The authors investigated mechanisms for the transport of the essential vitamin folate in freshly isolated Müller glial cells. Their laser scanning confocal microscopic and electron microscopic immunolocalization studies demonstrate for the first time colocalization of two proteins, folate receptor α and proton-coupled folate transporter, in the endosomes of Müller cells, suggesting that these proteins function coordinately to mediate folate acquisition in these cells.
Abstract
Purpose.
To analyze the mechanisms of folate uptake in retinal Müller cells.
Methods.
RT-PCR and Western blot analysis were performed in freshly isolated neural retina and RPE/eyecup, primary mouse Müller cells, and rMC-1 cells for the three known folate transport proteins folate receptor α (FRα), proton-coupled folate transporter (PCFT), and reduced folate carrier (RFC). Laser scanning confocal microscopy (LSCM) and immunoelectron microscopy were used to determine the subcellular location of FRα and PCFT in primary Müller cells. The pH dependence of the uptake of [3H]-methyltetrahydrofolate ([3H]-MTF) was assayed in Müller cells in the presence/absence of thiamine pyrophosphate, an inhibitor of RFC.
Results.
FRα and PCFT are expressed abundantly in the retina in several cell layers, including the inner nuclear layer; they are present in primary mouse Müller cells and rMC-1 cells. LSCM localized these proteins to the plasma membrane, nuclear membrane, and perinuclear region. Immunoelectron microscopic studies revealed the colocalization of FRα and PCFT on the plasma membrane and nuclear membrane and within endosomal structures. Müller cell uptake of [3H]-MTF was robust at pH 5.0 to 6.0, consistent with PCFT activity, but also at neutral pH, reflecting RFC function. RFC was expressed in mouse Müller cells that had been allowed to proliferate in culture, but not in freshly isolated primary cells.
Conclusions.
FRα and PCFT are expressed in retinal Müller cells and colocalize in the endosomal compartment, suggesting that the two proteins may work coordinately to mediate folate uptake. The unexpected finding of RFC expression and activity in cultured Müller cells may reflect the upregulation of this protein under proliferative conditions.
Folate, a water-soluble vitamin essential for the synthesis of DNA, RNA, and proteins, is required for cell survival. Folate deficiency has deleterious consequences on the retina. In nutritional amblyopia, which may occur in the presence of other vitamin deficiencies1,2 or in isolated folate deficiency (Schaible ER, et al. IOVS. 1993;34:ARVO Abstract 2516),3 an optic neuropathy develops in which the papillomacular fibers of the retina are damaged, resulting in central vision loss.4,5 In methanol-induced ocular toxicity, formate, a highly toxic byproduct of methanol metabolism, damages Müller cells, leading to blindness or serious visual impairment.6 Folate is necessary to convert formate to carbon dioxide.7 Folate deficiencies can precipitate accumulation of homocysteine,8 which has been implicated in retinal diseases such as maculopathy, open-angle glaucoma, and diabetic retinopathy.9–14
Although a deficiency of folate can occur through dietary insufficiency, impaired transport of folate into cells could have similar deleterious effects. Three cellular mechanisms for folate transport have been identified: folate receptors (FR), reduced folate carrier (RFC), and the newly described proton-coupled folate transporter (PCFT). FRs are anchored to the cell surface plasma membrane by glycosylphosphatidylinositol.15–18 Upon binding of folate to FR, the receptor-folate complex is internalized by endocytosis. There are four human isoforms of FR (α, β, γ, δ). In mice, the protein is referred to as folate binding protein (Folbp), and it has three isoforms (Folbp 1, 2 3) analogous to the α, β, and δ forms in humans. Depending on the isoform, FRs contain approximately 240 to 260 amino acids and have a molecular mass in the range of approximately 28 to 40 kDa, reflecting the extent of glycosylation. FRα has a much greater affinity for nonreduced folates, such as folic acid, than for reduced folates. RFC is a 57 to 65 kDa integral transmembrane and energy-dependent protein that exhibits a high affinity for N5-methyltetrahydrofolate (MTF; Moravek Biochemicals, Inc., Brea, CA), the predominant form of folate in blood (see Refs. 19 and20 for reviews). RFC (also known as reduced folate transporter and as folate transport protein) is a member of the SLC19 family of solute carriers (SLC19A1).20 RFC functions as an anion exchanger operating optimally at pH 7.4; its activity and folate-concentrating ability decrease as pH decreases. PCFT is a newly described folate transport protein that is the product of SLC46A1.21 Originally identified as heme carrier protein 1,22 PCFT mediates H+-coupled electrogenic transport of folate and its derivatives with similar affinity for the oxidized and reduced forms of folic acid.21 PCFT is reported to have a molecular weight of 50 to 65 kDa, depending on the extent of glycosylation.23 PCFT is a typical integral membrane transporter protein and spans the membrane 12 times.24 Folate transport by PCFT involves the influx of one H+ per transport cycle. Because the transport process is electrogenic, this stoichiometry suggests that the zwitterionic form of folate is recognized by the transporter as the substrate, and the involvement of one H+ indicates that folate is accepted as a substrate only in its electroneutral form.25
Previous studies from our laboratory examined the mechanism(s) by which folate is taken up by the RPE,26–31 a layer of epithelial cells that forms the outer blood-retinal barrier. Using intact retinal tissue, FRα and RFC demonstrated a polarized distribution such that FRα is placed on the basolateral retinal pigment epithelial surface, presumably poised to take up folic acid from the choroidal circulation, and RFC is located on the apical membrane and likely releases folic acid into the subretinal space.26–28 The activity of RFC in RPE is attenuated by hyperglycemia, nitric oxide, and hyperhomocysteinemia.29–31 In situ hybridization and immunohistochemical data suggested that though FRα is present in multiple retinal layers including the ganglion cell layer and some cells of the inner nuclear layer and inner segments of photoreceptor cells,27 the expression of RFC in intact retina is limited primarily to the RPE. Interestingly, folate uptake was examined using the conditionally immortalized rat retinal capillary endothelial cell line (TR-iBRB2), an in vitro model of the inner blood retinal barrier. RFC was expressed, and functional studies suggested that it played the major role in folate uptake in these cells.32 Initial studies of PCFT in retina show that the mRNA encoding this protein is expressed in neural retina, RPE/eyecup, and in ganglion, Müller, and retinal pigment epithelial cells in vitro.25 The distribution of PCFT in mammalian retina and its subcellular location have not been examined.
In this study, we investigated mechanisms of folate transport in Müller cells, the major retinal glial cell. Müller cells span the entire retinal thickness, contacting and ensheathing retinal neurons. Many retinal diseases are associated with reactive Müller cell gliosis. Müller cells play a crucial role in neuronal survival by providing trophic substances and precursors of neurotransmitters to neurons.33 Studies of the transport properties of Müller cells have centered on the uptake of neurotransmitters such as glutamate34–37 and GABA38 and the abundant retinal amino acid taurine.39 Nothing is known about the uptake of folate in these cells. To fill that void, molecular and cell biology methods were used to determine which folate transport proteins were present in freshly isolated mouse Müller cells37,40 and the rMC-1 Müller cell line.41 We investigated whether FRα and PCFT are localized in the endosomal compartment, as has been suggested in studies of nonretinal cell types.42,43 Our data show unequivocally the presence of FRα and PCFT in retinal Müller cells and demonstrate at the ultrastructural level the colocalization of these proteins in endosomal compartments.
