Abstract
A matured megakaryocyte releases thousands of platelets through a drastic morphological change, proplatelet formation (PPF). The megakaryocyte/erythrocyte-specific transcription factor, p45 NF-E2, is essential for initiating PPF, but the factor regulating PPF has not been identified. Here we report that estradiol synthesized in megakaryocytes triggers PPF. We demonstrate that a key enzyme for steroid hormone biosynthesis, 3β-hydroxysteroid dehydrogenase (3β-HSD), is a target of p45 NF-E2, and rescues PPF of p45 NF-E2-deficient megakaryocytes. We also show that estradiol is synthesized within megakaryocytes, and that extracellular estradiol stimulates PPF, inhibition of 3β-HSD activity blocks PPF, and estrogen receptor antagonists inhibit platelet production in vivo. We conclude that autocrine estradiol action regulates platelet production by triggering PPF.
Keywords: Sex steroid hormone, p45 NF-E2, proplatelet, 3β-HSD, hematopoiesis
Platelets are produced from pluripotent hematopoietic stem cells in bone marrow through a unique differentiation process (Kaushansky 1995). Thrombopoietin (TPO) stimulates the differentiation of stem cells to megakaryocytes by polyploidization and cytoplasmic maturation. At the last stage of megakaryocyte differentiation, matured polyploid (16N-128N) megakaryocytes produce several lengthy beaded cytoplasmic processes, and this drastic morphological change, called proplatelet formation (PPF), results in the release of thousands of platelets at once (Radley and Scurfield 1980; Shivdasani et al. 1995; Nagahisa et al. 1996; Italiano et al. 1999; Lecine et al. 2000; Rojnuckarin and Kaushansky 2001). It has been shown that TPO is not required for PPF (Gurney et al. 1994; Shivdasani and Orkin 1995; Shivdasani et al. 1995, 1997; Nagahisa et al. 1996; Bunting et al. 1997; Rojnuckarin and Kaushansky 2001; Oda et al. 2003), and thus the identity of another, as yet unidentified humoral factor that regulates PPF was sought. The search for this PPF-stimulating factor or shedding factor has been unsuccessful to date; thus, the mechanism of PPF and platelet release remains unresolved.
As described below, several lines of evidence suggested that an unidentified intracellular factor, whose expression is up-regulated by the megakaryocyte/erythrocyte-specific transcription factor p45 NF-E2, controls PPF. The identification of this key factor has been sought for a long time. p45 NF-E2 is expressed mainly in megakaryocyte and erythroid lineage. Although p45 NF-E2-deficient (p45-/-) mice displayed only mild red blood cell abnormality (Shivdasani and Orkin 1995), p45-/- mice produced megakaryocytes with premature cytoplasm from which platelets could not be released, and thus lacked circulating platelets and died of hemorrhage (Shivdasani et al. 1995, 1997). In TPO receptor-deficient (c-Mpl-/-) mice, the number of platelets as well as megakaryocytes was decreased (Gurney et al. 1994; Alexander et al. 1996). The megakaryocytes and platelets remaining in c-Mpl-/- mice, however, were functionally normal, and these mice did not exhibit severe bleeding abnormalities (Bunting et al. 1997). In addition, a delay between TPO injection and peak circulating platelet number has been observed in vivo (Farese et al. 1995). These observations strongly suggested that TPO is not required for PPF, but that the expression of another as yet unidentified factor is up-regulated by p45 NF-E2 in megakaryocytes, and it is this factor that triggers PPF. We therefore attempted the identification of this key factor.
Results and Discussion
Establishment of p45-/- and p45+/+ ES cells and defect of PPF in p45-/- megakaryocytes
To identify the target genes of p45 NF-E2 and to study their roles in PPF, it was essential to establish p45-/- ES cells, because most p45-/- mice die soon after birth and the required number of megakaryocytes is not available for experimentation. The genotypes of established p45-/- ES cells as well as p45+/+ ES cells were confirmed by PCR analysis (Fig. 1A). In the presence of TPO, p45-/- ES cells on OP9 stromal cells differentiated into polyploid megakaryocytes but did not display PPF (Fig. 1B, left panel), whereas the majority of p45+/+ ES-derived megakaryocytes (p45+/+ Meg) did exhibit PPF (Fig. 1B, right panel). These data are consistent with in vivo results using p45-/- mice (Shivdasani et al. 1995).
Figure 1.
