A single intracameral dose of an adenovirus carrying glucocorticoid-inducible MMP1 reduces and prevents elevated IOP induced by steroids in a sheep model.
Abstract
Purpose.
To investigate whether intracameral injection of the adenovirus vector AdhGRE.MMP1 would reduce or prevent elevated intraocular pressure (IOP) induced by corticosteroids in living animals.
Methods.
Glucocorticoid-inducible adenovirus vectors carrying wild-type or mutant forms of human metalloproteinase 1 (MMP1 and mutMMP1) cDNAs were generated. An adenovirus carrying no gene (Ad5.CMV.Null) was used as an additional control. Sheep were injected intracamerally with 30 μL of each vector, either previously or after the induction of increased IOP with topical prednisolone or sub-Tenon triamcinolone under various protocols. IOP was measured with a Perkins tonometer. Inflammation was monitored by visual inspection.
Results.
In eyes in which IOP was already elevated to 24 to 30 mm Hg, injection of AdhGRE.MMP1 reduced IOP by 70% in 24 hours and to 10 to 13 mm Hg in 48 hours. In eyes with normal IOP (9–11 mm Hg), preinjection of the virus protected against the increase in IOP normally produced by the corticosteroid. IOP remained at a level of approximately 12 mm Hg for 5 days despite the continuous application of the corticosteroid. Injections of the control viruses had no hypotensive effects. There were no signs of ocular inflammation or discomfort to the animals.
Conclusions.
A single dose of a gene therapy vector carrying an inducible metalloproteinase human gene can both protect against the IOP increase produced by corticosteroid instillation in the sheep model and quickly reverse the IOP increase previously elicited by the corticosteroid. These results are a first step toward a treatment of steroid-glaucoma with inducible overexpression of extracellular matrix modulator genes.
Glucocorticosteroids such as prednisolone exhibit therapeutic versatility given their common use as anti-inflammatory, immunosuppressive, and antiangiogenic agents.1,2 However, glucocorticosteroids also elicit adverse ocular effects such as cataract formation and increased IOP.3 Persons susceptible to increased intraocular pressure (IOP) may require treatment for glaucoma. The phenomenon of glucocorticosteroid-induced ocular hypertension has been recognized for decades,4 and a number of predisposing risk factors have been identified among patients receiving various corticosteroid treatments.5,6 Yet the mechanisms by which glucocorticosteroids induce IOP elevation have not been determined. It is recognized that this adverse effect is the result of reduced trabecular aqueous humor outflow associated with morphologic and biochemical changes in the trabecular meshwork.5,6 As such, a thorough understanding of the cellular processes eliciting corticosteroid-induced ocular hypertension may shed light on the cause of primary open-angle glaucoma.
We recently demonstrated the effectiveness of using Corriedale sheep (Ovis aries) as an animal model for glucocorticosteroid-induced ocular hypertension.7 The IOP of these animals increased ≈2.5-fold within 2 weeks of topical application of 0.5% prednisolone acetate three times daily. This intraocular pressure elevation occurred with a 100% incidence in the corticosteroid-treated eyes. After the discontinuation of corticosteroid instillation, the IOP of the treated eyes declined to baseline values over the course of 1 to 3 weeks. Similar IOP elevations were obtained in all sheep receiving the corticosteroid triamcinolone acetonide, applied as a single sub-Tenon injection rather than as a topical application (our unpublished observations, 2009).
The 100% incidence of corticosteroid-induced ocular hypertension in O. aries and the docile nature of the animals, which readily submit to manipulations such as those required for in vivo outflow facility measurements, render this species an ideal model for both examining the mechanisms underlying corticosteroid-induced glaucoma and testing possible IOP-lowering agents. In this work, we tested the newly developed glucocorticoid-inducible matrix metalloproteinase 1 (MMP1) gene therapy vector (see Ref. 8) for its ability to reduce corticosteroid-induced ocular hypertension in the sheep model. We found that a single dose of a gene therapy vector carrying an inducible metalloproteinase human gene can protect against the increase in IOP normally produced by corticosteroid instillation in the sheep model and can quickly reverse the IOP increase elicited by corticosteroid pretreatments.
