These results showing that oral administration of sildenafil and tadalafil increased sheep IOP (with a longer-lasting IOP elevation with the latter agent) are novel findings. The results are discussed within the context of a modified model for AH dynamics that may be of particular importance to senior individuals, the group most likely to be treated with these compounds for vascular diseases.
Abstract
Purpose.
To determine the effects of vasodilators on intraocular pressure (IOP) and the protein content of sheep aqueous humor (AH), because the vasodilators may increase fluid leakage from the fenestrated capillaries of the ciliary body to the extracellular tissue and directly to the anterior chamber (AC) via the iris, and some senior patients (older than 70) treated with sildenafil have exhibited elevated IOP.
Methods.
Experiments were performed on domestic sheep residing on a ranch in Argentina. These docile and compliant animals readily swallowed tablets of sildenafil (50 and 100 mg) and tadalafil (20 mg). IOP was monitored by Perkins applanation tonometry in 21 normal sheep receiving orally administered drugs. In addition, paracentesis was performed on six sheep to quantify changes in AH protein levels.
Results.
Ingestion of both sildenafil and tadalafil increased sheep IOP from normal levels of ∼9 to 11 mm Hg within 1 hour. The IOP elevation was ∼1.6-fold with both doses of sildenafil. IOP returned to control values within 4 hours. With the longer-lasting vasodilator tadalafil, IOP remained 1.6- to 1.9-fold higher than normal for at least 48 hours and returned to control levels within 4 days. The AH protein content was approximately 39% higher in sheep given 100 mg sildenafil.
Conclusions.
These data are consistent with a vasodilator-evoked increase in plasma-like fluid in the AC, which likely accounts for the IOP elevation. The results are discussed with a model for AH dynamics that may be of importance to senior individuals treated for vascular diseases with these compounds.
It has been commonly assumed that all aqueous humor (AH) is secreted across the ciliary epithelium (CE), reaches the posterior chamber (PC), and then enters the anterior chamber (AC) via the pupil. From the AC, AH leaves the eye via the conventional trabecular meshwork (TM) outflow pathway and the uveoscleral pathway (UVP). Because of the absence of an epithelial restriction between the stroma of the ciliary body (CB) and the AC, there is the possibility that extracellular tissue fluid in the CB stroma will directly enter the AC via the iris root and anterior iris surface. This idea was originally posited by Bill,1,2 who also thought that the magnitude of such flow would not contribute significantly to the movement of fluid into the AC. We hypothesize that this possible flow changes in magnitude under conditions that increase the hydrostatic pressure difference between the CB stroma and the AC. The systemic administration of a vasodilator to an individual may be one such condition. In this situation, the pressure difference between the CB stroma and AC could be augmented due to an enhanced leak of plasmalike fluid from the CB capillaries.
Freddo et al.3 demonstrated that proteins directly enter the AC by way of the iris from the CB stroma. This finding is consistent with the absence of an anatomic barrier between the CB stroma and the AC, and as such, the possibility of a net fluid movement via this pathway could also occur.
In the present study, because sildenafil and tadalafil are potent vasodilators of the small precapillary arteries, we measured their effects on the IOP of sheep to test the hypothesis that these agents may induce an increased hydrostatic pressure difference between the CB stroma and the AC and result in elevated IOP. In addition, we measured the protein levels in the AC before and after the sheep were orally treated with sildenafil. We selected sildenafil and tadalafil as test compounds because of their recognized potency for inducing systemic vasodilating effects that include the dilation of vessels within the eye.4,5 Both agents are inhibitors of phosphodiesterase type 5 (PDE5), with the former originally designed to treat cardiac ischemic conditions.6,7 Endothelium-derived relaxing factors (e.g., NO) diffuse into the smooth muscle and increase cGMP levels, which in turn produces muscle relaxation and dilation of blood vessels. These PDE5 inhibitors potentiate the muscle-relaxing effects of NO and cGMP.
