Abstract
The mouse retina has been used extensively over the past decades to study both physiologic and pathologic angiogenesis. Over time, various mouse retina models have evolved into well-characterized and robust tools for in vivo angiogenesis research. This article is a review of the angiogenic development of the mouse retina and a discussion of some of the most widely used vascular disease models. From the multitude of studies performed in the mouse retina, a selection of representative works is discussed in more detail regarding their role in advancing the understanding of both the ocular and general mechanisms of angiogenesis.
In 2006, intraocular anti-VEGF therapy was ranked among the top 10 science breakthroughs of the year,1 reflecting the increased attention retinal angiogenesis has received in recent years. Epidemiologic studies provide evidence that this attention is well deserved: Proliferative diabetic retinopathy (PDR) accounts for the highest incidence of acquired blindness in the working-age population2,3; choroidal (subretinal) neovascularization in age-related macular degeneration (AMD) represents the leading cause of blindness in people over the age of 65,4,5 and retinopathy of prematurity (ROP) is a major cause of acquired blindness in children.6 In addition, there are many other ocular diseases that have a vascular component, such as Norrie disease (ND), familial exudative vitreoretinopathy (FEVR), Coats' disease, sickle cell retinopathy, retinitis pigmentosa, and many others. Various animal models have been developed in the effort to understand the underlying mechanisms of these sight-threatening conditions. Among the numerous animal species used in these models, the mouse offers a unique combination of affordable cost, well-established platforms for creating systemic or conditional transgenic strains, and access to a wide availability of recombinant proteins and antibodies. In this article, we review mouse models of retinal angiogenesis and provide brief discussions of some of the most relevant lessons learned from these models.
Developmental Retinal Angiogenesis in Humans
Any animal model that is intended to advance the understanding of a human disease state must be interpreted with regard to the clinical picture. In humans, the retina begins to vascularize in utero at approximately 16 weeks of gestational age. During the early stages, vascular supply to the eye is partially provided by the hyaloid vasculature originating from the optic nerve and traversing the primitive vitreous toward the anterior segment.7 To attain a transparent visual axis, the hyaloid vasculature must regress when ocular development proceeds.8 Hyaloid regression is usually completed before 34 weeks of gestation in humans. By this time, development of the intraretinal vasculature is already well advanced. The nasal retina becomes fully vascularized at approximately 36 weeks of gestational age, and the temporal vessels reach the ora serrata at approximately week 40.7 A human term infant is thus born with fully developed retinal vessels and with regressed hyaloid vasculature. Several developmental disorders, however, can interfere with normal growth or maturation of the retinal vasculature. One example is Norrie's disease, an inherited eye disease that is associated with persistent hyaloid vessels, incomplete development of the retinal vasculature, and pathologic retinal neovessel (NV) proliferation, often resulting in blindness from birth.9
Besides developmental disorders, premature birth is the most common condition leading to altered retinal vascular development. Human preterm infants with ROP show delayed maturation of the retinal vasculature that can be associated with pathologic extraretinal vessel formation. In recent years, there has been an increase in the prevalence of ROP, probably as a result of improved survival among the most premature infants.10 This alarming increase in ROP represents a challenge for clinicians who care for these infants and a challenge for researchers to further intensify their efforts to understand the mechanisms behind ROP. Clinically, ROP is characterized by two stages: an early phase of vascular injury with obliteration of immature vessels and a second phase of vascular repair. In most premature infants, the retina successfully vascularizes during this second phase of repair. However, in some (usually the most immature ones with the least vascularization of the retina at birth), there is pathologic NV formation with angiogenic sprouting of abnormal vessels from the retina into the vitreous. This NV sprouting leads to complications such as plasma leakage, bleeding, and, in some cases, tractional retinal detachment and blindness.11–14 The severity of pathologic NV formation is dictated to a large extent by the amount of avascular retina present in an individual infant.15 Consequently, in infants with only limited nonvascularized retina, the sequence of events from initial vascular injury to the deleterious consequences of NV formation does not develop to its full extent16 and the pathologic NVs either never fully form or regress over time.17
Even if pathologic neovascularization develops, the initially damaged intraretinal vasculature often resumes physiologic growth during the second phase of ROP, and vascularization of the avascular areas with functional vessels may ensue.18 Ultimately, the final functional outcome of ROP in eyes is largely dependent on which of the two processes, pathologic NV formation or physiologic vascularization, prevails. However, it is important to note that even in cases of mild ROP, in which no or only minimal overt NV formation occurs, physiologic vascularization of the retina is significantly delayed compared with that in term-born infants. Although retinal vascularization is complete by 40 weeks' gestational age in term infants, complete retinal vascularization in infants with ROP can be delayed until 48 to 52 weeks of gestational age.7 In this context, the same growth factors deemed responsible for pathologic NV formation are likely to be crucial for physiologic vascularization of the retina, albeit with different timing and at different levels.19–24
Developmental Retinal Angiogenesis in Mice
In contrast to humans, mouse (and other rodent) pups have an immature retinal vasculature and persistent hyaloid vessels at birth.25 The highly organized pruning of hyaloid vessels in the postnatal mouse eye has been a very useful tool in the study of mechanisms of physiologic vessel regression. In 2005, seminal work from the Lang laboratory26 identified WNT7b as a short-range paracrine signal for vessel regression using the mouse hyaloid system, and important work from Liu and Nathans27 identified an essential role for the putative WNT-receptor frizzled 5 in hyaloid regression. Of interest, two human conditions with disturbed retinal development, FEVR and ND, have both been linked to defects in the Wnt signaling cascade.9,28–34
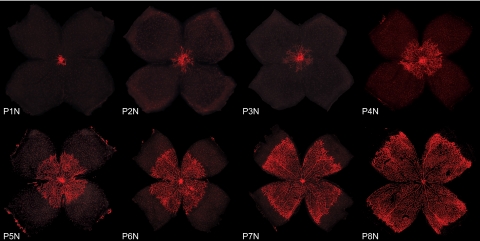
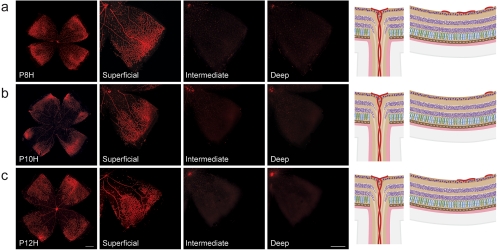
Parallel to hyaloid regression, development of the intraretinal vasculature in mice occurs postnatally in a tightly regulated temporal and spatial pattern that can readily be observed and manipulated experimentally.35 Most of the data obtained to date were collected from different wild-type strains, among which C57Bl/6 is the most widely used. It is important to note that the time course of normal vascular development in wild-type mice varies considerably among the strains. Normal retinal vascular development in BALB/cByJ mice, for example, is delayed by a few days compared with that in C57Bl/6 mice.36 The time course illustrated in Figures 1 and 2 therefore applies only to retinal vascular development in C57Bl/6 mice: In this strain, the superficial vascular plexus forms during the first week after birth by radial outgrowth of vessels from the optic nerve into the periphery, reaching the retinal edges at approximately P8 (Fig. 1). From P7 onward the superficial capillaries start sprouting vertically to form first the deep and then the intermediate vascular plexus (Fig. 2 and Movie S1, http://www.iovs.org/cgi/content/full/51/6/2813/DC1). The deep plexus, located in the outer plexiform layer, forms rapidly and reaches the retinal periphery at approximately P12 (Fig. 2b), followed by the intermediate plexus in the inner plexiform layer between P12 and P15 (Figs. 2b, 2c). By the end of the third postnatal week, all three vascular layers are fully mature with multiple interconnecting vessels between layers (Figs. 2e, 2f).
Figure 1.
Development of the superficial vascular plexus in C57Bl/6 mouse retinas. Retinal whole mounts from postnatal day (P)1 to P8 were stained for endothelial cells with isolectin B4-Alexa 594 (red). N (normoxia) signifies normal development, as opposed to the hyperoxia time course shown in Figures 4 and 5. At P1N, the mouse retina is almost completely devoid of blood vessels. The superficial vascular plexus can be seen originating from the optic nerve head. During the first week of postnatal development, the superficial plexus extends radially from the optic nerve head into the surrounding tissue, reaching the retinal periphery at ∼P8N.
Figure 2.
