The authors show that contact lens–related Pseudomonas aeruginosa keratitis can occur in vivo after a single inoculation of the lens. The data suggest that biofilm formation on the posterior lens surface, or other bacterial adaptations to the in vivo environment, are involved in the pathogenesis of infection. The data also show that fluorescein staining of the cornea does not predict increased susceptibility to infection.
Abstract
Purpose.
Contact lens wear predisposes to Pseudomonas aeruginosa keratitis, but the mechanisms involved remain unclear. An in vivo model was used to study lens inoculation conditions enabling disease.
Methods.
Custom-made hydrogel contact lenses were fitted to rats after incubation in P. aeruginosa approximately 1011 cfu/mL (3 hours) or approximately 103 cfu/mL (24 hours). Another group was inadvertently inoculated with a suction pen previously used with high inocula, but rinsed in ethanol and stored dry (6 months). Some corneas were tissue paper–blotted to cause fluorescein staining before lens fitting. Contralateral eyes were untreated. Twenty-four hours after disease detection, lenses were transferred to naive rats or examined by confocal microscopy before homogenization to quantify viable bacteria. After lens removal, corneas were washed to collect nonadherent bacteria and were analyzed by immunohistochemistry.
Results.
All eyes challenged with unworn contaminated lenses developed keratitis after approximately 7 to 10 days. Disease delay and severity were unaffected by inoculum parameters or tissue blotting but occurred sooner with lenses transferred from infected eyes (∼2 days). Worn lenses and corneal washes contained infecting bacteria. Posterior, not anterior, lens surfaces harbored P. aeruginosa biofilms that penetrated the lens matrix. Diseased corneas showed an infiltration of phagocytes and T-lymphocytes.
Conclusions.
P. aeruginosa induces keratitis in this lens-wearing model after a single inoculation. Delayed disease onset was interesting considering the greater keratitis risk during extended wear. Infection did not require the disruption of corneal barrier function before lens wear and occurred without exposure to lens care solutions. The data suggest that keratitis involves biofilm formation or other bacterial adaptations in vivo.
Contact lens wear is the most common predisposing factor for corneal infection caused by Pseudomonas aeruginosa. However, the mechanisms by which contact lens wear predisposes the otherwise resistant cornea to infection by this or any other pathogen are not well understood. Importantly, the availability of daily disposable contact lenses, and of lens materials that are hyperpermeable to oxygen, has not eliminated the risk for contact lens–related infection.1–4 Thus, factors other than lens deposits and hypoxia appear to be involved in pathogenesis of this disease.
Our understanding of the factors that initiate contact lens–related infectious keratitis has been limited, in part, by the difficulty of obtaining lenses that fit the eyes of rodents. Although researchers have studied lens wear in rabbits and guinea pigs, these animals are expensive and awkward to work with. Rabbit models involve surgical removal of nictitating membranes, or suture-mediated eyelid closure, to overcome lens dislodgement and to maximize lens effects such as hypoxia.5 Guinea pig models (lens-wearing guinea pigs without surgical interventions) are of value for studying the ocular safety of lenses6 and the effects of extended lens wear on corneal physiology,7 but these animals are not susceptible to P. aeruginosa infection. Accordingly, most of our knowledge to date about how P. aeruginosa and other microbes infect the cornea has necessarily been derived from experiments using cultured corneal epithelial cells or in vivo rodent models in which lenses are not used. To enable susceptibility to infection without contact lens wear, researchers have heavily used a scarification model, or they have performed intrastromal injection of the infectious agent (for reviews see Refs. 8–10). Although these models are useful tools for elucidating the host responses to an already established infection or for exploring the role(s) of bacterial virulence factors in maintaining persistence within an infected cornea, they are of limited value for studying how lens wear enables disease susceptibility or for elucidating factors involved in the initiation of disease in the case of lens-related infection.
A rat model of extended hydrogel lens wear has been published.11 Experiments using this model have shown the induction of P. aeruginosa keratitis, the upregulation of proinflammatory cytokines, and dendritic cell and neutrophil infiltration of the cornea.11 The model has also been used to show increased proinflammatory responses and increased susceptibility to P. aeruginosa infection for low- versus high-Dk lenses.12 Thus, this model has advanced our understanding about P. aeruginosa infection once it has been initiated and about lens parameters that contribute to risk. Because the method requires repeated bacterial inoculation to enable infection, and consequently confusion in the timing of challenge, it is less useful for studying initiating events related to the bacteria or the early corneal response to them.
The purpose of the present study was to determine whether the previously published rodent model could be modified to enable P. aeruginosa keratitis using a single inoculation event (synchronizing the inoculum) so that conditions leading to disease susceptibility/initiation could be better defined. We hypothesized that the adjustment of bacterial growth conditions and the disruption of host epithelial barrier function (to fluorescein) by tissue paper blotting of the cornea might allow P. aeruginosa keratitis to develop after a single inoculation if given sufficient time. Surprisingly, results revealed that disease occurred under all conditions tested with a significant delay that was reduced only when the inoculum vehicle was a lens transferred from an already infected eye. Taken together, the data suggest that bacterial adaptation to the in vivo environment, possibly enabled by biofilm formation found on the posterior surface of lenses removed from infected eyes, is involved in the pathogenesis of contact lens–related keratitis.