Potent PDE5 inhibitors (i.e., sildenafil, tadalafil) are commonly administered to patients as effective treatments for erectile dysfunction and various vascular diseases, including pulmonary hypertension.7 Given the widespread application of these agents, several studies have been conducted to examine the effects of PDE5 inhibitors on ocular blood flow and IOP in both normal human subjects5 and patients with age-related macular degeneration.8 In general, no adverse effects of sildenafil on ocular circulation and IOP have been reported.4,5,9–11 However, one report mentioned elevated IOP in two senior patients (>70 years of age) who had taken sildenafil.10 Because of the possibility that potent vasodilators increase IOP in a particular group of individuals, we decided to test the effects of sildenafil and tadalafil on IOP in an animal model.
Sheep (Ovis aries) were used as experimental subjects based on our experience in handling this species, which we found could serve as an ideal model for examining the mechanisms underlying corticosteroid-induced glaucoma.12 In addition, sheep have eyes with dimensions and volume similar to that in humans (e.g., an anterior–posterior axis of ∼27 mm and an equatorial axis of ∼30 mm). As in other ruminants, sheep have a TM and an aqueous plexus that is equivalent to Schlemm's canal. We report that when sheep received orally administered sildenafil (50 and 100 mg), their IOP increased by ∼65% within 1 hour and approximately doubled within 2 hours of tadalafil ingestion. These results are discussed within the context of a modified model for AH dynamics that may be of particular importance to senior individuals, the group most likely to be treated with these compounds for vascular diseases.
Materials and Methods
Animal Care and Husbandry
All animal experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Twenty-one healthy sheep (Corriedale breed; 19 female, 2 male) between 12 and 24 months of age and weighing 35 to 40 kg, were selected from a local ranch in Corrientes, Argentina. The eyes and general health of the animals were considered normal by an ophthalmologist and a veterinarian, respectively. The sheep were tagged with a number for individual identification on their ear lobes and herded from pasture whenever it was necessary to (1) orally administer a vasodilator, (2) measure IOP by applanation tonometry, or (3) perform paracentesis of the AC to quantify the protein content of the AH. For these maneuvers, the sheep were guided into a funnel corral ending in a loose-fitting yoke.12 This arrangement allowed movement and holding of the head by one person while another administered the drugs, measured IOP, or sampled AH. Between all procedures, the sheep were free to return to pasture.
Drug administration entailed the placement of the appropriate pill in the animal's mouth (either 50 or 100 mg of sildenafil citrate [Vorst; Laboratorios Bernabo, Argentina], or 20 mg of tadalafil [Cialis; Eli Lilly and Co., Indianapolis, IN]) followed by water, to force the animal to swallow. The docile nature of the sheep rendered them remarkably compliant to this procedure. The pharmaceuticals used were purchased without prescription at a local pharmacy in Argentina.
Measurement of IOP of Conscious Sheep
The animals were led to the funnel corral, and their heads were suitably oriented within the neck yoke to enable an ophthalmologist to measure IOP with a Perkins applanation tonometer. Before the IOP measurement, 2 drops of topical 0.5% proparacaine (Alcon Argentina, Tortuguitas, Argentina) followed by 2 drops of 0.25% fluorescein were instilled. Two sets of measurements were taken on each eye, alternating first one eye and then the other. The Perkins tonometry readings were converted to millimeters of mercury, as described in detail elsewhere.12
Paracentesis of the AC and Protein Assay
Before the needle was inserted through the cornea, a local anesthetic was applied to the ocular surface (1–2 drops Alcaine [proparacaine] 0.5%; Alcon Argentina). Approximately 120 μL of aqueous was drawn from the AC with a 29-gauge needle (attached to a 500-μL syringe) that was inserted through the cornea near the angle and perpendicular to the visual axis. Care was taken to avoid touching the iris or lens. The procedure took less than 30 seconds. Withdrawn fluids were promptly transferred to opaque plastic vials that were capped and kept on ice during transport (∼60 minutes) before processing in the laboratory of the School of Medicine at UNNE. Some duplicate samples were brought to Mount Sinai in New York (with appropriate permit of the U.S. Department of Agriculture), to corroborate the data.
