Activation of Toll-like receptors during microbial infection plays an important role in the pathogenesis of autoimmune diseases such as uveitis.
Abstract
Purpose.
Induction of tissue-specific experimental autoimmune diseases involves the use of complete Freund adjuvant containing Mycobacterium tuberculosis, whose recognition by the innate immune system depends on Toll-like receptors (TLRs) that signal through the adaptor molecule MyD88. The authors' previous study showed that MyD88−/− mice, but not TLR2−/−, TLR4−/−, or TLR9−/− mice, were resistant to experimental autoimmune uveitis (EAU).
Methods.
The EAU induction in mice deficient in TLR3 or mice double deficient in TLR2+4, TLR2+9, and TLR4+9 was examined and the role of the TLR agonists in the adjuvant effect involved in the induction of EAU was assessed.
Results.
TLR3-deficient and TLR2+4, TLR2+9, and TLR4+9 double-deficient mice were as susceptible to EAU as their control littermates. However, in mice immunized with a low-dose EAU regimen, TLR4 agonist lipopolysaccharide (LPS) enhanced EAU scores, delayed-type hypersensitivity responses, and antigen-specific T-cell proliferation. Antigen-specific IL-17 and IFN-γ production by T lymphocytes was markedly increased in the LPS-treated group. The effects of LPS on EAU were abolished by treatment with an LPS deactivator polymyxin B. Inclusion of agonists for TLR2, TRL3, or TRL9 in immunization also enhanced EAU scores.
Conclusions.
These results suggest that signaling of TLR2, TRL3, TRL4, and TRL9 is highly redundant in the adjuvant effect needed to induce EAU and that diverse microbial infections may contribute to the pathogenesis of diseases such as uveitis.
Toll-like receptors are a family of pattern recognition receptors that recognize distinct molecular patterns associated with microbial pathogens.1–4 Thus far, 11 TLRs in human and 13 TLRs in mouse have been identified, and many of their ligands are known. For example, TLR2 is involved in the response to microbial lipoproteins and peptidoglycan, such as those in mycobacteria, Gram-positive bacteria, and yeasts.2 TLR3 recognizes double-stranded RNA such as poly(I:C) mimicking viral dsRNA, which is a molecular pattern found in most viruses.5–7 TLR4 is required for the recognition of endotoxin of Gram-negative bacteria.2 TLR9 recognizes unmethylated CpG DNA motifs in both viruses and bacteria.7–10 These receptors constitute the first line of defense against many pathogens and play a crucial role in the function of the innate immune system by activating NF-κB and other signaling pathways to produce inflammatory cytokines.2,3
Activation of the innate immune system by TLRs influences the development of adaptive immune responses. TLR4 cognate ligand LPS, the most studied and well-characterized pathogen-associated molecular patterns, mediates much of its pleiotropic effects on antigen-presenting cells. In vivo coadministration of LPS, along with soluble proteins, results in lasting antigen-specific T-cell memory.11,12 LPS enhances antigen presentation and costimulatory molecule expression by DCs and induces the synthesis and release of proinflammatory mediators.12,13 Moreover, animals deficient in the TLR signal adaptor MyD88 molecule failed to generate proinflammatory and Th1 responses when stimulated with TLR ligands.14–16 These animals are highly susceptible to infection with a wide variety of different pathogens, including Staphylococcus aureus,17 Listeria monocytogenes,18,19 Toxoplasma gondii,20 and Mycobacterium tuberculosis (MTB).21,22 MyD88-deficient mice are also highly susceptible to Leishmania major infection associated with a polarized Th2 response.23 Study with an intracellular infection model by pathogen Chlamydia muridarum showed that MyD88-dependent early IL-17 production was important in protection of mice against airway infection.24 These findings have led to the conclusion that MyD88 plays a critical role in host resistance to microbial infection and in adaptive immunity.
Induction of experimental autoimmune diseases, such as experimental autoimmune encephalomyelitis (EAE), experimental autoimmune orchitis, and experimental autoimmune uveitis (EAU), by immunization of susceptible animals with the respective organ-specific autoantigens involves an obligatory adjuvant effect. Traditionally, this has been achieved by emulsifying the antigen in complete Freund adjuvant (CFA) containing MTB,25–27 which drives a predominantly cell-mediated immune response, characterized by the production of IFN-γ and other proinflammatory cytokines. TLR2 and TLR4 have been implicated in the immune response to MTB.28–30 In addition, it is reported that CpG oligonucleotides, such as are present in bacterial DNA, are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo.31 Many, but not all, strains of mice also require pertussis toxin (PT) as an additional adjuvant for animal models. We showed previously that PT enhances the Th1 response when used as part of a disease-inducing immunization regimen32,33 and that it binds to TLR4 on dendritic cells.34 Therefore, the components of MTB and PT acting as ligands to stimulate TLRs are the sources of the adjuvant effect necessary to facilitate induction of experimental autoimmune diseases. Our previous study showed that mice with MyD88 or IL-1R deficiency were resistant to EAU and had profoundly altered immunologic responses. However, mice deficient in single-gene TLR2, TLR4, or TLR9 were fully susceptible to the disease and had essentially unaltered immunologic responses in comparison with wild-type animals.16 These results raised the possibility that signaling through TLR2, TRL4, or TRL9 is either unnecessary or redundant for EAU induction.
