The activities of the gelatinases MMP-2 and MMP-9 were evaluated with gel and in situ zymography to assess possible gelatinase modulation after vessel targeting therapy with anecortave acetate (AA), an antiangiogenic drug, in LHBETATAG retinal tumors. In the present study, gelatinase activity decreased after treatment with AA and correlated with a significant decrease in tumor burden. Gelatinase activity may be a useful adjuvant strategy to target retinoblastoma.
Abstract
Purpose.
Gelatinases, matrix metalloproteinase (MMP)-2, and MMP-9 are known for their importance in angiogenesis and tumor biology. The purpose of this study was to test the hypothesis that anecortave acetate (AA) decreases transgenic retinoblastoma (RB) tumor burden by modulating gelatinase activity.
Methods.
To assess the possible gelatinase modulation after AA treatment, a single subconjunctival injection of AA (300 μg) was delivered to the right eyes of 10-week-old LHBETATAG mice. Eyes were evaluated for gelatinase expression and activity by gel and in situ zymography at 24 hours, 48 hours, and 1 week after treatment.
Results.
Gel zymography of whole eye extracts and in situ zymography of retinal tumors showed strong gelatinase expression and activity within transgenic RB tumors. AA treatment in RB transgenic mice resulted in a significant decrease of gelatinase activity 1 week after AA treatment. Surprisingly, there was an initial transient upregulation of MMP-9 activity in whole eye extracts at 24 and 48 hours after AA treatment in both LHBETATAG transgenic and wild-type mice. This increase was not observed in the tumors.
Conclusions.
As suggested by our data, inhibition of gelatinase activity appears to be a mechanism of action of AA. AA treatment results in a decrease in gelatinase activity that correlates with the significant decrease in tumor burden shown by the authors' previous studies. However, the significance of the initial, transient upregulation of gelatinase by AA injection is unknown, and further studies are warranted. Combining antiangiogenic agents with multiple mechanisms of action has the potential to enhance RB tumor control.
Retinoblastoma (RB) is the most common intraocular tumor of childhood.1 Although current treatments are effective, they lead to a number of local and systemic complications.2–4 Recent research efforts have focused on developing adjunctive treatment modalities that concentrate on improving local tumor control and reducing the toxicity of systemic chemotherapy. Chemotherapy failures require either radiotherapy, with an increased risk for second cancers,5,6 or permanent removal of one or both eyes.7 Even in successful cases, current chemotherapeutic regimens produce significant morbidity, including bone marrow suppression, resulting in unplanned hospitalization, transfusion, or both in up to 75% of patients.8 Several treatment strategies are being investigated, including the use of vessel-targeting therapy and glycolytic inhibitors. Vessel-targeting therapy has been shown to be an effective treatment for reducing tumor burden in the LHBETATAG mouse model of RB and is promising as future translational adjuvant therapy.9–12 Our recent studies using this mouse model of RB have shown that advanced tumors contain regions of hypoxia that can be selectively targeted using 2-deoxy-d-glucose, a glycolytic inhibitor.13
The angiogenic capacity of RB tumors has been demonstrated,14–16 and it is correlated with invasive growth and metastasis.17–19 We have shown that a single periocular injection of the antiangiogenic agent anecortave acetate (AA) significantly reduces tumor burden in the LHBETATAG transgenic mouse model of RB.9 LHBETATAG transgenic mice develop bilateral, heritable retinal tumors with the histologic and clinical features of human RB.20 AA is a cortisene, a steroid derivative that has been shown to inhibit blood vessel growth in a number of preclinical models of angiogenesis without typical glucocorticoid side effects such as intraocular pressure elevation, cataract, and anti-inflammatory activity.21 However, the mechanism of action of AA is not fully understood. In this study, gel and in situ zymography techniques were used to asses whether gelatinase modulation may be a mechanism of tumor reduction in transgenic RB eyes treated with AA.