The protein content of each AH sample was measured in duplicate in 50-μL aliquots of the withdrawn fluid by a protein assay (Pierce Protein Research, Rockford, IL)13 that is not affected by the levels of reducing agents in the normal aqueous. Briefly, each 50-μL aliquot of AH sample was diluted in half with water and the resulting 0.1 mL was mixed with 1.5 mL of assay reagent solution (as received from Thermo Fisher Scientific).13 After a 5-minute incubation at RT, the absorbance of the solution was measured at 660 nm and compared to the absorbance obtained from bovine serum albumin standards after subtraction of the reagent background.
Data Analysis
The significance of experimentally elicited changes in IOP and AH protein levels were analyzed by using Student's t-test for paired and unpaired data, respectively, with α = 0.05 chosen as the level of significance.
Results
Effects of Oral Sildenafil and Tadalafil on IOP of Normal Sheep
Drugs known to systemically relax smooth muscle and dilate arterioles were administered to sheep on the premise that such agents may also elicit a dilation of the vessels in the CB stroma. If so, an increased leak of proteins and fluid from the fenestrated capillaries of the CB could be expected. From this, an increase in parallel flow to the AC with a consequent higher IOP could occur. To test this concept, we measured the IOP of normal sheep immediately before and 1 hour after the administration of sildenafil (Table 1) and measured the IOP of normal sheep immediately before and up to 2 days after the administration of the longer lasting dilator tadalafil (Table 2).
Table 1.
Effect of Oral Sildenafil on IOP of Normal Sheep Measured with Applanation Tonometry
| Sheep No. |
Mean IOP | n | SEM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 054 |
055 |
056 |
057 |
371 |
371 |
||||||||||
| OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | ||||
| Sex | F | M | F | F | F | M | |||||||||
| Low-dose experiment | |||||||||||||||
| Baseline IOP (0 h) | 9.4 | 9.4 | 11.6 | 11.6 | 11.6 | 11.6 | 13.7 | 14.8 | 9.4 | 9.4 | 10.5 | 9.4 | 11.0 | 12 | 0.5 |
| IOP (1 h) | 13.7 | 12.7 | 21.3 | 18.1 | 18.1 | 20.2 | 20.2 | 20.2 | 18.1 | 15.9 | 22.4 | 18.1 | 18.3* | 12 | 0.9 |
| High-dose experiment | |||||||||||||||
| Baseline IOP (0 h) | 10.5 | 9.4 | 11.6 | 10.5 | 11.6 | 9.4 | 11.6 | 10.5 | 10.6 | 8 | 0.3 | ||||
| IOP (1 h) | 17.0 | 17.0 | 17.0 | 15.9 | 18.1 | 20.2 | 18.1 | 15.9 | 17.4* | 8 | 0.5 | ||||
Oral sildenafil (50 mg) was administered to six sheep within 1 minute of the recording of baseline IOP, and IOP was remeasured 1 hour after drug ingestion. The experiment was repeated 2 days later at a higher dose (100 mg) in four of the six sheep. Recorded values with applanation tonometer were converted to mm Hg according to a calibration curve for sheep eyes (Gerometta et al.12). IOP returned to baseline values within 4 hours of sildenafil ingestion.
Significantly higher than respective baseline value as paired data, P < 2 × 10−5.
Table 2.
Effect of Oral Tadalafil on IOP of Normal Sheep Measured with Applanation Tonometry
| Sheep No. |
Mean IOP | n | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 033 |
034 |
035 |
036 |
||||||||
| OD | OS | OD | OS | OD | OS | OD | OS | ||||
| Sex | F | F | F | F | |||||||
| Baseline IOP (0 h) | 9.4 | 9.4 | 9.4 | 9.4 | 10.5 | 9.4 | 9.4 | 9.4 | 9.6 | 8 | 0.1 |
| IOP at 1 h | 13.7 | 13.7 | 14.8 | 17.0 | 15.9 | 13.7 | 15.9 | 15.9 | 15.1* | 8 | 0.4 |
| IOP at 2 h | 18.1 | 19.1 | 17.0 | 18.1 | 19.1 | 18.1 | 18.1 | 18.1 | 18.2* | 8 | 0.2 |
| IOP at 48 h | 17.0 | 15.9 | 12.7 | 14.8 | 17.0 | 13.7 | 13.7 | 15.9 | 15.1* | 8 | 0.6 |
Tadalafil (20 mg) was administered orally within 1 minute of the recording of baseline IOP, IOP was remeasured 1, 2, and 48 hours after drug ingestion. Recorded values with Perkins tonometer were converted to mm Hg according to a calibration curve for sheep eyes (Gerometta et al.12). IOP returned to baseline within 4 days of tadalafil ingestion.