Development of the deep and intermediate vascular plexus in C57Bl/6 mouse retinas. Retinal whole mounts and cross sections from postnatal day (P)9 to P25 were stained for endothelial cells with isolectin B4-Alexa 594 (red) and for cell nuclei with DAPI (blue). N (normoxia) signifies normal development, as opposed to the hyperoxia time course shown in Figures 4 and 5. (a) At P9N, the superficial plexus has fully extended to the peripheral retina. The deep vascular plexus begins forming centrally from vertical vessels diving down from the superficial plexus. The intermediate vascular plexus has not begun to form yet. On cross section, the three neuronal layers of the retina and the superficial vascular plexus can be identified. Representative drawings on the right illustrate both superficial and deep vascular plexus in the immediate vicinity of the optic nerve, whereas in the periphery the superficial plexus remains the only vascular network. (b) At P12N, the intermediate vascular plexus becomes visible on retinal whole mounts. The deep vascular plexus is nearly fully developed. On cross section, vertical sprouting of vessels toward the intermediate plexus can be observed. Representative drawings illustrate the beginning of a three-layered vascular plexus around the optic nerve and superficial and deep vascular plexus with interconnecting vessels in the periphery. (c) At P15N, the intermediate vascular plexus continues to develop throughout the retina as illustrated both on retinal whole mounts and cross sections. Representative drawings illustrate complete formation of superficial and deep plexus with continued development of the intermediate vascular plexus in the periphery. (d) At P17N, the superficial, intermediate, and deep plexus can be seen both centrally and in the peripheral retina. Cross sections and representative drawings illustrate the three-layered vascular system in all parts of the retina along with multiple interconnecting vessels. (e, f) Between P21N and P25N, further maturation of especially the intermediate plexus can be observed. Cross sections and representative drawings illustrate the mature retinal vasculature. Note that isolectin B4 binds to choroidal vessels as well as nonspecifically to RPE and the scleral wall in some of the cross sections (a–f).
The Mouse Retina as a Model for Physiologic Angiogenesis
The cellular and molecular mechanisms underlying the developmental time course just described have been studied extensively.37–41 One of the major advantages of studying postnatal retinal development in mice lies in the ease of accessibility of the developing vasculature for imaging and intervention without the difficulties associated with investigating embryonic development.36,37,42,43 In pioneering work from the Keshet laboratory,22 for example, the developing mouse retina was used to meticulously analyze VEGF expression during developmental angiogenesis. The Carmeliet and D'Amore groups44 further investigated the specific roles of different VEGF isoforms during retinal vascular development and Gerhardt et al.45 investigated how endothelial cells respond to VEGF gradients by tip cell formation and guided migration in the developing mouse retina. More recently, the important role of the notch signaling pathway in vascular development has been extensively studied in the mouse retina model.46–48
As postnatal vascular development in the retina proceeds in a very tightly regulated and organized pattern, it also allows for reliable detection of any developmental abnormality, both in transgenic animals and pharmacologic studies.41 A very useful tool in this respect is an analysis of global gene expression during normal retinal development published by the Friedlander group.49 The same group has also used the developing mouse retina to investigate the role of bone-marrow–derived stem cells in angiogenic development, which has significantly advanced our understanding of how bone-marrow–derived cells contribute to retinal vessel formation and how endothelial progenitor cells (EPCs) can attenuate vessel regression in the rd/rd mouse model of retinal degeneration.50–52
As organized vascularization is essential not only for physiologic development of the retina but also for the normal development of most other tissues, many observations made in the developing mouse retina also apply to developmental angiogenesis in other organs. In this respect, the eye has been used by many researchers to decipher the general angiogenic mechanisms related to developmental angiogenesis or tumor vascularization.53,54
Animal Models of ROP
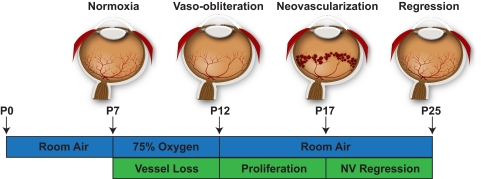
Besides modeling normal development, the mouse retina can be used as a tool to investigate conditions in which normal vascular development becomes unbalanced, leading to sight-threatening diseases, as occurs in human ROP. Successful modeling of human ROP in animals necessitates replication of most of the hallmarks of the human disease. The animal model should accurately reproduce the two phases of ROP—initial vaso-obliteration (VO), and subsequent neovascularization (NV)—along with some of the more frequent complications like vascular leakage. An adequate model system should furthermore display revascularization of the avascular area with functional intraretinal vasculature and regression of pathologic neovascularization during the later disease stages. Several animal models have been developed that fulfill these criteria. All these models use exposure of neonatal animals to high oxygen tension to induce loss of immature retinal vasculature.55 Because of the causal role of oxygen exposure and considering that the animals used in these models were not born prematurely, oxygen-induced retinopathy (OIR) is a more accurate term than ROP for describing these models. However, because term neonates in these species are born with an immature retinal vasculature, they replicate the cardinal aspects of human ROP and the two characteristic phases of ROP: vaso-obliteration followed by neovascularization. These two phases have been reported in feline,56–58 canine,59 rat,60 and mouse models of OIR.61 The mouse model of OIR will be described in more detail, along with a review of some of its most relevant contributions to in vivo angiogenesis research.
The Mouse Model of OIR
One of the most widely used animal models of ROP is the mouse model of OIR,61 with more than 15,000 publications since it was first published in 1994 (Google Scholar search on keywords “oxygen induced retinopathy mice,” June 15, 2009). Using the mouse as a model animal enables access to a wide range of genetically manipulated animals, recombinant proteins, and antibodies. In addition, animal costs and procedure times in the mouse model compare favorably to those of other animal species, and the mouse retina very reliably develops pathologic vaso-obliteration and neovascularization. However, certain caveats should be kept in mind when using the OIR mouse model: Different severities of OIR have been found to develop in different wild-type strains.62,63 Similarly, OIR severity can vary within one wild-type strain because of vendor-related substrain differences. This variability has also been observed in the rat OIR model.64 From our experience with the OIR mouse model, C57Bl/6 wild-type mice from Jackson Laboratories (Bar Harbor, ME) display slightly higher retinal NV than do their C57Bl/6 counterparts from Taconic Farms (Germantown, NY). The reason for these differences is most likely the many generations of inbreeding at an individual vendor's facility during which spontaneous mutations can occur that persist in the colony undetected and translate into measurable phenotypic differences in the OIR model. On the basis of this possibility, it can be speculated that even animals from the same vendor may vary, depending, for example, on whether the animals were bought from the same vendor's Asian, European, or American breeding facility. The differences in wild-type strains, however, can easily be controlled by using the same strain and vendor source for all animals throughout an entire experimental protocol in both treatment and control groups. For studies involving transgenic mice, however, it becomes crucial to obtain the correct wild-type control animal, based on the genetic background of each transgenic strain investigated.
Another variable that should be carefully controlled in the OIR mouse model is postnatal weight gain during the OIR period. Clinical observations from ROP infants have identified postnatal weight gain as a strong predictor of the severity of ROP.65–71 The same appears to be true in the OIR mouse model.72 Therefore, weights should be measured both at P7 and P17, and all experimental groups in the OIR mouse model should be adjusted for equal body weights. Ideally, weight recordings should be reported in publications. In addition, to control for other confounding variables, sufficient animal numbers per experimental group are essential. To account for possible litter-specific differences, at least 10 eyes from three different litters should be collected per experimental group (based on power analysis with α = 0.05, β = 0.2, treatment effect = 25%, and SD = 23%).
Using the OIR Mouse Model
When these variables are controlled, the OIR mouse model61 produces robust and reliable data in a straightforward and time-efficient experimental setting. It also reproduces the defining disease characteristics observed during the development of human ROP. An extensive step-by-step protocol for the OIR mouse model has recently been published.73 In brief, neonatal mice are exposed to 75% oxygen from postnatal day (P)7 until P12 and returned to room air (21% oxygen) from P12 to P17 (Fig. 3). During the first phase of hyperoxic exposure (P7–P12), retinal vessels constrict to regulate retinal Po2 levels74 and immature capillaries in the central retina regress leading to a central zone of vaso-obliteration (Figs. 4a–c). As hyperoxia exposure overlaps with the phase of physiologic vertical sprouting of vessels from the superficial layer into the deep and intermediate plexus, a marked delay in the formation of deeper retinal vasculature can also be observed. With regard to the central VO area, it is important to note that the oxygen-induced vaso-obliteration is rapid, with peak VO being reached 48 hours after onset of oxygen exposure.75 Although still exposed to oxygen, revascularization of the VO zone begins from P9 to P12,75,76 likely reflecting increasing oxygen demands of the developing retina.77 On return to room air at P12, the VO area becomes hypoxic78 and significant upregulation of Hif-1α-dependent, proangiogenic pathways ensues.20,77,79 Of note, the avascular retina in the VO zone of OIR mice differs from the avascular retina during normal development in regard to oxygen tension: whereas the VO zone stains positively for conjugated pimonidazole (Hypoxyprobe; Chemicon, Temecula, CA), a marker for tissue oxygen tensions of less than 10 mm Hg, the peripheral avascular retina during normal development remains unstained.78,80 These findings indicate a balance between oxygen supply and demand in the avascular areas of the developing peripheral retina, as opposed to an oxygen undersupply in the VO zone of the OIR model.
