
Physical map of plasmid pBEE99 showing the putative ORFs and their orientations. The molecular size of pBEE99 was determined to be 80,600 base pairs and 95 ORFs were identified using the FGENESB software. Putative genes are labeled with the closest match (lowest E value) from protein BLAST searches. Genes in which no hits were found are labeled with the ORF number (column 1 from Table 1). Pseudo-genes or interrupted ORFs are labeled with the original gene name and an asterisk.
A set of open reading frames that potentially encode a bacteriocin similar to the Bacteriocin 41 from pYI14 (Tomita et al., 2008) were identified at position 43,207 to 49,672 on pBEE99. These open reading frames include two copies of bacL1 (the second a possible pseudogene), bacL2, two hypothetical proteins, bacA, and bacI with identities ranging from 77% to 100% to the pYI14 genes. Finally, pBEE99 possesses 24 hypothetical proteins, and although no antibiotic resistance genes were readily apparent on pBEE99, we cannot rule out the possibility that these hypothetical genes could potentially encode novel resistance determinants.
3.2 pBEE99 does not encode a mating response to the peptide sex pheromone cCF10
Given the degree of identity and similarity to pCF10, we sought to determine whether pBEE99 specifies a mating response to the sex pheromone cCF10 by performing a cell aggregation assay in the presence and absence of 10 ng/ml cCF10. Overnight cultures of E. faecalis strains OG1RF (pCF10) and E99 were diluted 1:100 in 5 mL THB broth containing 10 ng/mL of cCF10 peptide, or no peptide, and incubated at 37°C. As expected, cell aggregation was observed after 2 hours of incubation for strain OG1RF (pCF10) in the presence, but not in the absence of 10 ng/ml cCF10 (data not shown). On the contrary, E99 was not observed to clump in the presence of cCF10, indicating that pBEE99 does not encode a mating response to this pheromone. Moreover, in our previous work, transfer of pBEE99 from E99 to OG1RF was not detected in liquid broth, suggesting that pBEE99 might have lost the ability to specify a response to sex pheromones. Alternatively, transfer of pBEE99 from the E99 background might be inefficient. Whether broth transfer from the OG1RF background to another recipient of a different strain background can occur remains to be determined.
3.3 E. faecalis strain OG1RF (pBEE99) does not elaborate detectable bacteriocin activity against E. faecalis strain FA2-2
A feature that distinguishes pBEE99 from pTEF2 and pCF10, in addition to the bee locus, is a set of genes encoding a putative bacteriocin similar to the Bacteriocin 41 from pYI14. Bacteriocin 41 consists of two lytic subunits, BacL1 and BacL2, an activator component, BacA, and was demonstrated to only be active against E. faecalis (Tomita et al., 2008). Two additional open reading frames, ORF9 and ORF10 are found in between the bacL2 and bacA genes on pYI14, and likewise two hypothetical proteins are similarly situated between the putative bacL2 and bacA genes of pBEE99. Functions for ORF9 and ORF10 on pYI14 have not yet been elucidated. Producer self-protection is afforded by BacI, a homolog of which is also found on pBEE99. While the pBEE99 bacL1 gene possesses a frameshift mutation located towards the 3′ end at position 44,724 that could potentially result in a nonfunctional polypeptide, we nonetheless sought to test whether the pBEE99 plasmid specifies bacteriocin production. We compared strain OG1RF to a previously generated OG1RF transconjugant, designated IG9, possessing the pBEE99 plasmid (Tendolkar et al., 2006) for the ability to inhibit the growth of E. faecalis strain FA2-2, which was previously shown to be sensitive to Bac41 (Tomita et al., 2008). However, neither the parental strain OG1RF nor the transconjugant IG9 [OG1RF (pBEE99)] produced any zones of inhibition on a lawn of FA2-2 (data not shown), suggesting that neither strain produces bacteriocin activity against strain FA2-2 under the conditions tested. BacL1 from pYI14 shows a high degree of identity and similarity to lytic enzymes from gram-positive bacteria, such as muramidase and lysozyme (Lopez and Garcia, 2004), and possesses three characteristic domains: a signal sequence, catalytic, and ligand binding domain consisting of three proline-rich SH3 repeat regions (Tomita et al., 2008). Alignment of the pBEE99 BacL1 with the pYI14 BacL1 reveals that the pBEE99 version possesses all three domains; however, a frameshift mutation following amino acid 505 results in a truncated polypeptide missing the C-terminal 90 amino acids. This mutation deletes the third SH3 repeat region from the ligand binding domain and thus might alter or abrogate receptor binding. This might explain the lack of observed bacteriocin activity against strain FA2-2. Work is currently in progress to address this possibility, as well as other possible explanations for the observed lack of activity, such as whether the pBEE99 bacteriocin genes are expressed, that FA2-2 possesses a chromosomally-encoded immunity determinant, or whether the pBEE99 Bac41 possesses altered target specificity.
