We have developed an intact globe expansion method (GEM) that stresses the tissue by using an in vivo loading geometry and is capable of quantifying the effects of cross-linking treatments on both the cornea and sclera. We introduce rabbit kit eyes as a model of diseased tissue that can be subsequently strengthened to the level of normal tissue, as demonstrated with riboflavin/UVA and glyceraldehyde treatments.
Abstract
Purpose.
To measure the tissue mechanical response to elevated intraocular pressure (IOP) using intact globe expansion of rabbit eyes. This method examined rabbit kit (2–3 weeks old) eyes as a model for weakened tissue and evaluated riboflavin/UVA and glyceraldehyde cross-linking treatments.
Methods.
The ocular shape of enucleated eyes was photographed during a 24-hour period while a controlled IOP was imposed (either low IOP = 22 mm Hg or high IOP = 85 mm Hg). Untreated controls consisted of kit eyes tested at both low- and high IOP and adult eyes tested at high IOP. Treated kit eyes (dextran controls, riboflavin/UVA treatment of the cornea, and glyceraldehyde treatment of the entire globe) were tested at high IOP.
Results.
Low IOP elicited negligible creep of the sclera and very gradual creep of the cornea. In contrast, high IOP induced up to an 8% strain in the sclera and a 15% strain in the cornea of rabbit kit eyes. The expansion of adult eyes was less than one third that of kit eyes at the same, high IOP. Riboflavin/UVA treatment of corneas reduced expansion compared with that in both dextran-treated and untreated control corneas. Glyceraldehyde treatment prevented expansion of the cornea and sclera.
Conclusions.
The intact globe expansion method (GEM) imposes a loading geometry comparable to in vivo conditions and can quantify changes in mechanical stability as a function of testing conditions (e.g., IOP, tissue maturation, and therapeutic cross-linking) with small sample sizes and small variability. Rabbit kit eyes provide a model of weak tissue suitable for screening treatments that strengthen the cornea and sclera.
There is currently a high level of interest in strengthening the ocular coat (i.e., cornea and sclera). Diseases such as keratoconus and degenerative myopia lead to progressive visual loss due to inadequate mechanical stability of the cornea and sclera.1–3 In these diseases, changes in the biochemistry of the tissue (altered types and concentrations of enzymes, proteoglycans, and collagen) coincide with macroscopic structural changes (thinning, weakening, and increased susceptibility to deformation).4–6 To stabilize the shape of the globe, Wollensak et al.7 have pioneered the use of riboflavin and ultraviolet light (UVA) to cross-link collagen and enhance the mechanical properties of the tissue. This method shows promise for treatment of keratoconus8 and other cross-linking approaches (e.g., glyceraldehyde and nitroalcohols) may provide stabilization of scleral shape in progressive myopia9–11 or strengthening of peripapillary sclera in glaucoma.12
The effects of treatments on the strength of the cornea and sclera are manifested in the change in the rate and extent of deformation of the globe under the influence of intraocular pressure. Therefore, it is desirable to develop in vitro methods to quantitatively evaluate the change in mechanical stability due to treatment. Previously, researchers evaluating tissue mechanics have relied on methods such as tensile tests,13–17 button tests,18,19 and intact globe tests.20–23 The majority of work evaluating riboflavin/UVA and other cross-linking treatments has been done with tensile measurements (Spoerl E, et al. IOVS 1999;40:ARVO Abstract 1800).24–26 The tensile testing method is well established in biomechanics, but researchers have acknowledged problems. As summarized by Greene and McMahon,20 “Uniaxial stress-strain tests on a soft biological material such as sclera are plagued with many problems. First, the sample must be cut from the sclera, thus fraying the collagen fibrils at the edges of the sample. The mechanical effect of this procedure is difficult to assess, but it almost certainly weakens the sample. Second, the sample is initially curved, and must be unbent for the purpose of the uniaxial test. This unavoidably gives rise to a distribution of positive and negative stresses across the thickness of the sample, a situation quite different from the in vivo state of affairs. Third, the sample must be clamped in the jaws of a vise, thus giving rise to very large compressive stresses at the ends of the sample. Slippage at the jaws could easily be mistaken for plastic deformation or creep of the sample. Lastly, the normal state of stress in the sclera is biaxial, not uniaxial.” Ideally, a testing method to quantify changes in mechanical stability of the ocular coat would be repeatable with little variability, would be applicable to healthy or diseased tissue, and would mimic in vivo conditions. Significant features of the mechanics in vivo are biaxial loading due to a pressure difference between the interior and exterior of the globe, low total strain, and very low strain rates. Indeed, the therapeutic benefit of proposed treatments depends on their efficacy at low strain, particularly in preventing gradual creep (i.e., time-dependent extension under a constant load). A testing method capable of addressing these problems may enable the in vivo effects of therapeutic agents to be anticipated by in vitro assays, providing a tool for optimizing treatments.
We used the sensitivity of a new apparatus, together with a model of weakened cornea and sclera to enable in vitro, quantitative comparison of treatments that alter the mechanical stability of ocular tissues. Specifically, we devised an intact globe expansion apparatus that enables the study and quantification of a whole eye's ability to resist deformation while subjected to elevated intraocular pressure (IOP). In relation to diseases, rabbit kit (2–3 weeks old) eyes were examined as a suitable model for weak tissue that exhibits a propensity to deform under constant IOP. Together, the expansion apparatus and kit eyes were evaluated as a tool for determining the efficacy of possible treatments. Specifically, riboflavin/UVA-treated tissue was readily distinguishable from both untreated (negative) controls and glyceraldehyde cross-linked (positive) controls even at small strain (<10%) and extremely slow strain rates (<0.01%/min).
Methods
Tissue Specimens and In Vitro Treatment Protocols
Enucleated eyes from New Zealand White rabbits were used. Rabbits of different sex were included randomly. Two distinct age groups were examined. Enucleated eyes from young rabbit kits (2–3 weeks old, 0.75–1.0 kg) were provided by Keith Duncan (University of California, San Francisco). Within 3 hours of enucleation, the eyes were shipped in isotonic saline on ice. Enucleated eyes from adult New Zealand White rabbits (>6 months old, >2.7 kg) were purchased from Pel-Freez Arkansas, LLC (Rogers, AR). Prior studies confirmed that the epithelium was intact after shipping (application of 2% fluorescein drops failed to stain the corneal collagen).