Significantly higher than baseline IOP as paired data, P < 2 × 10−5.
Consistent with our hypothesis, both drugs increased IOP within 1 hour of ingestion. In the experiments with sildenafil, doses of 50 and 100 mg were administered (Table 1). The lower dose was given to six sheep, which exhibited a 65% increase in IOP 1 hour after drug ingestion (Table 1). Their IOP returned to baseline within 4 hours. Four of these sheep were given 100 mg of sildenafil 2 days later (Table 1). One hour after drug administration, the IOP was 64% higher than baseline, suggesting that the lower dose had elicited the maximum response.
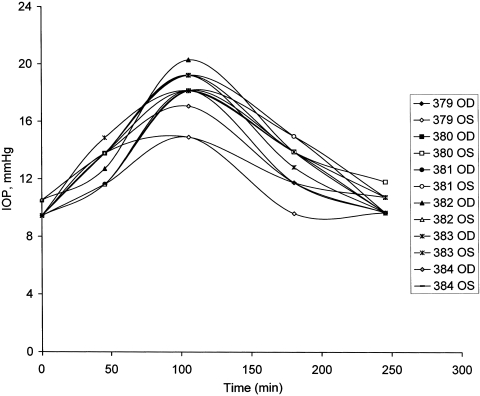
The time course of the IOP changes after sildenafil was demonstrated by giving a set of six additional sheep 100 mg of the vasodilator and recording the IOP at intervals of approximately 1 hour until the IOPs were not different from those measured at t = 0 (Fig. 1). In this set of animals, the IOP 245 minutes after drug ingestion (10.0 ± 0.2 mm Hg, n = 12 eyes) was not significantly different from baseline (9.6 ± 0.1 mm Hg, n = 12, P > 0.2, paired data). A maximum IOP of 17.9 ± 0.5 mm Hg was measured 105 minutes after sildenafil was administered (P < 2 × 10−9, compared with the baseline paired data).
Figure 1.
Time course of changes in sheep IOP on oral administration of 100 mg sildenafil. IOPs in each eye of six sheep are plotted. The sheep numbered 379 to 384 were used in the experiment.
With sheep given tadalafil, an elevated IOP persisted for at least 2 days (Table 2). In this set of four sheep, a maximum IOP nearly two times higher than baseline was recorded 2 hours after drug ingestion. The time frame of the effects of sildenafil and tadalafil on IOP coincides with the general effects on blood pressure and circulation described in the literature. That IOP remained elevated by 57% over baseline IOP 48 hours after tadalafil (Table 2) suggests to us that there was an increased flow of fluid in the proposed parallel pathway toward the AC via the iris.
Effects of Oral Sildenafil on AH Protein Content
A third set of six sheep was selected for determination of the possible effects of sildenafil on the AH protein levels of the AC. The protocol was designed so that AH was sampled from each eye only once. For this, AH was sampled from one eye and sildenafil was then administered, followed 80 minutes later by the sampling of AH from the second eye. IOP was also monitored during this protocol. As such, at t = 0 of the experiment, baseline IOP was recorded in all eyes (n = 12), and six samples of AH were withdrawn, with three right and three left eyes sampled (Table 3). Thereafter, 100 mg of sildenafil was administered orally, and IOP was remeasured 80 minutes after drug ingestion. At this point, IOP was 20.8 ± 0.6 mm Hg, a value ∼2.2-fold higher than baseline (9.5 ± 0.1 mm Hg; Table 3), and the IOPs of the eyes from which AH had already been sampled tended to be lower than that in the paired fellow eyes not yet exposed to the invasive protocol (19.8 ± 1.0 vs. 21.8 ± 0.6 mm Hg; P < 0.02, paired data). Table 3 shows the individual IOPs from the paired eyes at t = 80 minutes. Paracentesis was then performed on the second eye of each sheep. The protein content from this sampling of AH was 0.122 ± 0.023 μg/μL, which was on average 60% higher than the value obtained from the contralateral eye sampled before sildenafil ingestion (0.076 ± 0.013 μg/μL; P < 0.04, paired data; Table 3). No other drugs or doses were tested on this parameter.