Materials and Methods
Animals
All animal experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eighteen healthy (female) sheep (Corriedale breed) between 12 and 24 months of age, and weighing 35 to 40 kg each, were selected from a local ranch in Corrientes, Argentina, for this study. The eyes and general health of the animals were considered normal by an ophthalmologist and a veterinarian, respectively. Sheep were tagged for individual identification on their ear lobes and herded from pasture whenever it was necessary to topically instill prednisolone, inject a sub-Tenon depot of triamcinolone acetonide, inject adenoviral vectors carrying the MMP1 transgene intracamerally by way of the cornea, or measure IOP by applanation tonometry. To apply prednisolone, the sheep were guided into a funnel corral ending in a loose-fitting yoke.7 This arrangement allowed one person to move the animal's head and hold it while another instilled the drops. To measure IOP with a Perkins tonometer, the sheep were guided into the funnel corral and then into the neck yoke. For sub-Tenon injection of triamcinolone and the trans-corneal injections of adenoviral vectors, sheep were anesthetized topically. Between all procedures, the sheep were free to pasture.
Prednisolone Instillation Protocol
In sheep eyes in which prednisolone was used to induce ocular hypertension, the following general protocol was applied. After determining the baseline measurement of IOP over the course of several days, two drops of 0.5% prednisolone acetate (Ultracortenol; Novartis Ophthalmics, Hettlingen, Switzerland) was topically instilled three times daily at 7 am, 2 pm, and 7 pm for durations that lasted for 10 to 15 days, depending on the experiment. In some experiments, prednisolone was instilled bilaterally; in others, only one eye received the corticosteroid.
Sub-Tenon Injection of Triamcinolone in Topically Anesthetized Sheep Eyes
In those sheep in which triamcinolone was used to induce ocular hypertension, the following general protocol was applied. Two drops of proparacaine (0.5%) were topically instilled to the ocular surface. Then a single 1-mL injection of sterile triamcinolone acetonide (40 mg/mL or 4%; Bristol-Myers Squibb, Princeton, NJ) was administered by sub-Tenon injection using a 30-gauge needle inserted 5 mm from the limbus.
For injections, the distance from the limbus was determined from the width of a 5-mm × 30-mm Schirmer strip that was held between the limbus and the injection site. The conjunctiva at the injection site was grasped with fine forceps, and an initial oblique conjunctival puncture was made with the needle bevel facing upward. The needle was then pushed deeper (continuing at an oblique angle) to create another puncture in the Tenon capsule in such a manner that the Tenon puncture did not underlie the conjunctival puncture. The needle was pushed through the Tenon capsule until the tip reached the sclera (as determined by feel). Care was taken to avoid puncturing the sclera itself. In addition, because the Tenon capsule consists of several layers, care was taken to administer a sub-Tenon (to create a juxtascleral depot), not an intra-Tenon, injection. Immediately after the injection the needle was rapidly withdrawn. The volume injected (1 mL) yielded a characteristic 180° to 240° quasi-donut-shaped bolus of fluid around the limbus. Each injected eye then received 2 drops of tobramycin ophthalmic solution (Tobrex 0.3%; Alcon Laboratories, Inc., Forth Worth, TX).
Measurement of IOP of Conscious Sheep with the Handheld Perkins Applanation Tonometer
Animals were led to a funnel corral, and their heads were suitably oriented within a neck yoke to enable an ophthalmologist to measure IOP with the Perkins tonometer. Before the IOP measurement, 2 drops of topical 0.5% proparacaine (Alcon Argentina, Buenos Aires, Argentina) followed by 2 drops of 0.25% fluorescein were instilled. Two sets of measurements were taken on each eye, alternating first one eye and then the other. All IOP measurements were taken between 2 pm and 4 pm every 2 or 3 days. Perkins tonometry readings were converted to millimeters of mercury, as described in detail previously.7
Steroid-Inducible MMP1 Recombinant Adenoviruses
Details of the design, construction, characterization, and titration of the steroid-inducible MMP1 adenoviruses are given in Spiga and Borrás.8 Briefly, a full-coding MMP1 cDNA driven by a glucocorticoid-response element (GRE) and a basal promoter was engineered into a shuttle vector and electroporated into cells containing the adenovirus genome. The transgene viral DNA was produced by recombination, and the generation of the virus (AdhGRE.MMP1) was achieved by transfecting the viral DNA into QBI-HEK-293 cells. As a functionally negative control, an inducible vector carrying an MMP1 mutation in the catalytic site (AdhGRE.mutMMP1) was constructed in a similar manner. An additional control carrying no transgene (Ad5.CMV.Null) was purchased from Qbiogen (Montreal, QC, Canada) and was grown and purified in our laboratory. All recombinant adenoviruses were grown and purified to high-titer stocks, as previously described.9 Viral lots used in this study had concentrations of 3.1 × 1011 and 2.4 × 1011 virus genomes (vg)/mL (AdhGRE.MMP1), 4.0 × 1011 vg/mL (AdhGRE.MMP1), and 9.3 × 1011 vg/mL (Ad5.CMV.Null), which correspond approximately to twice the infectious units per milliliter (see Ref. 8).