On receipt, the eyes were stored in saline on ice until used, not sooner than 24 or later than 48 hours after enucleation. The fat and muscle were removed from all eyes with scissors to expose the sclera. A smooth ocular surface provided a well-defined perimeter in the images. Thirty-five eyes were used: 8 from adult rabbits and 27 from rabbit kits. The number of eyes for each protocol is indicated in the Results section.
Riboflavin/UVA.
Pairs of enucleated eyes from five rabbit kits were used to study riboflavin/UVA treatment according to the procedures outlined in Wollensak et al.24 Briefly, the epithelium was removed by scraping the corneal surface with a scalpel blade, and then drops of treatment solution containing 0.1% riboflavin-5′-monophosphate (Sigma-Aldrich, St. Louis, MO) in 20% dextran T500 (Pharmacosmos, Holbaek, Denmark) were applied to the cornea for 5 minutes followed by 30 minutes of UVA exposure (370 nm, 3 mW/cm2, evaluated with a 55PM Laser Power Meter; Liconix, Santa Clara, CA) with additional drops every 5 minutes. The light source built in our laboratory consisted of an array of seven LEDs (Roithner LaserTechnik, Vienna, Austria) located ∼2 cm from the cornea. Fellow eyes with the epithelium removed were used as the control; they received 20% dextran without riboflavin with drop application and irradiation as just described. After irradiation, each eye was rinsed in ∼10 mL of Dulbecco's phosphate-buffered saline (DPBS; D8662, 274–303 mOsm/kg; Sigma-Aldrich) solution for 30 minutes and then loaded into the intact globe expansion apparatus.
Glyceraldehyde.
Six rabbit kit eyes were used to establish the effect of glyceraldehyde cross-linking. The epithelium was removed with a scalpel blade, and whole eyes were soaked in 2% dl-glyceraldehyde (Sigma-Aldrich) in DPBS for 12 hours. The eyes were then placed in a DPBS bath for 12 hours to rinse off excess glyceraldehyde. Dextran-treated and untreated kits eyes were used as control samples.
Experimental Apparatus and Measurements
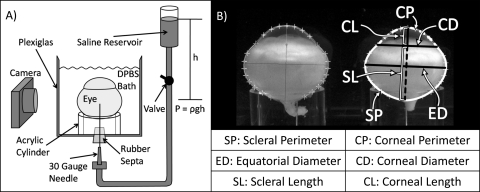
Intact globe expansion measurements were made in a transparent Plexiglas observation cell (Fig. 1). The bottom of the observation cell had two holes sealed with rubber septa, used for the insertion of hypodermic needles (30-gauge) to regulate the IOP. The pressure imposed through the needles was achieved with a DPBS reservoir held at a corresponding height (h) above the apparatus, imposing an IOP equal to the hydrostatic pressure (IOP = ρgh, where ρ is density and g is gravitational acceleration). Before the globes were mounted, the integrity of the fluid path was confirmed by briefly opening the valve to observe fluid flow from the reservoir through the needle. To ensure that the hydrostatic pressure was indeed the IOP in the globe, it was necessary to minimize leakage of fluid from the eyes that could result in a pressure drop across the needle. Therefore, small-diameter needles were used to minimize the risk of a leak around the needle after the sclera was pierced. The apparatus makes it immediately obvious when a leak occurs: changes in fluid level in the sample chamber are readily observed and are recorded in the sequence of images. Of the experiments reported herein, three fourths had no detectable leak, and one fourth had detectable but very gradual leaks (bath fluid level rose <0.3 mm/h, corresponding to a volumetric flow rate of <0.9 mL/h, and perturbing the imposed IOP by <2%). There was no rupture of the eyes within the 24 hours allocated to this experiment.
Figure 1.
(A) Apparatus for controlling IOP during intact globe expansion experiments. (Not to scale: The eye is approximately 14 cm in diameter, and the height of the reservoir ranges from 30 to 116 cm.) The height of the saline reservoir generates a hydrostatic pressure that is transferred to the eye through the hypodermic needle on opening the valve. A bath of DPBS maintains the equilibrated extent of hydration of the tissue. Digital photographs are taken throughout the 24 hours of testing. (B) Ocular dimensions are measured with a program written in commercial software (MatLab; The MathWorks, Natick, MA).
After specimen preparation, two enucleated eyes were loaded onto transparent acrylic cylinders in the DPBS bath (Fig. 1A). Because the tissues of interest are anisotropic and the eye is not symmetric, a consistent orientation was used; the eyes were aligned with the optical axis vertical, with the major axis of the equator parallel to the imaging plane, and with the optic nerve on the side closest to the camera. The eyes were nearly neutrally buoyant in the bath solution; therefore, loading was performed with the bath partially filled (fluid level slightly above the cylinder height), so that the weight of the eyes facilitated orienting the globes. The needles were then inserted through the posterior sclera. After insertion, friction between the needle and tissue was sufficient to hold the eyes in place without other methods of fixing the position. The bath was then filled with DPBS to maintain the state of hydration of the tissues. To minimize bacterial growth during the experiment, we added several antibiotic eye drops (neomycin, polymyxin B sulfate and gramicidin ophthalmic solution USP; Bausch & Lomb, Tampa, FL) to the solution in the observation cell. The eyes were allowed 15 minutes to reach bath temperature (∼22°C) before the valve was opened, and an IOP similar to the physiological state was reintroduced (see the Shape Restoration section). For creep experiments, the pressure was subsequently increased from low IOP to high IOP (see the Creep section).
Initially, experiments were performed by recording photographs from three orthogonal directions: along the optical axis of the globe and two projections orthogonal to the optical axis (in the plane of the major equatorial axis and in the plane of the minor equatorial axis). All three projections gave consistent results for the change of the perimeter of the sclera; and the two projections orthogonal to the optical axis gave consistent results for the change of the perimeter of the cornea. Therefore, a simplified method using a single projection orthogonal to the optical axis was adopted; specifically, the plane containing the optical axis and the major equatorial axis was chosen to characterize relative changes in eye shape for this article. Digital photographs of this projection were taken automatically every 15 minutes (PowerShot G3; Canon, Tokyo, Japan). A time series of images offers advantages over relying on initial and final photographs, such as permitting sensitive detection of leaks; tracking of anatomic features, such as the corneal–scleral intersection at the limbus; and evaluating changes in creep rates over the course of the experiment.
