High and low perfusion pressures and blood pressures are associated with a higher prevalence of open-angle glaucoma in adult Latinos.
Abstract
Purpose.
To examine the cross-sectional relationship between blood pressure, perfusion pressure, and prevalence of open angle glaucoma (OAG) in an adult Latino population.
Methods.
Participants aged 40 years and older (N = 6130) from the Los Angeles Latino Eye Study (LALES), a large, population-based study of self-identified adult Latinos, underwent an interviewer-administered questionnaire and a complete ocular and clinical examination. Logistic regression was used to evaluate the covariate-adjusted association of OAG with systolic, diastolic, and mean blood pressures and perfusion pressures. Covariates included age, intraocular pressure, history of glaucoma treatment including medications and surgery, and history of blood pressure and treatment of blood pressure including use of medications.
Results.
Low systolic (odds ratio [OR] = 2.5), diastolic (OR = 1.9), and mean (OR = 3.6) perfusion pressures and low diastolic blood pressure (OR = 1.9) were associated with a higher prevalence of OAG in LALES participants. Higher systolic blood pressure and mean arterial blood pressure were associated with a higher prevalence of OAG. There was no relationship between the prevalence of OAG and the presence of a history of cardiovascular disease.
Conclusions.
Low diastolic, systolic and mean perfusion pressures, low diastolic blood pressure, and high systolic and mean arterial blood pressures are associated with a higher prevalence of OAG in adult Latinos.
The pathogenesis of open-angle glaucoma (OAG) remains unknown. Although elevated intraocular pressure (IOP) is thought to play a major role in the development and progression of the disease, it is generally well understood that other factors, particularly those affecting the blood supply to the optic nerve head may play a significant role. Numerous studies have been conducted to investigate the relationship between OAG and vascular factors such as systemic hypertension, hypotension, atherosclerosis, and vasospasm.1–9 Study results, however, are conflicting, and the impact of high or low blood pressure on the development of OAG is still not fully understood. According to the vascular theory of OAG pathogenesis, low blood pressure (BP), particularly in the face of elevated IOP, can reduce perfusion pressure (PP) at the optic nerve head, causing ischemic damage to the retinal ganglion cells.10,11 In cases of chronically elevated BP, on the other hand, increased peripheral resistance and small-vessel disease can also reduce perfusion of the optic nerve head. In addition, IOP and BP are positively correlated4,12,13 resulting in further difficulty in interpreting the existing data and understanding the relationship between OAG and BP.
The objective of this study was to evaluate the cross-sectional relationship between BP, PP, and OAG in the Los Angeles Latino Eye Study (LALES). LALES is a population-based study of Latinos in Los Angeles County that was initiated to better understand health care and eye disease in this minority population.14
Methods
Design
The study population consisted of self-identified Latinos (primarily Mexican American), aged ≥40 years, living in the city of La Puente, California. The University of Southern California's Institutional Review Board approved the study, and all procedures were in accord with the tenets of the Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all study participants. Details of the study design, sampling plan, and baseline data are reported elsewhere.14 An in-home questionnaire and a complete clinical and ocular examination was administered to all eligible participants (N = 7789). In LALES, 82% (n = 6357) of those eligible persons completed the clinical examination.14 Participants with incomplete data (n = 227) were excluded from the analyses. Thus, our cross-sectional study cohort consisted of 6130 consecutively enrolled adult Latinos who had completed an in-home interview and a complete clinical and eye examination.
Clinical Procedures and Definitions
After informed consent was obtained, the participants underwent a complete ophthalmic examination, including visual acuity (VA) measurement, refraction, and slit lamp examination, as detailed elsewhere.14 Three measurements of IOP were obtained by Goldmann applanation tonometry (Haag-Streit, Bern, Switzerland) and averaged to yield a single value for each eye.
In addition, an interviewer-administered questionnaire was used to obtain demographic, ocular, and medical histories. Information pertinent to this study included a history of cardiovascular disease including a history of angina, myocardial infarction, heart failure, and cerebrovascular accident. Participants were asked whether they had a history of glaucoma and whether they had been (or were currently being) treated with medications or laser or incisional surgery. Participants were also asked whether they had been told by a physician that they had elevated BP and were being treated for it, including the use of BP-lowering medications.