Materials and Methods
Rat Contact Lenses
Low-Dk hydrogel contact lenses (69% water, Dk 26) were custom made to fit rat corneas and had the following dimensions: 5.34-mm diameter, 2.4-mm base curve, 80-μm center thickness, and 40-μm edge thickness. Integrity of the lenses was inspected under a stereomicroscope to ensure edges were smooth before lens fitting.
Bacteria Strain and Inoculum Preparation
P. aeruginosa invasive strain PAO1 expressing GFP on a pSMC2 plasmid (PAO1-GFP) was used in all experiments. Bacteria were grown on tryptic soy agar (TSA) plates supplemented with carbenicillin 300 μg/mL at 37°C for approximately 16 hours. For high-inoculum conditions, bacteria were suspended in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO) to a concentration of 1011 cfu/mL. For low-inoculum conditions, a single colony of bacteria was picked by a sterile toothpick and inoculated into 3.5 mL modified Mian's medium (7.5 mM NaH2PO4, 16.8 mM K2HPO4, 10 mM MgSO4, 0.2% NaNO3, 10 mM CH3COONa)13 contained within a contact lens case resulting in an initial concentration of 103 cfu/mL and reaching a final concentration of 106 cfu/mL after 24 hours at room temperature. Bacterial counts in the inocula were confirmed by viable counts. Contact lenses were incubated with either a high inoculum for 3 hours or a low inoculum for 24 hours at room temperature before placement on the left cornea of each rat. Lenses were not rinsed to remove nonadherent bacteria before insertion (∼10 μL soaking inoculum was carried over with the lens). Just before inoculation there were approximately 106 cfu adherent P. aeruginosa on each lens after incubation under high-inoculum conditions and approximately 105 cfu on each lens under low-inoculum conditions.
Lens-Wearing In Vivo Model
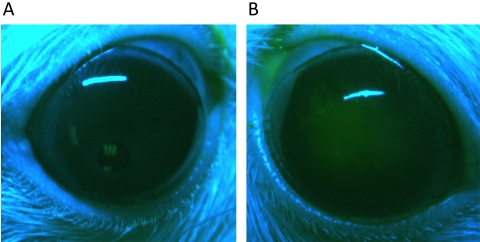
Three-month-old female Lewis rats were purchased from Charles River Laboratories Inc. (Wilmington, MA). During the experiment, animals were housed individually and were allowed normal activity without behavior restraints in a controlled environment (temperature at 72°F ± 4°F, humidity at 30%–40%, and 12-hour light/12-hour dark cycles). Before insertion and removal of inoculated contact lenses or examination and image-capturing of eyes, animals were subject to light anesthesia by administration of 2.5% isoflurane in medical-grade oxygen using a precision vaporizer. Corneas of some animals were blotted once with 1-ply tissue paper (Kimwipe; Kimberly-Clark, Irving, TX) before fitting with high-inoculum–soaked lenses. The blotting procedure sufficiently disrupted the corneal epithelium to allow extensive fluorescein staining (Fig. 1). In other experiments, bacteria were inadvertently introduced into the lens-wearing rat eye during handling with a previously “disinfected” suction pen. The pen had been used 6 months previously for high-inoculum experiments, ethanol-rinsed, air-dried, and stored dry for 6 months before use for fitting lenses that were otherwise sterile.
Figure 1.
Fluorescein staining of the normal rat cornea after tissue paper blotting injury. Corneas of Lewis rats were stained with fluorescein sodium solution (0.35%) to observe epithelial disruption. An unblotted rat cornea shows no staining, as expected (A). In contrast, corneas blotted with tissue paper show extensive staining, indicating epithelial disruption (B).
Each experimental group consisted of at least three animals. Contralateral eyes served as controls and were not fitted with lenses or inoculated with bacteria. Animals were monitored daily for corneal pathology. Twenty-four hours after a faint opacity was observed on the cornea, images were collected using a three-chip cooled camera (Optronics, Goleta, CA) attached to a stereomicroscope (Stemi 2000-C; Carl Zeiss, Thornwood, NY). A 5-point extended grading system (0–4) that assesses four characteristics of the pathology was used as described previously.14 This involved scoring the area of the opacity, density of the central and peripheral opacities, and epithelial surface quality. The calculated sum of scores for these four characteristics ranges from 0 (no infection) to a maximum of 16 (severe infection). When lens dislodgement occurred before disease initiation, the lens was not reinserted, but animals were observed for the remainder of the study.