The images were analyzed to evaluate changes in ocular dimensions using a computer program written by one of the authors (MSM; MatLab; The MathWorks, Natick, MA). The code allows the user to select points along the perimeter of the eye defining the boundary of the cornea and sclera and uses cubic spline interpolation to trace the outline of the eye. Using the traces, we computed three measures of the sclera (scleral perimeter, SP; equatorial diameter, ED; and scleral length, SL) and three measures of the cornea: corneal perimeter, CP; corneal diameter, CD; and corneal length, CL (Fig. 1B). The ratios of the scleral and corneal perimeters at time t to their respective values at time 0, SP(t)/SP(0) and CP(t)/CP(0), are analogous to strain measurements in tensile tests. The corneal and scleral lengths are similar to the axial length measurements typical in biometry. The acrylic cylinders of known diameter (∼12.7 mm) provided a scale inside the bath for calibration of pixels to millimeters in each image sequence.
Uncertainty in the measured length ratios is small and predominantly due to animal-to-animal variability. Three potential sources of uncertainty are (1) inaccuracy of point placement during image analysis, (2) image distortion through the bath and the camera optics, and (3) errors in calibration. The first two were evaluated using images of grids with known line spacing placed in the DPBS bath. First, to characterize the uncertainty in point placement during image analysis, we performed repeat placement of points along a 1-mm line. Repeatability was good to the nearest pixel (SD ∼0.25 pixel); the resulting uncertainty in the measured length ratios was an order of magnitude less than the observed experimental uncertainty (e.g., for a 15% increase in CP(t)/CP(0), point placement accounts for <0.015% uncertainty, whereas the overall experimental uncertainty is ±2%). Second, the images of the uniform grid were used to characterize distortion resulting from refraction at the air–bath interface and from the optics of the low-cost cameras that were used for the experiments. The number of pixels per millimeter interval on the grid varied <3% from intervals near the center of a specimen to intervals near their perimeter. This image distortion has very little effect on the measured length ratio, since it affects the numerator and denominator almost identically (e.g., for a 15% increase in CP(t)/CP(0), the resulting error is <0.03%). Since the actual uncertainty due to animal-to-animal variability is approximately 10-fold greater, no benefit could be gained by improving the resolution of the camera or the quality of the lenses, or by implementing correction factors for refraction at the air–bath interface. Last, although uncertainty in the calibration of pixels per millimeter has no effect on the measured length ratios, it is characterized for completeness. From uncertainty in measuring the acrylic cylinder diameter, the number of micrometers/pixel was found to vary up to 0.3% (i.e., ±0.1 μm/pixel of 28 μm/pixel).
Experimental Protocol
Shape Restoration.
Enucleated eyes are not stabilized by physiological IOP, and so handling them during shipping, removing fat and muscle from their surface, and mounting the globes results in a variable shape. Normal IOP in rabbits is known to fluctuate throughout the day (lowest, 12.6 ± 0.5 mm Hg; highest, 17.6 ± 1.3 mm Hg).27 In an effort to reverse the effects of handling, an IOP slightly above the physiological state (22 mm Hg) was imposed for 1 hour. Ocular dimensions measured at the end of this 1-hour restoration period were taken as the initial state of the eye for all other experiments.
Creep Measurements.
The slow, time-dependent deformation of a sample subjected to a constant applied stress is referred to as creep, which has particular relevance to the cornea and sclera, as they are continually subjected to IOP. Despite their nonuniform thickness, nonuniform curvature, and nonuniform mechanical properties, the cornea and sclera in an intact globe geometry exhibit reproducible nominal creep rates under the influence of a constant, elevated IOP. For simplicity, we refer to the observed “nominal creep” as creep. Analogous to strain, nominal creep is expressed as a change in length divided by an initial length.
The effect of imposed IOP was examined in rabbit kit eyes with intact epithelium. After loading and shape restoration, five eyes were maintained at low IOP (22 mm Hg) and six eyes were subjected to high IOP (85 mm Hg) for 23 hours.
To examine the effect of tissue maturation, we used eight adult rabbit eyes with intact epithelium. After loading and shape restoration, the eyes were subjected to high IOP for 23 hours.
The effect of riboflavin/UVA treatment of the cornea was examined relative to negative (untreated and dextran) and positive (glyceraldehyde treated) controls. Specimens were loaded after receiving the in vitro treatment protocols described earlier. After shape restoration, the eyes were subjected to high IOP for 23 hours.
Results
Shape Restoration
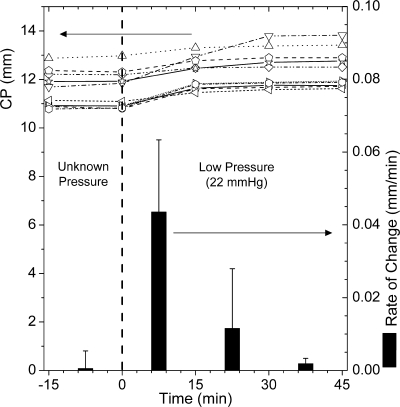
Initially, the enucleated eyes had a variable shape caused by postmortem handling (e.g., enucleation, shipping, and removal of fatty tissue) while their IOP was 0. Applying an IOP near the physiological level (low IOP, 22 mm Hg) restored the shape of the globe (Fig. 2). The 11 rabbit kit eyes used in the low (n = 5)- and high (n = 6)-pressure creep experiments all received the same preparation before loading (intact epithelium, no treatment). One eye was excluded from the analysis because there was no photograph preceding the application of low pressure. The rate of change in CP was computed from the average change over a given 15-minute time interval. During the first 15 minutes of low pressure, the changes in each ocular dimension (Fig. 1) were rapid and highly variable (for example, the corneal perimeter CP increased at a rate of 0.044 ± 0.020 mm/min, n = 10). In the next 15 minutes, the changes were slower on average (0.012 ± 0.016 mm/min for CP, n = 10) but still variable, which can be seen from the individual traces of CP for each eye (Fig. 2, open symbols). In the next 15 minutes, the eyes had stabilized, showing much smaller shape changes with little variability (0.002 ± 0.001 mm/min for CP, n = 10). Similar results were obtained for all ocular dimensions. Therefore, the ocular dimensions of eyes after 1 hour at low IOP were taken to represent complete shape restoration (Table 1).