Intraocular Injection of Adenoviral Vectors into the Anterior Chambers of Sheep
Adenoviral vectors were constructed in the laboratory of TB (University of North Carolina, Chapel Hill, North Carolina) and were shipped on dry ice to New York. OAC (Mount Sinai School of Medicine, New York, NY) delivered the vectors to Corrientes for the experiments. Until needed, the parcels containing the lots of viral vectors were always maintained either on dry ice or in a freezer at −70°C. At the time of viral administration, the appropriate Eppendorf tube containing the frozen virus suspension was thawed in the field as each sheep was immobilized within a narrow passage ending in a yoke. Two drops of topical proparacaine 0.5% (Alcon Argentina) were instilled on the eyes as an anesthetic. After this, ≈ 30 μL virus suspension was injected into the eye using a Hamilton syringe with a 28-gauge needle. The needles were inserted diagonally through the cornea (a few millimeters inside the limbus) into the anterior chamber without touching the iris. The injection procedure took less than 30 seconds.
Statistical Analysis
The significance of experimentally elicited changes in IOP were analyzed using Student's t-test as either paired or unpaired data, with α = 0.05 chosen as the level of significance. If both eyes from the same animal react equally to a treatment, paired analysis can be used. On the other hand, there is evidence that fellow eyes are not identical and in that case unpaired tests should be used. To avoid uncertainties, we used both tests.
Results
Administration of Corticosteroids
The IOP in both eyes of the normal sheep used in this study was measured before any treatment to establish the baseline values. The measured Perkins tonometry readings and the equivalent IOP as determined from a calibration curve indicated baseline pressures between ≈ 9 and 11 mm Hg, values similar to those obtained earlier.7
The present experiments were designed to determine whether the intracameral administration of adenoviral vectors carrying an active human MMP1 transgene could both prevent IOP elevation in a sheep model of glucocorticosteroid-induced ocular hypertension and reduce the elevation in IOP after its establishment by pretreatments of corticosteroid. Prednisolone was administered as one of the IOP-elevating agents, as used previously.7 This corticosteroid was administered by thrice-daily topical instillations. With this agent, IOP will remain elevated for as long as the instillation regimen is maintained.7 The second corticosteroid used in the present study was triamcinolone, which was administered as a single sub-Tenon injection. Its advantage is the less tedious application because the agent is introduced only once, and subsequent daily administrations are avoided. A disadvantage is that it is difficult to accurately assess when the administered triamcinolone depot has been depleted. In experiments described in this study, the triamcinolone depots appeared to subsist for periods of ≈2 to 3 weeks, as determined by the IOP.
Hypotensive Effect of a Single Dose of Glucocorticoid-Inducible MMP1 Adenovirus on Prednisolone-Induced Ocular Hypertension
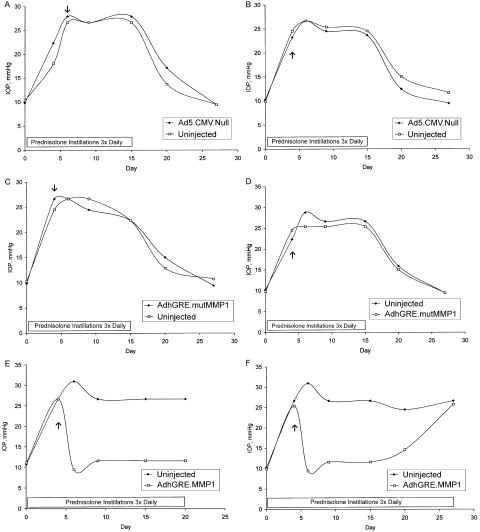
In the first set of experiments (Fig. 1), six normal sheep were treated topically with prednisolone three times a day in both eyes beginning on day 0. Subsequently, as IOP increased, one eye of each sheep was injected with 1 of 3 adenoviral (Ad) vectors. The active adenoviral vector (AdhGRE.MMP1) carried the wild-type MMP1 transgene. MMP1 was chosen because it encodes for a well-known trabecular meshwork (TM) enzyme that breaks down extracellular matrix components. The other two vectors (used as controls) included a null adenoviral vector (without transgene) (Ad5.CMV.Null) and a vector carrying a mutated transgene with an inactive catalytic site (AdhGRE.mutMMP1). These vectors also carried the inducible GRE element so that transgene expression would be activated only in the presence of steroids. Among the six sheep, two received one of the three adenoviral vectors prepared. Three to 5 days after adenoviral vector injection, elevated IOP was reduced in eyes receiving the MMP1 transgene but not in either of the two control eyes.
