DHA downregulates basal and cytokine-induced ASMase and NSMase activity in human retinal endothelial cells, and inhibition of sphingomyelinases in endothelial cells prevents cytokine-induced inflammatory response.
Abstract
Purpose.
The authors have previously demonstrated that DHA inhibits cytokine-induced inflammation in human retinal endothelial cells (HRECs), the resident vasculature affected by diabetic retinopathy. However, the anti-inflammatory mechanism of docosahexaenoic acid (DHA) is still not well understood. Sphingolipids represent a major component of membrane microdomains, and ceramide-enriched microdomains appear to be a prerequisite for inflammatory cytokine signaling. Acid sphingomyelinase (ASMase) and neutral sphingomyelinase (NSMase) are key regulatory enzymes of sphingolipid metabolism, promoting sphingomyelin hydrolysis to proinflammatory ceramide. The authors address the hypothesis that DHA inhibits cytokine-induced inflammatory signaling in HRECs by downregulating sphingomyelinases.
Methods.
ASMase and NSMase activity was determined by sphingomyelinase assay in primary cultures of HRECs. The expression of ASMase, NSMase, ICAM-1, and VCAM-1 was assessed by quantitative PCR and Western blot analysis. Gene silencing of ASMase and NSMase was obtained by siRNA treatment.
Results.
Inflammatory cytokines TNFα and IL-1β induced cellular adhesion molecule (CAM) expression and rapid increase in ASMase and NSMase activity in HRECs. DHA decreased basal and cytokine-induced ASMase and NSMase expression and activity and the upregulation of CAM expression. Anti-inflammatory effects of DHA on cytokine-induced CAM expression were mimicked by inhibition/gene silencing of ASMase and NSMase. The sphingomyelinase pathway rather than ceramide de novo synthesis pathway was important for inflammatory signaling in HRECs.
Conclusions.
This study provides a novel potential mechanism for the anti-inflammatory effect of DHA in HRECs. DHA downregulates the basal and cytokine-induced ASMase and NSMase expression and activity level in HRECs, and inhibition of sphingomyelinases in endothelial cells prevents cytokine-induced inflammatory response.
The retina has a unique fatty acid profile and the highest level of polyunsaturated fatty acids (PUFAs) in the body, especially docosahexaenoic acid (DHA).1 DHA deficiency is associated with a number of retinal degenerative diseases, including retinitis pigmentosa, retinopathy of prematuritym, and age-related macular degeneration (see Ref. 2 for review). DHA is decreased in the plasma of children with diabetes3 and in the retinas of diabetic eyes.4 Recent data showed a beneficial effect of dietary DHA in reducing pathologic retinal angiogenesis, thus preventing the development of oxygen-induced retinopathy.5 Yet, the DHA protective mechanism in retinopathy remains poorly understood.
The aim of our study was to investigate the anti-inflammatory mechanism of DHA in human retinal endothelial cells (HRECs), the resident vasculature affected by diabetic retinopathy. It is hypothesized that very early-stage diabetic retinopathy represents a low-grade chronic inflammatory disease that involves leukocyte adhesion to the retinal vasculature, a process mediated by adhesion molecules expressed on the endothelial cell surface, especially intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1).6–8 Proinflammatory cytokines, including TNFα and IL-1β, are increased in diabetic eyes9–11 and induce the upregulation of adhesion molecule expression.12 Our group has previously shown that DHA inhibits cytokine-induced cellular adhesion molecule expression in HRECs through cholesterol displacement from caveolae/lipid raft membrane microdomains.12
HRECs contain lipid rafts and particular plasma membrane microdomains known as caveolae that are considered to have an important role in regulating vascular permeability,13 lipid trafficking, cholesterol homeostasis,14,15 and, in particular, signal transduction.16,17 Caveolae/lipid raft membrane microdomains are dynamic assemblies of cholesterol, sphingolipids (sphingomyelin, ceramide), and glycerophospholipids.18 Sphingolipids may have vital roles in the membrane microdomain structure and are now known to act as messengers in signaling pathways mediating inflammation, apoptosis, cell differentiation, and proliferation.19 Ceramide can be generated by catabolism of sphingomyelin by either neutral or acid sphingomyelinases or by de novo synthesis.