Table 3.
Effects of Oral Sildenafil on IOP and AH Protein Levels of Normal Sheep
| Sheep No. |
Mean | n | SEM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 392 |
393 |
040 |
041 |
045 |
034 |
||||||||||
| OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | ||||
| Baseline IOP | 9.4 | 9.4 | 9.4 | 9.4 | 9.4 | 9.4 | 9.4 | 10.5 | 9.4 | 9.4 | 9.4 | 9.4 | 9.5 | 12 | 0.1 |
| Baseline protein content (μg/μL) | 0.057 | 0.075 | 0.056 | 0.127 | 0.042 | 0.096 | 0.076 | 6 | 0.013 | ||||||
| IOP (at 80 min) | 19.1 | 15.9 | 21.3 | 18.1 | 20.2 | 22.4 | 22.4 | 20.2 | 22.4 | 22.4 | 22.4 | 23.4 | 20.8* | 12 | 0.6 |
| Protein content (μg/μL) | 0.171 | 0.086 | 0.094 | 0.198 | 0.048 | 0.132 | 0.122† | 6 | 0.023 | ||||||
Paracentesis of the anterior chamber for the protein assay was performed on one eye of six female sheep, followed by oral administration of sildenafil (100 mg) within 3 minutes of the recording of baseline IOP. IOP was remeasured 80 minutes after drug ingestion. IOP values in bold indicate the eye from which AH was removed. Paracentesis was performed on the fellow eye immediately after the second IOP measurement. The IOP of the sampled fellow eye is shown in bold. In this manner, AH was sampled from each eye only once, and the effects of sildenafil on AH protein levels were determined by comparing the protein contents of the anterior chambers from the contralateral eyes.
IOP significantly higher as paired data, P < 3 × 10−9.
Protein content significantly higher as paired data, P < 0.04.
However, the paired statistical comparison presumes that AH protein concentrations in any one animal are equal in both eyes. Although this may be likely in principle, in practice, more or less protein may be withdrawn from a given eye by paracentesis, especially since there may be a gradient of protein from the angle to the front of the pupil.3 As such, an unpaired comparison between the protein levels of the fellow eyes is more appropriate. Applying this analysis to the protein data in Table 3 results in P = 0.112 for the unpaired two-tailed data. Because we also had additional control data on the AH protein levels that were obtained from one eye of other sheep used for another study, we also compared this set of expanded control values with those from the six eyes of the sildenafil-treated animals shown in Table 3. This group comparison is shown in Table 4 and indicates that the mean level in 20 control eyes was significantly less (at the 5% level) than the mean in the six sildenafil-treated eyes. In this group comparison, the sildenafil-treated values are, on average, approximately 39% higher than the control levels. Overall, the tendency for there to be higher protein concentrations in the AH withdrawn from animals treated with sildenafil is consistent with an increased flow of plasmalike fluid into the AC with the associated diffusion of protein after administration of the vasodilator.
Table 4.