We expanded this study to examine EAU induction in mice with a single TLR3 gene deficiency or double TLR2+4, TLR2+9, or TLR4+9 gene deficiency. We then assessed the outcome of augmenting TLR signaling on EAU induction using the TLR4 agonist LPS and the TLR2, TRL3, or TRL9 agonist pam3cys, poly I:C, or CpG DNA, respectively. The results demonstrated that each one of the agonists was capable of enhancing EAU scores in a suboptimal immunization regimen, suggesting that TLR2, TRL3, TRL4, and TRL9 signaling is redundant in the adjuvant effect needed to induce EAU. Our data also support the notion that the activation of diverse TLRs after microbial infection may contribute to the pathogenesis of autoimmune diseases such as uveitis.
Materials and Methods
Animals
MyD88−/−, TLR2−/−, TLR4−/−, or TLR9−/− mice were originally generated by Shizuo Akira (Osaka University, Osaka, Japan).35 TLR3−/− mice were purchased from Taconic Farms (Germantown, NY). These mice were backcrossed 10 or more generations onto the C57BL/6 background and were then intercrossed to obtain the knockout (KO) genotypes and wild-type (WT) mice (as control). TLR2+4, TLR2+9, and TLR4+9 double-gene knockout mice were generated by backcross of these single KO mice. Littermates of both sexes between 8 and 12 weeks old were used in all experiments. WT B10.RIII mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were kept in a specific pathogen-free facility and given water and standard laboratory chow ad libitum. Animal care and use were in compliance with institutional guidelines and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antigen and Reagents
CFA and incomplete Freund adjuvant were purchased from Difco Laboratories (Sparks, MD). MTB strain H37RA was purchased from Sigma-Aldrich (St. Louis, MO). Purified LPS from Escherichia coli 011:B4 strain was purchased from Invivogen (San Diego, CA). Purified Bordetella PT and polymyxin B were purchased from Sigma-Aldrich. Pam3cys, poly I:C, and CpG ODN were purchased from Apotech (San Diego, CA). Interphotoreceptor retinoid-binding protein (IRBP) was isolated from bovine retinas, as described previously, using Con A-Sepharose affinity chromatography and fast performance liquid chromatography.36 IRBP preparations were made into aliquots and stored at −70°C.
EAU Induction and Scoring
EAU in C57BL/6 background mice was induced by active immunization with 150 μg IRBP in PBS emulsified 1:1 vol/vol in CFA that had been supplemented with MTB to 2.5 mg/mL. For the purpose of testing the enhancing effect of TLR agonist on mice deficient in TLR3 or TLR4 (C57BL/6 background), EAU was induced with a suboptimal regimen of 100 μg IRBP in PBS-emulsified 1:1 vol/vol in CFA with MTB to 1 mg/mL. EAU in B10.RIII background mice was induced by immunization with 3 μg IRBP in PBS-emulsified 1:1 vol/vol in CFA (MTB 1 mg/mL). A total of 200 μL of emulsion was injected subcutaneously, divided among the base of the tail and both thighs. For C57BL/6 mice, PT (0.5 μg/mouse) in PBS containing 2% normal mouse serum was given by intraperitoneal injection concurrently with immunization as additional adjuvant. Clinical EAU was evaluated by funduscopic analysis under a binocular microscope after dilation of the pupil and was graded on a scale of 0 to 4 using criteria based on the extent of inflammatory lesions, as described in detail elsewhere.37 Eyes harvested 21 days after immunization were prefixed in 4% phosphate-buffered glutaraldehyde for 1 hour (to prevent artifactual detachment of the retina) and then transferred to 10% phosphate-buffered formaldehyde until processing. Fixed and dehydrated tissue was embedded in methacrylate, and 4- to 6-μm sections were stained with standard hematoxylin and eosin. Eye sections cut through pupillary-optic nerve planes were scored in a masked fashion. Severity of EAU was graded on a scale of 0 to 4 in half-point increments using the criteria described previously, based on lesion type, number, and size.37,38
Delayed-type Hypersensitivity
To assess delayed-type hypersensitivity (DTH), 10 μg IRBP in 10 μL PBS was injected into the ear pinna. Ear thickness increment was measured 48 hours later using a spring-loaded micrometer. The specific response was calculated as the difference between ear thickness after IRBP injection minus ear thickness before IRBP injection.
Lymphocyte Proliferation Assay
Draining (inguinal and iliac) lymph nodes were collected 21 days after immunization and were pooled within the group. Triplicate 0.2-mL cultures containing 5 × 105 cells were seeded in round-bottom, 96-well microtiter plates. RPMI 1640 (BioWhittaker, Walkersville, MD) was supplemented with mouse serum, mercaptoethanol, antibiotics, glutamine, and nonessential amino acids, as described,16 and contained gradient IRBP started at 20 μg/mL as stimulant. The cultures were incubated for a total of 60 hours. Tritiated thymidine (1 μCi/well) was added during the last 18 hours. The data are shown as counts per minute.
Cytokine Assays
For detection of antigen-induced cytokines in cell culture supernatants, inguinal and iliac lymph node cells from immunized mice were cultured in 24-well, flat-bottom plates (5 × 106 cells/mL culture medium per well) either alone or with IRBP at 20 μg/mL. Supernatants were collected after 48 hours and were kept frozen in small aliquots at −70°C. Cytokines were measured by multiplex ELISA (SearchLight; Pierce Boston Technology, Woburn, MA)39 (http://www.searchlightonline.com).