Materials and Methods
Culture of rMC-1 Cells and Freshly Isolated Mouse Müller Cells
The rMC-1 (rat Müller) cell line was a generous gift from Vijay P. Sarthy (Northwestern University, Evanston, IL)36; cells were cultured as described.40 Mouse Müller cells were isolated from 5-day-old mice and were cultured in accordance with our method.25,37,40 Treatment of mice conformed to policies set forth in the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. In one experiment, primary ganglion cells were isolated from mouse retina in accordance with our method 37 and were used for immunocytochemistry.
Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from mouse neural retina, RPE/eyecup, rMC-1 cells, and freshly isolated Müller cells using reagent (TRIzol; Invitrogen, Carlsbad, CA).40 Table 1 provides the sequences for the primers used in these experiments. The 18S primer/competimer ratio for FRα was 5:5, and for PCFT it was 3:7. PCR was performed for 30 cycles with a denaturing phase of 1 minute at 94°C, an annealing phase of 1 minute at 52°C for FRα, 62°C for PCFT, and 55° for RFC, and an extension phase of 1 minute at 72°C. Samples were reverse transcribed and subjected to PCR for 30 cycles. PCR products were confirmed by isolating RNA from the neural retina and eyecup. RT-PCR was performed, and the PCR products were ligated into vector (T-Easy; Promega, Madison, WI) and transformed into Escherichia coli cells (JM109; Promega). The white colony was grown in LB medium overnight, the plasmid was extracted, and the inserted band was confirmed by EcoRI enzyme digestion. Plasmid sequences were analyzed by the Medical College of Georgia DNA sequencing core. Sequences were confirmed by BLAST analysis against the National Center for Biotechnology Information gene database.
Table 1.
Sequences of Primers for Folate Transport Proteins
| Gene | NCBI Accession No. | Primer Sequence | Predicted Band Size |
|---|---|---|---|
| Mouse primers (used with primary mouse Müller cells) | |||
| FRα | NM_008034 | Forward: 5′-GAAGACGAATTCCTGCTGT-3′ | 419 |
| Reverse: 5′-TGAGCTTGTAGGAGTGACT-3′ | |||
| PCFT | NM_026740 | Forward: 5′-TGCTAGCCCCTCCGTGTTTGC-3′ | 567 |
| Reverse: 5′-CCATCCGGAAGGTGCGACTGT-3′ | |||
| RFC | NM_031196 | Forward: 5′-TCTTTCTAAAGCGCCCTAA-3′ | 626 |
| Reverse: 5′-GATACAGGTCTTAAGCGCAGTAGC-3′ | |||
| Rat primers (used with rat-derived rMC-1 cells) | |||
| FRα | NM_133527 | Forward: 5′-GTGGATGGCCGAATGTGC-3′ | 638 |
| Reverse: 5′-AGAACCTCGCCACTTCCTCGTT-3′ | |||
| PCFT | BC_089868 | Forward: 5′-CACACAGTACATTTGGCACCGCAT-3′ | 646 |
| Reverse: 5′-TGGGACCACATACAGCTGGACAAT-3′ | |||
| RFC | NM_017299 | Forward: 5′-CCCTCTTCCTAAAGCGTC-3′ | 724 |
| Reverse: 5′-CAGATGATGGACAGCGTCAG-3′ | |||
Preparation of Antibody against PCFT
There is no antibody commercially available for PCFT. We used the Antheprot software program to determine the peptide sequence for mouse PCFT best suited for generation of an antibody. The peptide sequence CFGETVKEPKSTRLF, corresponding to residues 241 to 255 of mouse PCFT, was considered most antigenic. It was synthesized in rabbit by GenScript Corporation (Piscataway, NJ). Antiserum was dialyzed overnight and purified by affinity chromatography with a purification kit (Melon Gel IgG; Pierce, Rockford, IL).
Immunodetection of FRα and PCFT in Tissues and Isolated Cells
Cryosections of 3-week-old Balb/c mouse eyes were prepared for immunohistochemistry according to our method.26 Sections were incubated with goat anti–FRα antibody (1:25; Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti–PCFT antibody (1:500). Negative control sections were treated identically in the absence of primary antibodies or in the presence of antibody preadsorbed with an excess of antigenic peptide. Sections were rinsed and incubated for 1 hour with donkey anti–goat and donkey anti–rabbit IgG-conjugated Alexa Fluor 488 and Alexa Fluor 555 (Invitrogen), respectively, and were counterstained with DAPI to label nuclei. Sections were examined by epifluorescence with a microscope (Axioplan-2; Carl Zeiss, Göttingen, Germany) equipped with digital image processing software (Axiovision, version 4.7; Carl Zeiss). The cellular location of FRα and PCFT was analyzed by laser scanning confocal microscopy (LSCM) in freshly isolated Müller cells prepared for immunocytochemistry according to our method.40 They were incubated overnight at 4°C with FRα or PCFT antibodies (1:500), anti-vimentin (1:25; glial cell marker; Calbiochem, San Diego, CA), anti-lamin A (nuclear membrane marker, 1:100), or anti-PDI (ER marker, 1:100). Cells were incubated for 30 minutes with goat anti–rabbit IgG coupled to Alexa Fluor 568 and goat anti–mouse IgG coupled to Alexa Fluor 488 (1:1000) and were examined by LSCM using a confocal microscope (LSM 510; Carl Zeiss) equipped with Meta imaging software. Immunocytochemical detection of FRα, RFC, or CRALBP (generous gift from John Saari, University of Washington, Seattle, WA) in freshly isolated mouse Müller cells used a microscope (Axioplan-2; Carl Zeiss) equipped with digital image processing software (Axiovision, version 4.7; Carl Zeiss) and a camera (HRM; Carl Zeiss). Unless otherwise specified, antibodies and chemical reagents were from Sigma Chemical Corp. (St. Louis, MO).
Immunogold Electron Microscopy
Müller cells were fixed in 2% paraformaldehyde/2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), postfixed in 4% osmium tetroxide, and processed for embedding in LR White resin. Thin sections were cut with a diamond knife on an ultramicrotome (EM UC6; Leica Microsystems, Inc., Bannockburn, IL) and collected on nickel grids. Sections were incubated with primary antibody overnight and stained with gold-labeled secondary antibody, followed by uranyl acetate. FRα was labeled with a secondary antibody conjugated to an 18-nm gold particle, and PCFT was labeled with a secondary antibody conjugated to a 10-nm gold particle. Cells were examined under a transmission electron microscope (JEM 1230; JEOL USA. Inc., Peabody, MA) at 110 kV and imaged (UltraScan 4000 CCD camera/First Light Digital Camera Controller; Gatan Inc., Pleasanton, CA).