After the development of corneal pathology, lenses were removed, and infected corneas were immediately washed with 10 μL PBS to collect nonadherent bacteria for quantification. Worn lenses of diseased animals were examined by laser scanning confocal microscopy before homogenization in 100 μL PBS. In some experiments, these lenses (from the infected low-inoculum group) were transferred to naive rats. Viable bacteria in ocular washes and on worn lenses were quantified by serial dilution and subsequent plating on nonselective TSA, with selective media inoculated in parallel: 300 μg/mL carbenicillin-supplemented TSA to select plasmid-bearing bacteria, MacConkey agar to select Gram-negative bacteria, and Cetrimide agar to select P. aeruginosa. Animals were killed by inhalation of 5% isoflurane before enucleation of eyes, which were processed for frozen-sectioning and immunofluorescence microscopy. All procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee of the University of California, Berkeley.
Immunohistochemistry of Corneal Tissue
Whole eyes were fixed in 4% paraformaldehyde (wt/vol) for 1 hour at room temperature, infiltrated with 30% sucrose in PBS (wt/vol) overnight at 4°C, and frozen in optimal cutting temperature embedding medium before sectioning to 5- to 8-μm thickness with a cryostat (CM1900; Leica Microsystems, Bannockburn, IL). To block nonspecific staining, sections were incubated in PBS containing 3% bovine serum albumin (BSA), 3% goat serum, and 0.1% Triton X-100 for 1 hour at room temperature. Sections were incubated in mouse monoclonal antibody raised against rat CD11b/c (labels the CR3 complement receptor found on most professional phagocytes), ED-1 (a monocyte/macrophage marker), αβ T-cell receptor or CD4 (BD Pharmingen, San Jose, CA; 1:100 dilution in blocking buffer) overnight at 4°C and then were washed three times for 5 minutes each in PBST (0.1% BSA and 0.1% Triton X-100 in PBS). Rhodamine-Red–conjugated goat anti-mouse IgG secondary antibody (Invitrogen, Carlsbad, CA; 1:5000 dilution in blocking buffer) was used for 1 hour in room temperature, followed by four washes for 10 minutes each in PBST. Samples were mounted and counterstained with mounting medium containing DAPI (Vectashield; Vector Laboratories, Burlingame, CA) to label cell nuclei (blue) and were viewed under an epifluorescence microscope (IX70; Olympus, Center Valley, PA).
Laser Scanning Confocal Microscopy of Contact Lenses
Contact lenses removed from infected animals were imaged with an upright confocal system (LSM 510; Carl Zeiss GmbH, Jena, Germany) using the 488-nm laser line to study bacterial binding as represented by the GFP signal. To enable viewing, contact lenses were cut in half, moistened with PBS, and flatmounted on glass slides. To enable both the anterior and the posterior sides of each lens to be studied and to avoid possible signal detection differences along the z-depth of thick samples, the lens halves were oriented in opposite directions so that both the posterior and the anterior surfaces were facing the objective and could be viewed in the same plane. Stacks of horizontal-plane (x-y) images from three random fields were acquired at 1-μm intervals down the z direction until signals diminished. Image projections and vertical cross-sections (x-z and y-z planes) were generated (LSM Image Browser; Zeiss). Pilot studies confirmed that bacteria attached to contact lenses removed from infected corneas retained the GFP plasmid.
Statistical Analysis
Numerical data are expressed as median values with lower and upper quartiles (Q1:Q3). The nonparametric Mann-Whitney U test was used to compare differences between two groups and the Kruskal-Wallis test for three or more groups. P < 0.05 was considered statistically significant.
Results
Effect of Inoculum Parameters on Contact Lens–Induced P. aeruginosa Keratitis
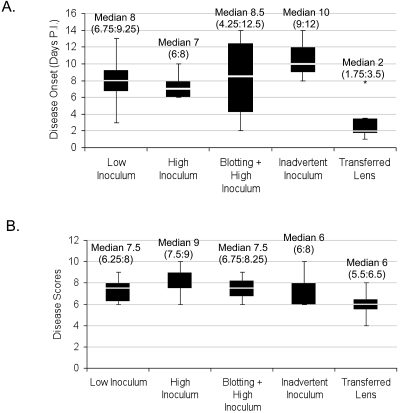
All contact lens–wearing rat eyes developed corneal opacity after a single inoculation with P. aeruginosa at the time of lens fitting, irrespective of inoculum conditions or corneal blotting. In each instance, initiation of disease was delayed (Fig. 2A). There was no significant difference in the median delay in disease onset between the low-dose inoculation group (8 days) and the high-dose group (7 days) (P = 0.617; Mann-Whitney U test). Inadvertent inoculation during lens handling with a previously “sterilized” suction pen (see Materials and Methods) also enabled disease with a comparable onset delay (10 days) that was not significant different from that of low- and high-dose inocula (P = 0.240; Kruskal-Wallis test). Transferred lenses from previously diseased eyes showed a trend toward a shorter delay in keratitis onset (median 2 days), which was statistically significant compared to the inadvertent inoculum group (P = 0.048; Mann-Whitney U test) (Fig. 2A). Blotting of rat corneas with tissue paper before inoculation (high dose) and lens fitting to allow fluorescein staining (Fig. 1) did not impact the timing of disease onset (median, 8.5 days) (P = 1.000; Mann-Whitney U test compared with a high-dose inoculum without blotting).