Figure 3.
The mouse model of OIR. Neonatal mice and their nursing mother are kept at room air from birth through postnatal day (P)7. From P7 to P12, the mice are exposed to 75% oxygen, which induces loss of immature retinal vessels and slows development of the normal retinal vasculature, leading to a central zone of vaso-obliteration (VO). After returning mice to room air at P12, the central avascular retina becomes hypoxic, triggering both normal vessel regrowth and a pathologic formation of extraretinal neovascularization (NV). Maximum severity of NV is reached at P17. Shortly thereafter, NV starts to regress and by P25 almost no VO or NV remains visible. This figure was first published in Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathologic angiogenesis. Nat Protoc. 2009;4:1565–1573.
Figure 4.
Vessel loss during hyperoxia in the OIR model. Retinal whole mounts from postnatal day (P)8 to P12 were stained for endothelial cells with isolectin B4-Alexa 594 (red). H for hyperoxia is used to signify the OIR time course as opposed to the normal development shown in Figures 1 and 2. (a) At P8H, only 24 hours after onset of hyperoxia, a central zone of vaso-obliteration (VO) develops in the superficial vascular plexus. As hyperoxia coincides with formation of the intermediate and deep vascular plexus, development of these layers is significantly impeded. Right: representative drawings of the central VO zone showing preserved superficial vessels in the periphery and the absence of deep and intermediate vessel formation. (b) At P10H, the central VO zone has increased in size, extending farther into the peripheral retina. Formation of both deep and intermediate plexus is suppressed. (c) At P12H, the central VO zone has slightly decreased in size, probably due to revascularization in response to increased oxygen demand of the developing retina. The deep and intermediate plexus, however, are still absent.
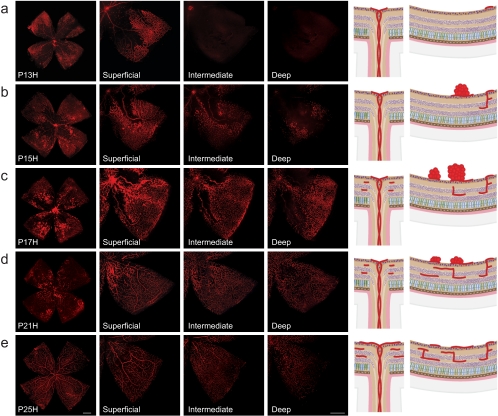
The hypoxia-triggered angiogenic growth factors in the second phase of OIR are not only essential for revascularization of the VO zone but also induce formation of pathologic NV (Figs. 5a–c). These pathologic neovessels leave the highly organized, layered structure of the retina and grow into the normally avascular vitreous cavity to form unorganized, small-caliber vessels, termed preretinal tufts, that resemble the pathologic neovascularizations seen in human ROP or PDR.61 Whereas neovascular tufts form in the superficial layer, the intermediate and deep layers also show considerable vascular abnormalities compared with normoxic age-matched control retinas (see Fig. 2 for comparison). In the OIR mouse model, the maximum severity of the proliferative phase is reached at P17, marked by the greatest extent of pathologic NV and associated plasma leakage from the preretinal neovessels. From P17 onward, neovessels start to regress, until at P25, almost no VO or NV remains visible81 (Figs. 5d, 5e). The tight interdependence of VO and NV, with the former dictating the time course and severity of the latter to a considerable degree, emphasizes the importance of publishing data on both VO and NV, with the OIR mouse model.
Figure 5.
Neovascularization during relative hypoxia in the OIR model. Retinal whole mounts from postnatal day (P)13 to P25 were stained for endothelial cells with isolectin B4-Alexa 594 (red). H for hyperoxia is used to signify OIR time course as opposed to the normal development shown in Figures 1 and 2. (a) At P13H, 24 hours after moving mice back to room air, the superficial vascular plexus shows a central area of vaso-obliteration (VO) without morphologic signs of neovascularization (NV). Intermediate and deep vascular plexus are absent. (b) At P15H, the VO zone has decreased in size through revascularization by normal vessels. At the same time, NV formation in the superficial vascular plexus starts to emerge between the VO zone and the peripheral vascularized retina. Vertical vessels diving down to form the deep and intermediate plexus can be observed in the peripheral retina where the superficial plexus was preserved during hyperoxia. Both this growth pattern and the time course of deeper plexus formation differ significantly from normal development. Representative drawings on the right illustrate vessel regrowth and extraretinal NV formation in the superficial plexus along with development of the deep and intermediate plexus. (c) At P17H, revascularization of the VO zone has progressed farther and pathologic NV formation is at its maximum. The intermediate and deep vascular plexus have continued to form, covering now the same area as the superficial plexus. The remaining central VO zone is devoid of all three vascular layers. (d) At P21H, revascularization of the VO area has progressed farther in all layers and NV has begun to regress. (e) At P25H, the VO area is fully revascularized in all layers and NV has completely resolved.
Quantifying VO and NV in the OIR Mouse Model
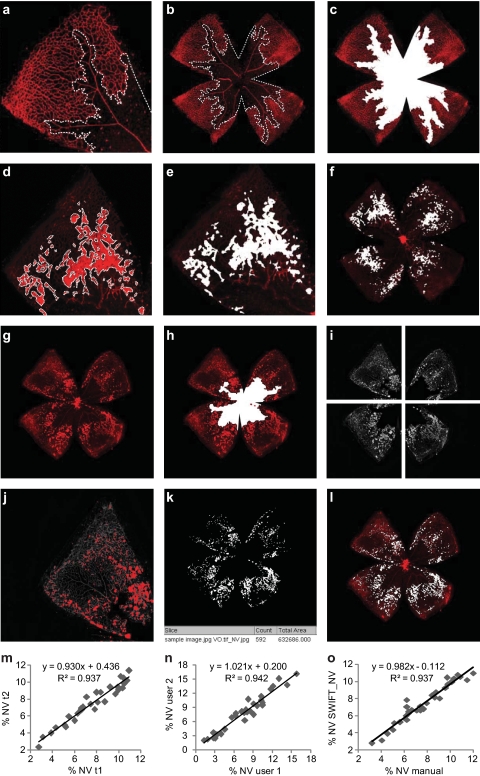
The two main features to quantify in the OIR mouse model are VO and subsequent NV. VO develops during the first, hyperoxic, phase of the model, and NV formation follows from P14 onward. In studies designed to investigate vasoprotective strategies, the extent of VO can be measured at P12 (or even as early as P8), to determine the amount of vasculature lost during hyperoxia. For most other studies, VO is measured together with neovascularization at P17, when the severity of the proliferative change is at its maximum.61 Regrowth of normal vasculature occurs together with NV formation between P12 and P17. Two groups with identical VO areas at P12 may therefore have different VO areas at P17 if they differ in their capability to repair physiologic vasculature. In studies of vascular regrowth, the extent of VO can therefore be compared between P12 and P17. VO is most frequently measured by outlining the vaso-obliterated area with image-processing software (e.g., Phtotoshop; Adobe Systems, San Jose, CA) in relation to total retinal area (%VO; Figs. 6a–c).73
Figure 6.