Western Blot Analysis for Folate Transport Proteins
Protein was extracted from mouse RPE/eyecup, neural retina, and intestine or from Müller cells as described.37 Protein samples were subjected to SDS-PAGE and, after transfer to nitrocellulose membranes, were incubated with antibody against FR-α, PCFT, or RFC overnight at 4°C, followed by incubation with horseradish peroxidase–conjugated goat anti–rabbit IgG antibody. After washing, proteins were visualized with the ECL Western blot detection system (Thermo Scientific, Waltham, MA). β-Actin served as the loading control.
Transport Experiments
The uptake of [3H]-MTF (specific radioactivity, 25 Ci/mmol) was measured in mouse Müller cells following the second passage after the cells had reached confluence. The culture medium was removed, and cells were washed once with warm Na+-containing uptake buffer. PCFT operates optimally at pH 5.0 to 5.5, whereas RFC functions optimally at a more neutral pH.44 To assess the influence of pH on the transport process, uptake buffers of varying pH (5.0–8.0) were prepared by mixing the following two buffers: 25 mM Mes/Tris (pH 5.0), 140 mM NaCl, 1.8 mM CaCl2, 5.4 mM KCl, 0.8 mM MgS04, 5 mM D(+)-glucose and 25 mM Tris/HEPES (pH 8.0), 140 mM NaCl, 1.8 mM CaCl2, 5.4 mM KCl, 0.8 mM MgS04, 5 mM D(+)-glucose. Uptake was initiated by adding 250 μL uptake medium containing [3H]-MTF. Cells were incubated for 15 minutes at 37°C. Then the medium was removed, and cells were washed with ice-cold uptake buffer at pH 7.5, solubilized with 0.5 mL of 1% SDS-0.2N NaOH, and used for determination of radioactivity by liquid scintillation spectrometry. Thiamine pyrophosphate (TPP), which inhibits the function of RFC45–47 but not of PCFT, was used in experiments to assess the relative contribution of RFC to [3H]-MTF uptake by Müller cells.
Results
Gene and Protein Analysis of FRα and PCFT in Isolated Retinal Samples
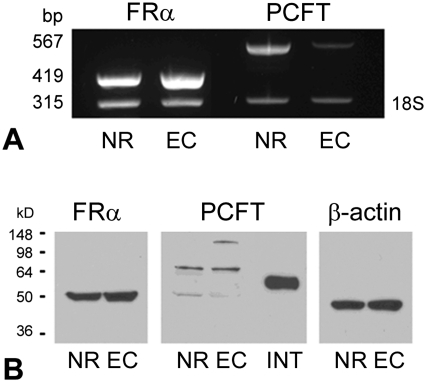
Neural retina and RPE/eyecup were isolated from mice, and the expression of the genes encoding FRα and PCFT was analyzed by RT-PCR (Fig. 1A). Both genes were expressed abundantly in neural retina. FRα expression was robust; PCFT expression was qualitatively lower in the RPE/eyecup (Fig. 1B). Western blot analysis detected FRα and PCFT in neural retina and RPE/eyecup. PCFT detected in retinal samples had a molecular weight of approximately 64 kDa, whereas PCFT from duodenum migrated with a molecular weight of approximately 55 kDa as reported.21 The intestine was used as a positive control because this was the first tissue in which PCFT was identified.21 The differences in band size between intestine and retinal samples and the minor bands detected in the retinal samples may reflect differences in the extent of protein glycosylation.23
Figure 1.
FRα and PCFT expression in mouse retina. (A) Total RNA was isolated from mouse neural retina (NR) and RPE/eyecup (EC). RT-PCR analysis was performed using primers specific for FRα (expected product size, 419 bp) and PCFT (567 bp). FRα and PCFT were expressed in NR and RPE/EC. 18S (315 bp) served as an internal standard. (B) NR, RPE/EC, and intestinal (INT) protein lysates were prepared from mouse and used for Western blot analysis. FRα (∼50 kDa) and PCFT (50–64 kDa) were detected in NR and RPE/EC. PCFT harvested from mouse duodenum migrated at a molecular weight of 55 kDa. β-Actin (∼45 kDa) served as the loading control.
Immunolocalization of FRα and PCFT in Mouse Retina
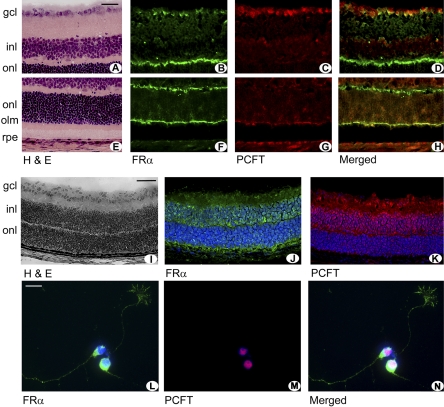
Earlier studies from our laboratory had demonstrated FRα in several layers of the retina27; however, the retinal localization of PCFT protein has not been reported. To screen for PCFT distribution in the retinal layers, immunolocalization studies were performed in intact mouse retina using commercially available antibodies to detect FRα and antibodies from our laboratories (IDG) to detect PCFT. Figures 2A and 2E provide retinal cryosections showing hematoxylin and eosin (H&E)–stained sections for comparison with fluorescence detection in remaining panels. FRα was detected abundantly in cells of the ganglion cell layer and was present in the outer limiting membrane (Figs. 2B, 2F). Müller cell processes are an integral component of a diverse group of intercellular contacts that compose the outer limiting membrane, a readily identifiable line of demarcation in retinal sections.33 The outer plexiform layer, representing synaptic connections between neurons of the outer and inner nuclear layers, was intensely immunopositive for FRα. There was faint green fluorescence in the inner plexiform layer, light labeling around cell soma within the inner nuclear layer, and intense labeling of the outer limiting membrane. FRα was detected in RPE, confirming earlier reports.27 To examine which retinal cells were positive for PCFT, immunodetection was performed using a red fluorescing secondary antibody (Figs. 2C, 2G). As with FRα, PCFT was present in the ganglion and retinal pigment epithelial cell layers. The somas of some cells in the inner nuclear layer were immunopositive for PCFT. The merged image shows the areas of colocalization of the two proteins (Figs. 2D, 2H). The ganglion cell layer and the Müller processes of the outer limiting membrane were intensely positive for both proteins. These experiments were performed in retinas of 3-week-old mice, an age when retinal layers are distinct. We asked whether the somas of cells in the inner nuclear layer (which would include cell bodies of Müller cells) would be positive for FRα and PCFT at earlier ages and examined the location of FRα and PCFT in retinas of 5-day-old mice. Figure 2I shows an H&E-stained section of mouse retina depicting the emerging nuclear layers during retinal development. FRα and PCFT are present in somas of cells within the inner nuclear layer (Figs. 2J, 2K). Thus, it appears that the proteins are present in cells of the inner nuclear layer during early retinal development and that, as the retina matures, the distribution is concentrated in the outer limiting membrane (of Müller cell origin) and in the ganglion cell layer, outer plexiform layer, and RPE.