3.4 pBEE99 enhances ultraviolet radiation resistance of OG1RF and E99
The presence of open reading frames with a high degree of identity and similarity to genes involved in UV resistance suggests that pBEE99 might enhance the ability of E. faecalis to survive UV radiation and therefore enhance the fitness of E. faecalis in the environment. In order to determine whether the pBEE99 plasmid confers an increased resistance to UV, we initially compared the UV sensitivity of strain OG1RF to strain IG9. We performed a UV irradiation killing assay essentially as described by Scott et al (Scott et al., 2008). As shown in Fig. 2, strain IG9 was approximately an order of magnitude more resistant to ultraviolet radiation than OG1RF. In order to quantify the difference between these two strains, an aliquot was taken at each time point and CFU/ml determined by track dilution. As shown in Fig. 3, OG1RF demonstrated a more precipitous decline in viability over time relative to IG9, and by 60 seconds was statistically significantly lower (P = 0.048). These results support a role for the pBEE99 plasmid in enhancing the resistance of strain OG1RF to ultraviolet radiation. To further show that pBEE99 imparts an enhanced UV resistance phenotype, we cured strain E99 of pBEE99 by repeated passaging of E99 at 45°C. The resultant strain, designated KDS01, demonstrated significantly greater sensitivity to UV light than the parental strain E99 after 30 seconds of UV exposure (Fig. 3; P = 0.038). At 120 seconds, the CFU per milliliter for all 4 strains was below the threshold of detection (<103 CFU/ml), and therefore this time point is not shown on the graph. While these results ascribe a role for pBEE99 as a whole in enhancing UV resistance of the host, the role(s) of specific gene(s) in this process will be the subject of future studies.
Fig. 2.
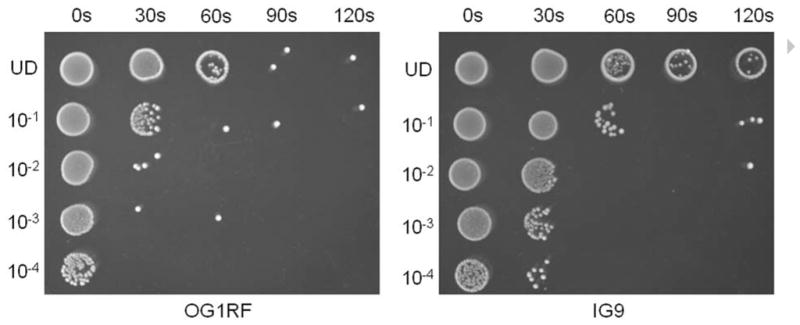
Comparison of the UV resistance of strain OG1RF to that of strain IG9 (an OG1RF transconjugant possessing pBEE99). IG9 demonstrated approximately an order of magnitude greater UV resistance than the parental strain OG1RF. Shown is a representative of two independent experiments.
Fig. 3.
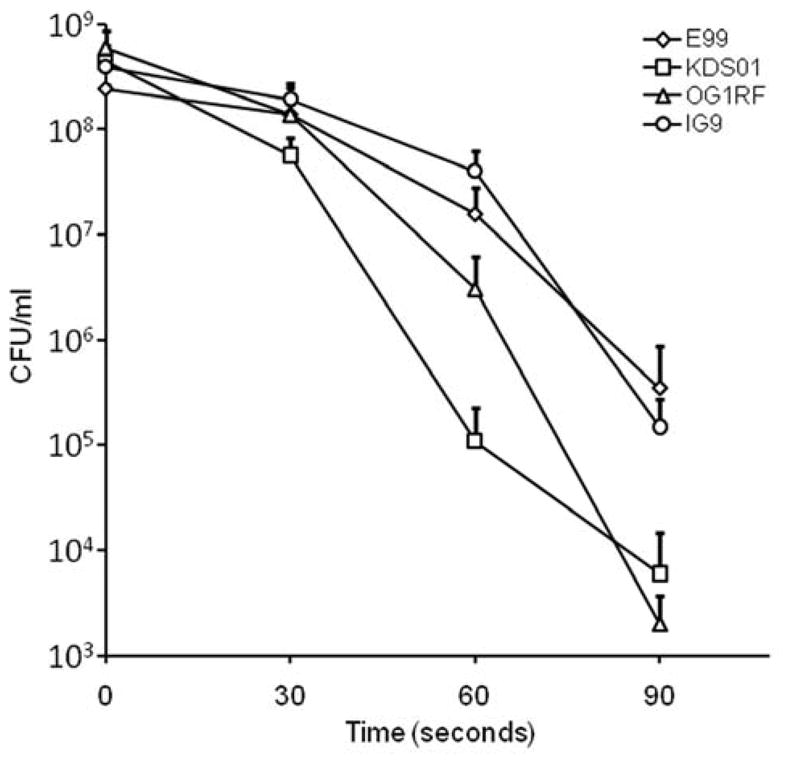
Plasmid pBEE99 specifies enhanced resistance to ultraviolet radiation. Strain E99 demonstrated significantly greater resistance than the pBEE99-cured strain KDS01 (P = 0.038), and strain IG9 was significantly more resistant to UV than was the parental strain OG1RF (P = 0.048). Experiments were conducted in triplicate and the error bars indicate the mean ± standard deviation. Levels of significance were determined using a Student’s t-test.