Reproducibility and Statistical Analysis
Experiments were repeated at least twice. Results were highly reproducible. Figures show pooled data from repeat experiments or from representative experiments, as indicated. Statistical significance of differences in disease scores was calculated using Snedecor and Cochran's test40 for linear trend in proportions, with each mouse (average of both eyes) as one statistical event. This is a nonparametric test that generates its probability values by frequency analysis of the number of individuals at each possible score, thus taking into account both severity and incidence of disease. Delayed hypersensitivity and lymphocyte proliferation data were analyzed using an independent t-test. P ≤ 0.05 was considered significant.
Results
EAU Induction in TLR3 Single Gene– or TLR2+4, TLR4+9, TLR2+9 Double Gene–Deficient Mice
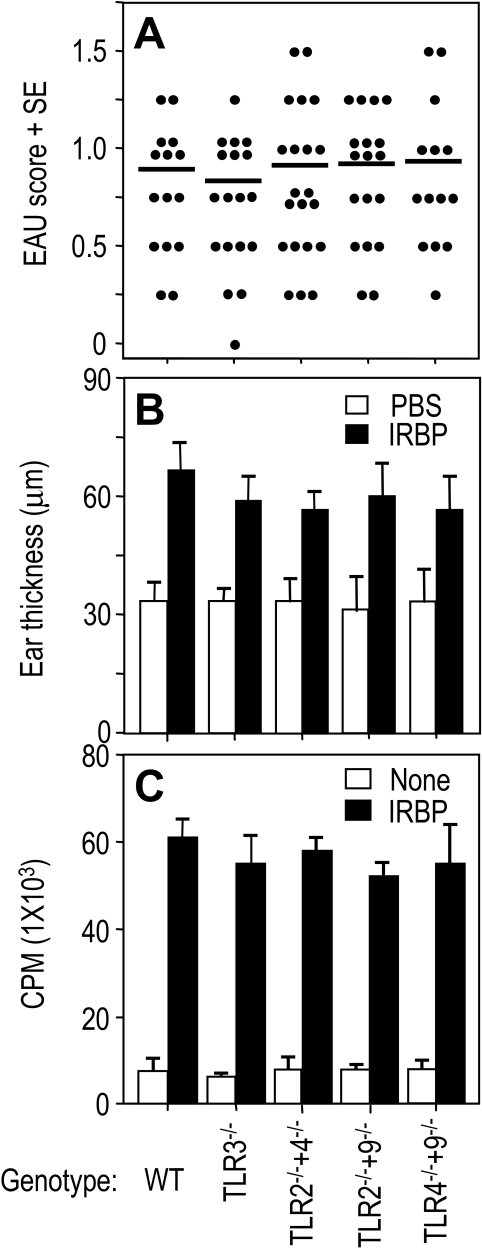
Our previous report showed that MyD88−/− mice failed to develop EAU. However, mice deficient in TLR2, TLR4, or TLR9 developed similar disease compared with control normal homozygous littermates.16 In this study, we examined EAU induction in mice deficient in TLR3 single gene or TLR2+4, TLR4+9, TLR2+9 double genes on the C57BL/6 background, immunized with 150 μg retinal antigen IRBP in CFA and given 0.5 μg/mouse of PT as additional adjuvant. Evaluation of eyes collected for histopathology on day 21 showed that EAU scores in all TLR3 single-gene knockout mice and in TLR2+4, TLR4+9, and TLR2+9 double-gene knockout mice were similar to those of control normal littermates (Fig. 1A). These data indicated that there was no discernible effect of deleting TLR2, TLR3, TLR4, or TLR9 on EAU induction.
Figure 1.
EAU and associated cellular responses in TLR-deficient mice. The mice deficient in TLR3 single gene or TLR2+4, TLR2+9, and TLR4+9 double genes were immunized with 150 μg IRBP in PBS emulsified 1:1 vol/vol in CFA (2.5 mg/mL). At the same time, 0.5 μg PT as additional adjuvant was injected intraperitoneally. (A) EAU scores of eyes, as evaluated by histopathology 21 days after immunization. Each point represents the EAU score of one mouse (average of both eyes). (B) DTH scores of mice challenged with 10 μL IRBP into the ear pinna 19 days after immunization. Scores represent ear thickness 48 hours after antigen or PBS injection. (C) Antigen-specific proliferation of draining lymph node cells 21 days after immunization. Shown are counts per minute of 3H thymidine (±SE) of triplicates. (A) Pooled data of three experiments. (B, C) Representative results of three repeat experiments.
EAU-associated DTH response and IRBP-specific T-cell proliferation in these mice were examined. DTH responses of TLR3−/−, TLR2−/−+4−/−, TLR2−/−+9−/−, and TLR4−/−+9−/−–deficient mice and their normal littermates immunized for EAU were elicited by ear challenge 19 days after immunization, as described in Materials and Methods, and the responses were read on day 21. Similar to EAU scores, DTH responses to IRBP of all TLR3−/−, TLR2−/−+4−/−, TLR2−/−+9−/−, and TLR4−/−+9−/−–deficient mice were similar to those of their wild-type littermate controls (Fig. 1B). In keeping with the DTH responses, proliferation to IRBP of draining lymph node cells collected 21 days after immunization was similar in both WT and deficient littermates of the TLR3, TLR2+4, TLR2+9, TLR4+9 lines (Fig. 1C).
TLR Agonists Enhanced EAU and Associated Immunologic Responses under Conditions of a Suboptimal Immunization Regimen
Results with TLR knockout mice indicated that signaling through TLR2, TLR3, TLR4, and TLR9 might have been redundant for EAU induction. To clarify these questions, we took advantage of a highly susceptible mouse EAU model in B10.RIII mice to evaluate the role of these TLRs using their agonists, in the adjuvant effect involved in the induction of EAU.