BP was measured by random zero sphygmomanometer with the participant in the sitting position. Two consecutive measurements of systolic and diastolic BP were obtained, and the average was used in the analysis. Hypertension was defined as systolic BP ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg and/or current antihypertension therapy. Borderline hypertension was defined as SBP between 120 and 140 and/or DBP between 80 and 90. Hypotension was defined as SBP ≤90 and/or DBP ≤60. Mean arterial BP (MABP) was defined as ⅓SBP + ⅔DBP. Systolic, diastolic, and mean PPs were defined as systolic, or diastolic or mean arterial blood pressure minus IOP, respectively.
The criteria for diagnosing glaucoma in LALES are described in detail elsewhere.15 In brief, definite OAG was defined as the presence of an open angle, with evidence of characteristic or compatible glaucomatous optic nerve damage on stereoscopic fundus photography and congruent, characteristic, or compatible glaucomatous visual field (VF) in at least one eye of a participant. Probable OAG was defined as the presence of an open angle and one of the following four criteria: (1) end-stage disease with VA of ≤20/200 and a cup–disc ratio of 1.0, with absence of VF data; (2) at least one abnormal VF test result with characteristic/compatible glaucomatous VF defects and no evidence of optic disc damage; (3) characteristic/compatible glaucomatous optic disc damage with no evidence of VF abnormality; and (4) other combinations of VF (lack of perfect congruence between the two or three VFs) and optic disc abnormalities that are both compatible with glaucomatous damage. The IOP level was not considered in establishing the diagnosis of OAG, and, thus, no differentiation was made between low- and high-tension OAG. Two glaucoma specialists used a stereoscopic viewer (Asahi viewer; Pentax, Englewood, CO) to examine simultaneous stereoscopic optic disc photographs and characterize optic nerve findings in terms of vertical and horizontal cup-disc ratios, cup-disc ratio asymmetry between the two eyes, disc and peripapillary nerve fiber layer hemorrhage, peripapillary atrophy, diffuse thinning of the neural rim (remaining neural rim <0.1), and notching of the neural rim (remaining neural rim in a localized area <0.1). Glaucomatous optic nerve damage was classified as characteristic if it met two or more of the following criteria and compatible if it met one of the following: horizontal or vertical cup-disc ratio ≥0.8, notching of the neural rim, localized or diffuse loss of the neural rim with a maximum remaining neural rim of <0.1, or nerve fiber layer defect in the arcuate bundles. Furthermore, two glaucoma specialists graded the VF loss as characteristic or compatible with glaucoma; caused by other nonglaucomatous/neurologic disease or artifact; or not determinable or not applicable based on the optic disc evaluation, clinical examination data, and evaluation of disc and fundus photographs. VF defects corresponding to the nerve fiber bundle pattern, which included nasal steps (either superior or inferior, but not both), paracentral defect, arcuate defect, and central island, temporal island, and absolute defect were defined as characteristic of glaucoma. VF defects that conformed to nerve fiber bundle loss but deviated in some manner from the characteristic defects, including altitudinal loss, both superior and inferior nasal steps, and defects with fair convergence, including a VF defect present in one VF but not in the second VF test (defects in the nasal, arcuate, or paracentral regions) were defined as compatible with glaucoma.
Statistical Analysis
The study sample was divided into cases (OAG) and controls (no OAG). Frequencies of cases and controls across various strata for each demographic characteristic were calculated. Covariate-adjusted logistic regression analyses were performed to estimate odds ratios (ORs) for having OAG across: (1) different classifications of SBP and DBP (hypotension, borderline hypertension, and hypertension); (2) different categories of SBP and DBP; and (3) different categories of SPP, DPP, and MPP. BPs and PPs were collapsed into categories of 10 and 20 mm Hg, respectively, so that trends across categories could be evaluated more easily. Covariates included age, IOP, history of glaucoma treatment, and history of elevated BP (including history of medical treatment). The Wald test was performed to analyze the trend of ORs across the various categories of each variable. Logistic regression was also used to assess the relationship between the prevalence of OAG and cardiovascular factors such as histories of angina, myocardial infarction, CVA/brain hemorrhage, and heart failure, after adjustment for age, IOP, and BP.