Recently, we reported that the pBEE99 plasmid harbors the novel bee gene cluster that encodes the ability to enhance biofilm formation by E. faecalis (Tendolkar et al., 2006) and thus pBEE99 represents a conjugative plasmid capable of disseminating a trait associated with the pathogenesis of this species (Mohamed et al., 2004). This locus, consisting of a cluster of 5 genes encoding three putative surface proteins and two sortases, respectively, bears a striking similarity to the gene clusters encoding pili in a number of pathogenic Gram-positive organisms (Tendolkar et al., 2006; Lauer et al., 2005; Ton-That and Schneewind, 2003). BLAST analysis of this gene cluster suggested that the three structural genes might have been acquired from Leuconostoc mesenteroides (Tendolkar et al., 2006). Recent genetic, mutational, and complementation analyses by our laboratory have confirmed that the bee1, bee2, and bee3 genes encode surface-localized proteins that are polymerized into a pilus structure (manuscript in revision).
4. Conclusions
In summary, we sequenced and characterized in E. faecalis, a novel 80 kb conjugative plasmid (pBEE99) that specifies an enhanced ability to form biofilms and resistance to ultraviolet radiation. This plasmid bears global similarity to pCF10; however, pBEE99 differs from pCF10 in that it is missing the Tn925 element and possesses a gene cluster that might have been acquired from L. mesenteroides (Tendolkar et al., 2006), as well as an operon potentially specifying a Bac41-like bacteriocin. The discovery of a conjugative plasmid that enhances the ability of E. faecalis to form a biofilm and to resist ultraviolet radiation represents another mechanism for enterococcal strains to disseminate the genes necessary to persist in the hospital setting or in susceptible patients.
Acknowledgments
This work was supported, in part, by the Oklahoma Center for the Advancement of Science and Technology (OCAST) through Health Research Program Grant HR06-104, and by NIH R01 AI059673 to N.S. We thank Helmut Hirt for performing the cCF10 clumping assay, Keith Weaver for analysis of the RepA-encoding region of pBEE99, and to Catherine King for performing the spot ultraviolet sensitivity test. We also thank Tamara Hunt for technical support and Mark Huycke for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clewell DB, Dunny GM. Conjugation and Genetic Exchange in Enterococci. In: Gilmore MS, Clewell DB, Courvalin P, Dunny GM, Murray BE, Rice LB, editors. The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance. Washington, D.C.: ASM Press; 2002. pp. 265–300. [Google Scholar]
- Clewell DB, Yagi Y, Dunny GM, Schultz SK. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974;117:283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn PS, Baghdayan AS, Dolan GT, Shankar N. Horizontal transfer of virulence genes encoded on the Enterococcus faecalis pathogenicity island. Mol Microbiol. 2007;63:530–544. doi: 10.1111/j.1365-2958.2006.05520.x. [DOI] [PubMed] [Google Scholar]
- De Boever EH, Clewell DB, Fraser CM. Enterococcus faecalis conjugative plasmid pAM373: complete nucleotide sequence and genetic analyses of sex pheromone response. Mol Microbiol. 2000;37:1327–1341. doi: 10.1046/j.1365-2958.2000.02072.x. [DOI] [PubMed] [Google Scholar]
- Dunny G, Funk C, Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981;6:270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Dunny GM. The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution. Philos Trans R Soc Lond B Biol Sci. 2007;362:1185–1193. doi: 10.1098/rstb.2007.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny GM, Brown BL, Clewell DB. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia MV, Fujimoto S, Tille P, Weaver KE, Clewell DB. Replication of Enterococcus faecalis pheromone-responding plasmid pAD1: location of the minimal replicon and oriV site and RepA involvement in initiation of replication. J Bacteriol. 2004;186:5003–5016. doi: 10.1128/JB.186.15.5003-5016.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia MV, Haas W, Wirth R, Samberger E, Muscholl-Silberhorn A, Gilmore MS, Ike Y, Weaver KE, An FY, Clewell DB. Completion of the nucleotide sequence of the Enterococcus faecalis conjugative virulence plasmid pAD1 and identification of a second transfer origin. Plasmid. 2001;46:117–127. doi: 10.1006/plas.2001.1533. [DOI] [PubMed] [Google Scholar]