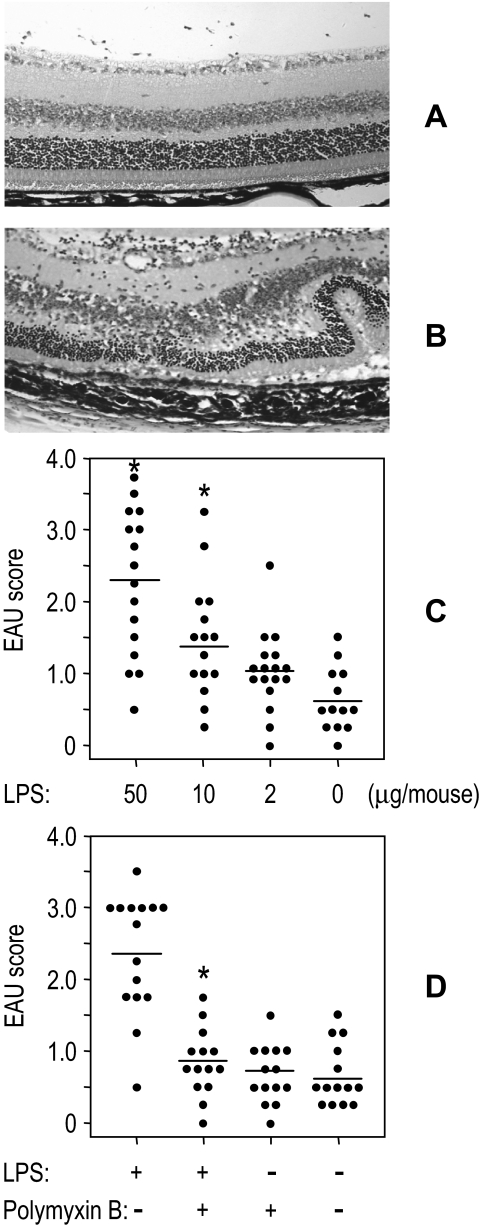
We initially examined the TLR4 ligand LPS. EAU induction in the moderately susceptible C57BL/6 requires challenge with high doses of IRBP (150 μg/mouse) and MTB (2.5 mg/mL) in CFA, in addition to intraperitoneal injection of PT as an additional adjuvant. Similar EAU scores in B10.RIII mice were achieved by immunization with as little as 3 μg/mouse IRBP and 1 mg/mL MTB in CFA. This suboptimal regimen in B10.RIII mice typically results in EAU scores ranging from 0.5 to 1, which are easily discernible on clinical observation and histopathologic examination (Fig. 2A), and allows visualization of the enhanced disease severity by a given treatment (Fig. 2B). The data showed that LPS incorporated into CFA during immunization facilitated the development of EAU. The effect on EAU induction was dose-dependent, reaching threefold enhancement of scores at a dose of 50 μg/mouse compared with control mice (Fig. 2C). To confirm that the enhancing effect on EAU induction was elicited by LPS rather than by some other contaminant in the reagent, polymyxin B, a cyclic cationic polypeptide antibiotic known to specifically block the biological effect of LPS,41 was included in the IRBP/CFA emulsion. A significant decrease in EAU scores was observed in the group treated with LPS+PMB compared with LPS alone (P < 0.01; Fig. 2D), confirming that LPS played a role in the enhanced EAU induction.
Figure 2.
Enhancement of EAU by LPS in B10.RIII mice. B10.RIII mice were immunized with 3 μg IRBP and the indicated doses of LPS or with or without polymyxin B (50 μg) in CFA (1 mg/mL) administered subcutaneously. EAU scores of eyes as evaluated by histopathology 21 days after immunization. (A, B) Ocular histopathology of EAU score 0.5 (A) and 3 (B) (hematoxylin and eosin; magnification, ×100). (C, D) Enhancement of EAU by LPS (C) and blockage by polymyxin B (D). Each point represents the EAU score of one mouse (average of both eyes). *P < 0.05; statistically significant difference in values from PBS group (C) or LPS-treated group (D).
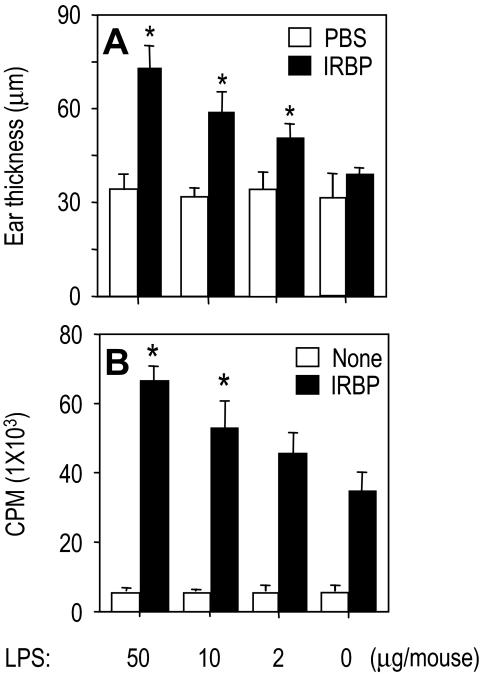
To examine EAU-associated cellular immune responses in vivo and in vitro, IRBP-immunized and LPS-included B10.RIII mice were challenged 2 days before the termination of the experiment for IRBP-specific DTH by ear assay. Any mouse for which LPS was included in immunization exhibited a dose-dependent enhancement of DTH (Fig. 3A). Similar to the DTH, lymphocyte proliferation was markedly enhanced by the inclusion of LPS in the immunization regimen (Fig. 3B). Thus, LPS was able to enhance the DTH response and antigen-specific lymphocyte proliferation.