LOWESS (locally weighted regression) plots of the predicted prevalence of OAG and the level of BP and PP were created (Stata, ver. 9.0; StatSoft, College Station, TX), after adjusting for the covariates mentioned above. The covariate-adjusted predicted prevalance of OAG were derived for each independent variable (BP or PP) by logistic regression and tested for goodness-of-fit against the actual data with the Hosmer-Lemeshow goodness-of-fit test (SAS, ver. 9.1; SAS Institute, Cary, NC).
Results
Of the 6130 participants with a complete set of data pertaining to this study, 287 (4.7%) met the LALES criteria for definite or probable OAG and 5843 participants had no OAG. Detailed demographic and clinical characteristics of this study population have been published15 and are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics Study Stratified by Presence of Open-Angle Glaucoma (OAG)
| OAG n (%) | No OAG n (%) | |
|---|---|---|
| Age, y (mean ± SD) | 65.4 (11.7) | 54.4 (10.6) |
| 40–49, n (%) | 31 (10.7) | 2332 (39.9) |
| 50–59 | 54 (18.2) | 1799 (30.8) |
| 60–69 | 88 (30.3) | 1107 (18.9) |
| >70 | 117 (40.3) | 613 (10.5) |
| Sex, n (%) | ||
| Male | 138 (47.6) | 2419 (41.3) |
| Female | 152 (52.4) | 3432 (58.7) |
| Hypertension, n (%)* | ||
| Yes | 137 (47.8) | 1633 (28.0) |
| No | 150 (52.2) | 4210 (72.0) |
| History of elevated BP (including history of medical treatment), n (%)* | ||
| Yes | 136 (47.4) | 1721 (29.5) |
| No | 151 (52.6) | 4108 (70.5) |
| SBP, mm Hg (mean ± SD) | 133.5 (23.8) | 122.9 (19.0) |
| DBP, mm Hg (mean ± SD) | 76.4 (12.2) | 75.7 (11.0) |
| IOP, mm Hg (mean ± SD) | 17.3 (5.4) | 14.4 (3.5) |
Total OAG, n = 290; No OAG, n = 5851. BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; IOP, intraocular pressure.
Missing data (OAG/No OAG): hypertension (3/8), history of elevated BP (3/22).
Tests of trend revealed that both SBP and DBP differed significantly across strata with respect to IOP (data not shown). IOP was higher in persons with higher BP; this relationship was significant for both SBP and DBP (P < 0.001) and was independent of age. A 10-mm Hg higher SBP was associated with a 0.33-mm Hg higher IOP, and a 10 mm Hg higher DBP was associated with a 0.44-mm Hg higher IOP. Both IOP and SBP were higher in older Latinos than in younger Latinos (P < 0.001). Diastolic blood pressure was not associated with age (P = 0.38).
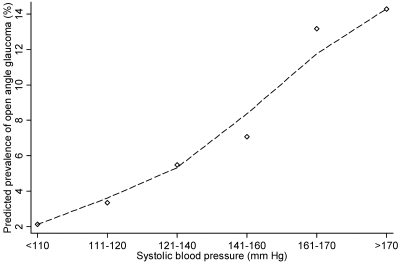
Analysis of the covariate-adjusted association between OAG and conventional broad categories of hypotension, borderline hypertension, and hypertension yielded no significant relationship between hypertension (as defined conventionally) and the prevalence of OAG (data not shown). Further evaluation of the covariate-adjusted association of OAG across ranges of BP, however, revealed a significant association between a higher prevalence of OAG and higher SBP and MBP (P < 0.01, Wald test; Table 2). Those with SBP higher than 160 mm Hg or a MABP higher than 110 mm Hg had a higher prevalence of OAG than those with lower BP (OR ≥ 2.0 and 1.6, respectively). Those with lower DBP (≤60 mm Hg) were also had a higher prevalence of OAG (Table 2, OR = 1.9). Figures 1, 2, and 3 show the relationships between the predicted probability of OAG and SBP, DBP, and MABP, respectively. The probability of a higher prevalence of OAG was higher at higher levels of SBP and MABP. However, although the prevalence of OAG was higher on average at both higher and lower levels of DBP, there was wide variability in the likelihood of having OAG across study participants. The relationship between higher DBP and prevalence of OAG is not statistically significant.