Generation of p45-/- and p45+/+ ES cells and PPF assay. (A) Genomic PCR of established p45-/- and p45+/+ ES cells. Map of exon 3 region of mouse p45 NF-E2 genomic DNA and the locations of the primers used for PCR (left panel). Agarose gel electrophoresis of PCR products of p45+/+, p45+/-, and p45-/- ES cells as indicated (right panel). The primers used are 5′-GTTAACTTGCCGGTAGATGACTTT-3′ and 5′-AGACCAGCTCAATCTGTAGCCTCC-3′. (B) PPF assay of p45-/- Meg and p45+/+ Meg. p45-/- and p45+/+ ES cells were cocultured on OP9 stromal cells with TPO. (Left) p45-/- Meg, from which no proplatelet could be formed. (Right) A typical PPF produced from p45+/+ Meg.
3β-HSD is expressed onlyin p45+/+ Meg
By suppressive subtractive hybridization of cDNAs from p45-/- ES-derived megakaryocytes (p45-/- Meg) and those from p45+/+ Meg, the cDNA fragments encoding 3β-hydroxysteroid dehydrogenase (3β-HSD), which is a key regulatory enzyme of all steroid hormone biosynthesis (Abbaszade et al. 1997; Peng et al. 2002), were most frequently isolated (∼16% among the subtracted genes). The cDNAs of both 3β-HSD I and 3β-HSD VI were isolated, but the type VI isoform cDNA was the major isoform detected in a ratio of 15 to 1. To date, only two genes have been identified as targets of p45 NF-E2 in megakaryocytes (Deveaux et al. 1997; Lecine et al. 2000). One of these is a hematopoietic-specific β1-tubulin gene (Lecine et al. 2000), which was the second most abundant gene (5% of the subtracted genes) among the isolated cDNA in our subtraction system. The other is megakaryocyte/platelet differentiation marker, thromboxane synthase gene (Deveaux et al. 1997), which was less abundant among the cDNAs isolated. Several other putative p45 NF-E2 target genes were also isolated. The 3β-HSD gene is, therefore, the third gene that was identified as a p45 NF-E2 target.
Northern blot analysis clearly showed that 3β-HSD transcripts were abundant in p45+/+ Meg (Fig. 2A, lane 1), but the transcripts were completely absent in p45-/- Meg (Fig. 2A, lane 2). The transcripts of type I and VI isoforms are not distinguishable by Northern blot analysis. The use of RT-PCR followed by specific restriction enzyme digestion (Abbaszade et al. 1997) demonstrated that both type I and VI isoforms were expressed in p45+/+ Meg (Fig. 2B, left panel), whereas only the type I isoform was found in bone marrow megakaryocytes (Fig. 2B, right panel).
Figure 2.
Expression of 3β-HSD in p45+/+ Meg and bone marrow megakaryocytes but not in p45-/- Meg. (A) Expression of 3β-HSD transcripts in p45+/+ Meg but not p45-/- Meg. Northern blot analysis was performed with 3β-HSD cDNA as a probe. (Lane 1) p45+/+ Meg; (lane 2) p45-/- Meg. (B) Expression of 3β-HSD I and/or VI transcripts in p45+/+ Meg and bone marrow megakaryocytes. RT-PCR products of p45+/+ Meg (left panel) and bone marrow megakaryocytes (right panel) were digested with unique restriction enzymes, and the isoform types of 3β-HSD transcripts were determined. Roman numerals below indicate 3β-HSD isoform types. (Lane 1) Molecular weight marker. Arrows indicate the digested cDNA fragments, showing the presence of the designated isoform cDNA in PCR products. (C) Immunoblot analysis with 3β-HSD I- and VI-specific antibodies. Total cell extracts prepared from testis (lanes 2), p45+/+ undifferentiated ES cells (lanes 3), p45+/+ Meg (lanes 4), p45-/- Meg (lanes 5), and p45-/- Meg expressing 3β-HSD VI (lanes 6) were probed with anti-3β-HSD I-specific antibody (left panel). The same filter was reprobed with anti-3β-HSD VI-specific antibody (right panel). (Lane 1) Molecular weight marker.
To confirm 3β-HSD expression, we prepared anti-3β-HSD I-as well as anti-3β-HSD VI-specific antibodies. The immunoblot analysis with these specific antibodies clearly demonstrated that neither type I nor type VI isoform was present in p45-/- Meg (Fig. 2C, lanes 5), but both isoforms were expressed in p45+/+ Meg (Fig. 2C, lanes 4). In undifferentiated p45+/+ ES cells, however, neither isoform was detected (Fig. 2C, lanes 3), confirming that 3β-HSD is one of the p45 NF-E2-inducible gene products during megakaryocyte differentiation. Only the type I isoform was expressed in bone marrow megakaryocytes (data not shown). Testis extracts were used as controls (Fig. 2C, lanes 2), because it has been shown that both isoforms are expressed in testes (Baker et al. 1999). These data demonstrated that 3β-HSD is expressed only in PPF-producing megakaryocytes. We therefore proceeded to study further the role of 3β-HSD in PPF.