Figure 1.
(A–F) IOP measurements in six sheep treated with adenoviral vectors. Each graph represents data from one animal. All animals were treated with 2 drops of 0.5% prednisolone acetate in both eyes starting on day 0. Prednisolone instillation continued three times daily until the day indicated. Arrows: day the animals received a unilateral, intracameral injection of an adenoviral (Ad) vector; contralateral eyes were not treated with viral vectors (uninjected). Two animals received a null adenoviral vector (without transgene; A, B), two received an adenoviral vector that carried a mutated MMP1 transgene with out catalytic activity (C, D), and two received an active human MMP1 transgene (E, F). One animal (E) was killed on day 20. Note that in the eyes that received the active MMP1 transgene (E, F), IOP returned to normal levels for at least 15 days.
In the two eyes receiving the active MMP1 transgene (Figs. 1E, F), IOP returned to normal levels for at least 15 days. In one sheep, IOP was monitored for 20 days (Fig. 1E); in the second one, IOP was continuously monitored until day 27 (Fig. 1F). At this point, IOP increased to a level identical with that of the fellow eye not exposed to the adenoviral vector, suggesting either that the induced MMP1 activity was transitory or that it was overwhelmed by the continuous daily prednisolone applications.
No clinical adverse effects were noted in any of the eyes treated with adenoviral vectors. There were no signs of conjunctival hyperemia, inflammation, or irritation. Similarly, the cornea remained clear without signs of edema in response to viral injection. Presumably, the viral dose administered to the sheep (5–6 × 109 vg, corresponding to approximately 2.5 to 3 × 109 infectious units) was not sufficient to trigger inflammation in this species. Interestingly, a similar dose caused a strong inflammatory response in cynomolgus monkeys, whereas minimal or no signs of inflammatory reactions were noted when the monkey was injected with 107 plaque-forming units of adenoviral vectors.10
Hypotensive Effect of a Single Dose of Glucocorticoid-Inducible MMP1 Adenovirus on Triamcinolone-Induced Ocular Hypertension
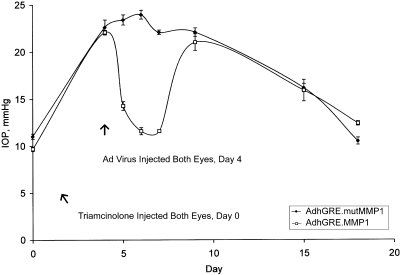
Triamcinolone was used as the IOP-elevating agent in the second set of experiments (Fig. 2). For this, four normal sheep received bilateral sub-Tenon injections of triamcinolone on day 0, which caused the IOP to approximately double within 4 days. IOP increased from 11.0 ± 0.3 to 22.6 ± 0.8 mm Hg (n = 4) in the eyes to which adenoviral vectors carrying the mutated MMP1 transgene were subsequently injected, and from 9.7 ± 0.2 to 22.1 ± 0.2 mm Hg (n = 4) in the eyes to which the adenoviral vectors carrying the active MMP1 transgene were subsequently injected (P < 3 × 10−4, as paired data for these IOP increases between days 0 and 4). After the IOP was recorded on day 4, both eyes of each animal were injected with the respective vectors. On days 6 and 7 (or 2 and 3 days after the virus injections), IOP in the eyes receiving the active form of the transgene were significantly lower than in fellow eyes (P < 2 × 10−5, as paired data). For example, on day 7, the IOP of the eye injected with adenoviral vector with the mutated form of MMP1 was 22.1 ± 0.2 mm Hg (n = 4), but in the fellow eye receiving the active form it was 11.6 ± 0.4 mm Hg (n = 4). Thereafter, the IOP of the latter eye increased to a level nearly identical with that of the fellow eye by day 9 (P > 0.15, as paired data). The reason for this increase was not determined. It is not clear why the apparent transitory MMP1 activity persisted for at least 15 days in the experiments shown in Figure 1 and for only 3 days in experiments shown in Figure 2. In the experiments shown in Figure 2, the triamcinolone depot appeared to be nearly consumed by day 18, as judged by the IOP measurements. On this day, the IOP readings of the fellow eyes were 10.5 ± 0.4 mm Hg (n = 4) and 12.4 ± 0.2 mm Hg (n = 4).