Table 2.
Frequency Distributions and Odds Ratios (ORs) for the Relationship between Blood Pressure and Prevalence of Open-Angle Glaucoma (OAG) in the Los Angeles Latino Eye Study
| OAG n (%) | No OAG n (%) | OR* | |
|---|---|---|---|
| SBP, mm Hg | |||
| ≤110 | 36 (12.6) | 1534 (26.3) | 0.84 (0.5–1.4) |
| 111–120 | 50 (17.4) | 1422 (24.3) | 1.0 (reference) |
| 121–140 | 109 (37.6) | 1905 (32.6) | 1.2 (0.8–1.7) |
| 141–160 | 58 (34.1) | 764 (13.0) | 1.1 (0.7–1.7) |
| 161–170 | 17 (5.9) | 115 (2.0) | 2.0 (1.01–4.0) |
| >170 | 17 (5.9) | 103 (1.8) | 2.1 (1.1–4.0) |
| Total | 287 (100) | 5843 (100) | — |
| DBP, mm Hg | |||
| ≤60 | 33 (11.5) | 446 (7.6) | 1.9 (1.1–3.0) |
| 61–70 | 61 (21.3) | 1386 (23.7) | 1.1 (0.8–1.6) |
| 71–80 | 86 (30.0) | 2197 (37.6) | 1.0 (reference) |
| 81–90 | 75 (26.1) | 1323 (22.6) | 1.4 (1.0–1.9) |
| 91–100 | 23 (8.0) | 388 (6.6) | 1.5 (0.9–2.5) |
| >100 | 9 (3.1) | 103 (1.8) | 2.0 (0.8–4.7) |
| Total | 287 (100) | 5843 (100) | — |
| MBP, mm Hg | |||
| ≤70 | 7 (2.4) | 201 (3.4) | 1.0 (0.3–2.7) |
| 71–80 | 33 (11.4) | 848 (14.5) | 1.2 (0.7–1.8) |
| 81–90 (reference) | 70 (24.1) | 1835 (31.4) | 1.0 (reference) |
| 91–100 | 93 (32.1) | 1734 (29.6) | 1.2 (0.9–1.7) |
| 101–110 | 46 (16.0) | 822 (14.1) | 1.1 (0.8–1.7) |
| >110 | 38 (13.3) | 402 (6.9) | 1.6 (1.1–2.6) |
| Total | 287 (100) | 5843 (100) | — |
Total OAG, n = 290; No OAG, n = 5851. BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; IOP, intraocular pressure.
Based on logistic regression models and adjusted for age, IOP, history of glaucoma treatment, history of elevated BP and treatment of BP (including use of BP lowering medications). Data in bold are statistically significant.
Figure 1.
The relationship between systolic blood pressure (SBP) and the predicted prevalence of open-angle glaucoma (OAG) in participants in the Los Angeles Latino Eye Study. The locally weighted regression plot illustrates the independent relationship between SBP and the prevalence of OAG after adjustment for covariates.
Figure 2.
The relationship between diastolic blood pressure (DBP) and the predicted prevalence of open-angle glaucoma (OAG) in participants of the Los Angeles Latino Eye Study. The locally weighted regression plot illustrates the independent relationship between DBP and prevalence of OAG after adjustment for covariates.
Figure 3.
The relationship between mean arterial blood pressure (MABP) and the predicted prevalence of open-angle glaucoma (OAG) in participants of the Los Angeles Latino Eye Study. The locally weighted regression plot illustrates the independent relationship between MABP and prevalence of OAG after adjustment for covariates.