Figure 3.
Enhancement of EAU-associated immunologic response by LPS in B10.RIII mice. B10.RIII mice were immunized with 3 μg IRBP and the indicated doses of LPS in CFA (1 mg/mL) administered subcutaneously. The data in each panel are representative of three or more repeat experiments. (A) DTH scores of mice challenged with 10 μL IRBP into the ear pinna 19 days after immunization. Scores represent ear thickness 48 hours after antigen or PBS injection. (B) Antigen-specific proliferation of draining lymph node cells 21 days after immunization. Shown are counts per minute of 3H thymidine (±SE) of triplicates. *P < 0.05; statistically significant difference in values from PBS group.
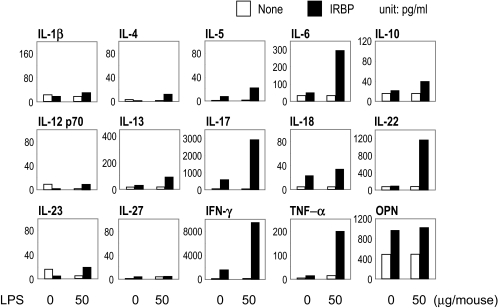
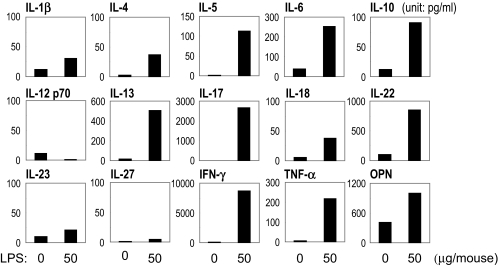
To assess the phenotype of the adaptive immune response to IRBP in LPS-treated mice, draining lymph node cells were collected 21 days after immunization and were stimulated in culture with IRBP. Supernatants collected after 48 hours were assayed for cytokine content by multiplex ELISA (SearchLight; Pierce Boston Technology). Although control mice had moderate increases only in IL-17 and IFN-γ, IFN-γ and IL-17 were strongly enhanced in the LPS-treated group (Fig. 4). IL-6, IL-22, TNF-α, and osteopontin (OPN) were increased, but there were no distinct changes in IL-1β, IL-12 p70, IL-18, IL-23, or IL-27 (Fig. 4).
Figure 4.
LPS upregulates antigen-specific cytokine production in B10.RIII mice. B10.RIII mice were immunized with 3 μg IRBP and the indicated doses of LPS in CFA (1 mg/mL) administered subcutaneously. Lymph node cells harvested 21 days after immunization from IRBP-immunized mice were pooled within groups, and cultures of 5 × 106 cells/mL were stimulated with IRBP (10 μg/mL). Shown is cytokine content in pooled supernatants of three experiments collected after 48-hour culture as assayed by multiplex ELISA.
Bacterial products tend to have strong effects on cells of the innate immune system; this, in turn, helps to determine the effector choices of the adaptive response that follows. To assess the innate response to LPS in an EAU model, we collected lymph node cells 2 days after immunization with or without LPS and determined the cytokine production ex vivo without further stimulation. Cytokines in supernatants from naive lymph nodes were undetectable (data not shown). Cytokines in the culture supernatants from groups treated with low-dose IRBP in CFA without LPS were low. In contrast, numerous cytokines (IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, IL-22, IFN-α, TNF-α, and OPN) were increased in the LPS-treated group (Fig. 5).
Figure 5.
Innate immune response induced by LPS in B10.RIII mice. B10.RIII mice were immunized with 3 μg IRBP with or without 50 μg LPS in CFA administered subcutaneously. Inguinal and iliac lymph node cells were collected at day 2 after immunization. Cells (5 × 106/mL) were cultured in 24-well flat-bottomed plates alone for 24 hours. Cytokine protein levels in the pooled supernatants of three experiments were measured by multiplex ELISA.