Quantification of vaso-obliteration (VO) and neovascularization (NV) in the OIR mouse model. Retinal whole-mounts from postnatal day (P)17 were stained for endothelial cells with isolectin B4-Alexa 594 (red), with VO and NV outlined in white. (a–c) For measurement of VO, the central avascular area was outlined with image-processing software (Photoshop; Adobe Systems, San Jose, CA) in relation to total retinal area (%VO). (d–f) For manual quantification of neovascularization, each individual neovascularization tuft and cluster was outlined using image-processing software (Photoshop; Adobe Systems) and the total neovascular area is expressed in relation to total retinal area (%NV). (g, h) For computer-aided NV quantification, both the original image and the VO image were imported into NIH's free-access ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). (i) The macro set SWIFT_NV divides the VO image into four quadrants, isolates the red color channel, and subtracts background fluorescence. (j) SWIFT_NV then allows the user to set a fluorescence threshold for each quadrant, marking NV tufts but not normal vessels. (k, l) Using the user-defined thresholds, SWIFT_NV quantifies all NV pixels from all four quadrants, reports the result as neovascular total area and creates an overlay of NV and original image. (m–o) Results obtained with the SWIFT_NV macros show reliable intra- and interindividual reproducibility and very good correlations to the established hand measurement protocols. Parts (a) through (f) of this figure were first published in Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathologic angiogenesis. Nat Protoc. 2009;4:1565–1573; parts (g) through (o) were reproduced with permission from Stahl A, Connor KM, Sapieha P, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. © Springer.
Similar to VO, quantification of NV is performed on retinal whole mounts, usually at the peak of proliferative retinopathy at P17. It is important to note that NV formation and regression are rapid processes in the OIR mouse model: At P14, almost no NV is visible, peak NV is observed only 3 days later, and by P25 NV has completely regressed (Smith laboratory, unpublished observations, 2009). For NV quantification, various approaches have been developed over time. In the earliest OIR studies, retinal cross sections were used to count preretinal nuclei of extraretinal neovessels.20,61,79,82 Subsequent studies moved to using retinal whole mounts to score retinal NV in a grading system.83,84 In 2006, Banin et al.85 introduced a quantification method, using image-processing software (e.g., Photoshop; Adobe) to manually outline neovascular tufts on retinal whole mounts (Figs. 6d–f). Recently, a computer-aided technique has been published that allows semiautomated quantification of retinal NV (Figs. 6g–l).86 This approach greatly accelerates NV quantification along with reliable interindividual reproducibility and very good correlations to the established hand measurement protocols (Figs. 6m–o and Movie S2, http://www.iovs.org/cgi/content/full/51/6/2813/DC1).
The OIR Mouse Model in Angiogenesis Research
Over the past 15 years, the OIR model61 has been used to investigate many relevant pathways involved in pathologic retinal angiogenesis: Several different groups have for example used the OIR mouse model to investigate the effects of reactive oxygen species like NO,87–90 leukocytes, and their inflammatory mediators like tumor necrosis factor (TNF)-α91–94 or angiogenic modulators like angiopoietin 2 (Ang-2).95–98 Of the large number of important pathways investigated, some work will be discussed in more detail in the following sections.
Vascular Endothelial Growth Factor
During the first phase of OIR (hyperoxic phase from P7 to P12), retinal VEGF expression is significantly suppressed, contributing to vessel loss.19,21,99 During the second, hypoxic phase of the OIR model, however, VEGF becomes upregulated mostly in glial and Müller cells of the inner retina77 and strongly contributes to pathologic NV formation.19,20,100,101 Early studies using the OIR model showed the beneficial effects of anti-VEGF treatment on hypoxia-driven retinal neovascularization.82,102–104 In addition, differential roles for the two main VEGF receptors, VEGFR1 and -2 were identified during vaso-obliteration in the OIR model.105,106 Together, these studies have significantly advanced our understanding of VEGF in pathologic neovascularization and have helped to lay the foundations for today's clinical application of anti-VEGF therapies in patients.
Erythropoietin
Another potent angiogenic factor expressed in the retina is erythropoietin (Epo).107 Epo, like VEGF, is induced via Hif-1α in a hypoxia-dependent fashion108 and has been found to have neuroprotective properties in the rat model of retinal ischemia and reperfusion injury.109 In the OIR mouse model, Epo shows expression patterns similar to those of VEGF, with initial downregulation during the hyperoxic phase and subsequent upregulation in relative hypoxia.110 Exogenous Epo administration during the early phase of vessel loss was found to reduce the area of VO and subsequent neovascularization. However, when administered during the later stages of retinal hypoxia, Epo conversely appears to contribute to pathologic neovascularization.110 This biphasic role with protective Epo effects during VO and proangiogenic effects during neovascularization was further confirmed in a subsequent study showing that siRNA-mediated knockdown of ocular Epo during the neovascular phase attenuates NV formation.78 The relevance of this work for clinical applications is illustrated by the fact that in patients with proliferative retinopathy and retinal vein occlusion vitreal levels of Epo are significantly elevated.107,111,112
Insulin-like Growth Factor and Insulin-like Growth Factor–Binding Protein
While VEGF is rightfully considered a master switch for angiogenesis,113 both the major proliferative retinopathies—ROP and PDR—have underlying pathomechanisms that are regulated by extensive metabolic pathways, both locally in the retina and systemically. It is therefore essential to investigate the underlying mechanisms of ROP and PDR that function upstream of VEGF expression. One of the mediators that has been found to act upstream of retinal VEGF is IGF-1. In ROP, IGF-1 appears to act as a permissive factor for retinal neovascularization, with inhibition of IGF-1 during the neovascular phase reducing hypoxia-induced retinal NV despite high levels of intraocular VEGF.79 Similarly, vessel-specific knockout of either insulin or IGF-1 receptors was found to protect from retinal neovascularization in the OIR mouse model.114 However, the complete role of IGF-1 in ROP appears to be significantly more complex: During the early phases of VO, low levels of IGF-1 seem to be associated with poor retinal vascularization, precipitating increased hypoxia. Since premature infants have very low levels of serum IGF-1 and the level is inversely correlated with the severity of ROP,67,115,116 the goal during neonatal care must be early normalization of IGF-1 levels, matching as closely as possible those levels found during normal development in utero. Normalization may also be critical during proliferative disease, as abnormally high IGF-1 levels during the proliferative stages can have equally detrimental effects.67,115–117 One important regulator of IGF-1 is the main IGF-binding protein, IGFBP-3. In the OIR mouse model, systemic IGFBP-3 administration was successfully used to reduce retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth.118 IGFBP-3 was also found to be significantly increased in neovascular tufts of the OIR retina, suggesting not only systemic but also possibly local regulatory functions of IGFBP-3 in the retina.119 In addition, recent work from the Grant laboratory120 found that IGFBP-3 prevents cell death of endothelial cells in the retina during OIR. Combined, these observations have helped lay the foundation for clinical trials intended to correct IGF-1 deficiency in premature infants by using equimolar combinations of IGF-1 and IGFBP-3.121
Bone-Marrow–Derived Progenitor Cells
Similar to the regulatory roles of IGF-1/IGFBP-3, the search for modifiable pathomechanisms of proliferative retinopathies upstream of VEGF have led to research into the role of bone-marrow–derived progenitor cells during OIR. One of the homing factors for bone-marrow–derived endothelial progenitor cells (EPCs) to the retina is IGFBP-3.122 Once recruited to the retina, bone-marrow–derived cells can facilitate normalization of retinal vasculature after OIR and reduce the hypoxic VO area and subsequently attenuate pathologic NV.63 However, in a mouse model of retinal vein occlusion and ocular VEGF overexpression, progenitor cells were found to play a role both in physiologic revascularization as well as in pathologic neovascularization.123 The possible therapeutic implications of targeting bone-marrow–derived progenitor cells for retinal vascular repair have been reviewed in more detail by Friedlander et al.124
Lipid-Derived Mediators of Angiogenesis
The ω-3 polyunsaturated fatty acids (ω-3 PUFAs) are essential fatty acids and key structural components of retinal cell membranes that are most abundant in the rod and cone outer segments. Besides their crucial role in photoreceptor function, ω-3 PUFAs have been found to bind to nuclear receptors involved in transcriptional regulation of pro- and anti-inflammatory mediators.125 With regard to ROP, ω-3 PUFAs are essential fatty acids that are abundant during intrauterine development, but are deficient after premature birth.126 Increasing ω-3 PUFAs in the OIR mouse model either by dietary supplementation from birth or by using transgenic fat-1 mice that are able to convert ω-6 into ω-3 PUFAs resulted in a 50% reduction of pathologic NV through improved regrowth of physiologic vasculature and increases in resolvins and neuroprotectins.127 Cyclooxygenase (Cox)-2, one of the key metabolizing enzymes for ω-3 and ω-6 PUFAs, has been independently identified as a strong modulator of proliferative retinopathy in the OIR retina128; Serhan et al.129 showed that Cox-2 modulation via aspirin can generate anti-inflammatory lipid mediators of the resolvin class. If clinical trials that are currently in the planning phase, find that increasing ω-3 PUFA levels in preterm infant nutritional intake is effective at reducing the severity of ROP, this highly cost-effective intervention could benefit millions of prematurely born infants.