- Gilmore MS, Coburn PS, Nallapareddy SR, Murray BE. Enterococcal virulence. In: Gilmore MS, Clewell DB, Courvalin P, Dunny GM, Murray BE, Rice LB, editors. The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance. Washington, D.C.: ASM Press; 2002. pp. 301–354. [Google Scholar]
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Hirt H, Manias DA, Bryan EM, Klein JR, Marklund JK, Staddon JH, Paustian ML, Kapur V, Dunny GM. Characterization of the pheromone response of the Enterococcus faecalis conjugative plasmid pCF10: complete sequence and comparative analysis of the transcriptional and phenotypic responses of pCF10-containing cells to pheromone induction. J Bacteriol. 2005;187:1044–1054. doi: 10.1128/JB.187.3.1044-1054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- Koch WH, Henrikson E, Eisenstadt E, Cebula TA. Salmonella typhimurium LT7 and LT2 strains carrying the imp operon on colIa. J Bacteriol. 1995;177:1903–1905. doi: 10.1128/jb.177.7.1903-1905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer P, Rinaudo CD, Soriani M, Margarit I, Maione D, Rosini R, Taddei AR, Mora M, Rappuoli R, Grandi G, Telford JL. Genome analysis reveals pili in Group B Streptococcus. Science. 2005;309:105. doi: 10.1126/science.1111563. [DOI] [PubMed] [Google Scholar]
- Lopez R, Garcia E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol Rev. 2004;28:553–580. doi: 10.1016/j.femsre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:3658–3663. doi: 10.1128/IAI.72.6.3658-3663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Tanimoto K, Fujimoto S, Tomita H, Ike Y. Cloning and genetic analysis of the UV resistance determinant (uvr) encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pAD1. J Bacteriol. 1997;179:7468–7475. doi: 10.1128/jb.179.23.7468-7475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, Tettelin H, Dodson RJ, Umayam L, Brinkac L, Beanan M, Daugherty S, DeBoy RT, Durkin S, Kolonay J, Madupu R, Nelson W, Vamathevan J, Tron B, Upton J, Hansen T, Shetty J, Khouri H, Utterback T, Radune D, Ketchum KA, Dougherty BA, Frase CM. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- Sarov-Blat L, Livneh Z. The mutagenesis protein MucB interacts with single strand DNA binding protein and induces a major conformational change in its complex with single-stranded DNA. J Biol Chem. 1998;273:5520–5527. doi: 10.1074/jbc.273.10.5520. [DOI] [PubMed] [Google Scholar]
- Schwarz FV, Perreten V, Teuber M. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid. 2001;46:170–187. doi: 10.1006/plas.2001.1544. [DOI] [PubMed] [Google Scholar]
- Scott J, Thompson-Mayberry P, Lahmamsi S, King CJ, McShan WM. Phage-associated mutator phenotype in group A Streptococcus. J Bacteriol. 2008;190:6290–6301. doi: 10.1128/JB.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BT, Walker GC. Mutagenesis and more: umuDC and the Escherichia coli SOS response. Genetics. 1998;148:1599–1610. doi: 10.1093/genetics/148.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard P, Wishart DS. Circular genome visualization and exploration using CGView. Bioinformatics. 2005;21:537–539. doi: 10.1093/bioinformatics/bti054. [DOI] [PubMed] [Google Scholar]
- Tendolkar PM, Baghdayan AS, Shankar N. Putative surface proteins encoded within a novel transferable locus confer a high-biofilm phenotype to Enterococcus faecalis. J Bacteriol. 2006;188:2063–2072. doi: 10.1128/JB.188.6.2063-2072.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Kamei E, Ike Y. Cloning and genetic analyses of the bacteriocin 41 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI14: a novel bacteriocin complemented by two extracellular components (lysin and activator) J Bacteriol. 2008;190:2075–2085. doi: 10.1128/JB.01056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton-That H, Schneewind O. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol Microbiol. 2003;50:1429–1438. doi: 10.1046/j.1365-2958.2003.03782.x. [DOI] [PubMed] [Google Scholar]
- Wang Z. Translesion synthesis by the UmuC family of DNA polymerases. Mutat Res. 2001;486:59–70. doi: 10.1016/s0921-8777(01)00089-1. [DOI] [PubMed] [Google Scholar]