Figure 2.
CP (lines and open symbols, left axis) and its rate of change (filled bars, right axis) measured before and during imposition of an IOP of 22 mm Hg in 10 kit eyes. The size of the eyes increased after the transition from no pressure to low pressure. The rate of change was greatest during the first 15 minutes. Most changes occurred within the first 30 minutes of shape restoration. Results for CP were exemplary of all globe dimensions during the first hour of low pressure.
Table 1.
Dimensions of the Eye Measured after 1 Hour of Low IOP
| n | SP | ED | SL | CP | CD | CL | |
|---|---|---|---|---|---|---|---|
| Kit, 2 wk | 11 | 28.5 ± 1.3 | 13.8 ± 0.7 | 9.6 ± 0.4 | 12.2 ± 1.1 | 10.1 ± 0.8 | 3.0 ± 0.4 |
| Adult, 6+ mo | 10 | 40.3 ± 1.3 | 20.6 ± 0.6 | 13.6 ± 0.6 | 20.0 ± 0.8 | 16.1 ± 0.5 | 5.1 ± 0.4 |
Ocular dimensions of the eye measured after 1 hour at 22 mm Hg (average ± SD in millimeters).
Creep
Nominal creep was measured with respect to the restored shape after 1 hour at low IOP (defined as t = 0). Although images were acquired at 15-minute intervals, measurements are shown at eight selected time points for clarity (0, 1, 3, 7, 11, 15, 19, and 23 hours, Figs. 3, 4).
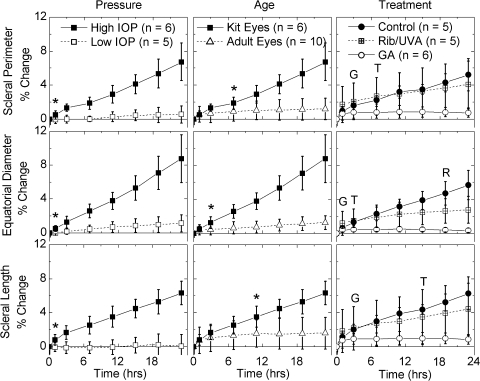
Figure 3.
Change of scleral dimensions with time (t = 0 at the end of the restoration period). Left: rabbit kit eyes subjected to low (22 mm Hg) and high (85 mm Hg) pressures; *the first time point at which the two differed significantly (P < 0.05, Student's t-test). At low pressure, the rabbit kit sclera remained stable; at high pressure, the sclera expanded. Middle, rabbit kit eyes and adult rabbit eyes subjected to high pressures (85 mm Hg); *the first time point at which the two differed significantly. The sclera of kit eye expanded much more than that of the adult eye. Right: rabbit kit eyes subjected to high pressure after treatment. Dextran control specimens expanded similarly to untreated kit eyes. Riboflavin/UVA (Rib/UVA) treatment was intentionally localized to the cornea, and so it had little effect on scleral expansion. Glyceraldehyde-treated eyes resisted expansion. R, the first point at which the difference between riboflavin/UVA treatment and the dextran control is statistically significant; G, the first point of significant difference between glyceraldehyde treatment and the control; and T, the first point of significant difference between the two treatments.
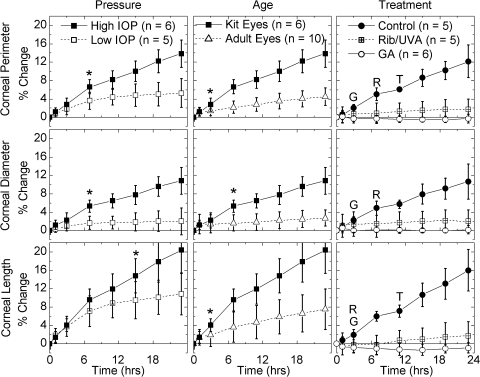
Figure 4.
Change of corneal dimensions with time (t = 0 at the end of the restoration period). Left: rabbit kit eyes subjected to low (22 mm Hg) and high (85 mm Hg) pressures; *the first time point at which the two differed significantly. The cornea was more susceptible to creep than was the sclera (compare to scaling of Fig. 3). The kit cornea expanded to nearly a plateau level at low pressures; at high pressure, it expanded continuously. Middle: kit and adult rabbit eyes subjected to high pressures (85 mm Hg); *the first time point at which the two differed significantly. The cornea of the adult eye resisted expansion compared to that of the kit eye. Right: rabbit kit corneas subjected to high pressure after treatment. Dextran controls expanded similarly to untreated kit eyes. Rib/UVA-treated corneas resisted corneal expansion. Glyceraldehyde-treated eyes resisted any expansion. Asterisk and R, G, and T: significant differences as described in Figure 3.
Low versus High Pressure.
Rabbit kit eyes that were subjected to low IOP for 23 hours showed negligible expansion of the sclera (SP, ED, and SL change <1.2%; Fig. 3, left column, open symbols), but did exhibit small increases in CP and axial protrusion (CL; Fig. 4, left column, open symbols). Changes in CD were consistently less than those in CP and CL, in accordance with anchoring cornea at the limbus to the stiffer sclera. The changes in CP, CD, and CL showed strain-hardening character, approaching plateau values. High IOP induced significantly greater expansion than low IOP. Creep was significant for both the sclera (Fig. 3, left, filled versus open symbols) and the cornea (Fig. 4), increasing linearly from 7 to 23 hours.
Adult versus Kit Eyes.
The initial size of the adult rabbit eyes was 50% larger than that of the kit eyes (Table 1). The adult eyes expanded under high pressure, but significantly less than the kit eyes under the same conditions (Figs. 3, 4, middle). The sclera expanded less than the cornea, as was seen in the kit eyes (compare scales of Figs. 3, 4). Beyond 7 hours, negligible further creep occurred in the sclera of the adult eyes (Fig. 3, middle), which was also manifested in the corneal diameter remaining fixed for t > 7 hours (Fig. 4, middle). Therefore, the difference in scleral deformation between the kit and adult eyes increased significantly for these parameters (SP, ED, SL, and CD) between 7 and 24 hours. The cornea of the adult eyes continued to creep for the duration of the experiment (both CP and CL increased steadily, Fig. 4). Due to the constraint at the limbus, the protrusion of the cornea CL was exaggerated relative to the increase in CP. Nevertheless, the total deformation of the cornea in the adult eyes was significantly less than that in the kit eyes (roughly one third, based on CP at 23 hours).