Figure 2.
Time of disease onset (A) and disease severity scores (B) of contact lens–induced P. aeruginosa keratitis in rats after a single inoculation with bacteria. Diseased eyes were scored (scaled 0–16)14 at 24 hours after observation of a faint corneal opacity. There was no significant difference in timing of disease onset (P = 0.240) or in disease severity (P = 0.427) (Kruskal-Wallis test) among low-dose (105 cfu total adherent and planktonic bacteria) (n = 4 animals), high-dose (109 cfu total adherent and planktonic bacteria) (n = 5 animals), and inadvertent inoculum (n = 3 animals) groups. Blotting of corneal epithelia before lens fitting had no effect on disease timing or severity (P = 1.000, P = 0.330, respectively; Mann-Whitney U test) (n = 4 animals in high-dose, blotted group). Transfer of lenses from previously diseased corneas trended toward shorter delays in disease onset compared with low- and inadvertent-inoculum groups (P = 0.074 and 0.048, respectively; Mann-Whitney U test) (n = 4 animals). *Outlier beyond 1.5 interquartile ranges above the third quartile.
Disease severity scores (Fig. 2B) ranged from a median of 6 (transferred lens) to 9 (high inoculum) out of a maximum of 16. There was no significant difference in disease severity scores among the low-, high-, and inadvertent inoculum groups (P = 0.427; Kruskal-Wallis test) or between the blotted and unblotted lens-wearing eyes at high inocula (P = 0.33; Mann-Whitney U test). None of the untreated contralateral eyes or eyes in a control group wearing sterile lenses for up to 14 days showed corneal disease.
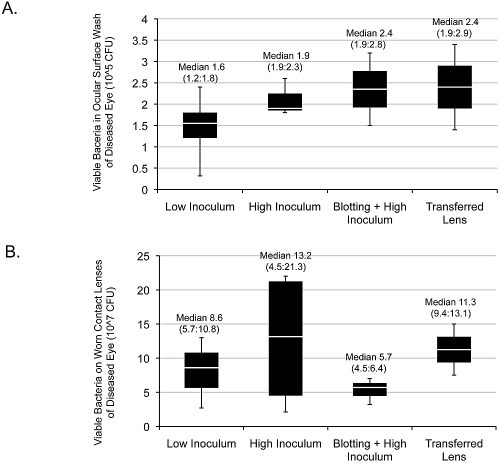
As shown in Figures 3A and 3B, ocular washes and lens homogenates from infected eyes revealed surprisingly consistent numbers of bacteria in each location (∼1.5–2.4 × 105 cfu and ∼0.57–1.3 × 108 cfu, respectively, for all animals). For both parameters, there was no significant difference among any of the groups (P = 0.659, P = 0.688, respectively).
Figure 3.
Viable bacterial counts of GFP-expressing P. aeruginosa strain PAO1 recovered from the ocular surface washes (A) or the homogenates of worn contact lenses (B) of rat eyes with P. aeruginosa keratitis. There was no significant difference among any of the groups in each panel (P = 0.659 and P = 0.688, respectively; Kruskal-Wallis test). The number of animals in each group was the same as described for Figure 2.
Selective media were used in control experiments to verify that bacteria recovered from infected eyes were from the inoculum introduced at the time of lens insertion. This was done by simultaneously plating ocular surface washes and homogenates of lenses removed from infected eyes on both nonselective TSA and selective media (see Materials and Methods). Similar colony numbers were recovered on these media for lens homogenates and surface washes (data not shown). Further, only one colony type was noted growing on TSA plates. These results confirmed retention of the GFP-plasmid in the lens-wearing eye in vivo and recovery of the inoculated bacteria with no significant colonization by any other type of recoverable bacterium. These data are consistent with our previous study showing the retention of a similar plasmid by P. aeruginosa in an infected murine cornea in vivo.15
Gross Pathology and Microscopy of Diseased Corneas
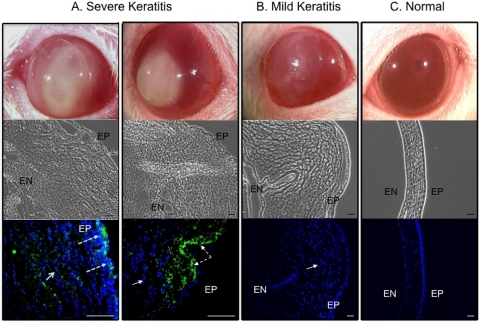
Cross-sections of diseased and control corneas were examined macroscopically and using phase-contrast and fluorescence microscopy (Fig. 4). Two examples of eyes with severe disease (clinical scores 10 or greater) are shown in Figure 4A. In each instance, there was a large central corneal opacity (top panels, one showing the lens in situ), gross disruption of all layers of the cornea with significant epithelial swelling (middle panels), and extensive cellular infiltration (blue cells, solid arrows). Notably, there was significant penetration of GFP-expressing P. aeruginosa (green, dashed arrows) in each infected cornea (lower panels). An example of eyes with less severe pathology (clinical scores lower than 10) is shown in Figure 4B. A large central corneal opacity was observed (top panel, with lens in situ) but was fainter than that observed with severely diseased eyes. There was a similar disruption of all corneal layers, corneal epithelial swelling, and cellular infiltrate (middle and bottom panels). However, GFP-expressing bacteria were not observed in corneas with mild disease (bottom panel), suggesting that bacteria had not entered the cornea or that they were cleared from the cornea at the time of kill.