Comparison of AH Protein Levels of Control Sheep with the Levels in the Sildenafil-Treated Sheep
| Sheep No. | Control Group | Sildenafil Group |
|---|---|---|
| 392 | 0.057 | 0.171 |
| 393 | 0.075 | 0.086 |
| 040 | 0.056 | 0.094 |
| 041 | 0.127 | 0.198 |
| 045 | 0.042 | 0.048 |
| 034 | 0.096 | 0.132 |
| 341 | 0.061 | |
| 342 | 0.116 | |
| 355 | 0.073 | |
| 356 | 0.096 | |
| 358 | 0.117 | |
| 360 | 0.124 | |
| 361 | 0.099 | |
| 366 | 0.089 | |
| 368 | 0.095 | |
| 369 | 0.072 | |
| 373 | 0.053 | |
| 377 | 0.122 | |
| 378 | 0.063 | |
| 390 | 0.118 | |
| Mean | 0.088 | 0.122* |
| SEM | 0.006 | 0.023 |
| n | 20 | 6 |
Value significantly greater than that of the control group (P < 0.05, as unpaired, two-tailed data).
Discussion
Our results showing that oral administration of sildenafil and tadalafil increased sheep IOP (with a longer-lasting IOP elevation with the latter agent) are novel findings. These potent vasodilators (particularly of arterioles) most likely affected IOP by increasing AH inflow, rather than by increasing the resistance to AH outflow. This postulated increase in AH production may have resulted from an increase in fluid flow via (1) the proposed parallel pathway, (2) an increase in fluid transport by the CE, or (3) a combination of both in an unknown proportion. In addition, PDE5-inhibitor ingestion may have increased choroidal volume in sheep, given the fenestrated capillaries of the choroid and that the choroid is a vascular tissue analogous to the corpus cavernosum.14 A putative increase in choroidal volume could have contributed to the elevation of IOP.15 However, the protein data do not appear to favor this possibility, as the PDE5 inhibitor-evoked IOP elevation occurred in tandem with an increase in the protein content of the AH in the AC. This increase is consistent with an increased flow of plasmalike fluid toward the AC via the iris in accordance with the model published by Freddo.3
There is a large variability in the literature regarding control protein concentrations in the AC, and to the best of our knowledge, the protein levels from sheep have not been measured. Moreover, we are unaware of any publication that has compared increases in IOP with the levels of AC proteins. After vasodilator ingestion, an increased volume, flow, and pressure in the precapillary arterioles could have different effects on IOP and AC protein levels. Pressure in the capillaries would be transmitted as a wave with some fluid flow, whereas proteins would actually have to diffuse and piggyback with the flow. Each CB stroma may also have different permeability for the flow and the diffusion of proteins. In addition, according to Freddo,3 the protein concentration at the angle is higher than in front of the pupil, and the rate of protein entry into the AC may be different during miosis and mydriasis. These multiple factors could have come into play and affected the levels of proteins withdrawn from the eye. As shown in Table 3, sheep 045 and 393 exhibited an increase in protein levels of approximately 13% and 15%, respectively, whereas IOP approximately doubled; in sheep 392, the large IOP increase occurred in tandem with a nearly threefold elevation of AC protein concentration. Nevertheless, because there was a tendency for the AC protein levels to be higher in the eyes of sheep receiving sildenafil, the apparent variability in AC protein values does not, in itself, negate the hypothesis of the parallel pathway.
Sildenafil and tadalafil have established effects on erectile function. The effectiveness of the former subsides in 2 to 3 hours, whereas the effectiveness of the latter persists for at least 48 hours. Thus, we have confirmed these different time courses on the IOP of sheep. Our results suggest that the effects of these agents on the muscles of the small arterioles of two different parts of the body are similar.
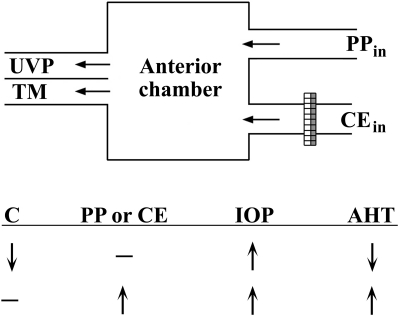
Given two possible ways in which IOP could increase (increased inflow or decreased outflow), the inflow increase could occur via the classic CE transport pathway or via our postulated parallel pathway (PP). The outflow could change via the conventional TM or UVP pathway. These possibilities are summarized in Figure 2.
Figure 2.