Methods
Animal Model
The study protocol was approved by the University of Miami School of Medicine Animal Care and Use Review Board (Miami, FL). All experiments in this study were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Transgenic LHBETATAG mice have been previously described to develop microscopic retinal tumors by age 4 weeks, small tumors by 8 weeks, medium tumors by 12 weeks, and large tumors that often fill the available globe space by16 weeks.22 These mice produce heritable ocular tumors with histologic, ultrastructural, and immunohistochemical features identical with those of human retinoblastoma.20
Periocular Injections
LHBETATAG mice were treated at 10 weeks of age, an age when small to medium-sized tumors are present. Previous studies with AA from this laboratory showed a significant reduction in tumor burden in mice treated at this age.9 Mice received a single subconjunctival injection of 300 μg AA (Alcon Pharmaceuticals, Forth Worth, TX) or vehicle to the right eye in a 20-μL volume (n = 21). Injections were delivered with a 33-gauge needle inserted into the superotemporal subconjunctival space. Control groups for the different experiments included litter-matched wild-type and LHBETATAG transgenic mice (n = 18); a control group positive for LHBETATAG retinal tumors, which received a single subconjunctival injection of 300 μg saline (n = 6); a control group negative for LHBETATAG retinal tumors, which received a single subconjunctival injection of 300 μg saline (n = 6); and the left untreated eyes of the treatment group (n = 21). After euthanatization with CO2, eyes were enucleated for analysis at different time points (24 hours, 48 hours, and 1 week after treatment).
To localize the gelatinase activity to tumoral or extratumoral locations, periocular injections of AA were performed for in situ gelatinase studies in litter-matched wild-type and LHBETATAG transgenic mice (n = 6 per group). Eyes were enucleated and evaluated by in situ gelatinase assays.
SDS-PAGE Zymography
Quantitation of MMP-2 and MMP-9 expression and activity was performed using a modified standard zymography protocol from Invitrogen (Carlsbad, CA). After enucleation of the eyes, the crystalline lens, periocular tissue, and blood vessels were removed. Serial eye sections from 10-week-old LHBETATAG mice were reviewed with confirmatory microscopic histopathology, documenting in this animal model that at the time evaluated in the study, tumor cells were not noted to the extent of the sclera. All dissections were performed with dissecting microscope to establish appropriate tissue planes for analysis. The cleaned globe was placed in an Eppendorf tube with PBS and centrifuged for 5 minutes at 4°C. The pellet was suspended in 80 μL PBS cold lysis buffer (1% Triton X-100, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 50 μM PMSF, and 1.5 μM benzamidine in PBS) and vortexed every 10 minutes for a total of 45 minutes, with the sample kept on ice. The homogenized sample was then centrifuged at −4°C for 30 minutes at 10,000 rpm. Samples were stored at −80°C until use.
Protein quantification was performed with an assay kit (BCA Protein Assay Kit; Pierce, Rockford, IL) according to the manufacturer's directions. For gel zymography, samples were normalized to contain equal amounts of protein (10 μg/lane). Briefly, loading buffer (5% SDS; 0.4 M Tris, pH 6.8; 20% glycerol; and 0.03% bromophenol blue) and PBS were added to samples before loading onto 10% gelatin precast gels (Invitrogen, Carlsbad, CA). After separation of proteins, the gels were incubated in 2.5% Triton X-100 at room temperature for 1 hour while rocking. The gels were then incubated in low-sodium gelatinase buffer (10× buffer: 5 M NaCl, 1 M Tris pH 8.0, 0.5 M CaCl2 dihydrate, 30% Brij, pH adjusted to 7.5) at 37°C for 48 hours. The gel was incubated in staining solution (0.5% Coomassie G-250, 30% methanol, 10% acetic acid) at room temperature for 1.5 hours. It was destained in a solution containing 30% methanol and 10% acetic acid for 1 hour at room temperature. Digital images were acquired by scanning the zymography gels with a flatbed scanner. Image analysis was performed using Scion Image software (National Institutes of Health, Bethesda, MD; available at http://www.nist.gov/lispix/imlab/prelim/dnld.html) to quantify the relative densities of the bands. Mouse MMP-2 and MMP-9 zymography standards were used as positive controls, as described in other studies.23–25
In Situ Zymography
After enucleation, fresh, unfixed eyes (n = 10) were embedded in optimal cutting temperature (OCT), flash frozen in liquid nitrogen, and serially sectioned (8 μm). Sections were incubated with (FITC)-labeled DQ-gelatin (fluorescein isothiocyanate-labeled dye-quenched-gelatin; 40 mg/mL; Molecular Probes, Eugene, OR) in 1× reaction buffer (Molecular Probes) for 2 hours at room temperature in a humidity chamber. DQ-gelatin is gelatin heavily labeled with FITC molecules, so its fluorescence is quenched. When DQ-gelatin is cleaved by gelatinolytic activity, fluorescent peptides are released that are visible against a weakly fluorescent background and that represent net proteolytic activity.26 Sections were rinsed three times in PBS and mounted (Gel/Mount; Biomeda Corp. Foster City, CA) reagent (Vectashield with DAPI; Vector Laboratories, Burlingame, CA) for fluorescence microscopy. To test the specificity of gelatinase activity 1 mM 1,10-phenatroline (PHEN; Sigma Aldrich, St. Louis, MO), a potent zinc chelator and a specific gelatinase inhibitor, was added simultaneously with DQ-gelatin to adjacent retinal sections. To detect background fluorescence, control sections were incubated with 1× reaction buffer or PHEN in 1× reaction buffer only.
Immunohistochemistry
Eyes were processed in sucrose (30%, 20%, and 10% for 30 minutes each), fixed with 4% paraformaldehyde (2 hours; 25°C), frozen in OCT (−80°C), and serially sectioned (8 μm). Immunohistochemical analyses were performed on the samples to measure MMP-9 activity levels with rabbit anti-MMP-9 polyclonal antibody (1:500; Abbiotec, LLC, San Diego, CA). Alexa Fluor 546 goat–anti-rabbit was used as a secondary antibody (1:500; Invitrogen). Secondary antibody was used alone as a negative control for nonspecific binding. Cell nuclei were stained for 5 minutes with 4′,6′ diamidino-2-phenylindole (DAPI, 1:5000; Invitrogen, Carlsbad, CA).
Scanning of Immunofluorescence and Image Processing
Serial cross-sections of the tumors were examined for gelatinase activity with an upright fluorescence microscope (BX51; Olympus American Inc., Melville, NY). Images were processed with image analysis software (Discovery; Image-Pro, Media Cybernetics, Bethesda, MD) at 100× magnification using different filters for the DAPI and Alexa Fluor 488 signals. Measured parameters (e.g., immunostaining intensities) were evaluated as the average from at least five different adjacent sections per tumor per eye. Results from all the different sections were averaged. Areas of interest within the LHBETATAG retinal tumors were selected blindly using DAPI staining. Only cells that had clearly labeled nuclei with DAPI were incorporated in the analyses.
Statistical Analysis
The relationship between AA-treated groups and nontreated or saline-treated control groups was assessed with repeated-measures analysis of variance and post hoc paired t-tests. Values were considered significant with P ≤ 0.05.