Figure 2.
Immunodetection of FRα and PCFT in mouse retina. (A, E) Mouse retinal cryosections (harvested from 3-week-old mice) were stained with H&E to demonstrate retinal layers for comparison with cryosections subjected to immunofluorescence detection of FRα (green, B, F) and PCFT (red, C, G). (Yellowish-orange, merged image, D, H) Areas of antibody coimmunofluorescence. (I) Mouse retinal cryosections (harvested from 5-day-old mice) were stained with H&E to demonstrate retinal layers for comparison with cryosections subjected to immunofluorescence detection of FRα (green, J) and PCFT (red, K). (L–N) Ganglion cells isolated by immunopanning from neonatal mouse retinas were subjected to immunocytochemistry to detect FRα and PCFT. FRα (green, L) is present in the cell body and processes extending from the cells; PCFT (red, M) is present in the cell body/nucleus of these neurons with minimal detection in processes. The merged image for these two proteins is shown in (N). Scale bars: 20 μm, (A–H); 50 μm (I–K); 7 μm (L–N). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; OLM, outer limiting membrane; RPE, retinal pigment epithelium.
FRα and PCFT labeling detected in the ganglion cell layer could represent labeling of the cell bodies of neurons in this layer or the endfeet of Müller cells (inner limiting membrane), or both. We examined whether these two proteins were present in freshly isolated ganglion cells harvested from neonatal mouse retina and detected FRα abundantly in the cell body and in the processes projecting from the cell body (Fig. 2L). PCFT was present only in the cell body of the ganglion cell (Fig. 2M). Although not the focus of the present study, the data clearly show the colocalization of these folate transport proteins in this retinal neuronal cell type (Fig. 2N).
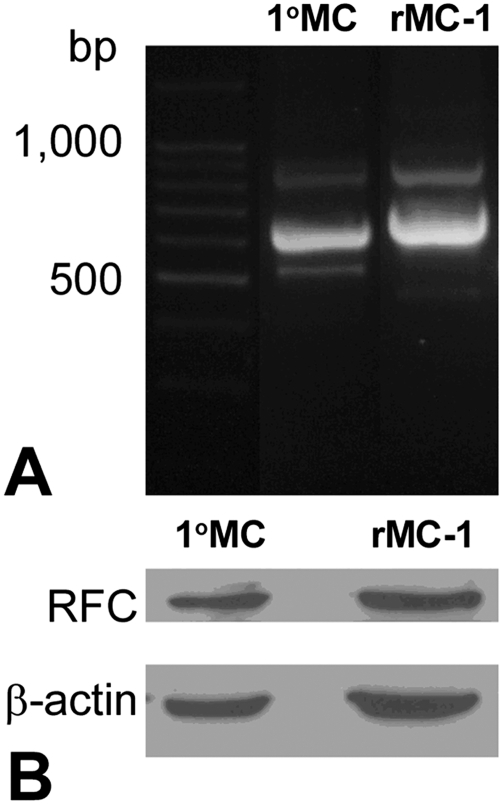
RT-PCR and Western Blot Analysis of FRα and PCFT in Freshly Isolated Müller Cells and rMC-1 Cells
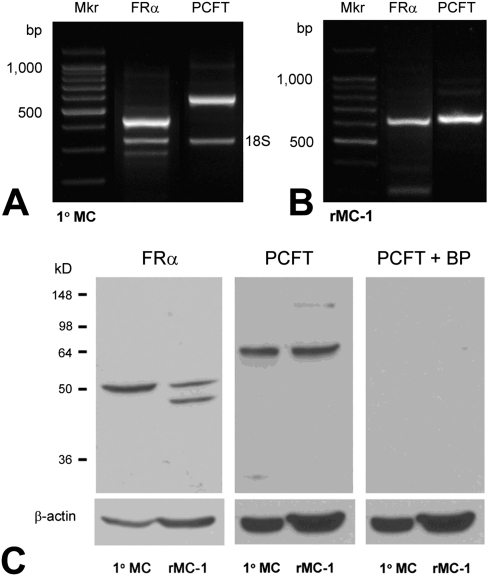
The presence of FRα and PCFT in the inner nuclear layer and in the outer limiting membrane is consistent with Müller cell localization. To analyze expression in these cells in detail, Müller cells were isolated from mouse retinas at postnatal day 5. Total RNA was prepared from these cells and rMC-1 cells. RT-PCR was performed using primers designed from the nucleotide sequences of the appropriate species (mouse or rat; Table 1). RT-PCR performed in the primary Müller cells amplified products of the expected size (419 bp and 567 bp) for FRα and PCFT, respectively (Fig. 3A). Companion studies using rat primers in the rMC-1 cells amplified products of the expected size (638 bp and 646 bp, respectively) for FRα and PCFT (Fig. 3B). To determine whether the FRα and pcft gene products were present in these cells, immunoblotting was performed. Proteins isolated from the primary Müller and rMC-1 cells were subjected to SDS-PAGE, and immunoblotting was performed using anti–FRα or anti–PCFT antibodies. Both proteins were detected in the primary Müller cells and the Müller cell line (Fig. 3C). We note that in analysis of FRα, one band is detected in the primary Müller cells, whereas two are present in the rMC-1 cells, possibly reflecting variations in the extent of glycosylation. To confirm the specificity of the PCFT antibody, we preadsorbed the antibody with an excess of the antigenic peptide and used this for immunoblotting. As shown (Fig. 3C, right panel), incubating the membrane with this preadsorbed antibody eliminated the bands completely suggesting that our affinity-purified antibody against PCFT is specific.
Figure 3.
RT-PCR and Western blot analysis of FRα and PCFT in Müller cells. Total RNA was isolated from cells and subjected to RT-PCR using primers specific for mouse FRα and PCFT (419 bp and 567 bp, respectively) in primary Müller cells (A) and for rat FRα and PCFT (638 bp and 646 bp) in rMC-1 cells (B). 18S served as an internal standard. (C) Western blot analysis of FRα and PCFT in primary Müller cells (1°MC) and rMC-1 cells. FRα (∼50 kDa) was detected in both 1°MC and rMC-1 cells, as was PCFT (∼64 kDa). When membranes were incubated with PCFT antibody that had been preabsorbed using a PCFT blocking peptide (BP) and subjected to immunoblotting, no immunoreactive bands were detected. β-Actin (∼45 kDa) served as loading control.