Neurovascular Cross-talk
Clinicians have observed that PDR regresses after the onset of photoreceptor loss in diabetic patients with retinitis pigmentosa.130 Similarly, both experimental diabetic retinopathy and hypoxia-induced neovascularization are reduced in mouse models of outer retinal degeneration.131,132 These findings may be explained by the fact that retinal photoreceptors are among the most highly metabolically active cells in the body, consuming significant amounts of oxygen delivered to the retina.75 When photoreceptors are lost, more oxygen is available to the remaining retina that subsequently reduces its expression of hypoxia-mediated proangiogenic mediators.133–137 This interdependence of vascular and neurosensory retina is further illustrated by studies showing that rod photoreceptor function can predict vascular anomalies138 and vice versa, that retinal vascularization can determine neuronal and glial changes as evidenced, for example, by specific upregulation of GFAP in Müller cells of the vaso-obliterated zone.139
Similar to photoreceptors, the role of glial cells of the inner retina in maintaining or directing retinal vascularization has increasingly gained interest,140–144 and it has been suggested that protection of inner retinal glial cells has beneficial effects on the recovery of physiologic vasculature after VO.76 In a recent publication, Dorrell et al.145 found that maintaining retinal astrocytes normalizes physiological revascularization and reduces secondary NV in the OIR mouse model. Similarly, the specific importance of retinal ganglion cells (RGCs) for both physiologic and pathologic retinal angiogenesis has been elucidated by the work of Sapieha et al.146 Retinas of transgenic mice engineered to specifically lack RGCs were found to be completely devoid of a retinal vascular plexus. More important, RGCs were found to express the GPR91 receptor for the Krebs cycle intermediate succinate, which accumulates in the hypoxic retina. Knockdown of GPR91 on RGCs substantially diminished pathologic NV formation, suggesting a regulatory role for RGCs during the proliferative phase of the OIR model.146
Other Models of Pathologic Angiogenesis in the Mouse Retina
Besides the OIR mouse model, many other models replicate various aspects of pathologic angiogenesis in the mouse retina. Some of the most relevant of these models will be discussed in the following sections.
Proliferative Diabetic Retinopathy
To date, there is no perfect animal model of diabetic retinopathy (DR).36 Models using streptozotocin-induced diabetes is used or genetic models resembling type II diabetes replicate aspects of the early stages of DR—namely, leukocyte adherence, capillary dropout, and pericyte loss—but do not proceed to the proliferative stages of DR.147–150 Many insightful reviews cover these models of non-PDR.151–154 However, as these models lack the proliferative stage seen in diabetic patients, results from other animal models of proliferative retinopathies have frequently been extrapolated to human PDR.36 One of the most frequently used models of proliferative retinopathies is the OIR mouse model. Although OIR mice do not exhibit the metabolic changes associated with DR, they share key features of human PDR. Most important, both in the OIR model and in human PDR, pathologic neovascularization develops as a consequence of earlier vessel loss.155 Similarly, breakdown of the blood–retinal barrier and plasma leakage can be observed in both the OIR model and human DR.156,157 Finally, both the OIR models and PDR show glial and neuronal damage associated with the vascular disease.145,158,159 The feasibility of this approach of using nondiabetic surrogate models for PDR has been repeatedly confirmed by clinical studies that have shown many of the same mechanisms to be involved in PDR that had earlier been identified in the OIR mouse model.160–162
Wet AMD
The mouse model of laser-induced choroidal neovascularization has been extensively used to study the pathogenesis and possible treatment of subretinal neovascularization commonly associated with the wet form of AMD.36 Different from all other model systems described in this article, the laser-CNV model induces subretinal (choroidal) neovascularization rather than neovessels originating from the intraretinal vasculature. Although laser-induced CNV has been performed in various animal species,163–165 it is now most widely used in the mouse, and important insights into both the pathogenesis of choroidal neovascularization and the general angiogenic mechanisms have been gained from the mouse model of laser-induced CNV.166,167 Work from both the Cousins and Grant groups,168–171 for example, have illustrated the role of bone-marrow–derived progenitor cells during choroidal neovascularization and a recent study from the D'Amato group172 identified genetic loci that control the size of laser-induced CNV. Similarly, the Ambati laboratory has used the laser CNV mouse model to investigate the role of macrophages and their inflammatory mediators in CNV formation.173–175 The same group also identified the chemokine CCR3 as an important contributor to laser-induced CNV formation.176 Of interest, CCR3 was found to be specifically expressed in choroidal neovascular endothelial cells from human patients with wet AMD, and blocking CCR3 in the mouse model has been found to be more effective at reducing CNV than is inhibition of VEGF.176
In addition to the laser-induced model of CNV formation, several genetic models have been described that replicate aspects of AMD. The Ambati group reported that mice deficient in the macrophage chemokine CCL2 or its receptor CCR2 develops cardinal features of AMD.177 This was the first mouse model of spontaneous AMD and promoted the concept that inflammation is critical in AMD pathogenesis. This work also spurred later reports of AMD-like disease in mice deficient in related macrophage chemoattractants.178,179 Work from the Chan laboratory found that ω-3 fatty acids can slow and even reverse the progression of AMD-like neovascular lesions in these models,180 thus confirming earlier results obtained in epidemiologic AMD studies181 and in the OIR mouse model.127
Retinal Vascular Occlusion and Ischemia–Reperfusion Injury
Retinal vascular occlusion in the mouse retina can be achieved by laser photocoagulation of retinal vessels. The resulting reduction in retinal blood flow leads to retinal ischemia, vascular leakage and retinal neovascularization resembling the clinical picture of patients with retinal vein occlusions.123 Scheppke et al.,182 for example, have used this model to investigate the effect of a novel VEGFR2/Src kinase inhibitor on retinal vascular permeability. Another model for impeded retinal circulation uses instillation of sterile saline into the anterior chamber to increase the intraocular pressure until retinal circulation ceases.183 After normalization of intraocular pressure, reperfusion of the retina ensues, and postischemic injury/repair mechanisms can be investigated. The Kern laboratory,184 for example, has used this model to show that death of retinal ganglion cells (RGCs) can precede vascular degeneration. Similarly, the Grant laboratory185 has shown that ischemia–reperfusion injury can be repaired by healthy, but not diabetic endothelial progenitor cells (EPCs).
Transgenic Mice Overexpressing VEGF or IGF-1
Elevated levels of VEGF have been reported both for ROP and PDR.18,186 The Campochiaro187–189 group developed a model with transgenic VEGF overexpression in the retina under the photoreceptor-specific rhodopsin promotor. With this driver, the neovascular changes mainly localize to intra- and subretinal areas of neovascularization. A mouse model with targeted very high overexpression of retinal IGF-1 resulting in very high VEGF expression has also been developed.190 This model has been found to reproduce some of the vascular changes observed in diabetic retinopathy—namely, loss of pericytes, thickening of basement membranes and microvascular abnormalities. These models overexpressing VEGF or IGF-1 can be useful to study the effects of genetic manipulation or antiangiogenic treatment in a controllable and defined in vivo system.
Conclusion
The mouse retina represents a well-characterized, readily accessible tissue for angiogenesis research. It allows the detailed investigation of both developmental and pathologic vessel growth in vivo. Because of the postnatal development of the retinal vasculature, both the formation and regression of blood vessels can be studied in a tightly controlled setting. The importance of various in vivo models using the postnatal mouse retina is emphasized by the large number of studies employing the mouse retina and their significant contribution to our understanding of the angiogenic pathomechanisms underlying some of the most sight-threatening human diseases.
Supplementary Material
Footnotes
Supported by Deutsche Forschungsgemeinschaft ([DFG] AS); the Canadian Institutes of Health Research and the Charles A. King Foundation (PS); the Knights Templar Eye Foundation, the Children's Hospital Boston Manton Center for Orphan Disease Research's Innovation Fund (MCODR) Innovation Fund, the Juvenile Diabetes Research Foundation International (JC); National Institutes of Health Grants EY008670, EY017017, EY14811, and EY017017, the Roche Foundation for Anemia Research, the V. Kann Rasmussen Foundation, Children's Hospital Boston Mental Retardation and Developmental Disabilities Research Center, National Institute of Child Health and Development Grant P01 HD18655, a Research to Prevent Blindness Senior Investigator Award, an Alcon Research Institute Award, and the MacTel Foundation (LEHS); and the William Randolph Hearst Award, the March of Dimes Foundation, Simeon Burt Wolbach Research Fellowship Fund, and National Eye Institute Grant F32 EY017789 (KMC).