Results
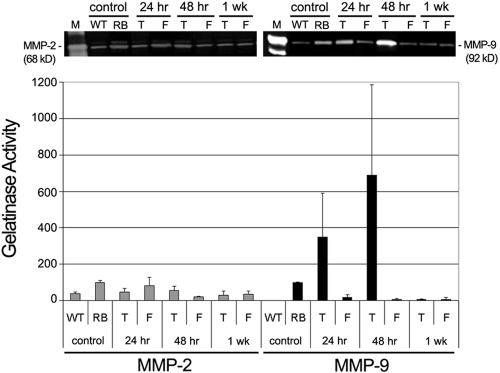
In the present study we tested the hypothesis that the antiangiogenic drug AA inhibits the upregulation of gelatinases MMP-2 and MMP-9 in tumor-positive LHBETATAG mice. MMP-2 is constitutively expressed, but there is almost no MMP-9 expression in wild-type mice. MMP-2 and MMP-9 levels are significantly increased in 10-week-old tumor-bearing LHBETATAG mice compared with wild-type littermate controls (P = 0.0063 and P < 0.001, respectively). A single subconjunctival injection of 300 μg AA resulted in a significant decrease in MMP-2 levels at all time points after injection (P = 0.045 at 24 hours; P = 0.024 at 48 hours; P = 0.031 at 1 week). AA injection also resulted in a significant decrease in MMP-9 levels at 1 week after (P < 0.001). AA injection resulted in a nonsignificant increase in MMP-9 levels at 24 and 48 hours after injection because of the variability (P = 0.21 [mean, 349; 95% confidence interval (CI), 72–626] and P = 0.11 [mean, 690; 95% CI, −107–1487], respectively). Although the 24-hour increase in MMP-9 was not statistically significant given the 95% CI provided, it suggested that MMP-9 was likely to increase. Interestingly, there was a decrease after injection in gelatinase expression and activity in the untreated fellow eyes (left eyes; P = 0.58 [mean, 82.5; 95% CI, −12.7–178], P < 0.001, and P = 0.025 at 24 hours, 48 hours, and 1 week for MMP-2 levels; P = 0.011, P = 0.0014, and P = 0.0033 at 24 hours, 48 hours, and 1 week for MMP-9 levels, respectively; Fig. 1).
Figure 1.
AA modulates MMP-2 and MMP-9 activity in LHBETATAG transgenic RB. A single subconjunctival injection of 300 mg AA was administered in the right eye (T) of the LHBETATAG transgenic RB mice, while the right fellow eye (F) remained untreated. Whole eye extracts were analyzed by gel zymography to measure MMP-2 ( ) and MMP-9 (■) activities at 24 hours, 48 hours, and 1 week after injection. Top: gelatin zymogram from a representative experiment. Mouse recombinant MMP-9 and MMP-2 were used as positive controls (M). Bottom: average results of three independent gel zymograms. Error bars represent standard deviations from the mean. WT, wild-type; RB, LHBETATAG.
) and MMP-9 (■) activities at 24 hours, 48 hours, and 1 week after injection. Top: gelatin zymogram from a representative experiment. Mouse recombinant MMP-9 and MMP-2 were used as positive controls (M). Bottom: average results of three independent gel zymograms. Error bars represent standard deviations from the mean. WT, wild-type; RB, LHBETATAG.
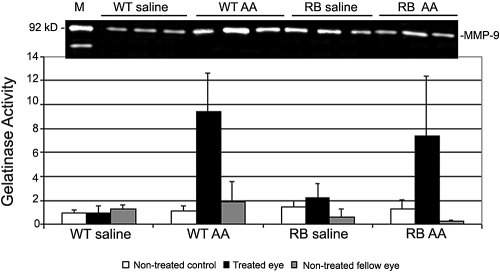
To determine whether the increase in MMP-9 expression and activity at 24 and 48 hours after AA injection in RB mice was caused by the injection, saline was used as a vehicle control. MMP-9 activity was higher in both wild-type and LHBETATAG AA-treated groups (P = 0.010 and P = 0.057, respectively) compared with either nontreated controls (P = 0.28 for wild-type and P = 0.31 for LHBETATAG), or nontreated fellow eye (P = 0.57 for wild-type and P = 0.42 for LHBETATAG; Fig. 2).
Figure 2.
MMP-9 expression and activity in LHBETATAG mice 24 hours after AA treatment. Top: gelatin zymogram from a representative experiment. Mouse recombinant MMP-2 and MMP-9 were used as positive controls. Bottom: average results of three independent gel zymograms. Saline was used as a vehicle control in both wild-type (WT) and LHBETATAG (RB) mice. Bars represent gelatinase activity relative to untreated controls. Error bars represent standard deviations from the mean.