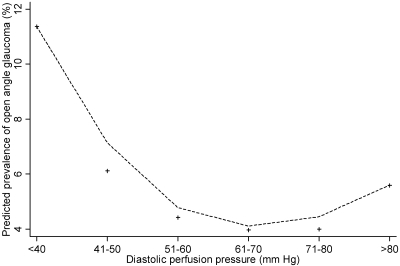
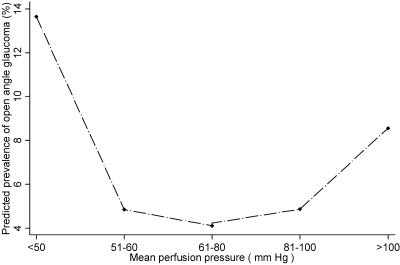
We also evaluated the covariate-adjusted relationship between PP and the prevalence of OAG. Low SPP, DPP, and MPP were all significantly associated with a higher prevalence of OAG (Table 3). Those with a MPP of 50 mm Hg or less were nearly four times more likely to have OAG than those with a mean PP higher than 50 mm Hg. Those with a high SPP (>150 mm Hg) also had a higher prevalence of OAG. There was a trend toward an association of OAG with higher MPP, although it did not reach statistical significance. Figures 4 and 5 show plots of the predicted probability of OAG in relation to DPP and MPP, respectively. The prevalence is linearly higher in persons with lower PP.
Table 3.
Frequency Distributions and Odds Ratios (ORs) for the Relationship between Perfusion Pressure and Prevalence of Open-Angle Glaucoma (OAG) in the Los Angeles Latino Eye Study
| OAG n (%) | No OAG n (%) | OR* | |
|---|---|---|---|
| SPP, mm Hg | |||
| ≤80 | 12 (4.2) | 222 (3.8) | 2.5 (1.2–5.2) |
| 81–90 | 23 (8.0) | 625 (10.8) | 1.5 (0.8–2.7) |
| 91–100 | 31 (10.8) | 1246 (21.5) | 1.0 (reference) |
| 101–120 | 112 (39.2) | 2387 (41.1) | 1.2 (0.8–1.9) |
| 121–140 | 70 (24.5) | 987 (17.0) | 1.4 (0.8–2.2) |
| 141–150 | 18 (6.3) | 183 (3.2) | 1.5 (0.8–3.0) |
| >150 | 20 (7.0) | 154 (2.7) | 2.0 (1.1–3.8) |
| Total | 286 (100) | 5804 (100) | |
| DPP, mm Hg | |||
| ≤40 | 20 (7.0) | 158 (2.7) | 1.9 (1.1–3.4) |
| 41–50 | 46 (16.1) | 694 (12.0) | 1.1 (0.8–1.7) |
| 51–60 | 86 (30.1) | 1839 (31.7) | 1.0 (reference) |
| 61–70 | 83 (29.0) | 1992 (34.3) | 0.9 (0.7–1.3) |
| 71–80 | 36 (12.6) | 867 (14.9) | 0.9 (0.6–1.4) |
| >80 | 15 (5.2) | 254 (4.4) | 1.3 (0.7–2.4) |
| Total | 286 (100) | 5804 (100) | |
| MPP, mm Hg | |||
| ≤ 50 | 9 (3.2) | 58 (1.0) | 3.6 (1.5–8.3) |
| 51–60 | 20 (7.0) | 376 (6.5) | 1.3 (0.8–2.1) |
| 61–80 | 144 (50.3) | 3325 (57.3) | 1.0 (reference) |
| 81–100 | 94 (32.9) | 1841 (31.7) | 0.9 (0.7–1.3) |
| >100 | 19 (6.6) | 204 (3.5) | 1.5 (0.9–2.6) |
| Total | 286 | 5804 (100) |
Total OAG, n = 290; No OAG, n = 5851. SPP, systolic perfusion pressure; DPP, diastolic perfusion pressure; MPP, mean perfusion pressure.
Based on logistic regression models and adjusted for age, IOP, history of glaucoma treatment, history of elevated blood pressure (including history of medical treatment). Data in bold are statistically significant.
Figure 4.