Disclosure: A. Stahl, None; K.M. Connor, None; P. Sapieha, None; J. Chen, None; R.J. Dennison, None; N.M. Krah, None; M.R. Seaward, None; K.L. Willett, None; C.M. Aderman, None; K.I. Guerin, None; J. Hua, None; C. Löfqvist, None; A. Hellström, None; L.E.H. Smith, None
References
- 1.Kennedy D. Breakthrough of the year. Science 2006;314:1841. [DOI] [PubMed] [Google Scholar]
- 2.Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552–563 [DOI] [PubMed] [Google Scholar]
- 3.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007;14:179–183 [DOI] [PubMed] [Google Scholar]
- 4.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122:477–485 [DOI] [PubMed] [Google Scholar]
- 5.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 6.Mechoulam H, Pierce EA. Retinopathy of prematurity: molecular pathology and therapeutic strategies. Am J Pharmacogenomics 2003;3:261–277 [DOI] [PubMed] [Google Scholar]
- 7.Paysse EA. Retinopathy of prematurity. UpToDate Online. Available at http://www.uptodate.com/home/index.html Accessed November 3, 2009
- 8.Mitchell CA, Risau W, Drexler HC. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: a role for vascular endothelial growth factor as a survival factor for endothelium. Dev Dyn 1998;213:322–333 [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004;116:883–895 [DOI] [PubMed] [Google Scholar]
- 10.Good WV, Hardy RJ. The multicenter study of Early Treatment for Retinopathy of Prematurity (ETROP). Ophthalmology 2001;108:1013–1014 [DOI] [PubMed] [Google Scholar]
- 11.Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity: The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1993;100:230–237 [DOI] [PubMed] [Google Scholar]
- 12.Andersen CC, Phelps DL. Peripheral retinal ablation for threshold retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev 2000;CD001693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LE. Pathogenesis of retinopathy of prematurity. Acta Paediatr Suppl 2002;91:26–28 [DOI] [PubMed] [Google Scholar]
- 14.Repka MX, Tung B, Good WV, et al. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity Study (ETROP). Arch Ophthalmol 2006;124:24–30 [DOI] [PubMed] [Google Scholar]
- 15.Flynn JT. Retinopathy of prematurity. Pediatr Clin North Am 1987;34:1487–1516 [DOI] [PubMed] [Google Scholar]
- 16.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity: The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991;98:1628–1640 [DOI] [PubMed] [Google Scholar]
- 17.Repka MX, Palmer EA, Tung B. Involution of retinopathy of prematurity: Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 2000;118:645–649 [DOI] [PubMed] [Google Scholar]
- 18.Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Semin Ophthalmol 2009;24:82–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995;1:1024–1028 [DOI] [PubMed] [Google Scholar]
- 20.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 1995;92:905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 1996;114:1219–1228 [DOI] [PubMed] [Google Scholar]
- 22.Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995;15:4738–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vannay A, Dunai G, Banyasz I, et al. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res 2005;57:396–398 [DOI] [PubMed] [Google Scholar]
- 24.Young TL, Anthony DC, Pierce E, Foley E, Smith LE. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS 1997;1:105–110 [DOI] [PubMed] [Google Scholar]
- 25.Gyllensten LJ, Hellstrom BE. Experimental approach to the pathogenesis of retrolental fibroplasia. I. Changes of the eye induced by exposure of newborn mice to concentrated oxygen. Acta Paediatr Suppl 1954;43:131–148 [DOI] [PubMed] [Google Scholar]
- 26.Lobov IB, Rao S, Carroll TJ, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 2005;437:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Nathans J. An essential role for frizzled 5 in mammalian ocular development. Development 2008;135:3567–3576 [DOI] [PubMed] [Google Scholar]
- 28.Warden SM, Andreoli CM, Mukai S. The Wnt signaling pathway in familial exudative vitreoretinopathy and Norrie disease. Semin Ophthalmol 2007;22:211–217 [DOI] [PubMed] [Google Scholar]
- 29.Robitaille J, MacDonald ML, Kaykas A, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 2002;32:326–330 [DOI] [PubMed] [Google Scholar]
- 30.Toomes C, Bottomley HM, Jackson RM, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet 2004;74:721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am J Hum Genet 2004;75:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ZY, Battinelli EM, Fielder A, et al. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet 1993;5:180–183 [DOI] [PubMed] [Google Scholar]
- 33.Shastry BS, Hejtmancik JF, Trese MT. Identification of novel missense mutations in the Norrie disease gene associated with one X-linked and four sporadic cases of familial exudative vitreoretinopathy. Hum Mutat 1997;9:396–401 [DOI] [PubMed] [Google Scholar]
- 34.Qin M, Hayashi H, Oshima K, Tahira T, Hayashi K, Kondo H. Complexity of the genotype-phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Hum Mutat 2005;26:104–112 [DOI] [PubMed] [Google Scholar]
- 35.Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res 2006;25:277–295 [DOI] [PubMed] [Google Scholar]
- 36.Aguilar E, Dorrell MI, Friedlander D, et al. Ocular models of angiogenesis. Methods Enzymol 2008;444:115–158 [DOI] [PubMed] [Google Scholar]
- 37.Otani A, Slike BM, Dorrell MI, et al. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci U S A 2002;99:178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connolly SE, Hores TA, Smith LE, D'Amore PA. Characterization of vascular development in the mouse retina. Microvasc Res 1988;36:275–290 [DOI] [PubMed] [Google Scholar]
- 39.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature 2005;438:960–966 [DOI] [PubMed] [Google Scholar]
- 40.Fruttiger M. Development of the retinal vasculature. Angiogenesis 2007;10:77–88 [DOI] [PubMed] [Google Scholar]
- 41.Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci U S A 2007;104:967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Amato R, Wesolowski E, Smith LE. Microscopic visualization of the retina by angiography with high-molecular-weight fluorescein-labeled dextrans in the mouse. Microvasc Res 1993;46:135–142 [DOI] [PubMed] [Google Scholar]
- 43.Ritter MR, Aguilar E, Banin E, Scheppke L, Uusitalo-Jarvinen H, Friedlander M. Three-dimensional in vivo imaging of the mouse intraocular vasculature during development and disease. Invest Ophthalmol Vis Sci 2005;46:3021–3026 [DOI] [PubMed] [Google Scholar]
- 44.Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest 2002;109:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003;161:1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benedito R, Roca C, Sorensen I, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 2009;137:1124–1135 [DOI] [PubMed] [Google Scholar]
- 47.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007;445:776–780 [DOI] [PubMed] [Google Scholar]
- 48.Lobov IB, Renard RA, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A 2007;104:3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorrell MI, Aguilar E, Weber C, Friedlander M. Global gene expression analysis of the developing postnatal mouse retina. Invest Ophthalmol Vis Sci 2004;45:1009–1019 [DOI] [PubMed] [Google Scholar]
- 50.Dorrell MI, Otani A, Aguilar E, Moreno SK, Friedlander M. Adult bone marrow-derived stem cells use R-cadherin to target sites of neovascularization in the developing retina. Blood 2004;103:3420–3427 [DOI] [PubMed] [Google Scholar]
- 51.Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest 2004;114:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 2002;8:1004–1010 [DOI] [PubMed] [Google Scholar]
- 53.Rennel ES, Harper SJ, Bates DO. Therapeutic potential of manipulating VEGF splice isoforms in oncology. Future Oncol 2009;5:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loges S, Roncal C, Carmeliet P. Development of targeted angiogenic medicine. J Thromb Haemost 2009;7:21–33 [DOI] [PubMed] [Google Scholar]
- 55.Madan A, Penn JS. Animal models of oxygen-induced retinopathy. Front Biosci 2003;8:d1030–d1043 [DOI] [PubMed] [Google Scholar]
- 56.Chan-Ling T, Stone J. Degeneration of astrocytes in feline retinopathy of prematurity causes failure of the blood-retinal barrier. Invest Ophthalmol Vis Sci 1992;33:2148–2159 [PubMed] [Google Scholar]
- 57.Chan-Ling T, Tout S, Hollander H, Stone J. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1992;33:2128–2147 [PubMed] [Google Scholar]
- 58.Phelps DL. Reduced severity of oxygen-induced retinopathy in kittens recovered in 28% oxygen. Pediatr Res 1988;24:106–109 [DOI] [PubMed] [Google Scholar]
- 59.McLeod DS, D'Anna SA, Lutty GA. Clinical and histopathologic features of canine oxygen-induced proliferative retinopathy. Invest Ophthalmol Vis Sci 1998;39:1918–1932 [PubMed] [Google Scholar]
- 60.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci 1993;34:576–585 [PubMed] [Google Scholar]
- 61.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–111 [PubMed] [Google Scholar]
- 62.Chan CK, Pham LN, Zhou J, Spee C, Ryan SJ, Hinton DR. Differential expression of pro- and antiangiogenic factors in mouse strain-dependent hypoxia-induced retinal neovascularization. Lab Invest 2005;85:721–733 [DOI] [PubMed] [Google Scholar]