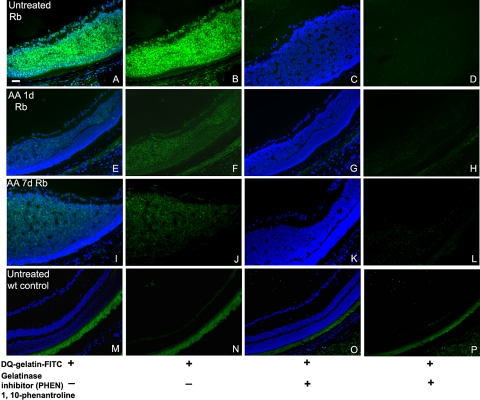
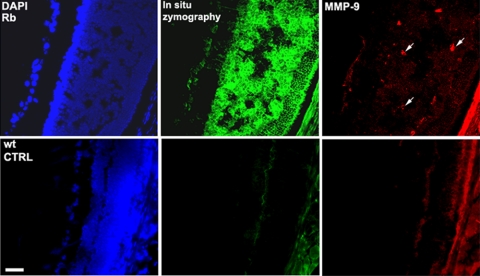
To assess whether the increase in gelatinase activity occurred within the retinal tumors, in situ zymography was performed on frozen sections (Fig. 3). Eye sections from LHBETATAG and control mice were incubated with the gelatinase substrate, DQ-gelatin FITC conjugate. The substrate is gelatin heavily labeled with FITC molecules so its fluorescence is quenched. When DQ-gelatin FITC is cleaved by the gelatinolytic activity in the tissue, fluorescent peptides are released that are visible against a weakly fluorescent background. As shown in Figures 3A and 3B, retinal tumors in nontreated LHBETATAG mice contained high levels of gelatinase activity. Tumor sections incubated with only the reaction buffer, without the fluorescent DQ-gelatin substrate, do not show gelatinolytic activity (data not shown). To test the specificity of the gelatinase activity in the tissue, we added a gelatinase inhibitor, PHEN, to the sections incubated with DQ-gelatin. Visible activity was reduced after treatment with PHEN; no fluorescent peptides were produced (Figs. 3C, 3D). There was no specific gelatinase activity in wild-type untreated eyes (Figs. 3M–P). In contrast to the early upregulation in gelatinase activity detected by gel zymography of whole eyes, tumor-specific gelatinase activity decreased at both 24 hours (Figs. 2E, 2F), and 1 week after injection of AA (Figs. 2I, 2J) compared with nontreated controls (Figs. 2A, 2B). This result suggests that the increase in gelatinase activity after AA injection does not localize to the tumor. Retinal tumors in the LHBETATAG mice show increases not only in gelatinolytic activity but also in MMP-9 expression, as shown by immunohistochemistry with a specific anti–MMP-9 antibody (Fig. 4).
Figure 3.
Gelatinase activity is decreased by AA treatment in RB tumors in LHBETATAG transgenic mice. Retinal tumors in saline-treated eyes contained high levels of gelatinase activity. The activity was reduced after treatment with the gelatinase inhibitor PHEN. Tumor-specific gelatinase activity decreased at both 24 hours and 1 week after injection.
Figure 4.
Retinal tumors in LHBETATAG mice have increased MMP-9 expression and gelatinolytic activity. Green: gelatinolytic activity; red: MMP-9 expression.