LSCM Immunolocalization of FRα and PCFT in Primary Müller Cells
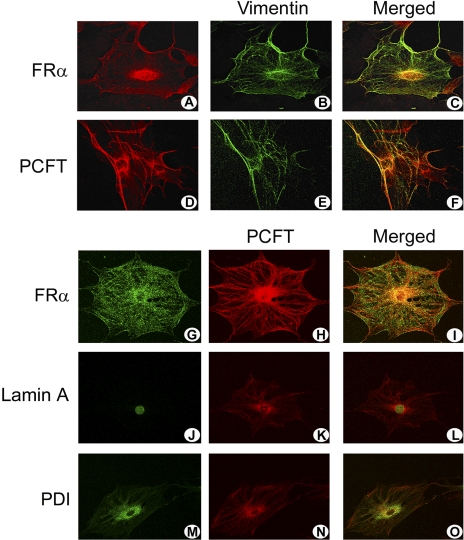
To explore how FRα and PCFT might function in mediating folate uptake in Müller cells, specific localization within these cells was assessed initially using LSCM on primary mouse Müller cells. The cells were grown on coverslips and were subjected to immunocytochemistry using antibodies against FRα, PCFT, and vimentin (Figs. 4A–F). Vimentin is an intermediate filament protein characteristically found in Müller cells. The cells clearly demonstrate this protein in a filamentous pattern of expression. FRα labeled the plasma membrane and the nuclear membrane (and perinuclear region); it colocalized in these regions with intermediate filaments labeled with vimentin (Figs. 4A–C). PCFT was also distributed along the plasma membrane, nuclear membrane, and perinuclear areas (Figs. 4D–F). There was robust colocalization of PCFT and FRα proteins along the plasma membrane and in the region of the nucleus (Figs. 4G–I). To examine the distribution of PCFT in the nuclear region, two cellular organelle markers were used: lamin A, which labels the nuclear membrane, and PDI, which labels the perinuclear endoplasmic reticulum. PCFT colocalized with both markers, consistent with expression in both cellular compartments (Figs. 4J–O).
Figure 4.
Laser-scanning confocal microscopic immunolocalization of FRα and PCFT in primary Müller cells. Müller cells that were freshly isolated from mouse retina were grown on coverslips and subjected to immunofluorescent detection of FRα (red, A) and vimentin (green, B) or PCFT (red, D) and vimentin (green, E). Areas of colocalization of the folate transport proteins with vimentin are shown (merged images, orange-red, C, F). Immunodetection of FRα (green, G), PCFT (red, H), and coimmunolocalization (merged image, orange, I). Detection of the nuclear marker lamin A (green, J), PCFT (red, K), and coimmunolocalization (merged image, orange, L) and the ER marker PDI (green, M), PCFT (red, N), and coimmunolocalization (merged image, orange, O).
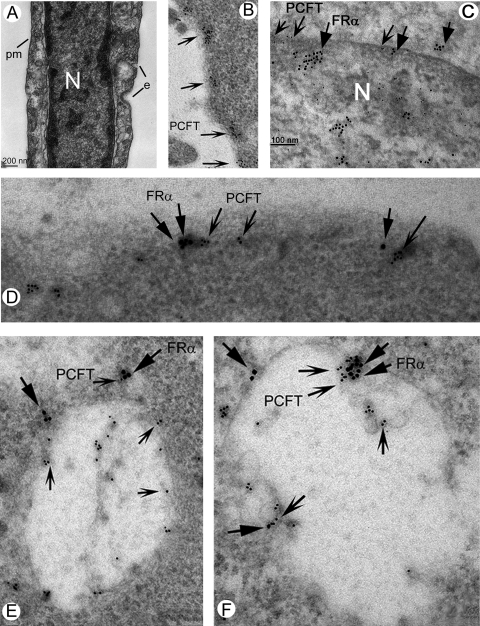
Electron Microscopy Immunolocalization of PCFT and FRα on the Endosomes of Müller Cells
Postembedding electron microscopy (EM) immunolocalization methods were used to determine the precise location of PCFT within Müller cells. Figure 5A (no immunolabeling) demonstrates many of the features of Müller cells, including the plasma membrane, nucleus, early endosomes, and late endosomes. Figures 5B to 5F show immunolabeling with gold particles for PCFT (10 nm), FRα (18 nm), or both. Although the placement of FRα on the plasma membrane is well known, the location of PCFT has not been characterized at the ultrastructural level. The labeling of PCFT along the plasma membrane is robust (Fig. 5B). Figure 5C shows the labeling of PCFT along the nuclear membrane colocalizing in many areas with FRα. Colocalization of PCFT with FRα on the plasma membrane is shown at high magnification in Figure 5D. We examined endosomes for the presence of PCFT and FRα. Figures 5E and 5F show endosomes in which the colocalization of PCFT and FRα is pronounced. These data provide the first ultrastructural evidence that PCFT and FRα colocalize on the plasma membrane and in endosomes.
Figure 5.
Electron microscopic immunolocalization of FRα and PCFT in Müller cells. Müller cells were fixed, and postembedding electron microscopy immunolocalization was used to detect PCFT (10-nm gold particle, indented arrows) and FRα (18-nm gold particle, flat arrows). (A) Electron microscopic photomicrograph (lower magnification) of a Müller cell. The nucleus (N) is prominent in the cell. e, Endosomes forming along the plasma membrane (pm). (B) PCFT immunolabeling on the plasma membrane. (C) PCFT and FRα immunolabeling along the nuclear membrane. (D) PCFT and FRα immunolabeling along the plasma membrane. (E, F) Immunodetection of PCFT and FRα in endosomes; note several areas in which the two proteins are in proximity.
Analysis of Contributions of PCFT versus RFC to [3H]-MTF Transport in Müller Cells
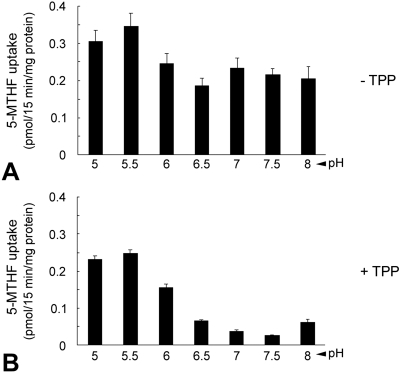
PCFT transports 5-methyltetrahydrofolate (5-MTHF), the predominant form of circulating folate in blood. It functions optimally at low pH (pH 5.0–5.5).44 We examined the uptake of 5-MTHF over a pH range of 5.0 to 8.0 in Müller cells isolated from neonatal mice that were passaged twice and grown to confluence. Robust uptake of 5-MTHF was observed at acidic pH 5.0 to 6.0 (Fig. 6A), consistent with PCFT activity. However, there was considerable uptake at neutral pH (pH 7.0–7.5) reflecting RFC rather than PCFT function. To further evaluate the contribution of RFC to folate uptake in the Müller cells, we used TPP, which is a good substrate for RFC but a poor substrate for PCFT.45–47 TPP eliminated > 80% of folate uptake at neutral pH. The lesser but significant inhibitory effect at low pH reflected some inhibition of PCFT from the very high TPP/5-MTHF concentration ratio used in these studies (Fig. 6B). These data suggest that RFC and PCFT contribute to the uptake of folate in these early-passage mouse Müller cells, the extent to which is determined by the pH at the membrane interface.