Figure 2.
IOP plot from four sheep treated with triamcinolone acetonide and adenoviral vectors according to the following regimen. Both eyes of each sheep received a single sub-Tenon injection of triamcinolone on day 0. On day 4, one eye of each sheep received a single intracameral injection of adenoviral vector that carried a mutated MMP1 transgene without catalytic activity (closed symbols), whereas the contralateral eye received adenoviral vector carrying an active human MMP1 transgene (open symbols). Points are mean ± SEM of the four eyes receiving the mutated transgene and of the four fellow eyes receiving the active transgene. In this set of experiments, treatment with the active transgene reduced IOP to normal levels for 3 days. Ad, adenoviral vector.
In tandem with the experiments shown in Figure 2, three other sheep were analyzed in parallel at the same time to check the effect of injecting null adenoviral vectors (without transgene) on the elevated IOP induced by triamcinolone (IOP plots not shown). For this test, each sheep unilaterally received a single sub-Tenon injection of triamcinolone on day 0. In two sheep, injections were made in the right eye, but in the third sheep, the injection was made in the left eye. On day 4, IOP readings of the control eyes were 10.9 ± 0.7 mm Hg (n = 3) and 25.2 ± 0.4 mm Hg (n = 3) in the eyes administered triamcinolone (P < 3 × 10−4, as either paired or unpaired data). After these readings, the triamcinolone-treated eyes were injected with null adenoviral vectors. Three days later, the IOP readings were 9.4 ± 0.0 mm Hg (n = 3) and 23.1 ± 0.4 (n = 3) for the control and steroid-treated eyes, respectively. The IOP of the latter was significantly lower statistically (P < 0.04, as paired data) 3 days after receipt of the null adenoviral vector, yet it was clear that the measured pressure difference was meager and that IOP remained 2.5-fold higher than in the control fellow eye. In contrast, in eyes receiving active MMP1 transgene, marked hypotensive effects can be measured within 24 hours of adenoviral vector injection, with reversions to the baseline IOP within 2 to 3 days (Figs. 1, 2).
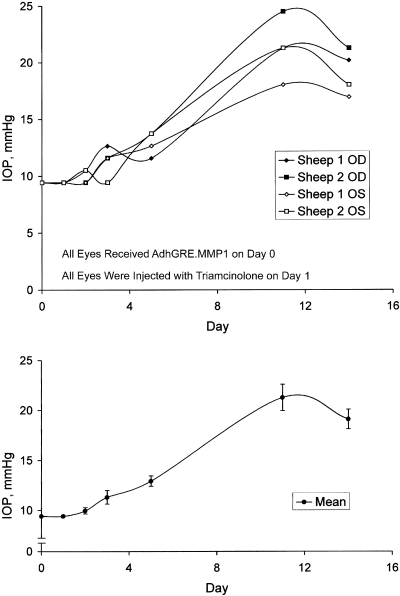
Protective Effect of a Single Dose of Glucocorticoid-Inducible MMP1 Adenovirus Injected before Triamcinolone Treatment
This protocol tested for preventive effects from pretreatments with MMP1 transgene on the elevated IOP induced by the triamcinolone depot. In these experiments, two sheep were bilaterally injected with adenoviral vector carrying the active transgene on day 0 and received sub-Tenon injections of triamcinolone in both eyes on day 1 (Fig. 3). In these four eyes, IOP remained near normal levels until at least day 5, at which point IOP was 12.9 ± 0.5 (n = 4). The day 5 IOP value was significantly higher than 9.4 ± 0.1 mm Hg (n = 4) measured on day 0 (P < 0.005, as paired data). At days 11 and 14, IOP readings were 21.3 ± 1.3 (n = 4) and 19.1 ± 1.0 (n = 4), respectively. These latter values were significantly higher than values at day 5 (P < 0.005, as paired data). The increase in IOP between day 5 and day 14 occurred with a time frame within which the triamcinolone depot likely subsisted. These results suggest that the active transgene offered protection against triamcinolone administration for at least 3 days. A protective effect is evident because the injections of triamcinolone in the control eye evoke a doubling of IOP within 4 days (Fig. 2).
Figure 3.