Discussion
Gelatinases are critically involved in the pathogenesis of numerous tumors.27,28 MMP-9, in particular, is linked to the progression of many tumors and is being evaluated as a biomarker for disease activity and as a potential therapeutic target.29,30 Gelatinases degrade the extracellular matrix (ECM), allowing the release of angiogenic factors and growth factors stored in the matrix. By degrading the ECM, gelatinases enable tumor cells and angiogenic endothelial cells to migrate, promoting tumor development, angiogenesis, and metastasis.27 MMPs play a role in the complex and highly dynamic process of angiogenesis. An initial response to locally produced angiogenic factors is followed by a rapid upregulation of ECM proteases, such as gelatinases and urokinase plasminogen activator [uPa]), which facilitate the breakdown of the capillary basal lamina. Novel blood vessels sprout through these openings in the basal lamina by endothelial cell proliferation and migration.27
In the present study, by using gel and in situ zymography as well as immunohistochemistry with specific anti–MMP-9 antibodies, we demonstrated that MMP-2 and, especially, MMP-9 are strongly u-regulated and that their activity is increased during tumorigenesis in LHBETATAG retinal tumors compared with wild-type littermate controls. This upregulation localizes to the retinal tumors and is successfully abrogated 1 week after a single 300-μg periocular injection of the antiangiogenic drug AA. In correlation with the current results, our laboratory previously demonstrated that a single periocular injection of the same dose of AA significantly reduced RB neovessels, and tumor burden 6 weeks after injection.9
Penn and others21,27 showed that AA treatment in a mouse model of retinopathy of prematurity results in the increased expression of retinal plasminogen activator inhibitor 1 (PAI-1) and, therefore, the inhibition of uPA activity. Given that uPA led to the plasminogen-mediated conversion of pro-MMP to active MMP, the decreases in MMP-9 and MMP-2 activity observed in retinoblastomas in our transgenic mouse model 1 week after AA treatment were the consequence of inhibition of posttranslational modification and activation of gelatinases, induced by uPA, which is indirectly inhibited by AA. Moreover, a decrease in pro-MMP levels has been detected in AA-treated human umbilical endothelial cells,31 suggesting that AA can also directly inhibit gelatinase transcription in LHBETATAG mice. The modulation of tumor gelatinase activity by AA may play a significant role in tumor burden reduction and inhibition of angiogenesis.
In the present study, we have also shown that MMP-9 levels transiently increased at 24 and 48 hours after AA injection. This early, transient up-regulation of MMP activity after AA injection was an interesting finding. Gelatinase up-regulation was not localized to the tumor and was present in non–tumor-bearing wild-type controls. The upregulation of gelatinase activity might have taken place in the periocular tissues close to the injection site. Because MMPs are involved in wound healing, the increase in gelatinase activity might have been attributed to an increase in MMP-9 activity in response to the subconjunctival injection. Saline was used as a vehicle control to evaluate whether the transient upregulation of gelatinase resulted from the injection itself. Surprisingly, the injection of vehicle did not induce this upregulation of gelatinase activity. Because an equivalent gelatinase upregulation occurred in both wild-type and tumor-bearing eyes, the transient upregulation of gelatinase was likely not caused by tumor-elaborated gelatinases. It is possible that AA induces a transient recruitment of leukocytes, cells known to constitutively express high levels of MMP-9.
Furthermore, clinical application of targeted MMP therapy has not been shown to increase metastatic potential. We believe that a transient increase in MMP level may have the potential to increase local tumor growth and to enhance metastatic disease and should be viewed with some caution. However, earlier work with AA injections have demonstrated decreased RB tumor burden in animals with at least 6 weeks of follow-up after injection. Additionally, we did not detect an increase in tumor-specific gelatinase activity, and no tumor cells were invading the sclera of these eyes on microscopic evaluation. Further evaluation within this animal model may provide insight into the hypothetical risk of a transient increase in MMP-9 activity after AA injection. Nonetheless, we believe that alteration in MMP levels may have the potential to increase local tumor growth and to enhance distant metastatic disease.
As suggested by our data, inhibition of gelatinase expression and activity appears to be a mechanism of action of AA and may prove to be an effective adjuvant therapy for RB. AA has been shown to inhibit pathologic retinal angiogenesis while not significantly affecting physiologic retinal microvasculature.21 LHBETATAG retinal tumors present with a heterogeneous vasculature that may have some implications in the effect of AA-targeting therapy.32 AA treatment, as well as radiotherapy and chemotherapy, have been shown in previous studies to cause cell death in the LHBETATAG mouse tumor through apoptosis.33 These antiangiogenic effects have already been reported to inhibit a variety of intraocular tumor growth in animal models, including a murine melanoma model30 and the LHBETATAG transgenic RB model.9 Combining antiangiogenic agents with multiple mechanisms of action could potentially enhance the effectiveness of tumor control.
Footnotes
Supported by Alcon Laboratories, Inc., National Institutes of Health Grant R01 EY013629 and Center Grant P30 EY014801, and an unrestricted grant to the University of Miami from Research to Prevent Blindness, Inc.