Expression of 3β-HSD VI gene rescues PPF of p45-/- Meg
The full-length 3β-HSD VI cDNA was transfected into p45-/- ES cells, and the transfectants were cloned by a limiting dilution. Immunoblot analysis confirmed that the cloned transfectants strongly expressed 3β-HSD VI (Fig. 2C, right panel, lane 6). Transfection of p45-/- ES cells with the 3β-HSD VI-expressing plasmid rescued PPF of p45-/- Meg (Fig. 3C), whereas the megakaryocytes derived from mock-transfected p45-/- ES cells did not display PPF (Fig. 3A,B). The p45-/- Meg exhibited two types of morphology (Fig. 3A,B), but neither type of megakaryocyte extended the cytoplasmic processes. The majority of p45-/- Meg expressing 3β-HSD VI did exhibit PPF, indicating that p45-/- Meg were induced to synthesize a steroid hormone through the expression of 3β-HSD VI and thus could regulate PPF by an autocrine mechanism. These data provide evidence that 3β-HSD is essential for PPF in megakaryocytes.
Figure 3.
Rescue of PPF ability by forced expression of 3β-HSD in p45-/- Meg and PPF inhibition by trilostane. Cells are shown at the same scale. p45-/- ES cells were transfected with 3β-HSD VI cDNA and/or β1-tubulin cDNA in expression vectors. The cloned transfectants were cocultured with OP9 cells with TPO, and megakaryocytes producing PPF were observed. (A,B) Mock-transfected p45-/- Meg, which cannot display PPF. (C) Two representative proplatelets formed from 3β-HSD VI-expressing p45-/- Meg. Arrows indicate beaded cytoplasmic processes. (D) A typical PPF of p45-/- Meg expressing both 3β-HSD VI and β1-tubulin. Arrows show long beaded cytoplasmic processes. (E) Trilostane-treated bone marrow megakaryocytes. (F) Dose-dependent inhibition of PPF by 3β-HSD-specific inhibitor, trilostane. Various concentrations of trilostane dissolved in DMSO were added to bone marrow megakaryocytes, and the number of PPFs formed was counted. Representative data (expressed as mean ± SEM of triplicate determination) from five independent experiments are shown.
Figure 3C shows two representative proplatelets formed from 3β-HSD-expressing p45-/- cells. These proplatelets can clearly be characterized as PPF; however, their cytoplasmic processes were not fully extended. Thus, the number of platelets released seemed insufficient, although beaded cytoplasmic processes ready to release platelets were observed (arrows in Fig. 3C). Forced expression of β1-tubulin in p45-/- ES cells was incapable of restoring PPF (data not shown). This is consistent with previous results obtained with p45-/- mouse fetal liver megakaryocytes (Lecine et al. 2000). Therefore, we transfected 3β-HSD VI and β1-tubulin together into p45-/- ES cells. Figure 3D shows a typical proplatelet formed from p45-/- Meg expressing both 3β-HSD VI and β1-tubulin. The proplatelets bearing several lengthy beaded cytoplasmic processes were produced as normal megakaryocytes, indicating that expression of 3β-HSD is indispensable for PPF and that, in addition, β1-tubulin is required for completing normal PPF.
3β-HSD inhibitor trilostane blocks PPF
The effect of trilostane, a known specific inhibitor of 3β-HSD (Potts et al. 1978), on PPF in megakaryocytes isolated from female and male mouse bone marrow was examined. As shown in Figure 3F, trilostane strongly blocked PPF in a dose-dependent manner, whereas the solvent (DMSO) used for dissolving the inhibitor had only a weak inhibitory effect. No gender difference was observed. Trilostane-treated megakaryocytes exhibited irregular shapes, which had several large round intracellular vesicles (Fig. 3E), similar to p45-/- Meg cells (Fig. 3A). They did not extend any cytoplasmic processes and with time underwent apoptosis. The above results also support that activity of 3β-HSD in megakaryocytes is a prerequisite for PPF, and that p45-/- Meg as a result of the induction of 3β-HSD acquire the capacity to synthesize steroid hormones. These observations indicate an autocrine mechanism in megakaryocytes leading to PPF.