Plots of the IOPs from two sheep treated with triamcinolone acetonide and adenoviral vectors according to the following regimen. All eyes received vectors carrying the active transgene on day 0 and were administered a single sub-Tenon injection of triamcinolone the next day. Top: individual IOP values from each eye. Bottom: mean ± SEM for the four eyes. Active transgene offered protection against triamcinolone administration for at least 3 days.
Hypotensive Effects of a Single Dose of Glucocorticoid-Inducible MMP1 Adenovirus on Sheep Simultaneously Administered Triamcinolone in One Eye and Prednisolone in the Other Eye
Additional experiments (composed of two separate protocols, each on one sheep) tested the effects of the injection of adenoviral vector carrying active MMP1 transgene on the IOP of contralateral eyes treated with unilateral triamcinolone and unilateral prednisolone. In the first protocol of this set, one eye of a sheep was administered triamcinolone by sub-Tenon injection 2 weeks before the start of IOP measurements. The point at which IOP measurements were initiated was designated day 0 of the experiment. The right eye, which received the triamcinolone, exhibited an IOP about twice that of the left eye (Fig. 4). After recording these measurements, thrice-daily instillations of prednisolone were begun on the left eye. Three days later, when the IOPs of both eyes were nearly identical, adenoviral vector carrying the active MMP1 transgene was bilaterally injected into the anterior chambers of the eyes. Subsequently, IOP declined in tandem in the fellow eyes (Fig. 4). At the point of virus injection, the IOP of the right eye had been elevated for 17 days; after virus injection, it was reduced up to day 24. It is also possible that triamcinolone lost its effectiveness, or that the depot was gradually depleted, between days 15 and 24 (Fig. 4). In the left eye, between days 15 and 24, IOP gradually increased, presumably because of a diminished effect from the injected MMP1 transgene in the face of continuous daily prednisolone instillations.
Figure 4.
Plot of the IOP from one sheep treated with triamcinolone acetonide, prednisolone acetate, and adenoviral vectors according to the following regimen. The right eye received a single sub-Tenon injection of triamcinolone on day −14 (not shown). IOP measurements began on day 0, at which point thrice-daily prednisolone instillations were begun on the left eye. Both eyes received intracameral injections of adenoviral vectors carrying active transgene on day 3.
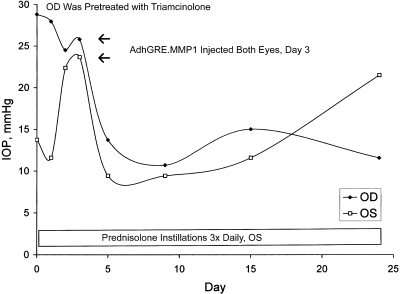
In the second protocol, the left eye of a sheep was injected with adenoviral vector carrying the active transgene on day 0, and the thrice-daily instillations of prednisolone were begun on day 3 (Fig. 5). The contralateral right eye was pretreated with a sub-Tenon injection of triamcinolone 2 weeks before the initiation of IOP measurements. On day 0, IOP measurements were initiated, and the right eye exhibited an IOP about twice that of the left eye (Fig. 5), indicating a typical response to corticosteroid administration. On day 1, the right eye was injected with adenoviral vector carrying active MMP1 transgene from the same lot as that given to the left eye; this injection resulted in an ocular hypotensive effect within 24 hours in the right eye. Thereafter, the IOP of the right eye remained low because of active protection from the MMP1 transgene or from a loss of effect of triamcinolone. The fellow left eye appeared to have received a protective effect from the MMP1 transgene administration up to at least day 15; thereafter, IOP gradually increased because of continuous prednisolone instillations (Fig. 5).
Figure 5.
Plot of the IOP from one sheep treated with triamcinolone acetonide, prednisolone acetate, and adenoviral vectors according to the following regimen. The right eye received a single sub-Tenon injection of triamcinolone on day −14 (not shown). Adenoviral vectors carrying the active transgene were injected intracamerally into the left eye on day 0 and into the right eye on day 1. Prednisolone was administered to the left eye during the days indicated.
Discussion
Sheep are docile and compliant animals that are particularly well suited for in vivo experiments such as those conducted in the present study. This advantage outweighs the lack of extensive genetic information on these animals. On the other hand, ovine physiology appears similar to human and other primate physiology with regard to aqueous secretion11 and trabecular meshwork.12,13 The major advantage to using an ovine steroid-induced model of IOP elevation is the consistency and robustness of the IOP response and its relatively low cost compared with studies in primates. Moreover, the sheep model for corticosteroid-induced ocular hypertension was preferable to other animal models. For example, only ≈50% of rabbits treated chronically with glucocorticoids such as dexamethasone develop ocular hypertension; in fact, dexamethasone-responding rabbits are commonly defined as those exhibiting IOP elevations of at least 5 mm Hg.14 In contrast, all treated sheep responded to prednisolone with ≈2.5-fold increases in IOP, as reported previously,7 and sub-Tenon injection of a triamcinolone depot was observed to be as effective as prednisolone in elevating ovine IOP in that all sheep administered triamcinolone exhibited ocular hypertension.