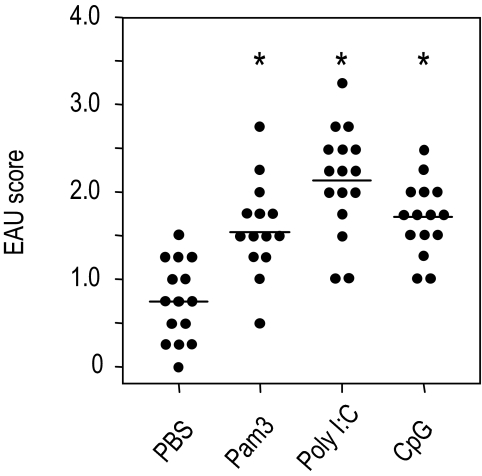
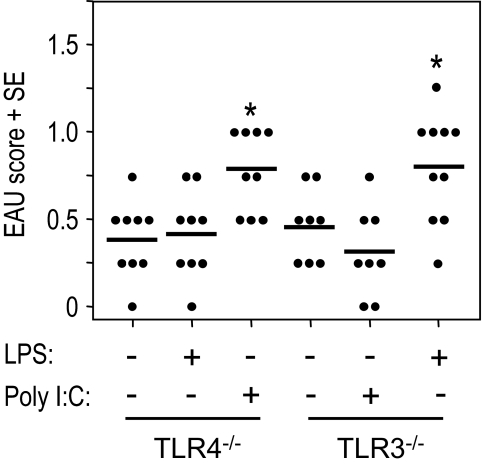
To examine whether the disease-enhancing effect was restricted to LPS, we tested the effects of additional agonists specific for other TLRs. B10.RIII mice were immunized with low doses of IRBP and MTB in CFA in the presence or absence of the TLR2, TLR3, and TLR9 agonist pam3cys, poly I:C, or CpG ODN. Eyes were harvested for histopathologic examination 21 days later. Figure 6 shows that pam3cys, poly I:C, or CpG ODN incorporated into the immunization mixture enhanced disease scores significantly (P ≤ 0.01) compared with the PBS-treated group. To test whether the agonist activated the respective receptor to enhance EAU, mice deficient in TLR4 or TLR3 were immunized with 100 μg IRBP and LPS (50 μg) or poly I:C (50 μg) and 0.2 μg/mouse of PT intraperitoneally as additional adjuvant. EAU scores of eyes were evaluated by histopathology 21 days after immunization. The results showed that LPS incorporated into CFA during immunization did not enhance EAU induction in TLR4-deficient mice and that poly I:C inclusion in immunization did not enhance EAU development in TLR3 knockout mice (Fig. 7). The data suggested that the enhancing effect of LPS or poly I:C on EAU induction occurred specifically by the activation of TLR4 or TLR3. These results support the notion that signaling through various TLRs is redundant for disease induction and suggest that diverse microbial infections may contribute to EAU pathology.
Figure 6.
Enhancement of EAU by pam3cys, poly I:C, and CpG in B10.RIII mice. B10.RIII mice were immunized with 3 μg IRBP and pam3cys (50 μg), poly I:C (50 μg), and CpG (20 μg) in CFA (1 mg/mL) administered subcutaneously. EAU scores of eyes as evaluated by histopathology 21 days after immunization. Each point represents the EAU score of one mouse (average of both eyes). These are the pooled data of three repeat experiments. *P < 0.05; statistically significant difference in values from the PBS group.
Figure 7.
Enhancement of EAU by LPS or poly I:C in TLR4−/− or TLR3−/− mice. Mice deficient in the TLR4 or TLR3 gene were immunized with 100 μg IRBP in PBS-emulsified 1:1 vol/vol in CFA (1 mg/mL). At the same time, 0.2 μg PT as additional adjuvant was injected intraperitoneally. EAU scores of eyes as evaluated by histopathology 21 days after immunization. Each point represents the EAU score of one mouse (average of both eyes). These are the pooled data of two repeat experiments. *P < 0.05; statistically significant difference in values from PBS group.
Discussion
There is a constant interplay between the innate and adaptive immune systems that leads to a protective immune response against pathogens and contributes effectively to self- and nonself-discrimination. The discovery of mammalian TLRs has provided new insights into the association between innate responses to microbes and adaptive responses and, consequently, their impact on autoimmune diseases. The activation of TLRs leads to the induction of antimicrobial pathways central to innate defense and to the upregulation of antigen-presentation molecules and the secretion of cytokines that influence the nature of the adaptive immune response. Inappropriate activation of TLR pathways by endogenous or exogenous ligands may lead to the initiation or perpetuation, or both, of autoimmune responses and tissue injury. MTB as a necessary adjuvant for the induction of experimental autoimmune diseases is an excellent model of the relationship between microbial infection and immunologically driven diseases.
Toll-like receptors involved in responses to MTB, the prototypic adjuvant that promotes tissue-specific autoimmunity, should be the ones involved in their adjuvant effects. It is reported that TLR2, TLR4, and TLR9 are critical receptors for protection against MTB infection.28–31 TLR3 is reported to be an endogenous sensor of tissue necrosis.42 Although published reports agree that animals deficient in MyD88 are resistant to disease challenge,43 studies in TLR gene knockout mice report conflicting results. For example, Prinz et al.44 show that MyD88-deficient mice are resistant to EAE and that this protection is partially the result of the engagement of TLR9. Gonnella et al.45 show that immunization with myosin induced greater severity of myocarditis in wild-type than in TLR-4−/− mice in an EAM model. However, Marta et al.46 showed that TLR4−/− and TLR9−/− mice exhibited more severe EAE symptoms than wild-type mice. Our previous study showed that none of the mice deficient in the TLR2, TLR4, or TLR9 gene showed inhibited EAU induction or changes in the associated immunologic responses (DTH, proliferation, and cytokine production).16
In this study, we examined EAU induction in mice deficient in the TLR3 single gene or the TLR2+4, TLR4+9, and TLR2+9 double genes. None of these mice showed inhibited EAU induction or changes in the associated immunologic responses (Fig. 1). These data suggest that TLR2, TLR3, TLR4, and TLR9 signaling either is not needed or, more likely, is redundant in the adjuvant effect needed to induce EAU but could not distinguish between these two possibilities. We reasoned that functions of missing TLR genes may be compensated for by other genes in animal models. Furthermore, mycobacteria are a complex mixture and contain TLR ligands in addition to TLR2 and TLR4, some of which may not yet be characterized. In addition, the local inflammation that is induced may generate endogenous ligands not only for TLRs but also for NLRs and other innate receptors. It is important to examine the effects of different TLRs in a positive fashion by using their cognate ligands. Therefore, we turned to a highly susceptible and robust EAU model in B10.RIII mice. When immunized with a suboptimal regimen of IRBP, this model allowed us to examine the enhancing effect of specific TLR agonists on the immunization mixture in vivo and ex vivo on disease and on associated immunologic responses. We were gratified to find that not only the TLR4 agonist LPS but also the TLR2 and TLR9 agonists pam3cys and CpG DNA enhanced disease. Furthermore, the TLR3 agonist poly I:C, which can signal through a pathway involving TRIF rather than MyD88, also enhanced EAU scores, suggesting that both MyD88-dependent and MyD88-independent pathways can participate in the pathogenesis of autoimmune diseases.