- 63.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest 2006;116:3266–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitzmann A, Leske D, Chen Y, Kendall A, Lanier W, Holmes J. Incidence and severity of neovascularization in oxygen- and metabolic acidosis-induced retinopathy depend on rat source. Curr Eye Res 2002;25:215–220 [DOI] [PubMed] [Google Scholar]
- 65.Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS 2000;4:343–347 [DOI] [PubMed] [Google Scholar]
- 66.Allegaert K, Vanhole C, Casteels I, et al. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS 2003;7:34–37 [DOI] [PubMed] [Google Scholar]
- 67.Lofqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol 2006;124:1711–1718 [DOI] [PubMed] [Google Scholar]
- 68.Hellstrom A, Ley D, Hansen-Pupp I, et al. New insights into the development of retinopathy of prematurity: importance of early weight gain. Acta Paediatr 2010;99:502–508 [DOI] [PubMed] [Google Scholar]
- 69.Lofqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol 2009;127:622–627 [DOI] [PubMed] [Google Scholar]
- 70.Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol 2009;247:831–836 [DOI] [PubMed] [Google Scholar]
- 71.Hellstrom A, Hard AL, Engstrom E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics 2009;123:e638–e645 [DOI] [PubMed] [Google Scholar]
- 72.Vanhaesebrouck S, Daniels H, Moons L, Vanhole C, Carmeliet P, De Zegher F. Oxygen-induced retinopathy in mice: amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr Res 2009;65:307–310 [DOI] [PubMed] [Google Scholar]
- 73.Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 2009;4:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 2003;121:547–557 [DOI] [PubMed] [Google Scholar]
- 75.Lange C, Ehlken C, Stahl A, Martin G, Hansen L, Agostini HT. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefes Arch Clin Exp Ophthalmol 2009;247:1205–1211 [DOI] [PubMed] [Google Scholar]
- 76.Gu X, Samuel S, El-Shabrawey M, et al. Effects of sustained hyperoxia on revascularization in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 2002;43:496–502 [PubMed] [Google Scholar]
- 77.Ozaki H, Yu AY, Della N, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 1999;40:182–189 [PubMed] [Google Scholar]
- 78.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci 2009;50:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith LE, Shen W, Perruzzi C, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 1999;5:1390–1395 [DOI] [PubMed] [Google Scholar]
- 80.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci 2008;49:1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davies MH, Stempel AJ, Powers MR. MCP-1 deficiency delays regression of pathologic retinal neovascularization in a model of ischemic retinopathy. Invest Ophthalmol Vis Sci 2008;49:4195–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A 1995;92:10457–10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higgins RD, Yu K, Sanders RJ, Nandgaonkar BN, Rotschild T, Rifkin DB. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr Eye Res 1999;18:20–27 [DOI] [PubMed] [Google Scholar]
- 84.Spierer A, Rabinowitz R, Pri-Chen S, Rosner M. An increase in superoxide dismutase ameliorates oxygen-induced retinopathy in transgenic mice. Eye 2005;19:86–91 [DOI] [PubMed] [Google Scholar]
- 85.Banin E, Dorrell MI, Aguilar E, et al. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci 2006;47:2125–2134 [DOI] [PubMed] [Google Scholar]
- 86.Stahl A, Connor KM, Sapieha P, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis 2009;12:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niesman MR, Johnson KA, Penn JS. Therapeutic effect of liposomal superoxide dismutase in an animal model of retinopathy of prematurity. Neurochem Res 1997;22:597–605 [DOI] [PubMed] [Google Scholar]
- 88.Ando A, Yang A, Mori K, et al. Nitric oxide is proangiogenic in the retina and choroid. J Cell Physiol 2002;191:116–124 [DOI] [PubMed] [Google Scholar]
- 89.Beauchamp MH, Sennlaub F, Speranza G, et al. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic Biol Med 2004;37:1885–1894 [DOI] [PubMed] [Google Scholar]
- 90.Kermorvant-Duchemin E, Sennlaub F, Sirinyan M, et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1-dependent microvascular degeneration. Nat Med 2005;11:1339–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol 2005;166:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med 2003;9:781–788 [DOI] [PubMed] [Google Scholar]
- 93.Kociok N, Radetzky S, Krohne TU, Gavranic C, Joussen AM. Pathological but not physiological retinal neovascularization is altered in TNF-Rp55-receptor-deficient mice. Invest Ophthalmol Vis Sci 2006;47:5057–5065 [DOI] [PubMed] [Google Scholar]
- 94.Yossuck P, Yan Y, Tadesse M, Higgins RD. Dexamethasone alters TNF-alpha expression in retinopathy. Mol Genet Metab 2001;72:164–167 [DOI] [PubMed] [Google Scholar]
- 95.Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate mmp-9 expression and retinal neovascularization. Lab Invest 2003;83:1637–1645 [DOI] [PubMed] [Google Scholar]
- 96.Feng Y, vom Hagen F, Pfister F, et al. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost 2007;97:99–108 [PubMed] [Google Scholar]
- 97.Feng Y, Wang Y, Pfister F, Hillebrands JL, Deutsch U, Hammes HP. Decreased hypoxia-induced neovascularization in angiopoietin-2 heterozygous knockout mouse through reduced MMP activity. Cell Physiol Biochem 2009;23:277–284 [DOI] [PubMed] [Google Scholar]
- 98.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol 2002;192:182–187 [DOI] [PubMed] [Google Scholar]
- 99.Gao G, Li Y, Gee S, et al. Down-regulation of vascular endothelial growth factor and up-regulation of pigment epithelium-derived factor: a possible mechanism for the anti-angiogenic activity of plasminogen kringle 5. J Biol Chem 2002;277:9492–9497 [DOI] [PubMed] [Google Scholar]
- 100.Geisen P, Peterson LJ, Martiniuk D, Uppal A, Saito Y, Hartnett ME. Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Mol Vis 2008;14:345–357 [PMC free article] [PubMed] [Google Scholar]
- 101.Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1996;37:290–299 [PubMed] [Google Scholar]
- 102.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A 1996;93:4851–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bainbridge JW, Mistry A, De Alwis M, et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther 2002;9:320–326 [DOI] [PubMed] [Google Scholar]
- 104.Ozaki H, Seo MS, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol 2000;156:697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A 2003;100:15859–15864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shih SC, Ju M, Liu N, Smith LE. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest 2003;112:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med 2005;353:782–792 [DOI] [PubMed] [Google Scholar]
- 108.Morita M, Ohneda O, Yamashita T, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J 2003;22:1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Junk AK, Mammis A, Savitz SI, et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2002;99:10659–10664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest 2008;118:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Inomata Y, Hirata A, Takahashi E, Kawaji T, Fukushima M, Tanihara H. Elevated erythropoietin in vitreous with ischemic retinal diseases. Neuroreport 2004;15:877–879 [DOI] [PubMed] [Google Scholar]
- 112.Stahl A, Buchwald A, Martin G, et al. Vitreal levels of erythropoietin are increased in patients with retinal vein occlusion and correlate with vitreal VEGF and the extent of macular edema. Retina In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 2009;29:789–791 [DOI] [PubMed] [Google Scholar]
- 114.Kondo T, Vicent D, Suzuma K, et al. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest 2003;111:1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hellstrom A, Engstrom E, Hard AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003;112:1016–1020 [DOI] [PubMed] [Google Scholar]
- 116.Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A 2001;98:5804–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab 2002;87:3413–3416 [DOI] [PubMed] [Google Scholar]
- 118.Lofqvist C, Chen J, Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A 2007;104:10589–10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lofqvist C, Willett KL, Aspegren O, et al. Quantification and localization of the IGF/insulin system expression in retinal blood vessels and neurons during oxygen-induced retinopathy in mice. Invest Ophthalmol Vis Sci 2009;50:1831–1837 [DOI] [PubMed] [Google Scholar]
- 120.Kielczewski JL, Jarajapu YP, McFarland EL, et al. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circ Res 2009;105:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lofqvist C, Niklasson A, Engstrom E, et al. A pharmacokinetic and dosing study of intravenous insulin-like growth factor-I and IGF-binding protein-3 complex to preterm infants. Pediatr Res 2009;65:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang KH, Chan-Ling T, McFarland EL, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci U S A 2007;104:10595–10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002;8:607–612 [DOI] [PubMed] [Google Scholar]