Riboflavin/UVA versus Negative and Positive Controls.
During high-pressure creep, negative control eyes (epithelium removed, dextran drops, and irradiated with UVA light) expanded in a fashion similar to the untreated kit eyes with intact epithelium (Figs. 3, 4, compare the dextran control in the right column to the kit eyes in the middle column). Treatment of the cornea with riboflavin/UVA significantly reduced the expansion of the cornea: the ocular dimensions CP, CD, and CL only expanded ∼2% after 23 hours, compared with 11% to 16% in the control corneas. As expected, treatment of the cornea had little effect on the sclera (SP and SL expanded nearly identically to the control corneas, whereas ED in the riboflavin-treated eyes expanded less than in the control eyes).
Treatment of the entire globe with glyceraldehyde cross-linked both the cornea and sclera, causing visible changes in color as well as mechanical stiffening that was even observable by hand. Expansion along every ocular dimension was significantly reduced relative to dextran control specimens. Even after 23 hours, expansion was indistinguishable from 0 for all ocular dimensions. Negative averages do not necessarily indicate shrinkage of the eyes, as the uncertainty does not permit distinction from no expansion (Table 2). For the cornea, the difference between the positive control (glyceraldehyde treated) and the riboflavin treatment is small (∼2%) but statistically significant (Fig. 4, open circles).
Table 2.
Percentage Change in Ocular Dimension after 23 Hours
| n | SP | ED | SL | CP | CD | CL | |
|---|---|---|---|---|---|---|---|
| Kit low IOP* | 5 | 0.5 ± 1.0 | 1.1 ± 1.0 | 0.02 ± 1.4 | 5.3 ± 3.2 | 2.0 ± 2.9 | 10.8 ± 4.6 |
| Kit high IOP† | 6 | 6.7 ± 2.2 | 8.9 ± 2.8 | 6.3 ± 1.4 | 13.9 ± 3.0 | 10.8 ± 2.9 | 20.4 ± 5.1 |
| Adult high IOP† | 8 | 1.2 ± 1.3 | 1.2 ± 0.8 | 1.6 ± 1.9 | 4.5 ± 1.9 | 2.7 ± 1.8 | 7.5 ± 4.4 |
| Dextran control† | 5 | 5.2 ± 1.9 | 5.7 ± 1.7 | 6.2 ± 2.0 | 12.2 ± 3.5 | 10.6 ± 3.8 | 16.0 ± 4.5 |
| Riboflavin/UVA† | 5 | 4.1 ± 2.7 | 2.8 ± 1.6 | 4.4 ± 3.1 | 1.7 ± 2.2 | 2.0 ± 2.5 | 1.7 ± 3.1 |
| Glyceraldehyde† | 6 | 0.7 ± 0.5 | 0.3 ± 0.3 | 0.9 ± 0.6 | −0.3 ± 0.9 | 0.2 ± 0.9 | −0.9 ± 1.1 |
Change in ocular dimension (average ± SD) was calculated as the percentage increase in the size of the eye during the 23 hours after shape restoration.
IOP = 22 mm Hg.
IOP = 85 mm Hg.
Discussion
We have developed a quantitative method of characterizing mechanical stability of the cornea and sclera in vitro that minimizes tissue damage during specimen preparation and mounting and retains the loading geometry that occurs in vivo. Digital imaging enables intact globe expansion measurements22 to be performed without mechanically constraining the globe20 or attaching a strain gauge.23 The present method incorporates desirable features of prior experimental methods, using image analysis to achieve sensitive, noncontact measurement of globe dimensions22 and controlling the environment around the eye to minimize changes in hydration during measurement.20 The measured parameters have correlates in vivo (corneal and scleral length are directly related to axial length)28 and in vitro (corneal and scleral perimeters are related to extension measured in tensile tests). The intact globe expansion method enables sensitive discrimination between specimens at slight strains and low strain rates. Application of this method reveals that rabbit kit eyes can serve as a model tissue mimicking the poor mechanical integrity characteristic of keratoconus and degenerative myopia. Treating this weak tissue with riboflavin/UVA or with glyceraldehyde strengthens it beyond the level of normal adult tissue.
We suggest that GEM with immature tissue could be used to optimize treatment parameters in vitro (e.g., to meet or exceed a benchmark in strength, such as the change produced by riboflavin/UVA treatment or the strength of normal tissue). The ability of GEM to discriminate between different treatments in small samples makes it an attractive tool for comparative studies of efficacy of collagen cross-linking.
Such in vitro studies could guide the selection of drug and irradiation combinations to advance to in vivo experiments. Furthermore, GEM may prove useful in conjunction with in vivo studies, providing a method of evaluating differences in mechanical stability between treated and fellow globes that were treated in vivo, then characterized postmortem. It is not intended to replace in vivo studies.
One challenge facing those conducting in vitro studies is the change in the hydration state of the tissue during transportation, dissection, and experimental measurement. Limited pachymetry readings on untreated kit eyes with intact epithelium showed that the corneas did swell during shipment in saline (received corneal thickness was 530 ± 50 μm), as expected based on prior literature.29 During shape restoration, the corneal thickness of untreated eyes decreased to 390 ± 20 μm (in vivo thickness is ∼300 μm).30 During expansion at high pressure, thickness measurements were not obtained; future experiments can be designed to facilitate thickness measurements without disturbing the eyes. However, there was no indication of swelling during the intact globe expansion (e.g., corneal transparency did not significantly change over the 23 hours of the experiment). Nor was there any indication that swelling markedly changed the tissue mechanical response (e.g., creep rates were indistinguishable for untreated kit eyes with intact epithelium and dextran controls that were de-epithelialized and given drops of 20% dextran for 1 hour). Therefore, we attribute the observed changes in mechanical stability to collagen cross-linking. Future experiments could evaluate the possible effects of swelling by modifying tissue handling and the environment (e.g., examine eyes immediately after enucleation and replace DPBS with corneal storage medium).