Interestingly, engaging TLR4 by incorporating LPS into the immunization emulsion maintained the balance between Th17- and Th1-related cytokine responses, enhancing both more or less equivalently. In addition, the cytokines that were increased included Th2 cytokines, which may play a counterregulatory role in restraining Th1/17 cell-mediated autoimmune diseases. This is consistent with findings of others that LPS drove Th2 cytokine production.47
With the exception of sympathetic ophthalmia, in which a penetrating wound to one eye and the release of normally sequestered antigens are felt to trigger autoimmunization leading to disease, the etiologic causes of autoimmune uveitis in humans are unknown. Antigenic mimicry between microbial components has been postulated. For example, infections with Klebsiella are felt to be associated with HLA B27–associated acute anterior uveitis.48 In the EAU model, there are several published reports of microbial antigen mimics that induce disease in mice or in rats, including sequences from yeast histone proteins, rotavirus, hepatitis B virus DNA polymerase, gag-pol polyprotein of Baboon endogenous virus and gag-pol polyprotein of AKV murine leukemia virus, and E. coli protein.49,50 Notably, all these peptide mimics had to be injected in CFA for EAU to be induced, suggesting that an adjuvant effect separate from antigenic recognition is required. We propose that infections with microbes expressing both a cross-reactive sequence and TLR agonist activity providing built-in adjuvanticity may be involved in the etiology of autoimmune uveitis.
In summary, our data in TLR2, TLR3, TLR4, and TLR9 single-gene knockout mice and TLR2+4, TLR2+9, and TLR4+9 double-gene knockout mice do not point to a unique role of these receptors in the induction of EAU. The ability of their cognate ligands pam3cys, poly I:C, LPS, and CpG to enhance the disease points to the conclusion that TLR2, TLR3, TLR4, and TLR9 have redundant roles in EAU induction. Our findings support the notion that activation of TLRs after microbial infection may be an important contributor to the pathogenesis of autoimmune diseases such as uveitis.
Acknowledgments
The authors thank Ji Ming Wang (Frederick Cancer Research Facility, National Cancer Institute) for his helpful critique of the manuscript.
Footnotes
Supported in part by National Basic Research Program of China Grant 2007CB512206, National Natural Science Foundation of China Grant 30772011, and Science and Technology Planning Project of Guangdong Province of China Grant 2006B36006004.
Disclosure: J. Fang, None; D. Fang, None; P.B. Silver, None; F. Wen, None; B. Li, None; X. Ren, None; Q. Lin, None; R.R. Caspi, None; S.B. Su, None
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003;21:335–376 [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol 2007;14:14.12 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Semin Immunol 2007;19:24–32 [DOI] [PubMed] [Google Scholar]
- 4.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol 2009;130:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiron D, Pellat-Deceunynck C, Amiot M, Bataille R, Jego G. TLR3 ligand induces NFκB activation and various fates of multiple myeloma cells depending on IFN-α production. J Immunol 2009;182:4471–4478 [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv Rev 2008;60:805–812 [DOI] [PubMed] [Google Scholar]
- 7.Li M, Zhou Y, Feng G, Su SB. The critical role of Toll-like receptor signaling pathways in the induction and progression of autoimmune diseases. Curr Mol Med 2009;9:365–374 [DOI] [PubMed] [Google Scholar]
- 8.Coch C, Busch N, Wimmenauer V, et al. Higher activation of TLR9 in plasmacytoid dendritic cells by microbial DNA compared with self-DNA based on CpG-specific recognition of phosphodiester DNA. J Leukoc Biol 2009;86:663–670 [DOI] [PubMed] [Google Scholar]
- 9.Yasuda K, Richez C, Uccellini MB, et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol 2009;183:3109–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crompton PD, Mircetic M, Weiss G, et al. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol 2009;182:3318–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity 1995;2:261–270 [DOI] [PubMed] [Google Scholar]
- 12.Yadav R, Zammit DJ, Lefrancois L, Vella AT. Effects of LPS-mediated bystander activation in the innate immune system. J Leukoc Biol 2006;80:1251–1261 [DOI] [PubMed] [Google Scholar]
- 13.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 1997;388:782–787 [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253–258 [DOI] [PubMed] [Google Scholar]
- 15.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998;9:143–150 [DOI] [PubMed] [Google Scholar]
- 16.Su SB, Silver PB, Grajewski RS, et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J Immunol 2005;175:6303–6310 [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000;165:5392–5396 [DOI] [PubMed] [Google Scholar]
- 18.Seki E, Tsutsui H, Tsuji NM, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol 2002;169:3863–3868 [DOI] [PubMed] [Google Scholar]
- 19.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol 2002;169:3869–3875 [DOI] [PubMed] [Google Scholar]
- 20.Scanga CA, Aliberti J, Jankovic D, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 2002;168:5997–6001 [DOI] [PubMed] [Google Scholar]