Simplified model for inflow and outflow pathways of AH. Each row of arrows and a dash qualitatively summarizes the relative changes in AH dynamics that in turn elicit a consequential increase in IOP. From the present work, it is hypothesized that vasodilators primarily increase PP inflow (PPin) and possibly inflow via the CE (CEin) without affecting C, the aqueous outflow facility (second row). AHT, aqueous humor turnover.
It is possible that the ingestion of PDE5 inhibitors increased cGMP levels within the tissues of the sheep eye. There are reports that cGMP inhibits transport in the isolated porcine CB16,17 and that it increases outflow facility in a dose-dependent manner in monkeys.18 However, reduction in fluid transport and increased outflow facility would have an effect on IOP opposite to the one that we observed. Because there is no indication of cGMP stimulation of the ciliary-originated fluid transport, nor of a cGMP-induced breakdown of the tight junctions between the CE cells, a possible explanation for the observed elevation in IOP is an increase in fluid flow via the PP.
The PP (ciliary capillaries to AC) acts as a pseudofacility pathway, because an increase in the IOP that is due to reduced facility of the TM would reduce the flow from the ciliary capillaries to the AC, since it should be pressure dependent.
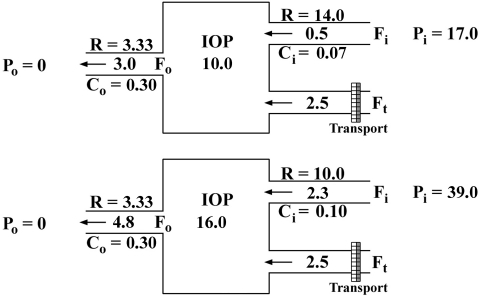
A graphic example (Fig. 3) clearly shows how a putative increase in capillary pressure from 17 to 39 mm Hg would affect IOP and conventional inflow and outflow. This is only a hypothetical example, with values similar to those obtained in the eye of sheep before and after sildenafil treatment. They do not represent actual measurements. It is a mathematical model that could be applied to any hydraulic circuit (pressure is in millimeters of mercury, flow in microliters per minute, and facility in microliters per minute per millimeters of mercury). The top diagram shows a steady state with an IOP of 10.0 with inflows and outflow of 3.0. The CE inflow pathway has a very high hydraulic resistance and is unresponsive to pressure gradients. The 0.5 inflow across the parallel pathway is driven by a pressure difference of 7.0 (17.0–10.0), so that its facility is 0.07. The outflow pathway (both the TM and UVP together) has a facility of 0.30 between the pressures of 10.0 to 0. Thus, 10 × 0.30 will drive a flux of 3. Now assume (as shown in the bottom diagram) that by the action of the vasodilator, Pi increases to 39.0, which will increase the parallel flow to a value (2.3) that in turn will increase IOP to a point (16.0) that will drive out of the eye 4.8, to equalize the input (2.3 + 2.5) and a new steady state. The CE inflow will not change, because it is unresponsive to pressure. The IOP stabilizes at a higher pressure because of mainly an increased inflow, which also drives an increased outflow.
Figure 3.
Quantitative theoretical model for the effect of vasodilators on the AH dynamics of sheep.
We are aware of several studies reporting minimal or no effect of sildenafil on IOP.4–5,9–11 One of the studies reported that sildenafil increased the IOP of two men older than 70 years of age.10 The increase in pressure required treatment or surgery. Reports of the effect of the long-lasting vasodilators also indicate no significant effect on IOP.9,19 To determine whether the vasodilators' effect on IOP is particular to sheep, the corresponding author of the study (OAC, who belongs to the senior group) took 100 mg of sildenafil. His IOP, which has been consistently 10 to 11 mm Hg for the past 6 years increased to 17 mm Hg 1 hour after the ingestion of the drug, whereas his systolic arterial pressure decreased to 95 mm Hg. Both parameters returned to baseline values in 3 hours. Since those who may take sildenafil regularly for vascular diseases are in the senior group, it is important that a more detailed study be performed on such individuals, and sheep may be a good animal model for this group.