In the present study, triamcinolone was used for convenience to avoid the necessity of applying prednisolone three times daily as the sole method for elevating IOP in all protocols. The triamcinolone acetonide preparation of the drug is minimally water soluble and is injected into sub-Tenon's space as a suspension. In this form, the lowered water solubility contributes to the formation of a relatively long-lasting depot, from which drug delivery into the eye occurs through the sclera.15,16 However, as discussed by Robinson et al.,17 many studies have observed intrasubject variability in intraocular drug levels after subconjunctival injection. These authors have suggested that a number of factors may influence drug release from the depot and intraocular entry, including differences in conjunctival lymphatic and capillary blood flow, scleral thickness, choroidal flow, and differences in the geometry of the depot itself.17 Presumably, the complex release kinetics of sub-Tenon triamcinolone might account for the varied degree and duration of the IOP elevations that were obtained in our protocols. For example, the IOP of the triamcinolone-treated eye was higher than 25 mm Hg on day 15 in some experiments (Figs. 4, 5), whereas an IOP lower than 20 mm Hg was observed 15 days after sub-Tenon drug injection (Fig. 2). Nevertheless, we tested the MMP1 transgene at the plateau of the elevated IOP to determine the potential usefulness of this approach in reversing the induced ocular hypertension, which was the central aim of this study.
Studies on cultured TM cells and on organ-cultured eyes, as well as ultrastructural studies on human specimens, suggest that the following general mechanisms may be involved in glucocorticosteroid-induced ocular hypertension: mechanical changes in the microstructure of the TM from the reorganization of actin stress fibers and the formation of reversible actin networks mediated by TM glucocorticoid receptors18,19; increase in the deposition of extracellular matrix material altering the ultrastructure of the outflow pathway20–22; reduction in the protease activities and phagocytic properties of the TM cells, thereby decreasing the breakdown of substances in the TM23–25; and reduction of water flow in the TM either transcellularly or paracellularly.26,27
Gene expression profile analysis of human TM cells exposed to prolonged dexamethasone treatment found that a group of actins and actin-associated proteins are involved in the development of cross-linked actin networks in the treated cells.28 In addition, a trend toward decreased expression of protease genes and increased expression of protease inhibitors in the dexamethasone-exposed cells was identified.28
A role for proteases is also suggested by the results of the present study. We determined that a single dose of a gene therapy vector carrying an inducible human MMP1 gene could both temporarily prevent the increase in IOP normally produced by glucocorticosteroid instillations in the sheep model and reverse the IOP increase previously induced by the glucocorticosteroids.
We did not observe cataract formation in any of the animals used in this or our previous study on sheep7 or in unpublished observations of sheep topically instilled prednisolone for 4 months. In the case of the triamcinolone depot, the drug appeared to subsist in the eye for ≈14 to 20 days, as judged by the IOP measurements.
Putatively, the triamcinolone depot might be a more effective experimental approach (compared with topical instillations) for elevating IOP because the drug is continuously present in the eye until the depot is totally dissipated. In contrast, there were 7- to 12-hour intervals between the administrations of the prednisolone drops. This difference in technique might explain the present observations that MMP1 activity induced by the adenoviral vector preparations seems to have persisted for at least 15 days in the case of the prednisolone experiments shown in Figure 1, and only 3 days in the case of the triamcinolone experiments shown in Figure 2. Additionally, the adenoviral vector preparations may not by necessity be identical. Further study is needed to explain this difference.
In short, our present work broaches the concept that it might be possible to treat steroid glaucoma with an inducible overexpression of extracellular matrix modulator genes. Given the common characteristics between steroid glaucoma and primary open-angle glaucoma, this potential therapy might have general applicability.
Footnotes
Supported by National Eye Institute Grants EY11906 (TB), EY13126 (TB), EY00160 (OAC), EY01867 (OAC), and EY13749 (OAC), and by unrestricted grants from Research to Prevent Blindness to the Departments of Ophthalmology at University of North Carolina and Mount Sinai School of Medicine.