Disclosure: M.L. Bajenaru, None; Y. Piña, None; T.G. Murray, None; C.M. Cebulla, None; W. Feuer, None; M.-E. Jockovich, None; M.-E. Marin Castaño, None
References
- 1.Tamboli A, Podgor MJ, Horm JW. The incidence of retinoblastoma in the United States: 1974 through 1985. Arch Ophthalmol 1990;108:128–132 [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol 2004;122:1316–1323 [DOI] [PubMed] [Google Scholar]
- 3.Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer 1996;77:1206–1213 [PubMed] [Google Scholar]
- 4.Rouic LL, Aerts I, Levy-Gabriel C, et al. Conservative treatments of intraocular retinoblastoma. Ophthalmology In press [DOI] [PubMed] [Google Scholar]
- 5.Eng C, Li FP, Abramson DH, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst 1993;85:1121–1128 [DOI] [PubMed] [Google Scholar]
- 6.Scott IU, Murray TG, Feuer WJ, et al. External beam radiotherapy in retinoblastoma: tumor control and comparison of 2 techniques. Arch Ophthalmol 1999;117:766–770 [DOI] [PubMed] [Google Scholar]
- 7.Chan HS, Gallie BL, Munier FL, Beck Popovic M. Chemotherapy for retinoblastoma. Ophthalmol Clin North Am 2005;18:55–63, viii [DOI] [PubMed] [Google Scholar]
- 8.Benz MS, Scott IU, Murray TG, Kramer D, Toledano S. Complications of systemic chemotherapy as treatment of retinoblastoma. Arch Ophthalmol 2000;118:577–578 [PubMed] [Google Scholar]
- 9.Jockovich ME, Murray TG, Escalona-Benz E, Hernandez E, Feuer W. Anecortave acetate as single and adjuvant therapy in the treatment of retinal tumors of LH(BETA)T(AG) mice. Invest Ophthalmol Vis Sci 2006;47:1264–1268 [DOI] [PubMed] [Google Scholar]
- 10.Escalona-Benz E, Jockovich ME, Murray TG, et al. Combretastatin A-4 prodrug in the treatment of a murine model of retinoblastoma. Invest Ophthalmol Vis Sci 2005;46:8–11 [DOI] [PubMed] [Google Scholar]
- 11.Tell S, Yi H, Jockovich ME, Murray TG, Hackam AS. The Wnt signaling pathway has tumor suppressor properties in retinoblastoma. Biochem Biophys Res Commun 2006;349:261–269 [DOI] [PubMed] [Google Scholar]
- 12.Jockovich ME, Bajenaru ML, Pina Y, et al. Retinoblastoma tumor vessel maturation impacts efficacy of vessel targeting in the LH (BETA) T (AG) mouse model. Invest Ophthalmol Vis Sci 2007;48:2476–2482 [DOI] [PubMed] [Google Scholar]
- 13.Boutrid H, Jockovich ME, Murray TG, et al. Targeting hypoxia, a novel treatment for advanced retinoblastoma. Invest Ophthalmol Vis Sci 2008;49:2799–2805 [DOI] [PubMed] [Google Scholar]
- 14.Albert DM, Tapper D, Robinson NL, Felman R. Retinoblastoma and angiogenesis activity. Retina 1984;4:189–194 [DOI] [PubMed] [Google Scholar]
- 15.Tapper D, Langer R, Bellows AR, Folkman J. Angiogenesis capacity as a diagnostic marker for human eye tumors. Surgery 1979;86:36–40 [PubMed] [Google Scholar]
- 16.Walton DS, Grant WM. Retinoblastoma and iris neovascularization. Am J Ophthalmol 1968;65:598–599 [DOI] [PubMed] [Google Scholar]
- 17.Marback EF, Arias VE, Paranhos A, Jr, Soares FA, Murphree AL, Erwenne CM. Tumour angiogenesis as a prognostic factor for disease dissemination in retinoblastoma. Br J Ophthalmol 2003;87:1224–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerimogglu H, Kiratli H, Dincturk AA, Soylemezoglu F, Bilgic S. Quantitative analysis of proliferation, apoptosis, and angiogenesis in retinoblastoma and their association with the clinicopathologic parameters. Jpn J Ophthalmol 2003;47:565–571 [DOI] [PubMed] [Google Scholar]