The relationship between diastolic perfusion pressure (DPP) and the predicted prevalence of open-angle glaucoma (OAG) in participants of the Los Angeles Latino Eye Study. The locally weighted regression plot illustrates the independent relationship between DPP and prevalence of OAG after adjustment for covariates.
Figure 5.
The relationship between mean perfusion pressure (MPP) and the predicted prevalence of open-angle glaucoma (OAG) in participants of the Los Angeles Latino Eye Study. The locally weighted regression plot illustrates the independent relationship between MPP and prevalence of OAG after adjustment for covariates.
In addition, we evaluated the association of OAG with other cardiovascular factors that commonly occur in those with elevated BP, such as angina, myocardial infarction, heart failure, and stroke. After adjustment for other risk factors associated with OAG in LALES, we found that none of these factors had any significant relationship with the prevalence of OAG (data not shown).
Discussion
The relationship between BP, PP, and OAG is poorly understood. There are several large epidemiologic studies in which this relationship has been investigated, most with conflicting reports.16 The Egna-Neumarkt,1 Rotterdam,3 and Blue Mountain Eye5 studies have reported significant associations between high systemic BP and OAG in their cross-sectional data, whereas the Barbados Eye17 and the Proyecto Ver18 studies have failed to demonstrate a significant relationship. Also, the longitudinal data from the Barbados Eye Study19 and the Early Manifest Glaucoma Trial20 do not support any association between elevated BP and incidence of OAG or progression of OAG. The variability in the results is not surprising, as the relationship between BP and OAG is quite complex and may be affected by numerous factors, such as the impact of BP on IOP, the use of BP- and IOP-lowering agents, the definition of hypertension, the inclusion or exclusion of IOP in the definition of OAG, the duration of hypertension, and even the variable susceptibility of people of different racial or ethnic backgrounds to the development of OAG.
The association of OAG with low PP, however, is more consistent, as most studies (cross-sectional and longitudinal) have shown a higher incidence and prevalence of OAG with lower PP.1,2,6,17,19,20 Our cross-sectional data provide some insight into this complex relationship in the Latino population.
Low Perfusion Pressure and Open-Angle Glaucoma
In our study, lower systolic, diastolic and mean perfusion pressures were associated with a higher prevalence of OAG. Our data are in agreement with the results of various other population-based, cross-sectional studies.1,2,18 The role of low PP in the development of OAG has also been confirmed in longitudinal studies. In the 4- and 9-year data from the Barbados Eye Study,17,19 low PPs were associated with higher incidence of OAG. Also, in the Early Manifest Glaucoma Trial,20 lower SPP was identified as a risk factor for the progression of OAG.
The vascular hypothesis for the development of glaucomatous optic nerve damage suggests that ischemia as a result of inadequate perfusion of the optic nerve head and the retinal ganglion cell layer is at least partly responsible. In systemic hypotension, reduction in BP causes a reduction of blood flow and PP to the optic nerve. This reduction, however, is not linear, because of the autoregulatory mechanisms that are normally in effect.21,22 At extremely low levels, however, PP can fall below the critical autoregulatory range and indeed cause a significant reduction in blood flow.21,23 Furthermore, glaucoma patients are believed to have abnormalities in their autoregulatory mechanisms that control blood flow to the optic nerve head.10,11
It is interesting to note that although we found a strong association between low PPs and higher prevalence of OAG, this association was not present in persons with low systolic and diastolic BPs. There indeed was a significant association with low DBP, but none with low SBP or MBP. This finding reflects the fact PP is not dependent on BP alone, but is influenced by IOP. Although it may be argued that the higher prevalence of OAG in those with a low PP can be explained by high IOP alone, in our analysis we controlled for IOP as well as for treatment for BP and IOP. Also, given our study results, it is likely that low PP is indeed associated with a higher prevalence of OAG, independent of the influence of IOP. Finally, low PP may be more relevant to the development of OAG than low BP (measured through the brachial artery), as PP may more closely reflect the level of blood flow to the optic nerve head.11,24
Our results, along with those of numerous other epidemiologic studies, provide evidence that low PP is an important vascular factor associated with a higher prevalence of OAG. In a recent study of patients without glaucoma, low DBP resulting from antihypertension therapy was found to be associated with increased cupping and decreased optic disc rim area, suggesting that low BP and PP can cause structural optic disc changes that may predispose to the development of glaucomatous damage.25 Thus, caution is needed in the medical management of BP, particularly in those who are either at risk of or already have glaucomatous damage. Specifically, the optic nerve and VF status of individuals with glaucoma should be monitored carefully, to avoid excessive reductions in BP and PP, particularly in those who are on antihypertension drugs.