Figure 4.
Representative photographs and cross-sectional phase-contrast and fluorescence micrographs of contact lens–induced P. aeruginosa keratitis in the rat model of extended wear. (A) Severely diseased eyes from two rats with total scores of 10 or above at day 5 (showing a lens still in place) and day 6 after inoculation, respectively, (B) a mildly diseased eye with a total score below 10 at day 7 after inoculation (showing the lens in place), and (C) an uninoculated normal eye (contralateral control). Corneal and infiltrating cell nuclei were stained with DAPI (blue). All diseased eyes showed large numbers of infiltrating cells within the cornea. Severely infected corneas had the worst structural disruption and showed GFP-expressing P. aeruginosa derived from original inoculum (green). EP, corneal epithelium; EN, endothelium. Scale bar, 50 μm. Images were taken from the experiments shown in Figure 2 (high-inoculum group).
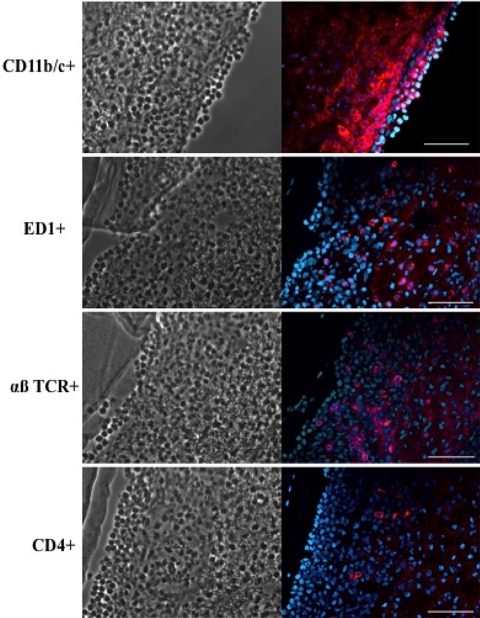
Figure 5 shows an example for a rat eye that had developed moderate P. aeruginosa keratitis after 3 days of inoculated lens wear. Immunostaining of corneal cross-sections showed infiltrates of cells that labeled with monoclonal antibodies reactive with CD11b/c (CR3 complement receptor), ED-1 (a monocyte/macrophage marker), the αβ T-cell receptor, and CD4. Although not shown, a similar pattern of immunolabeling was observed with severely diseased corneas at other times after inoculation. In all instances, eyes with lens-associated P. aeruginosa keratitis showed an infiltrate of CD11b/c+ cells, suggestive of professional phagocytes (e.g., PMNs and macrophages).
Figure 5.
Immunostaining of infiltrating cells (red) in cross-sections of a rat cornea at day 3 after inoculation with P. aeruginosa in the lens-wearing model. Keratitis was moderate. Right: infiltrating cells labeled with mouse monoclonal antibodies against rat CD11b/c (CR3 complement receptor, i.e., phagocytes), ED-1 antigen (monocytes, macrophages), αβ T-cell receptor (mature and immature αβ T cells), or CD4. Cell nuclei were counterstained with DAPI (blue). Left: phase-contrast images of the corresponding tissue sections. Scale bar, 50 μm. Images were taken from the experiments shown in Figure 2 (low-inoculum group).