- 19.Rossler J, Dietrich T, Pavlakovic H, et al. Higher vessel densities in retinoblastoma with local invasive growth and metastasis. Am J Pathol 2004;164:391–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conway RM, Wheeler SM, Murray TG, Jockovich ME, O'Brien JM. Retinoblastoma: animal models. Ophthalmol Clin North Am 2005;18:25–39, vii [DOI] [PubMed] [Google Scholar]
- 21.Penn JS, Rajaratnam VS, Collier RJ, Clark AF. The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2001;42:283–290 [PubMed] [Google Scholar]
- 22.Windle JJ, Albert DM, O'Brien JM, et al. Retinoblastoma in transgenic mice. Nature 1990;343:665–669 [DOI] [PubMed] [Google Scholar]
- 23.Ho TY, Yan W, Bagnell CA. Relaxin-induced matrix metalloproteinase-9 expression is associated with activation of the NFκB pathway in human THP-1 cells. J Leukoc Biol 2007;81:1303–1310 [DOI] [PubMed] [Google Scholar]
- 24.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis 2008;29:480–490 [DOI] [PubMed] [Google Scholar]
- 25.Marin-Castano ME, Striker GE, Alcazar O, Catanuto P, Espinosa-Heidmann DG, Cousins SW. Repetitive nonlethal oxidant injury to retinal pigment epithelium decreased extracellular matrix turnover in vitro and induced sub-RPE deposits in vivo. Invest Ophthalmol Vis Sci 2006;47:4098–4112 [DOI] [PubMed] [Google Scholar]
- 26.Otsuka K, Ohshima M, Kaku M, et al. An improved assay method for fibroblast gelatinolytic enzyme. J Nihon Univer Sch Dent 1997;39:182–190 [DOI] [PubMed] [Google Scholar]
- 27.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metast Rev 2006;25:9–34 [DOI] [PubMed] [Google Scholar]
- 28.Adithi M, Nalini V, Kandalam M, Krishnakumar S. Expression of matrix metalloproteinases and their inhibitors in retinoblastoma. J Pediatr Hematol Oncol 2007;29:399–405 [DOI] [PubMed] [Google Scholar]
- 29.Gossage JA, Humphries J, Modarai B, Burnand KG, Smith A. Adenoviral urokinase-type plasminogen activator (uPA) gene transfer enhances venous thrombus resolution. J Vasc Surg 2006;44:1085–1090 [DOI] [PubMed] [Google Scholar]
- 30.de Franceschi L, Malpeli G, Scarpa A, et al. Protective effects of S-nitrosoalbumin on lung injury induced by hypoxia-reoxygenation in mouse model of sickle cell disease. Am J Physiol 2006;291:L457–L465 [DOI] [PubMed] [Google Scholar]
- 31.Clark AF, Mellon J, Li XY, et al. Inhibition of intraocular tumor growth by topical application of the angiostatic steroid anecortave acetate. Invest Ophthalmol Vis Sci 1999;40:2158–2162 [PubMed] [Google Scholar]
- 32.Pina Y, Boutrid H, Schefler A, et al. Blood vessel maturation in human and LHBETATAG mouse model retinoblastoma tumors: spatial distribution of neovessels and mature vessels and its impact on ocular treatment. Invest Ophthalmol Vis Sci 2009;50:1020–1024 [DOI] [PubMed] [Google Scholar]
- 33.Jockovich ME, Suarez F, Alegret A, et al. Mechanism of retinoblastoma tumor cell death after focal chemotherapy, radiation, and vascular targeting therapy in a mouse model. Invest Ophthalmol Vis Sci 2007;48:5371–5376 [DOI] [PubMed] [Google Scholar]