Figure 6.
pH profile for 5-MTHF uptake in primary Müller cells. Extravesicular pH was varied from 5 to 8, and uptake was measured for 15 minutes at 37°C. Uptake of [3H]-5-MTHF (40 nM) was measured in the absence (A) and presence (B) of 400 μM TPP in primary mouse Müller cells. Results are mean ± SE of three determinations from at least two experiments.
RT-PCR and Western Blot Analysis of PCFT and RFC in Primary Müller and rMC-1 Cells
Our data (Figs. 3–5) indicated the presence of PCFT in Müller cells; hence, the observation of folate uptake at low pH (Fig. 6), consistent with PCFT function, was expected in these cells. The observation that Müller cells demonstrated folate uptake at neutral pH, which was inhibitable by TPP, suggested that RFC might be present and functional in Müller cells that had proliferated in culture. This finding was unexpected. In previous studies, the expression and localization of RFC were investigated comprehensively in intact mouse retina.26 In situ hybridization detected RFC mRNA transcripts only in the RPE, not in the neural retina. Laser scanning confocal immunodetection methods localized RFC to the basolateral membrane of the RPE but did not detect RFC in any layers of the neural retina. Because RFC activity was detected in the isolated Müller cell, we analyzed RFC expression in this experimental system. Müller cells were isolated from mouse retinas at postnatal day 5 and were grown to confluence and passaged twice to mimic those used in the uptake studies. Total RNA was prepared from these cells and from rMC-1 cells; RT-PCR was performed using primers designed from the nucleotide sequences of the appropriate species (mouse or rat; Table 1). RT-PCR amplified products of the expected size (724 bp and 626 bp) for rat and mouse RFC, respectively (Fig. 7A). In companion experiments, proteins were isolated from these cells and subjected to SDS-PAGE and immunoblotting using anti–RFC antibody.26 RFC was detected in mouse primary Müller cells and rMC-1 cells.
Figure 7.
RT-PCR and Western blot analysis of RFC in Müller cells. (A) Total RNA was isolated from cells and subjected to RT-PCR using primers specific for mouse RFC (626 bp) in primary Müller cells and for rat RFC (724 bp) in rMC-1 cells. (B) Western blot analysis of RFC in primary Müller cells (1°MC) and rMC-1 cells. RFC (∼60 kDa) was detected in both 1°MC and rMC-1 cells. β-Actin (∼45 kDa) served as loading control.
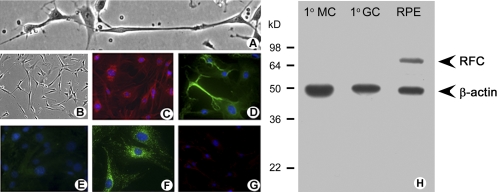
RFC expression in cultured Müller cells was not consistent with earlier findings in intact retina that reported RFC expression primarily in RPE with minimal expression in the neural retina,26 but it was consistent with evidence of RFC function (Fig. 6). A possible explanation for these observations is that when mouse Müller cells are allowed to proliferate in culture—an activity requiring DNA synthesis and therefore folate—they upregulate RFC. However, in their freshly isolated state, which more closely reflects the intact retina, RFC expression is minimal. To further evaluate this possibility, freshly isolated Müller cells were examined immunocytochemically for RFC and FRα expression (Fig. 8). Our results supported this explanation. Phase-contrast images show the expected angular appearance of the Müller cells and their extensive processes (Fig. 8A). Lower magnification phase-contrast images reveal the uniformity of the cultures (Fig. 8B). The cells were positive for Müller cell markers CRALBP (red fluorescence, Fig. 8C) and vimentin (green fluorescence, Fig. 8D) but negative for the neuronal marker neurofilament light protein (NF-L; Fig. 8E). Immunolabeling detected FRα in cell bodies and processes of the cells (bright green, Fig. 8F); however, there was minimal detection of RFC in the freshly isolated Müller cells (Fig. 8G). These data were confirmed by Western blot analysis (Fig. 8H).
Figure 8.
Immunocytochemical and Western blot analysis of FRα and RFC in freshly isolated retinal Müller cells. (A) Phase-contrast microscopic analysis of primary Müller cells in culture. (B) Lower magnification of the cells shown in (A). Cells were processed for immunofluorescence detection of (C) CRALBP (red), (D) vimentin (green), (E) NF-L (green, negligible in these cells), (F) FRα (green), and (G) RFC (green, negligible in these cells). In all immunofluorescence experiments, DAPI was used to stain the nuclei of the cells (blue). (H) Primary Müller cells, primary ganglion cells, and RPE/eyecup protein lysates were prepared from mouse and used to perform Western blot analysis. RFC (∼65 kDa) was detected in RPE, which served as a positive control. RFC was not expressed in the primary Müller cells (1°MC) or primary ganglion cells (1°GC). β-Actin (∼45 kDa) served as the loading control.
Discussion
This report represents the first study of folate transporter localization and function in retinal Müller cells. These cells have an absolute folate requirement and play a pivotal role in sustaining neurons of the retina. The data complement existing information concerning folate acquisition in retinal pigment epithelial26–31 and retinal endothelial cells.32 Three findings emerged from these studies. First, FRα and the newly discovered folate transporter PCFT are expressed at the message and protein levels in primary Müller cells and in the cell line, rMC-1, of Müller cell origin. Second, FRα colocalizes with PCFT by light and electron microscopy in Müller cell organelles, including endosomes. Third, RFC may also contribute to folate uptake, at least in proliferating Müller cells.
Immunohistochemical analysis in intact mouse retina detected FRα and PCFT in multiple retinal layers, including the inner nuclear layer, which contains the cell bodies of Müller cells. RFC was not investigated in these initial studies of Müller cells because our earlier comprehensive investigations of RFC (RT-PCR, in situ hybridization, immunocolocalization) indicated that it was present in RPE but not in neural retina.26 The data acquired in neural retina did not disclose whether FRα and PCFT were present specifically in retinal Müller cells; therefore, we isolated these cells to examine this question more closely. The Müller cell origin of these isolated cells has been documented.37 These cells were analyzed by RT-PCR and Western blot analysis for PCFT and FRα. The genes encoding these proteins were expressed in primary Müller cells and rMC-1 cells, as were the proteins. FRα and PCFT were located in close proximity to each other at several sites within Müller cells. In the double-labeling confocal immunolocalization studies, optical sectioning revealed robust colocalization of the two proteins on the plasma membrane, the nuclear membrane and the perinuclear region of primary Müller cells. Endosomal colocalization of FRα and PCFT was demonstrated by immunoelectron microscopy. To our knowledge, these data represent the first report of colocalization of these two folate transport proteins in any cell type. These findings are noteworthy because FRα is thought to internalize folate by receptor-mediated endocytosis.43,48 It has been postulated that FRα releases internalized MTF into endosomes by acidification of the endosomal milieu.49,50 Given that folate is lipophobic, a mechanism for its release from the endosomal compartment to the cytosolic space is required. The presence of a specific endosomal mechanism that facilitates the export of folates was first proposed by Kamen et al.49 However, it was not until PCFT was cloned that a specific folate transporter was identified that could mediate transport efficiently within an acidified environment.21 The elegant studies by Wollack et al.44 in choroidal plexus epithelial cells and by Zhao et al.42 in HeLa cell sublines provide compelling functional evidence supporting this hypothesis. There had been no direct evidence, however, of coexpression of the two proteins in endosomes. The ultrastructural data from Müller cells reported in this study fill that void and provide strong support that the two proteins function coordinately.