Shape Restoration
Precise characterization of tissue mechanics requires repeatable techniques that measure changes from a well-defined initial state. The biomechanics literature describes a plethora of techniques for establishing the initial conditions (e.g., applying a cyclic load or deformation, deforming the tissue until a selected stress is recorded or applying a small stress until deformation ceases). Although any of these methods could be implemented with GEM, we chose to restore in vivo shape in a simple manner. When the eye is enucleated, there is a drop in IOP, and handling the eyes without the stabilization provided by the IOP causes each specimen to have a perturbed shape, different from its in vivo shape. Using GEM with an intact globe allows its natural constraints to be used in restoring a shape analogous to that in vivo. The protocol we used is based on physiological data (we applied an IOP that is slightly above the physiological range). The results showed very good reproducibility, perhaps as a consequence of using an IOP that was high enough to eliminate any variations between eyes that had different IOPs in vivo, while not producing a gross continual creep. The time necessary for each eye to reach a stable shape was found to be less than 30 minutes for all 11 eyes examined (Fig. 2). After reaching a stable shape, the sclera did not undergo statistically significant creep (Fig. 3, left column) and the cornea exhibited very gradual creep (strain at <0.02%/min; Fig. 4, left column). If the shape restoration period is too short, then the initial creep measurements will show artificially high creep rates with larger variability. Therefore, we chose to allow more than 30 minutes, to ensure minimal variability in the initial condition while keeping the shape restoration time short enough that negligible creep had time to occur (hence, a 1-hour shape-restoration period was chosen).
Creep
Mechanical stability is of obvious clinical importance. In vivo, the eye sustains an IOP while maintaining its shape. Creep tests permit the study of how well the tissue can resist deformation under a constant IOP. In disease states associated with elevated pressure or with weakened tissue, the eye can become susceptible to distension. This inspired our decision to examine an intact globe using a creep test at a constant IOP instead of injecting specified volumes of liquid22 or ramping up the pressure.19,23 Rather than forcing the tissue to undergo a specific extent of deformation (e.g., a step strain imposed by injection of a known volume of liquid), we probed the ability of the tissue to resist deformation. Instead of imposing a brief window of time at each incrementally higher pressure in a continuous ramp, we allowed many hours of observation at a fixed IOP, to discover whether the tissue deforms continuously or reaches a steady shape. Treatments aimed at making the tissue stronger could be evaluated by using other mechanical methods, but creep tests are particularly appropriate for revealing whether a specific treatment can actually prevent expansion of the tissue when challenged at a particular IOP.
The IOP that the cornea can withstand without ongoing deformation is lower than that which the sclera can withstand (Figs. 3, 4), in accordance with current knowledge of corneal and scleral mechanical properties.19,22 Although the hydration state of the tissue may shift these thresholds, a clear trend is evident: At low IOP only the cornea continues to creep, whereas at high IOP, both the cornea and the sclera undergo creep, with expansion increasing linearly with time. For the purpose of discriminating between different proposed collagen cross-linking conditions, it is useful to find an imposed IOP that produces nominal strain that is substantially greater than the experimental uncertainty. Simply stated, if the control eyes do not deform, then the treated tissues cannot be distinguished from the control. Comparison of low and high IOP illustrates the criteria for selecting a suitable IOP to use for comparative evaluation of treatments: Low IOP did not induce adequate deformation of the control specimens (Figs. 3, 4, left, open symbols), whereas high IOP applied to the kit cornea induced a deformation that was approximately five times greater than the SD of the data (Fig. 4, filled squares).
At the same time, the magnitude of the strain at which the experiment achieves this level of confidence should be small enough to be physiologically relevant. Therefore, methods for comparative evaluation of corneal cross-linking protocols must have small experimental uncertainty. Examination of the results in kit corneas subjected to high IOP showed that a nominal strain of 6% was sufficient (SD less than one fifth of the mean) and that consistent results were obtained over the range from 6% to 20% nominal strain. Further reduction in the uncertainty of GEM may be possible by reducing animal-to-animal variability. The most obvious direction to pursue is pair-wise comparison of eyes from the same animal. Analysis of the instrumental uncertainty shows that little can be gained by improved image acquisition or processing until other sources of variability are reduced by an order of magnitude.
A final consideration illustrated by the response to high IOP is that it generates a significant degree of expansion within 24 hours without rupturing the eyes. The experiment is short enough that the tissue does not significantly deteriorate, yet long enough that the deformation occurs gradually (∼0.01%/min). It is hoped that choosing the in vitro IOP in this way will allow the experiment to reveal information pertinent to prevention of progressive changes in corneal and scleral shape that occur at normal IOP over much longer periods in diseases such as keratoconus and degenerative myopia.
In addition to selection of the appropriate IOP to impose in vitro, we considered the choice of tissue to use as a model. Andreassen et al.15 and others16,17 have shown that the cornea and sclera in keratoconus and myopia have reduced biomechanical stability compared with healthy tissue. In in vitro studies of the mechanical changes induced by prospective treatments for these ocular diseases, normal adult porcine eyes have been used primarily, with human donor tissue used in a few (Spoerl E et al. IOVS 1999;40:ARVO Abstract 1800).24,25 In a study of in vivo treatments and evaluation of their effects, postmortem mature rabbits with healthy ocular tissue were used.31 It is well known that mature, healthy tissue undergoes considerable enzymatic cross-linking of collagen, which increases the strength of the cornea and sclera. Collagen in young animals does not have the full complement of cross-links present in mature collagen.32 There is clinical evidence that immature ocular tissue is more susceptible to deformation: Marked axial enlargement of the globe occurs in infantile glaucoma; for example, although no such changes are noted in adult forms.33 Therefore, we propose that tissues in which this maturation process is incomplete serve as a model that is more representative of weaker, diseased tissue. The reasoning is distinct from in vivo young animal models of myopia that exploit the neurophysiological feedback system to remodel the tissue during development and emmetropization.16,17,34 Rather, the low mechanical strength of the young tissue mimics the weakness found in diseased tissue that is addressed by therapeutic collagen cross-linking.