- 21.Shi S, Nathan C, Schnappinger D, et al. MyD88 primes macrophages for full-scale activation by interferon-gamma yet mediates few responses to Mycobacterium tuberculosis. J Exp Med 2003;198:987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun 2004;72:2400–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraille E, De Trez C, Brait M, De Baetselier P, Leo O, Carlier Y. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol 2003;170:4237–4241 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Gao L, Lei L, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol 2009;183:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuohy VK. Peptide determinants of myelin proteolipid protein (PLP) in autoimmune demyelinating disease: a review. Neurochem Res 1994;19:935–944 [DOI] [PubMed] [Google Scholar]
- 26.Kerlero de Rosbo N, Mendel I, Ben-Nun A. Chronic relapsing experimental autoimmune encephalomyelitis with a delayed onset and an atypical clinical course, induced in PL/J mice by myelin oligodendrocyte glycoprotein (MOG)-derived peptide: preliminary analysis of MOG T cell epitopes. Eur J Immunol 1995;25:985–993 [DOI] [PubMed] [Google Scholar]
- 27.Mendel I., Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol 1995;25:1951–1959 [DOI] [PubMed] [Google Scholar]
- 28.Reiling N, Holscher C, Fehrenbach A, et al. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol 2002;169:3480–3484 [DOI] [PubMed] [Google Scholar]
- 29.Branger J, Leemans JC, Florquin S, Weijer S, Speelman P, Van Der Poll T. Toll-like receptor 4 plays a protective role in pulmonary tuberculosis in mice. Int Immunol 2004;16:509–516 [DOI] [PubMed] [Google Scholar]
- 30.Drennan MB, Nicolle D, Quesniaux VJ, et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol 2004;164:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal BM, Chang JT, Shevach EM. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J Immunol 2000;164:5683–5688 [DOI] [PubMed] [Google Scholar]
- 32.Su SB, Silver PB, Wang P, Chan CC, Caspi RR. Dissociating the enhancing and inhibitory effects of pertussis toxin on autoimmune disease. J Immunol 2003;171:2314–2319 [DOI] [PubMed] [Google Scholar]
- 33.Agarwal RK, Sun SH, Su SB, Chan CC, Caspi RR. Pertussis toxin alters the innate and the adaptive immune responses in a pertussis-dependent model of autoimmunity. J Neuroimmunol 2002;129:133–140 [DOI] [PubMed] [Google Scholar]
- 34.Wang ZY, Yang D, Chen Q, et al. Induction of dendritic cell maturation by pertussis toxin and its B subunit differentially initiate Toll-like receptor 4-dependent signal transduction pathways. Exp Hematol 2006;34:1115–1124 [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi O, Akira S. Genetic approaches to the study of Toll-like receptor function. Microbes Infect 2002;4:887–895 [DOI] [PubMed] [Google Scholar]
- 36.Pepperberg DR, Okajima TL, Ripps H, Chader GJ, Wiggert B. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol 1991;54:1057–1060 [DOI] [PubMed] [Google Scholar]
- 37.Caspi RR, Sun B, Agarwal RK, et al. T cell mechanisms in experimental autoimmune uveoretinitis: susceptibility is a function of the cytokine response profile. Eye 1997;11(pt 2):209–212 [DOI] [PubMed] [Google Scholar]
- 38.Chan CC, Caspi RR, Ni M, et al. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun 1990;3:247–255 [DOI] [PubMed] [Google Scholar]
- 39.Moody MD, Van Arsdell SW, Murphy KP, Orencole SF, Burns C. Array-based ELISAs for high-throughput analysis of human cytokines. BioTechniques 2001;31:186–190, 192–184 [DOI] [PubMed] [Google Scholar]
- 40.Snedecor GW, Cochran WG. Statistical Methods Ames, IA: Iowa State University Press; 1967. [Google Scholar]
- 41.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest 2007;117:2197–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 2008;205:2609–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin SF, Dudda JC, Bachtanian E, et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med 2008;205:2151–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prinz M, Garbe F, Schmidt H, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest 2006;116:456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonnella PA, Waldner H, Del Nido PJ, McGowan FX. Inhibition of experimental autoimmune myocarditis: peripheral deletion of TcR Vβ 8.1, 8.2+ CD4+ T cells in TLR-4 deficient mice. J Autoimmun 2008;31:180–187 [DOI] [PubMed] [Google Scholar]
- 46.Marta M, Andersson A, Isaksson M, Kampe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol 2008;38:565–575 [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee S, Chen LY, Papadimos TJ, Huang S, Zuraw BL, Pan ZK. Lipopolysaccharide-driven Th2 cytokine production in macrophages is regulated by both MyD88 and TRAM. J Biol Chem 2009;284:29391–29398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahly H, Podschun R, Kekow J, Nolle B, Gross WL, Ullmann U. Humoral immune response to Klebsiella capsular polysaccharides in HLA-B27-positive patients with acute anterior uveitis and ankylosing spondylitis. Autoimmunity 1998;28:209–215 [DOI] [PubMed] [Google Scholar]
- 49.Shinohara T, Singh VK, Tsuda M, Yamaki K, Abe T, Suzuki S. S-antigen: from gene to autoimmune uveitis. Exp Eye Res 1990;50:751–757 [DOI] [PubMed] [Google Scholar]
- 50.Wildner G, Diedrichs-Mohring M. Autoimmune uveitis and antigenic mimicry of environmental antigens. Autoimmun Rev 2004;3:383–387 [DOI] [PubMed] [Google Scholar]