Although immunocytochemical studies performed in freshly isolated Müller cells did not detect robust levels of RFC, it is clear that both RFC function and PCFT function are robust in isolated primary Müller cells. To assess folate uptake in primary Müller cells, the assay requires sufficient numbers of cells to detect the uptake of radiolabeled MTF requiring that the primary cells be permitted to proliferate in culture. The cells were seeded and passaged twice, after which the uptake of [3H]-MTF was analyzed. Given that PCFT functions optimally at approximately pH 5.0 to 6.0, we anticipated that the greatest uptake of MTF would be observed at this acidic pH level, with minimal uptake observed at neutral pH. To our surprise, we observed a bimodal distribution of folate uptake with peak uptake occurring at pH 5.0 to 6.0 and a second wave of uptake observed at neutral pH (7.0–8.0). Recognizing that folate uptake at neutral pH is consistent with RFC function rather than PCFT, we examined uptake in the presence of TPP, which prevents RFC activity but not PCFT activity.45–47 The use of TPP, which has good affinity for RFC but low affinity for PCFT, made it possible to distinguish between the contributions of these transporters over a broad pH range, with the expected dominance of PCFT at low pH and RFC at neutral pH. TPP treatment of Müller cells eliminated more than 80% of folate uptake at the neutral pH. Thus, the data are consistent with contributions by both RFC and PCFT to the uptake process. To confirm this, we isolated RNA and protein from second-passage Müller cells and performed RT-PCR and Western blot analysis for RFC. Indeed, in these second-passage Müller cells, RFC was expressed. It is important to recognize that the cells were dividing, and we suspect that, under proliferative conditions, the Müller cells might have upregulated this robust transporter to take up folate needed for cellular division. Hosoya et al.32 reported robust activity of RFC in their retinal endothelial cell line. Our immunocytochemical studies performed in freshly isolated Müller cells did not detect robust levels of RFC. It is possible that in normal retina, where Müller cell proliferation is minimal, RFC expression is modest. Under disease conditions in which Müller cells are proliferating (active gliosis), RFC expression may be increased. This possibility could be evaluated in future studies in animal models of retinal degeneration.
In summary, the present work represents the first investigation of the subcellular localization of folate transporters in retinal Müller cells and the characterization of their functional properties. Using native retinal tissues, isolated primary Müller cells, and the rMC-1 Müller cell line, FRα and PCFT were found to colocalize on the plasma, nuclear, and endosomal membranes appropriately positioned to coordinately facilitate folate uptake/delivery into cells by receptor-mediated endocytosis. Given that Müller glial cells are essential for the maintenance and nourishment of adjacent neurons, coupled with clinical evidence that lack of folate has significant consequences on visual acuity, the present findings will form the foundation for future investigations of the mechanisms by which Müller cells regulate folate transporter proteins in retinal health and disease, including oxidative stress, hyperglycemia, ER stress, and excitotoxicity. Additional investigations will examine whether RFC expression and activity are increased in retinal diseases characterized by Müller cell gliosis.
Acknowledgments
The authors thank Josh Farrow and Suvika Virani for assistance with preparation of the PCFT antibody.
Footnotes
Supported by National Institutes of Health Grant R01 EY 012830.
Disclosure: B.R Bozard, None; P.S. Ganapathy, None; J. Duplantier, None; B. Mysona, None; Y. Ha, None; P. Roon, None; R. Smith, None; I.D. Goldman, None; P. Prasad, None; P.M. Martin, None; V. Ganapathy, None; S.B. Smith, None
References
- 1.Knox DL, Chen MF, Guilarte TR, Dang CV, Burnette J. Nutritional amblyopia: folic acid, vitamin B-12, and other vitamins. Retina 1982;2:288–293 [PubMed] [Google Scholar]
- 2.Smiddy WE, Green WR. Nutritional amblyopia: a histopathologic study with retrospective clinical correlation. Graefe's Arch Clin Exp Ophthalmol 1987;225:321–324 [DOI] [PubMed] [Google Scholar]
- 3.Golnik KC, Schaible ER. Folate-responsive optic neuropathy. J Neurophthalmol 1994;14:163–169 [PubMed] [Google Scholar]
- 4.Miller NR. The optic nerve. Curr Opin Neurol 1996;9:5–15 [DOI] [PubMed] [Google Scholar]
- 5.Sadun A, Rubin R. Residual psychophysical deficits following recovery from the Cuban epidemic of optic neuropathy. In: Lakshminarayan V. ed. Basic and Clinical Applications of Vision Science Dordrecht: Kluwer; 1997:231–234 [Google Scholar]
- 6.Garner CD, Lee EW, Louis-Ferdinand RT. Müller cell involvement in methanol-induced retinal toxicity. Toxicol Appl Pharmacol 1995;130:101–107 [DOI] [PubMed] [Google Scholar]
- 7.Johlin FC, Swain E, Smith C, Tephly TR. Studies on the mechanism of methanol poisoning: purification and comparison of rat and human liver 10-formyltetrahydrofolate dehydrogenase. Mol Pharmacol 1989;35:745–750 [PubMed] [Google Scholar]
- 8.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr 2000;71:614S–620S [DOI] [PubMed] [Google Scholar]
- 9.Heuberger RA, Fisher AI, Jacques PF, et al. Relation of blood homocysteine and its nutritional determinants to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Clin Nutr 2002;76:897–902 [DOI] [PubMed] [Google Scholar]
- 10.Axer-Siegel R, Bourla D, Ehrlich R, et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol 2004;137:84–89 [DOI] [PubMed] [Google Scholar]
- 11.Tsina EK, Marsden DL, Hansen RM, Fulton AB. Maculopathy and retinal degeneration in cobalamin C methylmalonic aciduria and homocystinuria. Arch Ophthalmol 2005;123:1143–1146 [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Gensler G, Klein ML, Milton RC. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am J Ophthalmol 2006;141:201–203 [DOI] [PubMed] [Google Scholar]
- 13.Bleich S, Jünemann A, von Ahsen N, et al. Homocysteine and risk of open-angle glaucoma. J Neural Transm 2002;109:1499–1504 [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Lu J, Pan C. The impact of plasma homocysteine level on development of retinopathy in type 2 diabetes mellitus. Zhonghua Nei Ke Za Zhi 2002;41:34–38 [PubMed] [Google Scholar]