Autocrine synthesis of estradiol and expression of estrogen receptor β in megakaryocytes
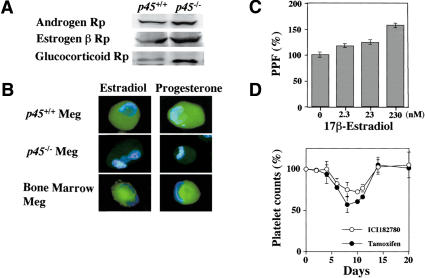
Immunoblot analysis confirmed that the receptors for androgen, estrogen (β type), and glucocorticoid were expressed in both p45+/+ Meg and p45-/- Meg (Fig. 4A), whereas the receptors for estrogen (α type) and progesterone were not detected (data not shown). The expression of steroid hormone receptors in bone marrow megakaryocytes has been described (Tarantino et al. 1994; Khetawat et al. 2000), although their function was not known.
Figure 4.
Expression of steroid hormones and their receptors in megakaryocytes, PPF stimulation by 17β-estradiol, and estrogen receptor antagonists inhibit platelet production. (A) Immunoblot analysis with antisteroid hormone receptor antibodies in megakaryocyte cell extracts. Cell extracts prepared from p45-/- Meg (right lanes) and p45+/+ Meg (left lanes) were probed with anti-androgen receptor, anti-estrogen receptor β, or anti-glucocorticoid receptor antibody, as indicated. (B) Immunostainings of megakaryocytes with anti-steroid hormone antibodies. Bone marrow megakaryocytes (lower panel), p45-/- Meg (middle panel), and p45+/+ Meg (upper panel) were stained with anti-estradiol, anti-testosterone, or anti-progesterone antibody (green) and with DAPI (blue). (C) 17β-Estradiol stimulates PPF. Various concentrations of 17β-estradiol were mixed with the culture medium of CD41+ c-Kit+ megakaryocyte progenitors with TPO for 4 d, and PPFs clearly formed were counted. Representative data (expressed as mean ± SEM of triplicate determination) from five independent experiments are shown. (D) Estrogen receptor antagonists inhibit platelet production in vivo. Mice were treated with tamoxifen (closed circles) or ICI182780 (open circles) for 9 d, and platelet counts were determined as indicated. The platelet counts in the mice treated with solvent alone (sesame oil containing 5% ethanol) are shown as 100%.
Furthermore, immunostainings of the cells demonstrated that estradiol and progesterone were actually synthesized in male as well as female mouse bone marrow megakaryocytes (Fig. 4B, lower panels) and in p45+/+ Meg (Fig. 4B, upper panels). Little estradiol and progesterone, however, was detected in p45-/- Meg (Fig. 4B, middle panels). Testosterone was not detected in any megakaryocytes (data not shown), and glucocorticoid synthesis could not be examined because of the unavailability of the antibody. Enzyme immunoassay revealed that male as well as female mouse bone marrow megakaryocytes and p45+/+ Meg, but not p45-/- Meg, secreted ∼140 ± 10 pg of estradiol per 105 cells into the medium during 44 h, and that little progesterone and no testosterone were detected in the media.
Extracellular 17β-estradiol but not testosterone, progesterone, or dexamethasone stimulates PPF
Because biosynthesis of estradiol and expression of its receptor in PPF-producible megakaryocytes was verified, we examined the effect of 17β-estradiol on PPF. Addition of 17β-estradiol to the culture medium of CD41+ c-Kit+ megakaryocyte progenitors in the presence of TPO demonstrated a clear stimulatory effect on PPF in a dose-dependent manner (Fig. 4C), suggesting that 17β-estradiol activates specific targets required for PPF or inactivates specific inhibitors for PPF within megakaryocytes. As described above, megakaryocytes can synthesize estradiol, and thus it was unexpected that the addition of extracellular 17β-estradiol could stimulate PPF. However, addition of 17β-estradiol did enhance PPF >50%, indicating that extracellular hormone could promote further the signals leading to PPF. In contrast, testosterone, progesterone, or the glucocorticoid analog, dexamethasone, had no effect on PPF (data not shown). Progesterone may be synthesized as an intermediate of estrogen, but its receptor is not expressed in megakaryocytes, so that megakaryocytes must be insensitive to progesterone. Although androgen receptor is expressed, testosterone has no effect on PPF and is not synthesized in megakaryocytes; thus, testosterone is not involved in the process of PPF, as is the case with glucocorticoid, because dexamethasone was found to be without effect.
Estrogen receptor antagonists inhibit platelet production in vivo
We next examined the effect of estrogen receptor antagonists on platelet production in vivo. ICI182780 or tamoxifen was injected daily for 9 d into mice, and platelet counts were determined in peripheral blood. The result shows that both antagonists inhibited platelet production in vivo (Fig. 4D). The platelet counts in peripheral blood began to decrease 5-6 d after initial injection, decreased further as long as the antagonist was supplied, and reached the lowest level at 8-11 d (Fig. 4D). The maximal decrease in platelet counts observed with ICI182780 and tamoxifen treatment was 25% and 45%, respectively. Following termination of the injection, platelet counts returned to the original level within 5 d. As the half-life of mouse platelets is ∼5-6 d, the delay of antagonist effect for 5-6 d suggests that the antagonists inhibited the release of newly produced platelets from the megakaryocytes into peripheral blood. These results demonstrate that estrogen receptor antagonists inhibit platelet production in vivo, and confirm that estradiol stimulates PPF and regulates platelet production.