P. aeruginosa Biofilm Formation on and within Contact Lenses Worn In Vivo
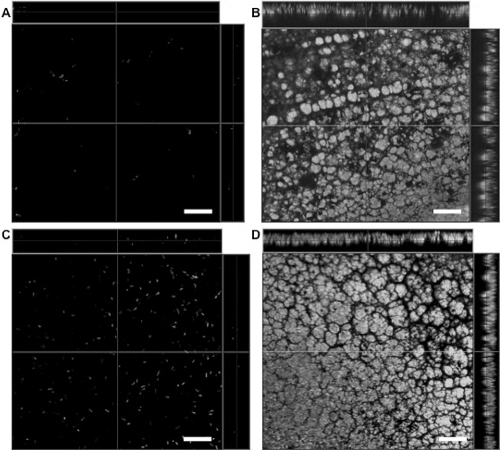
Laser scanning confocal microscopy of lenses removed from rat eyes with P. aeruginosa keratitis showed classical bacterial biofilm structures across the posterior lens surface (15- to 20-μm total thickness) (Figs. 6B, 6D). These biofilms were partially embedded within the lens (∼10–15 μm into lenses of approximately 80 μm total thickness), with the remaining (∼5 μm) of biofilm exposed on the lens surface. In contrast, the anterior surface of worn lenses showed only isolated bacteria embedded up to ∼10 μm beneath the surface (Fig. 6A, anterior surface of lens shown in Fig. 6B). Inoculated lenses, 103 cfu/mL PAO1-GFP for 24 hours (low inoculum; Fig. 6C) or 1011 cfu/mL for 3 hours (high inoculum; not shown) did not show biofilm structures before fitting.
Figure 6.
Laser scanning confocal microscopy of P. aeruginosa-inoculated contact lenses before and after in vivo wear in the rat contact lens model. The anterior surface of a worn lens (A) and posterior surface of the same lens (B) after 4 days of wear in the rat eye (low-inoculum group). Classical biofilm architecture of GFP-expressing P. aeruginosa strain PAO1 (derived from the original inoculum) was found on the posterior lens surface (B). Bacteria were found on the posterior lens surface at the time of fitting (C) (∼105 cfu). A classical biofilm of GFP-expressing P. aeruginosa strain PAO1 was also found on the posterior surface of lenses worn for 9 days (D) (high inoculum). Scale bar, 20 μm.
Discussion
The data presented in this report show that a single inoculum of P. aeruginosa introduced with a hydrogel contact lens can enable keratitis in rats over a period of 2 to 14 days of wear. Bacteria derived from the original inoculum were found on the lens and at the ocular surface (under the lens) in all diseased eyes. The bacteria were also found within some, but not all, of the diseased corneas. Pathology was associated with infiltration of professional phagocytes (CD11b/c+ and ED-1+ cells) and T-lymphocytes including CD4+ cells. Neither the delay in disease onset nor disease severity was significantly influenced by inoculum parameters (inoculum size or type) or the state of the epithelial barrier (to fluorescein). The delay was, however, significantly reduced when lenses were transferred from diseased eyes to naive animals. In all cases, there was extensive P. aeruginosa biofilms on, and embedded within, the posterior, but not the anterior, lens surfaces.
P. aeruginosa has a large genome with an unusual number of genes devoted to environmental adaptation.16 These include more than 70 two-component sensory-regulatory systems that enable it to adjust to a diverse array of environmental conditions. Some of these regulatory systems have been shown to impact virulence factor expression17 and corneal disease pathogenesis in vivo.18 The noted delay in disease onset, and the reduced delay when lenses were transferred from infected eyes to naive animals, suggests that pathogenesis of contact lens–related keratitis could involve bacterial adaptation to the in vivo environment, with accompanying changes to gene expression to enable a virulent bacterial phenotype/genotype. Also supporting bacterial adaptation to factors found in vivo are our (unpublished, 2009) data showing that P. aeruginosa can acquire an enhanced capacity to penetrate multilayered corneal epithelium on repeated exposure to epithelial cells. If adaptation does occur, and if this is critical to disease pathogenesis, then studies of the mechanisms involved (e.g., aspects of the in vivo environment that trigger these changes and the profile of bacterial genes impacted) could point toward new targets for disease prevention.
The biofilm formation found on posterior lens surfaces could also be involved in pathogenesis. That involvement could be direct, or indirect by enabling bacteria to persist in the eye so that there can be adaptation to the in vivo environment. Existence within a biofilm on the posterior surface would protect bacteria from the physical removal forces of blinking and tear flow. Further, it is well known that biofilm formation protects P. aeruginosa and other bacteria from killing by antimicrobial substances and host immune defenses while providing a niche within which bacteria can alter gene expression and transfer antibacterial resistance genes.19–21 Bacterial growth as biofilms on contact lenses in vitro has been shown to enhance resistance to contact lens disinfectants22 and to phagocytic cells.23 Thus, biofilm growth on the posterior lens surface in vivo could protect bacteria against both epithelial cell and tear-derived antimicrobial factors, such as complement, IgA, and defensin antimicrobial peptides. Further studies will be needed to delineate the relative contributions of biofilm and other in vivo adaptations in the initiation of contact lens–related keratitis.