- 15.Spiegelstein O, Eudy JD, Finnell RH. Identification of two putative novel folate receptor genes in humans and mouse. Gene 2000;258:117–125 [DOI] [PubMed] [Google Scholar]
- 16.Antony AC. Folate receptors. Annu Rev Nutr 1996;16:501–521 [DOI] [PubMed] [Google Scholar]
- 17.Lee HC, Shoda R, Krall JA, Foster JD, Selhub J, Rosenberry TL. Folate binding protein from kidney brush border membranes contains components characteristic of a glycoinositol phospholipid anchor. Biochemistry 1992;31:3236–3243 [DOI] [PubMed] [Google Scholar]
- 18.Shen F, Wu M, Ross JF, Miller D, Ratnam M. Folate receptor type gamma is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: protein characterization and cell type specificity. Biochemistry 1995;34:5660–5665 [DOI] [PubMed] [Google Scholar]
- 19.Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Ann Rev Nutr 1999;19:91–122 [DOI] [PubMed] [Google Scholar]
- 20.Ganapathy V, Smith SB, Prasad PD. SLC19: the folate/thiamine transporter family. Pflugers Arch 2004;447:641–646 [DOI] [PubMed] [Google Scholar]
- 21.Qiu A, Jansen M, Sakaris A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006;127:917–928 [DOI] [PubMed] [Google Scholar]
- 22.Shayeghi M, Latunde-Dada GO, Oakhill JS, et al. Identification of an intestinal heme transporter. Cell 2005;122:789–801 [DOI] [PubMed] [Google Scholar]
- 23.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta 2008;1778:1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao R, Goldman ID. The molecular identity and characterization of a proton-coupled folate transporter—PCFT: biological ramifications and impact on the activity of pemetrexed. Cancer Metast Rev 2007;26:129–139 [DOI] [PubMed] [Google Scholar]
- 25.Umapathy NS, Gnana-Prakasam JP, Martin PM, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci 2007;48:5299–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chancy CD, Kekuda R, Huang W, et al. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor α in mammalian RPE. J Biol Chem 2000;275:20676–20684 [DOI] [PubMed] [Google Scholar]
- 27.Smith SB, Kekuda R, Gu X, Chancy C, Conway SJ, Ganapathy V. Expression of folate receptor alpha in the mammalian RPE and retina. Invest Ophthalmol Vis Sci 1999;40:840–848 [PubMed] [Google Scholar]
- 28.Bridges CC, El-Sherbeny A, Ola MS, Ganapathy V, Smith SB. Transcellular transfer of folate across the RPE. Curr Eye Res 2002;24:129–138 [DOI] [PubMed] [Google Scholar]
- 29.Naggar H, Ola MS, Moore P, et al. Downregulation of reduced-folate transporter by glucose in cultured RPE cells and in RPE of diabetic mice. Invest Ophthalmol Vis Sci 2002;43:556–563 [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SB, Huang W, Chancy C, Ganapathy V. Regulation of the reduced folate transporter by nitric oxide in cultured human retinal pigment epithelial cells. Biochem Biophys Res Commun 1999;257:279–283 [DOI] [PubMed] [Google Scholar]
- 31.Naggar H, Fei YJ, Ganapathy V, Smith SB. Regulation of reduced-folate transporter-1 (RFT-1) by homocysteine and identity of transport systems for homocysteine uptake in retinal pigment epithelial (RPE) cells. Exp Eye Res 2003;77:687–697 [DOI] [PubMed] [Google Scholar]
- 32.Hosoya K, Fujita K, Tachikawa M. Involvement of reduced folate carrier 1 in the inner blood-retinal barrier transport of methyltetrahydrofolate. Drug Metab Pharmacokinet 2008;23:285–292 [DOI] [PubMed] [Google Scholar]
- 33.Sarthy V, Ripps H. The Retinal Müller Cell New York: Kluwer Academic/Plenum Publishers; 2001:14–116 [Google Scholar]
- 34.Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci 2002;43:3109–3116 [PubMed] [Google Scholar]
- 35.Wang Z, Li W, Mitchell CK, Carter-Dawson L. Activation of protein kinase C reduces GLAST in the plasma membrane of rat Müller cells in primary culture. Vis Neurosci 2003;20:611–619 [DOI] [PubMed] [Google Scholar]
- 36.Tomi M, Funaki T, Abukawa H, et al. Expression and regulation of L-cystine transporter, system xc−, in the newly developed rat retinal Müller cell line (TR-MUL). Glia 2003;43:208–217 [DOI] [PubMed] [Google Scholar]
- 37.Dun Y, Mysona B, Van Ells TK, et al. Expression of the glutamate-cysteine (xc−) exchanger in cultured retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tiss Res 2006;324:189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D, Kim MJ, Lee JH, et al. Concomitant distribution shift of glial GABA transporter and S100 calcium-binding proteins in the rat retina after kainate-induced excitotoxic injury. Neurosci Lett 2003;353:17–20 [DOI] [PubMed] [Google Scholar]
- 39.El-Sherbeny A, Naggar H, Miyauchi S, et al. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and Müller cells. Invest Ophthalmol Vis Sci 2004;45:694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang G, Mysona B, Dun Y, et al. Expression, subcellular localization and regulation of sigma receptor in retinal Müller cells. Invest Ophthamol Vis Sci 2006;47:5576–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci 1998;39:212–216 [PubMed] [Google Scholar]
- 42.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 2009;284:4267–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wollack JB, Makori B, Ahlawat S, et al. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem 2008;104:1494–1503 [DOI] [PubMed] [Google Scholar]
- 44.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 2009;11:e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao R, Gao F, Wang Y, Diaz GA, Gelb BD, Goldman ID. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J Biol Chem 2001;276:1114–1118 [DOI] [PubMed] [Google Scholar]
- 46.Nakai Y, Inoue K, Abe N, et al. Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther 2007;322:469–476 [DOI] [PubMed] [Google Scholar]
- 47.Yokooji T, Mori N, Murakami T. Site-specific contribution of proton-coupled folate transporter/haem carrier protein 1 in the intestinal absorption of methotrexate in rats. J Pharm Pharmacol 2009;61:911–918 [DOI] [PubMed] [Google Scholar]
- 48.Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, Strous GJ. Endocytosis of GPI-linked membrane folate receptor-α. J Cell Biol 1996;132:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamen AK, Smith AK, Anderson RGW. The folate receptor works in tandem with a probenecid-sensitive carrier in MA104 cells in vitro. J Clin Invest 1991;87:1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwiener RJ, Johnson CA, Anderson RGW, Kamen BA. Purified folate receptor-5-methyltetrahydrofolic acid interaction at neutral and acid pH. Cancer Res Ther Control 1992;3:37–42 [Google Scholar]