The present comparison of tissues from 2- to 3-week-old rabbit kits and adult rabbits (>6 mo) indicates significantly greater expansion of immature globes (Figs. 3, 4, middle columns). This difference cannot be explained simply by the difference in size of the globes. For a given imposed IOP, the stress in the tissue scaled linearly with a characteristic radial dimension and inversely with a characteristic tissue thickness. We found that the ratio of the globe radial size and the tissue thickness was very similar in 2- to 3-week-old and adult rabbits (within 5%–10%, depending on the position chosen for comparing the tissue thickness). The observed differences (300% for the cornea and up to 700% for the sclera) were much greater, indicating that the kit eyes are more distensible than the adult eyes. Therefore, we regard kit eyes as the better model of weak tissue susceptible to expansion.
Comparative Evaluation of Treatments
The clinical significance of riboflavin/UVA treatment is becoming increasingly evident, motivating the development of further refinements to reduce the treatment time (∼1 hour per eye), to reduce toxicity to keratocytes,35 to enable treatment of thin corneas without damaging the endothelium,36 and to enable collagen cross-linking of the sclera without retinal toxicity.26 Therefore, researchers are modifying the established riboflavin/UVA protocol (e.g., reducing light intensity, irradiation time, and concentration of riboflavin) and examining alternative agents (e.g., glyceraldehyde).9,10 In the treatment optimization process, GEM can be used for in vitro evaluation of the relative efficacy of different treatments. The present experiments readily distinguished between control corneas and corneas treated using the riboflavin/UVA procedure (Fig. 4, right column). Groups of four samples were sufficient to achieve statistically significant differences between riboflavin/UVA-treated and control eyes, based on a t-test tolerating a 5% chance of a false-affirmative and a 5% chance of a false-negative conclusion (statistical analysis program, G*Power).37
Clinically, interesting comparisons would be those between diseased tissue that has been strengthened and normal tissue that is stable. As discussed, we chose kit eyes as a model of weakened tissue compared with stable adult eyes. Riboflavin/UVA treatment strengthened the weak kit cornea well beyond the normal adult rabbit cornea (Fig. 4, middle, right columns). The clinical success of the riboflavin/UVA corneal collagen cross-linking technique as a treatment for keratoconus indicates that treatment achieves stability; however, a milder treatment might be sufficient based on our expansion results in rabbits, which indicate that riboflavin/UVA treatment may strengthen tissue beyond normal levels. It would be interesting to study the efficacy of milder treatments. Furthermore, the alternative treatment with glyceraldehyde demonstrated greater stability than the riboflavin/UVA treatment.
In evaluating small differences between optimized treatments the testing method must be subject to little variability and must be sensitive. The variability of GEM is minimized by eliminating the need to cut tissue and the variability due to clamping or securing the tissue and by providing a method of establishing the initial size of the eye. The uncertainty of the measurement technique is well below the animal-to-animal variability. Even with the observed animal-to-animal variability, we have demonstrated that the sensitivity of this method allows for discrimination between treatments that change the ultimate strain (24 hours) by as little as 2%. To further improve sensitivity, pairs of fellow eyes given different treatments can be compared. Alternatively, increasing the IOP may create larger distension of strengthened tissue, allowing greater discrimination among treatments. Together, these techniques may provide the capability of distinguishing between treatments of different concentration, exposure time, and light intensity, for example. Finally, rabbit kit tissue affords greater sensitivity to treatment than does adult tissue, which has been used in prior studies of corneal and scleral cross-linking. The inherent stability of normal adult tissue obscures the incremental change in strength due to treatment. Indeed, a weak tissue model such as kit eyes may prove useful in conjunction with other mechanical testing methods as well.
In relation to myopia treatments, we note that glyceraldehyde cross-linking prevents expansion of the entire eye (measured changes are indistinguishable from 0% expansion). The intact globe expansion apparatus is capable of measuring differences in scleral expansion (Fig. 3, right column), with sensitivity similar to that for corneal expansion. Further experiments and characterization of treatments on the sclera could provide insight for development of future treatments for degenerative myopia (e.g., glyceraldehyde and nitroalcohols)9–11 or glaucoma.12
Conclusions
In this study, we were able to demonstrate a quantitative method of examining the mechanical behavior of the cornea and sclera while maintaining an intact geometry similar to that of the in vivo state. Despite their nonuniform thickness, nonuniform curvature, and nonuniform mechanical properties, the cornea and sclera in an intact globe geometry exhibited reproducible nominal creep rates under the influence of an imposed IOP. This characteristic of the intact globe makes it well suited to quantitative characterization of the effect of changes in mechanical stability, such as the effect of a prescribed collagen cross-linking procedure. Consequently, the intact GEM affords quantitative evaluation of treatments when applied to treated and control specimens from animals of a given species and age. We validated a model of weak eye tissue and used this model to illustrate head-to-head treatment comparisons. The results show that statistically significant discrimination between different treatments was achieved with a small number of specimens.
The apparatus can be readily adapted for other research objectives. In addition to probing the mechanical integrity of the ocular coat at constant IOP, this method is designed to accommodate a variety of tests to examine dynamic and elastic properties of the eye (e.g., steps or ramps in IOP). Measurements are performed while the tissue is immersed in a bath, enabling studies of the mechanical behavior of tissue as a function of the chemical and physical environment, such as pH, temperature, osmolarity, and concentration of various chemical species (e.g., distinct salts). The method permits researchers to use their choice of bath fluid (e.g., if eyes were used immediately after enucleation, mineral oil might be used if some deswelling is acceptable).20 Mechanical behavior as a function of disease state could be studied by applying GEM to eyes in animal models (myopia: guinea pig,34 chick,16 and tree shrew16,17; glaucoma: mouse/rat).38 Furthermore, the method could be extended by incorporating multiple cameras, particle tracking, pachymetry, ultrasound biometry, and/or optical coherence tomography to reconstruct the three-dimensional surface of the eye and monitor thickness as the eye responds to an imposed IOP.
Despite the diversity of possible applications of this method, the intact globe expansion apparatus is relatively simple and provides reproducible results. Inexpensive components allow for the construction of multiple setups in the laboratory and minimize the barrier for other researchers wishing to study intact globes. Within the field of ocular biomechanics, access to an inexpensive apparatus to characterize the mechanical integrity of the cornea and sclera may foster studies of the eye's tissue mechanics and facilitate development, characterization, and in vitro validation of treatments that therapeutically modify the mechanical properties of these tissues.