Autocrine-synthesized estradiol triggers PPF of mature megakaryocytes
Taken together, these results support the mechanism that 3β-HSD expression is up-regulated by p45 NF-E2 during late stages of megakaryocyte differentiation, which, in turn, induces biosynthesis of estrogen in matured megakaryocytes; finally, autocrine estrogen action triggers the last stage of megakaryocyte maturation, PPF, within megakaryocytes. This sex-independent and differentiation stage-specific autocrine steroid action is an effective mechanism for platelet production, because a small quantity of estradiol can efficiently and rapidly bind to its receptor or other targets and act on PPF within the megakaryocytes in the localized microenvironment of bone marrow.
Our results demonstrate that a sex hormone, estradiol, produced in unexpected tissue hematopoietic cells regulates the last stage of its own cell differentiation by an autocrine mechanism. It was reported that progesterone synthesized in the nervous system stimulates myelin formation and neurite growth (Koenig et al. 1995, 2000). Sex steroids may play important roles in cell differentiation of various unexpected tissues in a sex-independent manner as an autocrine rather than paracrine fashion.
Several aspects of the mechanism of PPF have been described. PKCα plays an important role in PPF by altering actin dynamics (Rojnuckarin and Kaushansky 2001), and β1-tubulin is involved in PPF (Lecine et al. 2000), as also was observed in our study. These results demonstrate that regulatory components of the cytoskeleton are involved in PPF. Furthermore, overexpression of antiapoptotic protein BclxL or Bcl-2 in megakaryocytes reduced PPF (de Botton et al. 2002; Kaluzhny et al. 2002), and PPF was regulated by compartmentalized caspase 3-directed apoptosis (de Botton et al. 2002; Clarke et al. 2003). These results suggest that platelet production is an apoptotic process. Recently, it was suggested that N-methyl-D-aspartate (NMDA) receptor-mediated signaling is involved in megakaryocyte maturation and PPF (Hitchcock et al. 2003). Despite these findings, the upstream key factor triggering PPF has not been identified. We report here that estradiol synthesized in megakaryocytes is the factor regulating PPF. As in the case of a “nongenomic” action described in the central nervous system and cardiovascular system (McEwen et al. 2001; Simoncini and Genazzani 2003), estradiol may modulate NMDA receptor as a membrane receptor, transduce signals leading to PPF, and rapidly and effectively respond to the requirement of platelet production. There is now convincing evidence that paracrine-synthesized estradiol can modulate the functions of neural and vascular cells via nongenomic actions (Kelly and Levin 2001). The rapid response of estradiol as well as the rapid inhibition of 3β-HSD indicates a nongenomic action, suggesting that this system may function when an emergency requires a quick platelet production. Alternatively, estradiol may also act by binding to the nuclear receptor and up-regulate specific factors activating PPF or down-regulate specific inhibitor of PPF as a “genomic” action, as suggested by the detection of estrogen receptor β in megakaryocytes. In this case, a lag time is required until the action occurs, and thus this system may effectively function during nonemergency megakaryocyte differentiation. Both nongenomic and genomic actions may work together or cross-talk with each other in platelet production in vivo. Further studies are required for clarifying how autocrine steroid action correlates with the previous observations described above, and which gene products are up-regulated or down-regulated by estrogen receptor β in megakaryocytes as a genomic action.
Potential clinical applications of estradiol and 3β-HSD inhibitor/activator
Platelet transfusions are presently the only established therapy for preventing bleeding complications in severely thrombocytopenic individuals such as patients with aplastic anemia or cancer patients on chemotherapy. Furthermore, steroid therapy has been empirically applied for the treatment of various hematopoietic disorders, although its working mechanism was unknown (Negrev 1990; Ranganath et al. 1996; Bord et al. 2000). Our findings suggest that 17β-estradiol or an activator of 3β-HSD, in combination with a low dose of TPO, is effective for the treatment of thrombocytopenia. Furthermore, there is no useful drug for the treatments of thrombocythemia and thrombocytosis. Our data suggest that inhibitors of 3β-HSD such as trilostane and estrogen receptor antagonists may be applicable for the treatments of these diseases.