NSMase and ASMase are rapidly activated by diverse stress stimuli and promote the hydrolysis of sphingomyelin to ceramide and phosphorylcholine.20–22 NSMase is considered one of the major candidates for mediating stress-induced production of ceramide; it has been reported to be localized to the plasma membrane and palmitoylated on five cysteine residues by thioester bonds.23–25 ASMase was initially considered a strictly lysosomal enzyme because of its optimal pH at 4.5 to 5.0. Nevertheless, an ASMase isoform was recently shown to be enclosed into secretory vesicles close to the plasma membrane and to be secreted into the extracellular space on cell stimulation.26–28 Liu and Anderson22 described the plasma membrane form of ASMase in caveolae and showed that proinflammatory cytokine IL-1β may induce ASMase activation in this compartment.
Lipid analysis demonstrated that approximately 70% of total cellular sphingomyelin is found in membrane microdomains.29–33 Enrichment of sphingomyelin content in membrane microdomains makes them potential substrate pools for cellular sphingomyelinases to produce a high local concentration of ceramide. The generation of ceramide-enriched membrane microdomains profoundly alters the properties of the cellular membrane through their ability to spontaneously fuse to form ceramide-rich macrodomains34–37 that may be a critical factor for receptor clustering and downstream signaling.38,39 Clustering is shown to be an important feature used by several receptors that mediate inflammatory signaling pathways in diabetic retinopathy, such as TNFα and IL-1β pathways.40,41
In this study, we examined a potential anti-inflammatory mechanism of DHA through the inhibition of ASMase and NSMase activity, with a profound impact on inflammatory cytokine signaling in endothelial cells.
Materials and Methods
Reagents and Antibodies
DMEM and F12 culture medium, antibiotics, fetal bovine serum, trypsin, and other materials (Amplex Red Sphingomyelinase Assay Kit, NuPAGE Novex 10% Bis-Tris gels, Platinum SYBR Green qPCR SuperMix-UDG w/ROX) were obtained from Invitrogen (Carlsbad, CA). GW4869 was purchased from Calbiochem (San Diego, CA). Desipramine and commonly used chemicals and reagents were purchased from Sigma (St. Louis, MO). NSMase antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used. ASMase antibody was a generous gift from Richard Kolesnick (Sloan-Kettering Institute, New York, NY). TNFα and IL-1β were from R&D Systems (Minneapolis, MN). Target and nontarget pools (ON-TARGET plus SMART pool SMPD1 [ASMase], ON-TARGET plus SMART pool SMPD2 [NSMase], and ON-TARGET plus siCONTROL) were purchased from Dharmacon (Chicago, IL). For lipid extraction and mass spectrometry, all solvents used were HPLC grade. Methanol and water were purchased from J.T. Baker (Phillipsburg, NJ). Ammonium hydroxide and chloroform were from EMD Chemicals (Gibbstown, NJ). Isopropanol was from Fisher Scientific (Pittsburgh, PA). Lipid standards were obtained from Avanti Polar Lipids (Alabaster, AL).
Cell Culture
Primary cultures of HRECs were prepared from postmortem tissue (National Disease Research Interchange, Philadelphia, PA) cultured as previously described.42 Passages 1 to 5 were used in the experiments. For experimental treatment, cells were transferred to serum-free medium for 14 to 24 hours before stimulatory agents were added.
Fatty Acid Treatment
Fatty acid stocks were prepared by dissolving fatty acids (NuCheck Prep, Inc., Elysian, MN) in ethanol to a final concentration of 100 mM fatty acid, as described previously. The fatty acid stock solutions were diluted to 50 to 100 μM in serum-free medium containing 10 to 20 μM charcoal-treated, solvent-extracted, fatty acid–free bovine serum albumin (BSA; Serologica Inc., Norcross, GA) as a fatty acid carrier. The fatty acid/albumin molar ratio was maintained at 5:1.43 The final concentration of ethanol in the media was <0.1%. Cells were incubated for the times indicated in Results. Equivalent amounts of BSA and ethanol were added to control plates. Linoleic acid, an ω6 polyunsaturated fatty acid, was used as a lipid control for the following considerations. The most abundant polyunsaturated fatty acid classes in the retina microvessels are ω3 (especially DHA) and ω6 (linoleic acid, arachidonic acid).1 In this study, we wanted to compare the effects of the principal ω3 PUFA DHA with a ω6 PUFA (linoleic acid).