- 124.Friedlander M, Dorrell MI, Ritter MR, et al. Progenitor cells and retinal angiogenesis. Angiogenesis 2007;10:89–101 [DOI] [PubMed] [Google Scholar]
- 125.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 2005;24:87–138 [DOI] [PubMed] [Google Scholar]
- 126.Crawford MA, Costeloe K, Ghebremeskel K, Phylactos A, Skirvin L, Stacey F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am J Clin Nutr 1997;66:1032S–1041S [DOI] [PubMed] [Google Scholar]
- 127.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 2007;13:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sennlaub F, Valamanesh F, Vazquez-Tello A, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 2003;108:198–204 [DOI] [PubMed] [Google Scholar]
- 129.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sternberg P, Jr, Landers MB, 3rd, Wolbarsht M. The negative coincidence of retinitis pigmentosa and proliferative diabetic retinopathy. Am J Ophthalmol 1984;97:788–789 [DOI] [PubMed] [Google Scholar]
- 131.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci 2006;47:5561–5568 [DOI] [PubMed] [Google Scholar]
- 132.Lahdenranta J, Pasqualini R, Schlingemann RO, et al. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc Natl Acad Sci U S A 2001;98:10368–10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 2000;41:3117–3123 [PubMed] [Google Scholar]
- 134.Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci 2000;41:3999–4006 [PubMed] [Google Scholar]
- 135.Penn JS, Li S, Naash MI. Ambient hypoxia reverses retinal vascular attenuation in a transgenic mouse model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 2000;41:4007–4013 [PubMed] [Google Scholar]
- 136.Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. Br J Ophthalmol 2005;89:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Gooyer TE, Stevenson KA, Humphries P, et al. Rod photoreceptor loss in Rho−/− mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest Ophthalmol Vis Sci 2006;47:5553–5560 [DOI] [PubMed] [Google Scholar]
- 138.Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci 2007;48:4351–4359 [DOI] [PubMed] [Google Scholar]
- 139.Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol 2007;504:404–417 [DOI] [PubMed] [Google Scholar]
- 140.Fruttiger M, Calver AR, Kruger WH, et al. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron 1996;17:1117–1131 [DOI] [PubMed] [Google Scholar]
- 141.Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res 1997;65:555–568 [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci 1997;38:1653–1666 [PubMed] [Google Scholar]
- 143.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci 2002;43:3500–3510 [PubMed] [Google Scholar]
- 144.West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development 2005;132:1855–1862 [DOI] [PubMed] [Google Scholar]
- 145.Dorrell MI, Aguilar E, Jacobson R, et al. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 2010;58:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sapieha P, Sirinyan M, Hamel D, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med 2008;14:1067–1076 [DOI] [PubMed] [Google Scholar]
- 147.Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch Ophthalmol 1996;114:986–990 [DOI] [PubMed] [Google Scholar]
- 148.Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci 2005;46:4281–4287 [DOI] [PubMed] [Google Scholar]
- 149.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 150.Zheng L, Du Y, Miller C, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 2007;50:1987–1996 [DOI] [PubMed] [Google Scholar]
- 151.Alder VA, Su EN, Yu DY, Cringle SJ, Yu PK. Diabetic retinopathy: early functional changes. Clin Exp Pharmacol Physiol 1997;24:785–788 [DOI] [PubMed] [Google Scholar]
- 152.Feng Y, vom Hagen F, Lin J, Hammes HP. Incipient diabetic retinopathy: insights from an experimental model. Ophthalmologica 2007;221:269–274 [DOI] [PubMed] [Google Scholar]
- 153.Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol 2008;586:4401–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci 2009;14:3974–3987 [DOI] [PubMed] [Google Scholar]
- 155.Hardy P, Beauchamp M, Sennlaub F, et al. New insights into the retinal circulation: inflammatory lipid mediators in ischemic retinopathy. Prostaglandins Leukot Essent Fatty Acids 2005;72:301–325 [DOI] [PubMed] [Google Scholar]
- 156.Iliaki E, Poulaki V, Mitsiades N, Mitsiades CS, Miller JW, Gragoudas ES. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci 2009;50:4898–4904 [DOI] [PubMed] [Google Scholar]
- 157.Zhang SX, Ma JX, Sima J, et al. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol 2005;166:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fletcher EL, Downie LE, Hatzopoulos K, et al. The significance of neuronal and glial cell changes in the rat retina during oxygen-induced retinopathy. Doc Ophthalmol 2010;120:67–86 [DOI] [PubMed] [Google Scholar]
- 159.Cunha-Vaz J, Bernardes R. Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes. Prog Retin Eye Res 2005;24:355–377 [DOI] [PubMed] [Google Scholar]
- 160.Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol 1997;12:99–109 [PubMed] [Google Scholar]
- 161.Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001;42:2408–2413 [PubMed] [Google Scholar]
- 162.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 2003;19:442–455 [DOI] [PubMed] [Google Scholar]
- 163.Frank RN. Growth factors in age-related macular degeneration: pathogenic and therapeutic implications. Ophthalmic Res 1997;29:341–353 [DOI] [PubMed] [Google Scholar]
- 164.Husain D, Kim I, Gauthier D, et al. Safety and efficacy of intravitreal injection of ranibizumab in combination with verteporfin PDT on experimental choroidal neovascularization in the monkey. Arch Ophthalmol 2005;123:509–516 [DOI] [PubMed] [Google Scholar]
- 165.Ryan SJ. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans Am Ophthalmol Soc 1979;77:707–745 [PMC free article] [PubMed] [Google Scholar]
- 166.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci 2000;41:3158–3164 [PubMed] [Google Scholar]
- 167.Saishin Y, Saishin Y, Takahashi K, et al. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol 2003;195:241–248 [DOI] [PubMed] [Google Scholar]
- 168.Chan-Ling T, Baxter L, Afzal A, et al. Hematopoietic stem cells provide repair functions after laser-induced Bruch's membrane rupture model of choroidal neovascularization. Am J Pathol 2006;168:1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Espinosa-Heidmann DG, Caicedo A, Hernandez EP, Csaky KG, Cousins SW. Bone marrow-derived progenitor cells contribute to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44:4914–4919 [DOI] [PubMed] [Google Scholar]
- 170.Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44:4908–4913 [DOI] [PubMed] [Google Scholar]
- 171.Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci 2005;46:343–348 [DOI] [PubMed] [Google Scholar]
- 172.Nakai K, Rogers MS, Baba T, et al. Genetic loci that control the size of laser-induced choroidal neovascularization. FASEB J 2009;23:2235–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44:3578–3585 [DOI] [PubMed] [Google Scholar]
- 174.Sakurai E, Taguchi H, Anand A, et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44:2743–2749 [DOI] [PubMed] [Google Scholar]
- 175.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A 2006;103:2328–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 2009;460:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 2003;9:1390–1397 [DOI] [PubMed] [Google Scholar]
- 178.Combadiere C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 2007;117:2920–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tuo J, Bojanowski CM, Zhou M, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci 2007;48:3827–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tuo J, Ross RJ, Herzlich AA, et al. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol 2009;175:799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sangiovanni JP, Agron E, Meleth AD, et al. ω-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr 2009;90:1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Scheppke L, Aguilar E, Gariano RF, et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J Clin Invest 2008;118:2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harada T, Harada C, Watanabe M, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A 1998;95:4663–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci 2007;48:361–367 [DOI] [PubMed] [Google Scholar]
- 185.Caballero S, Sengupta N, Afzal A, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007;56:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol 2009;24:87–92 [DOI] [PubMed] [Google Scholar]
- 187.Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997;151:281–291 [PMC free article] [PubMed] [Google Scholar]
- 188.Tobe T, Okamoto N, Vinores MA, et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci 1998;39:180–188 [PubMed] [Google Scholar]
- 189.Ohno-Matsui K, Hirose A, Yamamoto S, et al. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol 2002;160:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ruberte J, Ayuso E, Navarro M, et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Invest 2004;113:1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.