Interestingly, in one set of experiments, eyes that were not deliberately inoculated became infected during lens wear. GFP-expressing P. aeruginosa that grew on all the selective media were isolated from the lens and eye washes of these infected eyes. The source of the bacterial inoculum was traced back to the suction pen used for inserting into eyes; the pen had been disinfected in ethanol 6 months earlier and then stored dry in the interim. Only a few colonies of P. aeruginosa were isolated from the suction pen, suggesting that there are circumstances when only a very small inoculum is required and that incubation time with the lens does not have to be extended before lens fitting for infection to ultimately develop. These findings also raise questions about disinfection and air-drying strategies for preventing microbial keratitis and may relate to the lack of a strong association between user compliance and infection risk.24
Although the data point to bacterial adaptation in pathogenesis, they do not preclude the involvement of lens-induced changes to ocular surface defenses in the initiation of keratitis in this model. Striking differences in bacterial biofilm formation were found between the anterior (very few bacteria, no biofilm) and posterior (mature biofilm) surfaces of lenses from infected rat corneas. The cleanliness of the anterior surface likely reflects the normal efficacy of blinking and tear flow that would help prevent bacterial colonization of the cornea when a lens is not worn.25 Heavy biofilm formation on the posterior surface and the presence of bacteria in the ocular surface washes show the inability of these defenses to function under a contact lens. There could also be changes to tear biochemistry that favor bacterial growth under a lens. Contact lens wear is known to reduce tear mixing at the corneal surface, and it also influences the volume of tears available at the cornea.26,27 It is likely that lacrimal gland or conjunctival-derived biochemical defense factors will have significantly reduced access to the cornea when a lens is worn. Corneal-derived tear defense factors could also be altered in concentration or integrity under a contact lens.
Contact lens effects on the corneal epithelium have also been shown, and this could be involved in the pathogenesis of lens-related infection.28 Indeed, our published in vitro studies show that exposure of corneal epithelial cells to soft contact lenses blocks the upregulation of the antimicrobial peptide human β-defensin 2 in response to P. aeruginosa antigens through effects on JNK/AP-1 signaling.29 Further, studies in vivo using a rabbit model have shown that rigid gas-permeable contact lens wear reduces corneal epithelial cell proliferation and apoptotic desquamation.30,31 In the same model, hypoxic effects of contact lens wear were found to induce P. aeruginosa internalization into surface corneal epithelial cells.5 Hypoxia and extended lens wear have also been linked to increased P. aeruginosa binding to exfoliated corneal epithelial cells.32,33 All these studies suggest that contact lens effects on the corneal epithelium (through hypoxia or otherwise) could influence corneal susceptibility to P. aeruginosa infection. However, the relationship between these lens-induced epithelial effects and actual susceptibility to infection has not been well defined.
Although it is intuitive that physical and biochemical barriers of the corneal epithelium play a role in defense against infection, the nature of those defenses and the influences of contact lens wear on those defenses are yet to be elucidated. Indeed, the data presented in this report show that tissue paper blotting of the corneal epithelium, sufficient to induce extensive fluorescein staining, had no notable impact on the timing or severity of contact lens–induced P. aeruginosa keratitis in rats. Staining was not required and did not worsen outcomes. If lens-induced epithelial disruption is involved in the pathogenesis of infection, markers other than fluorescein staining are needed to predict barrier function impairment that allows susceptibility to infection. Because lens care solutions (some shown to cause fluorescein staining) have been blamed for outbreaks of infection in recent years, it is worth noting that solution use was not required to enable contact lens–related corneal infection in rats.
In conclusion, contact lens–wearing animal models of microbial keratitis provide significant advantages over established models involving scarification or intrastromal injection by providing a clinically relevant means to study the combination of microbial and host factors that predispose to infection. The data shown in this study suggest that bacterial adaptation to the contact lens–wearing cornea, possibly involving the biofilm formation found on the posterior surfaces of lenses, contribute to the pathogenesis of this disease.
Until recently, research in this field had been predominantly patient or cell culture based, which has limited the approaches used to either US Food and Drug Administration–approved methods or to in vitro experiments. The result of those significant (and costly) efforts has been the development of products that have had little impact on the incidence of the sight-threatening complications of contact lens wear. Continued availability of appropriately manufactured contact lenses that fit animals will be critical for developing an effective means to prevent these iatrogenic and potentially blinding conditions.
Acknowledgments
The authors thank Gerald B. Pier (Harvard Medical School, Boston, MA) for providing the pSMC2 plasmid and Steven Ruzin and Denise Schichnes (Biological Imaging Facility, University of California, Berkeley) for use of the confocal microscopy system and for expert technical assistance.
Footnotes
Supported by National Eye Institute Grant R01-EY011221 (SMJF).