Sphingomyelinase Assay
Fatty acids (DHA and linoleic acid) were added to HRECs in serum-free medium to their concentration of 100 μM fatty acid, 20 μM charcoal-treated, solvent extracted, and fatty-acid-free bovine serum albumin (BSA) as a fatty acid carrier. Cells were incubated with fatty acids for specified time points at 37°C; equivalent amounts of BSA and ethanol were added to the control plates. Then cells were lysed in the acid lysis buffer (50 mM sodium acetate, pH 5; 1% Triton X-100; 1 mM EDTA) or neutral lysis buffer (20 mM Tris-HCl, pH 7.5; 1% Triton X-100; 1 mM EDTA) with freshly added protease inhibitor cocktail (Sigma). For sphingomyelinase assay in a cell-free system, 0.5 mU bacterial sphingomyelinase was incubated with 5 to 80 μM fatty acids (DHA and linoleic acid), whereas equivalent amounts of ethanol were added to the control wells. Sphingomyelinase activity was measured (Amplex Red Sphingomyelinase Assay Kit; Molecular Probes, Eugene, OR), as described in the manufacturer's protocol.
Western Blot Analysis
Cells were lysed in the lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, and 10% glycerol) with freshly added protease inhibitor cocktail (Sigma) and phosphatase inhibitors (1 mM Na3VO4, 100 μM glycerophosphate, 10 mM NaF, 1 mM Na4PPi). Protein concentration was measured with a fluorometer (Qubit Invitrogen, Eugene, OR), as described in the manufacturer's protocol. Proteins were resolved on gels (NuPAGE Novex 10% Bis-Tris; Bio-Rad, Hercules, CA), transferred to nitrocellulose membrane, and immunoblotted using appropriate antibodies followed by secondary horseradish peroxidase-conjugated antibody or infrared secondary antibodies (IRDye; Invitrogen, Molecular Probes). Immunoreactive bands were visualized with enhanced chemiluminescence (ECL kit; Amersham Pharmacia Biotech, Piscataway, NJ) or the Odyssey program. Blots were quantified by scanning densitometry with ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Gene Silencing of ASMase and NSMase
For silencing ASMase and NSMase expression, cultured HRECs were detached with trypsin, centrifuged at 100g for 5 minutes, and resuspended in electroporation solution (Amaxa Biosystems, Gaithersburg, MD) to a final concentration of 4 to 5 × 105 cells/100 μL. Then 100 μL cell suspension was mixed with 100 nM ASMase/NSMase siRNA into the electroporation cuvette, and HRECs were electroporated (Nucleofactor program M-030; Amaxa Biosystems). The electroporated cells were maintained in supplemented medium in 37°C/5% CO2 incubator for 48 hours before TNFα (10 ng/mL) and IL-1β (5 ng/mL) treatment for 6 hours. To determine the efficiency of gene silencing, RNA was extracted from 100 nM siRNA-treated HRECs and was used as a template for real time-PCR, as described.44 ASMase/NSMase siRNA treatment of HRECs induced gene silencing of 94% for ASMase and 86% for NSMase (data not shown).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from HRECs with or without DHA pretreatment.45 Specific primers for each gene were designed using IDT DNA PrimerQuest software (Coralville, IA): NSMase: forward, GAGCGCAATCATGGCAGT; reverse, CCCACTGAACCAGTCACCAT; ASMase: forward, CAACCTCGCGCTGAAGAA; reverse, TCCACCATGTCATCCTC AAA. First-strand cDNA was synthesized (SuperScript II RNase H-Reverse Transcriptase; Invitrogen Carlsbad). Synthesized cDNA was mixed with 2× master mix (SYBR Green PCR; Invitrogen Carlsbad, CA) and different sets of gene-specific forward and reverse primers and was subjected to real-time PCR quantification (ABI PRISM 7900 Sequence Detection System; Applied Biosystems). All reactions were performed in triplicate. Relative amounts of mRNA were calculated by using the comparative CT method (User Bulletin 2; Applied Biosystems). Cyclophilin was used as a control, and all results were normalized to the abundance of cyclophilin mRNA.