Materials and methods
p45-/- and p45+/+ ES cells and megakaryocyte differentiation
The p45-/- and p45+/+ ES cells were established from the blastocysts of p45+/- mice (Jackson Laboratory), and their genotypes were determined by genomic PCR. The pcDNA3.1-3β-HSD VI was transfected into ES cells by electroporation, and 3β-HSD VI expressing transfectants were cloned. The p45-/-, p45+/+ ES cells or transfectants were cocultured with OP9 stromal cells for 3 d in α-MEM containing 20% fetal calf serum (FCS), and cocultured for a further 8 d in the presence of recombinant mouse TPO (50 units/mL) with medium changes. Recombinant mouse TPO was prepared as described (Nagata et al. 1995).
Isolation of 3β-HSD cDNA
Total RNAs were isolated from p45-/- Meg and p45+/+ Meg using an RNeasy Mini Kit (QIAGEN). The PCR-Select cDNA subtraction kit (Clontech) was used for cDNA synthesis and suppressive subtractive hybridization, according to the recommended protocol. The cDNA of p45+/+ Meg was used as the tester sample, and that of p45-/- Meg as the driver sample. The full-length 3β-HSD VI cDNA was isolated and inserted into a pcDNA3.1 expression vector. The 3β-HSD VI sequence, in which several nucleotides were different from one in the database, was deposited in the database (accession number AB109387). Northern blot analysis was performed as described (Nagata et al. 2001). The nucleotides 877-1126 of 3β-HSD cDNA was used as a probe.
Determination of isoform type of 3β-HSD transcripts
Isoform types of 3β-HSD transcripts were determined by RT-PCR followed by restriction enzyme digestion procedure as described (Abbaszade et al. 1997). The primers used were 5′-CAGACCATCCTAGATGT-3′ and 5′-AGGAAGCTCACAGTTTCCA-3′. The RT-PCR products were digested at the unique restriction site of each isoform-specific sequence and separated by 2% agarose gel electrophoresis.
Preparation of megakaryocytes and PPF assay
Megakaryocytes from BDF1 mice (6-8-week-old females and males) bone marrow were purified by a modified two-step separation technique as described (Nagahisa et al. 1996). The megakaryocytes (3 × 103 cells/mL) were incubated in serum-free medium (S-clone; Sanko) with 1% BSA with or without trilostane (Mochida Pharmaceuticals) at 37°C for 24 h. The CD41+ c-Kit+ cells (2 × 103 cells/mL) prepared as described (Oda et al. 2003) were cultured in S-clone medium with 1% FCS and mouse TPO (50 units/mL) with or without steroid hormones at 37°C for 4 d. Megakaryocytes displaying clear, long cytoplasmic processes were counted.
Antibody preparation and immunoblot analysis
Polyclonal anti-3β-HSD I and anti-3β-HSD VI rabbit antisera were prepared by injecting the N-terminal fragment (amino acids 1-267) of 3β-HSD I and C-terminal peptide (amino acids 360-374) of 3β-HSD VI conjugated to KLH, respectively, and 3β-HSD VI-specific antibody was purified by antigen affinity chromatography. Immunoblot analysis was performed as described (Nagata et al. 1995). The cell extracts (75 μg) and testis extracts (50 μg) were applied. Antibodies against 3β-HSD I (1:10000 dilution) and 3β-HSD VI (1:100 dilution), and antibodies against steroid receptors (1:200 dilution; Santa Cruz Biotech) were used. Peroxidase-conjugated AffiniPure F(ab′)2 fragment donkey anti-rabbit IgG (1:20000 dilution; Jackson ImmunoResearch) was used as a secondary antibody.
Immunohistochemical analysis
Immunofluorescence microscopic analysis was performed as described (Nagata et al. 1997), except for the following points. Smear samples of megakaryocytes were fixed with 3.7% formaldehyde at room temperature for 15 min. Anti-estradiol antibody (1:20 dilution; Chemicon), anti-progesterone antibody (1:20 dilution; Chemicon), anti-testosterone antibody (Biogenesis), and Alexa Fluor488 goat anti-rabbit IgG F(ab′)2 fragment (1:600 dilution; Molecular Probes) were used.
Enzyme immunoassay of steroid hormones
An enzyme immunoassay kit (PANTEX) was used to measure quantitatively steroid hormones in the supernatants of megakaryocytes cultured in serum-free medium.
Platelet counts
For platelet counts, 100 μL of tamoxifen (2.5 mg/mL) or ICI182780 (2.5 mg/mL) dissolved in solvent (sesame oil:ethanol, 19:1), or solvent alone was injected into BDF1 mice (8-week-old males; n = 7) daily for 9 d, and the number of platelets in peripheral blood were counted.