Disclosure: R. Gerometta, P; M.-G. Spiga, P; T. Borrás, P; O.A. Candia, P
References
- 1.Clark AF. AL-3789: a novel ophthalmic angiostatic steroid. Expert Opin Investig Drugs 1997;6:1867–1877 [DOI] [PubMed] [Google Scholar]
- 2.Clark AF. Mechanism of action of the angiostatic cortisene anecortave acetate. Surv Ophthalmol 2007;52(suppl 1):S26–S34 [DOI] [PubMed] [Google Scholar]
- 3.Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology 2005;112:139–143 [DOI] [PubMed] [Google Scholar]
- 4.McLean J. Use of ACTH and cortisone. Trans Am Ophthalmol Soc 1950;48:293–296 [PMC free article] [PubMed] [Google Scholar]
- 5.Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol 2006;17:163–167 [DOI] [PubMed] [Google Scholar]
- 6.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye 2006;20:407–416 [DOI] [PubMed] [Google Scholar]
- 7.Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Invest Ophthalmol Vis Sci 2009;50:669–673 [DOI] [PubMed] [Google Scholar]
- 8.Spiga MG, Borrás T. Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma. Invest Ophthalmol Vis Sci 2010;51:3029–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrás T, Rowlette LL, Erzurum SC, Epstein DL. Adenoviral reporter gene transfer to the human trabecular meshwork does not alter aqueous humor outflow: relevance for potential gene therapy of glaucoma. Gene Ther 1999;6:515–524 [DOI] [PubMed] [Google Scholar]
- 10.Borrás T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med 2001;3:437–449 [DOI] [PubMed] [Google Scholar]
- 11.Gerometta RM, Malgor LA, Vilalta E, Leiva J, Candia OA. Cl- concentrations of bovine, porcine and ovine aqueous humor are higher than in plasma. Exp Eye Res 2005;80:307–312 [DOI] [PubMed] [Google Scholar]
- 12.Simoens P, DeGeest JP, Lauwers H. Comparative morphology of the pectinate ligaments of domestic mammals, as observed under the dissecting microscope and the scanning electron microscope. J Vet Med Sci 1996;58:977–982 [DOI] [PubMed] [Google Scholar]
- 13.Guyomard JL, Rosolen SG, Paques M, et al. A low-cost and simple imaging technique of the anterior and posterior segments: eye fundus, ciliary bodies, iridocorneal angle. Invest Ophthalmol Vis Sci 2008;49:5168–5174 [DOI] [PubMed] [Google Scholar]
- 14.Pang IH, Moll H, McLaughlin MA, et al. Ocular hypotensive and aqueous outflow-enhancing effects of AL-3037A (sodium ferri ethylenediaminetetraacetate). Exp Eye Res 2001;73:815–825 [DOI] [PubMed] [Google Scholar]
- 15.Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol 2007;52:503–522 [DOI] [PubMed] [Google Scholar]
- 16.Mora P, Eperon S, Felt-Baeyens O, et al. Trans-scleral diffusion of triamcinolone acetonide. Curr Eye Res 2005;30:355–361 [DOI] [PubMed] [Google Scholar]
- 17.Robinson MR, Lee SS, Kim H, et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res 2006;82:479–487 [DOI] [PubMed] [Google Scholar]
- 18.Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton 2005;60:83–95 [DOI] [PubMed] [Google Scholar]
- 19.Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 1994;35:281–294 [PubMed] [Google Scholar]
- 20.Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Invest Ophthalmol Vis Sci 1990;31:2568–2571 [PubMed] [Google Scholar]
- 21.Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res 1993;12:783–793 [DOI] [PubMed] [Google Scholar]
- 22.Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol 1996;40:379–390 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest Ophthalmol Vis Sci 1997;38:1902–1907 [PubMed] [Google Scholar]
- 24.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res 1999;18:629–667 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res 2007;84:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Underwood JL, Murphy CG, Chen J, et al. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am J Physiol 1999;277:C330–C342 [DOI] [PubMed] [Google Scholar]
- 27.Xiong X, Miao J, Xi Z, Zhang H, Han B, Hu Y. Regulatory effect of dexamethasone on aquaporin-1 expression in cultured bovine trabecular meshwork cells. J Huazhong Univ Sci Technolog Med Sci 2005;25:735–737 [DOI] [PubMed] [Google Scholar]
- 28.Rozsa FW, Reed DM, Scott KM, et al. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis 2006;12:125–141 [PubMed] [Google Scholar]