High Blood Pressure and Open-Angle Glaucoma
We found no association between OAG and conventionally defined systemic hypertension. The data, however, are more revealing when the relationship is examined across a range of BPs rather than by arbitrary divisions and definitions. Elevated systolic and mean arterial BPs were significantly associated with a higher prevalence of OAG, independent of the impact of IOP (Table 3, Figs. 1, 3).
That there are pathophysiologic mechanisms that may be responsible for increased damage to the optic nerve head blood supply with hypertension is certainly plausible. In systemic hypertension, chronically elevated BP may result in arteriosclerosis, changes in the size of the precapillary arterioles, and capillary dropout leading to increased resistance to blood flow and thus reduced perfusion.21 Also, disruption of the autoregulatory mechanisms of blood flow in the optic nerve head vascular beds at high levels of BP may further contribute to reduced perfusion.26
Our study results are in agreement with cross-sectional data from the Egna-Neumarkt 1 and the Blue Mountains Eye5 studies, which also found an association of elevated BP with OAG but contradicts both cross-sectional and longitudinal results from the Barbados Eye Study and Early Manifest Glaucoma Trial.13,17,19,20 This difference in study results may be partially explained by the difference in the criteria used to define hypertension, the inclusion or exclusion of IOP in the definition of OAG, the impact of IOP- or BP-lowering therapy, or the variable susceptibility of people of different ancestries to OAG.
The strengths of our study in analyzing the relationship between BP and OAG include its large population-based design; exclusion of IOP in the definition of OAG; controlling for factors such as IOP, IOP treatment, BP, and its treatment; and stratification of participants by specific BP levels.
Important potential limitations of our study include the fact that BP was obtained by averaging two consecutive measurements in a single session. Given the potential variability in BP and the impact of “white coat syndrome,”27 having a single elevated BP reading may not be representative of an individual's true BP status. Questions can certainly be raised regarding the validity of the association that we found between elevated BP and OAG, although it is likely that the amount of error introduced because of the white coat syndrome would be the same for participants with and without OAG. Another important limitation in our analysis is the impact of antihypertension therapy on BP and the potential deleterious impact of intermittent hypotension induced by these medications on blood flow to the optic nerve. Our study controlled for the impact of BP-lowering therapy, but residual confounding related to the use of specific medications is certainly possible and should be considered in interpreting our data.
Given the higher prevalence of OAG in participants with elevated BP in our study, it was reasonable to explore whether a relationship existed between OAG and other cardiovascular diseases such as myocardial infarction and stroke. After controlling for other variables associated with OAG, however, our results do not support such a relationship in our population. The value of this finding should be interpreted with caution, as the number of participants with these histories in each group was small, possibly not large enough for us to draw robust inferences.
Conclusions
The findings of our cross-sectional study have demonstrated that low PP and high SBP and MBP are associated with a higher prevalence of OAG in the LALES. BP and PP are modifiable, and it is conceivable that by maintaining BP and ocular PP at physiologic levels on a long-term basis, we may be able to reduce the risk of developing glaucomatous optic nerve damage.