Acknowledgments
We thank Hirotaka Haruta for FACS and ES cells, Akira Kato and Etienne-Emile Baulieu for discussion in early stage of this work, Benita Katzenellenbogen for helpful advice, Masaaki Oda for help, and Masahiro Nobuhara for trilostane. This work was supported by PRESTO of JST (Y.N.), by the Bioarchitect project of RIKEN (K.T.), and by NICHD, NIH cooperative agreement as part of the Specialized Cooperative Centers Program in Reproductive Research (A.H.P.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USCsection 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1128003.
References
- Abbaszade I.G., Arensburg, J., Park, C.H., Kasa-Vubu, J.Z., Orly, J., and Payne, A.H. 1997. Isolation of a new mouse 3β-hydroxysteroid dehydrogenase isoform, 3β-HSD VI, expressed during early pregnancy. Endocrinology 138: 1392-1399. [DOI] [PubMed] [Google Scholar]
- Alexander W.S., Roberts, A.W., Nicola, N.A., Li, R., and Metcalf, D. 1996. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 87: 2162-2170. [PubMed] [Google Scholar]
- Baker P.J., Sha, J.A., McBride, M.W., Peng L., Payne, A.H., and O'Shaughnessy, P.J. 1999. Expression of 3β-hydroxysteroid dehydrogenase type I and type VI isoforms in the mouse testis during development. Eur. J. Biochem. 260: 911-917. [DOI] [PubMed] [Google Scholar]
- Bord S., Vedi, S., Beavan, S.R., Horner, A., and Compston, J.E. 2000. Megakaryocyte population in human bone marrow increases with estrogen treatment: A role in bone remodeling? Bone 27: 397-401. [DOI] [PubMed] [Google Scholar]
- Bunting S., Widmer, R., Lipari, T., Rangell, L., Steinmetz, H., Carver-Moore, K., Moore, M.W., Keller, G.A., and de Sauvage, F.J. 1997. Normal platelets and megakaryocytes are produced in vivo in the absence of thrombopoietin. Blood 90: 3423-3429. [PubMed] [Google Scholar]
- Clarke M.C., Savill, J., Jones, D.B., Noble, B.S., and Brown, S.B. 2003. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J. Cell Biol. 160: 577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Botton S., Sabri, S., Daugas, E., Zermati, Y., Guidotti, J.E., Hermine, O., Kroemer, G., Vainchenker, W., and Debili, N. 2002. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood 100: 1310-1317. [DOI] [PubMed] [Google Scholar]
- Deveaux S., Cohen-Kaminsky, S., Shivdasani, R.A., Andrews, N.C., Filipe, A., Kuzniak, I., Orkin, S.H., Romeo, P.H., and Mignotte, V. 1997. p45 NF-E2 regulates expression of thromboxane synthase in megakaryocytes. EMBO J. 16: 5654-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese A.M., Hunt, P., Boone, T., and MacVittie, T.J. 1995. Recombinant human megakaryocyte growth and development factor stimulates thrombocytopoiesis in normal nonhuman primates. Blood 86: 54-59. [PubMed] [Google Scholar]
- Gurney A.L., Carver-Moore, K., de Sauvage, F.J., and Moore, M.W. 1994. Thrombocytopenia in c-mpl-deficient mice. Science 265: 1445-1447. [DOI] [PubMed] [Google Scholar]
- Hitchcock I.S., Skerry, T.M., Howard, M.R., and Genever, P.G. 2003. NMDA receptor-mediated regulation of human megakaryocytopoiesis. Blood 102: 1254-1259. [DOI] [PubMed] [Google Scholar]
- Italiano J.E., Lecine, P., Shivdasani, R.A., and Hartwig, J.H. 1999. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 147: 1299-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzhny Y., Yu, G., Sun, S., Toselli, P.A., Nieswandt, B., Jackson, C.W., and Ravid, K. 2002. BclxL overexpression in megakaryocytes leads to impaired platelet fragmentation. Blood 100: 1670-1678. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. 1995. Thrombopoietin: The primary regulator of platelet production. Blood 86: 419-431. [PubMed] [Google Scholar]
- Kelly M.J. and Levin, E.R. 2001. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 12: 152-156. [DOI] [PubMed] [Google Scholar]
- Khetawat G., Faraday, N., Nealen, M.L., Vijayan, K.V., Bolton, E., Noga, S.J., and Bray, P.F. 2000. Human megakaryocytes and platelets contain the estrogen receptor β and androgen receptor (AR): Testosterone regulates AR expression. Blood 95: 2289-2296. [PubMed] [Google Scholar]