Finally, an explanation is not apparent as to why all sheep in this study that received the PDE5 inhibitors exhibited elevated IOP, whereas few human patients who receive these agents as treatments for vascular diseases manifest such a symptom. A possible explanation that should be studied further is that senior patients become deficient in the autoregulation of ocular blood flow, and autoregulation of blood flow in normal sheep may differ from that of humans in good health.
Footnotes
Supported by National Eye Institute Grants EY00160, EY01867, and EY13749, and by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY.
Disclosure R. Gerometta, None; L.J. Alvarez, None; O.A. Candia, None
References
- 1.Bill A. The role of ciliary blood flow and ultrafiltration in aqueous humor formation. Exp Eye Res 1973;16:287–298 [DOI] [PubMed] [Google Scholar]
- 2.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev 1975;55:383–417 [DOI] [PubMed] [Google Scholar]
- 3.Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res 2001;73:581–592 [DOI] [PubMed] [Google Scholar]
- 4.Koksal M, Ozdemir H, Kargi S, et al. The effects of sildenafil on ocular blood flow. Acta Ophthalmol Scand 2005;83:355–359 [DOI] [PubMed] [Google Scholar]
- 5.Harris A, Kagemann L, Ehrlich R, Ehrlich Y, Lopez CR, Purvin VA. The effect of sildenafil on ocular blood flow. Br J Ophthalmol 2008;92:469–473 [DOI] [PubMed] [Google Scholar]
- 6.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol 1999;83:13C–20C [DOI] [PubMed] [Google Scholar]
- 7.Konstantinos G, Petros P. Phosphodiesterase-5 inhibitors: future perspectives. Curr Pharm Des 2009;15:3540–3551 [DOI] [PubMed] [Google Scholar]
- 8.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS. Effect of Viagra on the foveolar choroidal circulation of AMD patients. Exp Eye Res 2005;81:159–164 [DOI] [PubMed] [Google Scholar]
- 9.Cordell WH, Maturi RK, Costigan TM, et al. Retinal effects of 6 months of daily use of tadalafil or sildenafil. Arch Ophthalmol 2009;127:367–373 [DOI] [PubMed] [Google Scholar]
- 10.Yajima T, Yajima Y, Koppiker N, Grunwald JE, Laties AM. No clinically important effects on intraocular pressure after short-term administration of sildenafil citrate (Viagra). Am J Ophthalmol 2000;129:675–676 [DOI] [PubMed] [Google Scholar]
- 11.Grunwald JE, Siu KK, Jacob SS, Dupont J. Effect of sildenafil citrate (Viagra) on the ocular circulation. Am J Ophthalmol 2001;131:751–755 [DOI] [PubMed] [Google Scholar]
- 12.Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Invest Ophthalmol Vis Sci 2009;50:669–673 [DOI] [PubMed] [Google Scholar]
- 13.Antharavally BS, Mallia KA, Rangaraj P, Haney P, Bell PA. Quantitation of proteins using a dye-metal-based colorimetric protein assay. Anal Biochem 2009;385:342–345 [DOI] [PubMed] [Google Scholar]
- 14.Paris G, Sponsel WE, Sandoval SS, et al. Sildenafil increases ocular perfusion. Int Ophthalmol 2001;23:355–358 [DOI] [PubMed] [Google Scholar]
- 15.Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res 1994;58:529–543 [DOI] [PubMed] [Google Scholar]
- 16.Fleischhauer JC, Beny JL, Flammer J, Haefliger IO. NO/cGMP pathway activation and membrane potential depolarization in pig ciliary epithelium. Invest Ophthalmol Vis Sci 2000;41:1759–1763 [PubMed] [Google Scholar]
- 17.Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol 2006;148:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kee C, Kaufman PL, Gabelt BT. Effect of 8-br cGMP on aqueous humor dynamics in monkeys. Invest Ophthalmol Vis Sci 1994;35:2769–2773 [PubMed] [Google Scholar]
- 19.Taner P, Basar MM, Unal B, Batislam E. Effects of vardenafil on intraocular pressure and orbital hemodynamics. J Ocul Pharmacol Ther 2007;23:275–279 [DOI] [PubMed] [Google Scholar]