Disclosure: C. Tam, None; J.J. Mun, None; D.J. Evans, None; S.M.J. Fleiszig, None
References
- 1.Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea 2008;27:33–39 [DOI] [PubMed] [Google Scholar]
- 2.Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 1999;354:181–185 [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim YW, Boase DL, Cree IA. Epidemiologic characteristics, predisposing factors and microbiological profile of infectious corneal ulcers: the Portsmouth Corneal Ulcer Study. Br J Ophthalmol 2009;93:1319–1324 [DOI] [PubMed] [Google Scholar]
- 4.Morgan PB, Efron N, Hill EA, et al. Incidence of keratitis of varying severity among contact lens wearers. Br J Ophthalmol 2005;89:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto N, Jester JV, Petroll WM, Cavanagh HD. Prolonged hypoxia induces lipid raft formation and increases Pseudomonas internalization in vivo after contact lens wear and lid closure. Eye Contact Lens 2006;32:114–120 [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Kumar A, Ozkan J, et al. Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects. Optom Vis Sci 2008;85:292–300 [DOI] [PubMed] [Google Scholar]
- 7.Sankaridurg PR, Rao GN, Rao HN, Sweeney DF, Holden BA. ATPase-positive dendritic cells in the limbal and corneal epithelium of guinea pigs after extended wear of hydrogel lenses. Cornea 2000;19:374–377 [DOI] [PubMed] [Google Scholar]
- 8.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res 2004;23:1–30 [DOI] [PubMed] [Google Scholar]
- 9.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci 2007;84:273–278 [DOI] [PubMed] [Google Scholar]
- 10.Evans DJ, McNamara NA, Fleiszig SM. Life at the front: dissecting bacterial-host interactions at the ocular surface. Ocul Surf 2007;5:213–227 [DOI] [PubMed] [Google Scholar]
- 11.Szliter EA, Barrett RP, Gabriel MM, Zhang Y, Hazlett LD. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye Contact Lens 2006;32:12–18 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Gabriel MM, Mowrey-McKee MF, et al. Rat silicone hydrogel contact lens model: effects of high- versus low-Dk lens wear. Eye Contact Lens 2008;34:306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakkis C, Fleiszig SM. Resistance of Pseudomonas aeruginosa isolates to hydrogel contact lens disinfection correlates with cytotoxic activity. J Clin Microbiol 2001;39:1477–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EJ, Evans DJ, Fleiszig SM. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci 2003;44:5220–5227 [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci 2003;44:3892–3898 [DOI] [PubMed] [Google Scholar]
- 16.Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000;406:959–964 [DOI] [PubMed] [Google Scholar]
- 17.Zolfaghar I, Angus AA, Kang PJ, et al. Mutation of retS, encoding a putative hybrid two-component regulatory protein in Pseudomonas aeruginosa, attenuates multiple virulence mechanisms. Microbes Infect 2005;7:1305–1316 [DOI] [PubMed] [Google Scholar]
- 18.Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun 2006;74:3880–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 2001;9:34–39 [DOI] [PubMed] [Google Scholar]
- 20.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002;416:740–743 [DOI] [PubMed] [Google Scholar]
- 21.Prince AS. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med 2002;347:1110–1111 [DOI] [PubMed] [Google Scholar]
- 22.Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea 2009;28:918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume EB, Stapleton F, Willcox MD. Evasion of cellular ocular defenses by contact lens isolates of Serratia marcescens. Eye Contact Lens 2003;29:108–112 [DOI] [PubMed] [Google Scholar]
- 24.Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses: a case-control study. Microbial Keratitis Study Group. N Engl J Med 1989;321:773–778 [DOI] [PubMed] [Google Scholar]
- 25.Mun JJ, Tam C, Kowbel D, et al. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect Immun 2009;77:2392–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara NA, Polse KA, Brand RJ, et al. Tear mixing under a soft contact lens: effects of lens diameter. Am J Ophthalmol 1999;127:659–665 [DOI] [PubMed] [Google Scholar]
- 27.Lin MC, Chen YQ, Polse KA. The effects of ocular and lens parameters on the postlens tear thickness. Eye Contact Lens 2003;29:S33–S36; discussion S57–S39, S192–S194 [DOI] [PubMed] [Google Scholar]
- 28.Lin MC, Polse KA. Hypoxia, overnight wear, and tear stagnation effects on the corneal epithelium: data and proposed model. Eye Contact Lens 2007;33:378–381, discussion 382 [DOI] [PubMed] [Google Scholar]
- 29.Maltseva IA, Fleiszig SM, Evans DJ, et al. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp Eye Res 2007;85:142–153 [DOI] [PubMed] [Google Scholar]
- 30.Ladage PM, Yamamoto K, Ren DH, et al. Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear. Invest Ophthalmol Vis Sci 2001;42:2804–2812 [PubMed] [Google Scholar]
- 31.Yamamoto K, Ladage PM, Ren DH, et al. Effects of low and hyper Dk rigid gas permeable contact lenses on Bcl-2 expression and apoptosis in the rabbit corneal epithelium. CLAO J 2001;27:137–143 [PubMed] [Google Scholar]
- 32.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology 2001;108:1279–1288 [DOI] [PubMed] [Google Scholar]
- 33.Fleiszig SM, Efron N, Pier GB. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest Ophthalmol Vis Sci 1992;33:2908–2916 [PubMed] [Google Scholar]