- Koenig H.L., Schumacher, M., Ferzaz, B., Thi, A.N., Ressouches, A., Guennoun, R., Jung-Testas, I., Robel, P., Akwa, Y., and Baulieu, E.E. 1995. Progesterone synthesis and myelin formation by Schwann cells. Science 268: 1500-1503. [DOI] [PubMed] [Google Scholar]
- Koenig H.L., Gong, W.H., and Pelissier, P. 2000. Role of progesterone in peripheral nerve repair. Rev. Reprod. 5: 189-199. [DOI] [PubMed] [Google Scholar]
- Lecine P., Italiano Jr., J.E., Kim, S.W., Villeval, J.L., and Shivdasani, R.A. 2000. Hematopoietic-specific β1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood 96: 1366-1373. [PubMed] [Google Scholar]
- McEwen B., Akama, K., Alves, S., Brake, W.G., Bulloch, K., Lee, S., Li, C., Yuen, G., and Milner, T.A. 2001. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. 98: 7093-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahisa H., Nagata, Y., Ohnuki, T., Osada, M., Nagasawa, T., Abe, T., and Todokoro, K. 1996. Bone marrow stromal cells produce thrombopoietin and stimulate megakaryocyte growth and maturation but suppress proplatelet formation. Blood 87: 1309-1316. [PubMed] [Google Scholar]
- Nagata Y., Nagahisa, H., Aida, Y., Okutomi, K., Nagasawa, T., and Todokoro, K. 1995. Thrombopoietin induces megakaryocyte differentiation in hematopoietic progenitor FDC-P2 cells. J. Biol. Chem. 270: 19673-19675. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Muro, Y., and Todokoro, K. 1997. Thrombopoietin-induced polyploidization of bone marrow megakaryocytes is due to a unique regulatory mechanism in late mitosis. J. Cell Biol. 139: 449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Oda, M., Nakata, H., Shozaki, Y., Kozasa, T., and Todokoro, K. 2001. A novel regulator of G-protein signaling bearing GAP activity for Gαi and Gαq in megakaryocytes. Blood 97: 3051-3060. [DOI] [PubMed] [Google Scholar]
- Negrev N. 1990. Female sex hormones and thrombocytopoiesis. Eksp. Med. Morfol. 29: 57-62. [PubMed] [Google Scholar]
- Oda M., Kurasawa, Y., Todokoro, K., and Nagata, Y. 2003. Thrombopoietin-induced CXC chemokines, NAP-2 and PF-4, suppress polyploidization and proplatelet formation during megakaryocyte maturation. Genes Cells 8: 9-16. [DOI] [PubMed] [Google Scholar]
- Peng L., Arensburg, J., Orly, J., and Payne, A.H. 2002. The murine 3β-hydroxysteroid dehydrogenase (3β-HSD) gene family: A postulated role for 3β-HSD VI during early pregnancy. Mol. Cell. Endocrinol. 187: 213-221. [DOI] [PubMed] [Google Scholar]
- Potts G.O., Creange, J.E., Hardoing, H.R., and Schane, H.P. 1978. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids 32: 257-267. [DOI] [PubMed] [Google Scholar]
- Radley J.M. and Scurfield, G. 1980. The mechanism of platelet release. Blood 56: 996-999. [PubMed] [Google Scholar]
- Ranganath L.R., Christofides, J., and Semple, M.J. 1996. Increased mean platelet volume after oestrogen replacement therapy. Ann. Clin. Biochem. 33: 555-560. [DOI] [PubMed] [Google Scholar]
- Rojnuckarin P. and Kaushansky, K. 2001. Actin reorganization and proplatelet formation in murine megakaryocytes: The role of protein kinase Cα. Blood 97: 154-161. [DOI] [PubMed] [Google Scholar]
- Shivdasani R.A. and Orkin, S.H. 1995. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc. Natl. Acad. Sci. 92: 8690-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani R.A., Rosenblatt, M.F., Zucker-Franklin, D., Jackson, C.W., Hunt, P., Saris, C.J., and Orkin, S.H. 1995. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 81: 695-704. [DOI] [PubMed] [Google Scholar]
- Shivdasani R.A., Fielder, P., Keller, G.A., Orkin, S.H., and de Sauvage, F.J. 1997. Regulation of the serum concentration of thrombopoietin in thrombocytopenic NF-E2 knockout mice. Blood 90: 1821-1827. [PubMed] [Google Scholar]
- Simoncini T. and Genazzani, A.R. 2003. Non-genomic actions of sex steroid hormones. Eur. J. Endocrinol. 148: 281-292. [DOI] [PubMed] [Google Scholar]
- Tarantino M.D., Kunicki, T.J., and Nugent, D.J. 1994. The estrogen receptor is present in human megakaryocytes. Ann. NY Acad. Sci. 714: 293-296. [DOI] [PubMed] [Google Scholar]