Footnotes
Supported by That Man May See; National Institutes of Health Grant EY017484-01; the Jacobs Institute for Molecular Engineering for Medicine; and Caltech SURF (Summer Undergraduate Research Fellowship).
Disclosure: M.S. Mattson, None; J. Huynh, None; M. Wiseman, None; M. Coassin, None; D.M. Schwartz, None; J.A. Kornfield, None
References
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol 1984;28:293–322 [DOI] [PubMed] [Google Scholar]
- 2.Curtin BJ. The Myopias: Basic Science and Clinical Management Lippincott Williams & Wilkins; 1985:495 [Google Scholar]
- 3.Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998;42:297–319 [DOI] [PubMed] [Google Scholar]
- 4.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retinal Eye Res 2003;22:307–338 [DOI] [PubMed] [Google Scholar]
- 5.Smith VA, Easty DL. Matrix metalloproteinase 2: involvement in keratoconus. Eur J Ophthalmol 2000;10:215–226 [DOI] [PubMed] [Google Scholar]
- 6.Smith VA, Hoh HB, Littleton M, Easty DL. Over-expression of a gelatinase a activity in keratoconus. Eye 1995;9:429–433 [DOI] [PubMed] [Google Scholar]
- 7.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003;135:620–627 [DOI] [PubMed] [Google Scholar]
- 8.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg 2008;34:796–801 [DOI] [PubMed] [Google Scholar]
- 9.Wollensak G, Iomdina E. Long-term biomechanical properties after collagen crosslinking of sclera using glyceraldehyde. Acta Ophthalmol 2008;86:887–893 [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg 2008;34:651–656 [DOI] [PubMed] [Google Scholar]
- 11.Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. Aliphatic beta-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res 2008;87:279–285 [DOI] [PubMed] [Google Scholar]
- 12.Thornton IL, Dupps WJ, Roy AS, Krueger RR. Biomechanical effects of intraocular pressure elevation on optic nerve/lamina cribrosa before and after peripapillary scleral collagen cross-linking. Invest Ophthalmol Vis Sci 2009;50:1227–1233 [DOI] [PubMed] [Google Scholar]
- 13.Hoeltzel DA, Altman P, Buzard K, Choe KI. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng Trans ASME 1992;114:202–215 [DOI] [PubMed] [Google Scholar]
- 14.Zeng YJ, Yang J, Huang K, Lee ZH, Lee XY. A comparison of biomechanical properties between human and porcine cornea. J Biomech 2001;34:533–537 [DOI] [PubMed] [Google Scholar]
- 15.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res 1980;31:435–441 [DOI] [PubMed] [Google Scholar]
- 16.Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci 2000;41:2028–2034 [PubMed] [Google Scholar]
- 17.Siegwart JT, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res 1999;39:387–407 [DOI] [PubMed] [Google Scholar]
- 18.Sthelen R, McEwen WK. Rheology of human Sclera, 1: anelastic behavior. Am J Ophthalmol 1961;52:539–548 [DOI] [PubMed] [Google Scholar]
- 19.Woo SLY, Schlegel WA, Kobayash AS, Lawrence C. Nonlinear material properties of intact cornea and sclera. Exp Eye Res 1972;14:29–39 [DOI] [PubMed] [Google Scholar]
- 20.Greene PR, McMahon TA. Scleral creep vs temperature and pressure in vitro. Exp Eye Res 1979;29:527–537 [DOI] [PubMed] [Google Scholar]
- 21.Forster W, Kasprzak H, Vonbally G. Measurement of elastic-modulus of the central bovine cornea by means of holographic-interferometry, 2: results. Optom Vis Sci 1994;71:27–32 [DOI] [PubMed] [Google Scholar]
- 22.Pierscionek BK, Asejczyk-Widlicka M, Schachar RA. The effect of changing intraocular pressure on the corneal and scleral curvatures in the fresh porcine eye. Br J Ophthalmol 2007;91:801–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tittel PG, Richards RD. Distensibility measurements of rabbit eye. Invest Ophthalmol 1971;10:800–809 [PubMed] [Google Scholar]
- 24.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg 2003;29:1780–1785 [DOI] [PubMed] [Google Scholar]
- 25.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res 1998;66:97–103 [DOI] [PubMed] [Google Scholar]
- 26.Wollensak G, Iomdina E, Dittert DD, Salamatina O, Stoltenburg G. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol Scand 2005;83:477–482 [DOI] [PubMed] [Google Scholar]
- 27.Schnell CR, Debon C, Percicot CL. Measurement of intraocular pressure by telemetry in conscious, unrestrained rabbits. Invest Ophthalmol Vis Sci 1996;37:958–965 [PubMed] [Google Scholar]
- 28.Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci 2004;45:758–763 [DOI] [PubMed] [Google Scholar]
- 29.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol 2001;85:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doughty MJ. The cornea and corneal endothelium in the aged rabbit. Optom Vis Sci 1994;71:809–818 [DOI] [PubMed] [Google Scholar]
- 31.Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol 2009;87:193–198 [DOI] [PubMed] [Google Scholar]
- 32.Lee RE, Davison PF. Collagen composition and turnover in ocular-tissues of the rabbit. Exp Eye Res 1981;32:737–745 [DOI] [PubMed] [Google Scholar]
- 33.Chandler PA, Grant WM, Epstein DL, Allingham RR, Schuman JS. Chandler and Grant's glaucoma 4th ed Baltimore: Williams & Wilkins; 1997:600–603 [Google Scholar]
- 34.Howlett MHC, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res 2006;46:267–283 [DOI] [PubMed] [Google Scholar]
- 35.Wollensak G, Spoerl E, Wilsch M, Seiler T. Keratocyte apoptosis after corneal collagen cross-linking using riboflavin/UVA treatment. Cornea 2004;23:43–49 [DOI] [PubMed] [Google Scholar]
- 36.Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg 2003;29:1786–1790 [DOI] [PubMed] [Google Scholar]
- 37.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191 [DOI] [PubMed] [Google Scholar]
- 38.Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci 2002;43:402–410 [PubMed] [Google Scholar]