Footnotes
Supported by National Institutes of Health Grants NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. RV is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Disclosure: F. Memarzadeh, None; M. Ying-Lai, None; J. Chung, None; S.P. Azen, None; R. Varma, None
References
- 1.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 2000;107:1287–1293 [DOI] [PubMed] [Google Scholar]
- 2.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure and primary open-angle glaucoma. Arch Ophthalmol 1995;113:216–221 [DOI] [PubMed] [Google Scholar]
- 3.Dielemans I, Vingerling JR, Algra D, et al. Primary open-angle glaucoma, intraocular pressure and systemic blood pressure in the general elderly population. Ophthalmology 1995;102:54–60 [DOI] [PubMed] [Google Scholar]
- 4.McLeod SD, West SK, Quigley HA, Fozard JL. A longitudinal study of the relationship between intraocular and blood pressures. Invest Ophthalmol Vis Sci 1990;31:2361–2366 [PubMed] [Google Scholar]
- 5.Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: the Blue Mountains Eye Study. J Glaucoma 2004;13:319–326 [DOI] [PubMed] [Google Scholar]
- 6.Hulsman CAA, Vingerling JR, Hofman A, et al. Blood pressure, arterial stiffness and open-angle glaucoma. Arch Ophthalmol 2007;125:805–812 [DOI] [PubMed] [Google Scholar]
- 7.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 1994;117:603–624 [DOI] [PubMed] [Google Scholar]
- 8.Drance SM, Douglas GR, Wijsman K, et al. Response of blood flow to warm and cold in normal and low-tension glaucoma patients. Am J Ophthalmol 1988;105:35–39 [DOI] [PubMed] [Google Scholar]
- 9.Drance SM. Some factors in the production of low tension glaucoma. Br J Ophthalmol 1972;56:229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flammer J. The vascular concept in glaucoma. Surv Ophthalmol 1994;38:S3–S6 [DOI] [PubMed] [Google Scholar]
- 11.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21:359–393 [DOI] [PubMed] [Google Scholar]
- 12.Klein BEK, Klein R, Linton KLP. Intraocular pressure in an American community: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 1992;33:2224–2228 [PubMed] [Google Scholar]
- 13.Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol 1997;115:1572–1576 [DOI] [PubMed] [Google Scholar]
- 14.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology 2004;111:1121–1131 [DOI] [PubMed] [Google Scholar]
- 15.Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology 2004;111:1439–1448 [DOI] [PubMed] [Google Scholar]
- 16.Deokule S, Weinreb RN. Relationship among systemic blood pressure, intraocular pressure, and open-angle glaucoma. Can J Ophthalmol 2008;43:302–307 [DOI] [PubMed] [Google Scholar]
- 17.Leske MC, Connell AMS, We SY, et al. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol 1995;113:918–924 [DOI] [PubMed] [Google Scholar]
- 18.Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto Ver. Arch Ophthalmol 2001;119:1819–1826 [DOI] [PubMed] [Google Scholar]
- 19.Leske MC, Wu SY, Hennis A, et al. Risk factors for incident open-angle glaucoma. Ophthalmology 2008;115:85–93 [DOI] [PubMed] [Google Scholar]
- 20.Leske MC, Heigl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114:1965–1972 [DOI] [PubMed] [Google Scholar]
- 21.Hayreh SS. Role of nocturnal arterial hypotension in the development of ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol 1999;10:474–482 [DOI] [PubMed] [Google Scholar]
- 22.Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci 1981;21:34–38 [PubMed] [Google Scholar]
- 23.Farnett L, Mulrow CD, Lin WD, et al. The J-curve phenomenon and the treatment of hypertension: is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489–495 [PubMed] [Google Scholar]
- 24.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev 1975;55:383–417 [DOI] [PubMed] [Google Scholar]
- 25.Topouzis F, Coleman AL, Harris A, et al. Association of blood pressure status with optic disk structure in non-glaucoma subjects: The Thessaloniki Eye Study. Am J Ophthalmol 2006;142:60–67 [DOI] [PubMed] [Google Scholar]
- 26.Anderson DR. Introductory comments on blood flow autoregulation in the optic nerve head and vascular risk factors in glaucoma. Surv Ophthalmol 1999;43:S5–S9 [DOI] [PubMed] [Google Scholar]
- 27.Verdeechi P, Schillaci G, Borgioni C, et al. Prognostic significance of the white coat effect. Hypertension 1997;29:1218–1224 [DOI] [PubMed] [Google Scholar]