Lipid Extraction and Analysis by Nanoelectrospray Ionization Followed by Tandem Mass Spectrometry
Whole HRECs (3 × 106 cells) were subjected to lipid extraction and nanoelectrospray ionization followed by tandem mass spectrometry analysis of ceramide molecular species, as previously described.46,47
Statistical Analysis
Data are expressed as mean ± SD. Factorial ANOVA with post hoc Tukey test (Prism5; GraphPad Software, San Diego, CA) was used for comparing the data obtained from independent samples. Significance was established at P < 0.05.
Results
Dose Response of TNFα- and IL-1β–Induced Cellular Adhesion Molecule Expression in HRECs
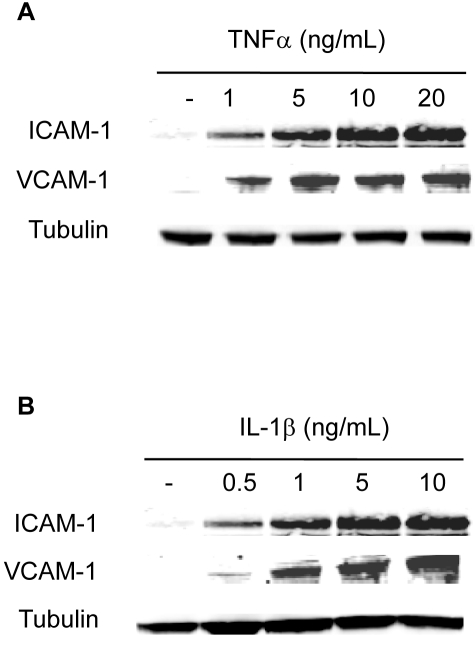
To determine the dose of cytokines to be used in the study necessary to induce an inflammatory response in HRECs, dose-response curves for TNFα (0–20 ng/mL) and IL-1β (0.5–10 ng/mL) were established using CAM (ICAM-1 and VCAM-1) expression as a measure. The induction of ICAM-1 and VCAM-1 by TNFα and IL-1β was assessed by immunoblot analysis (Fig. 1). Based on these data, we used 10 ng/mL TNFα and 5 ng/mL IL-1β in further studies.
Figure 1.
Dose-response of cytokine-induced CAM expression in HRECs. HRECs maintained in serum-free media overnight were stimulated with 0 to 20 ng/mL TNFα and 0 to 10 ng/mL IL-1β for 6 hours. The induction of ICAM-1 and VCAM-1 by TNFα (A) and IL-1β (B) was assessed by immunoblot analysis. Equal amounts of protein were added to each lane, as confirmed by tubulin level.
TNFα- and IL-1β–Induced ASMase and NSMase Activation in HRECs
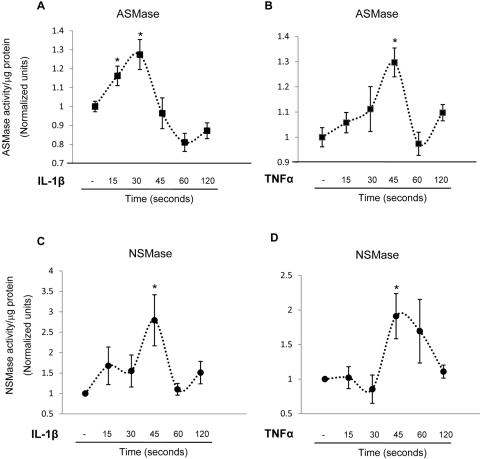
The effect of proinflammatory cytokines known to be increased in diabetic eyes, namely TNFα and IL-1β on ASMase and NSMase activity in HRECs, was first determined. Cells were stimulated with 5 ng/mL IL-1β and 10 ng/mL TNFα for 15 seconds to 2 minutes. The cells were then collected for ASMase and NSMase activity analysis using an assay kit (Amplex Red Sphingomyelinase; Invitrogen). IL-1β treatment increased ASMase activity in HRECs as early as 15 seconds of stimulation, and ASMase activity remained significantly higher during 30 seconds of stimulation (Fig. 2A). Similar results were obtained for NSMase activity; IL-1β stimulation induced maximum activation after 45 seconds of treatment (Fig. 2C). HREC treatment with proinflammatory cytokine TNFα also significantly induced ASMase and NSMase activation after 45 seconds of stimulation (Figs. 2B, 2D). Taken together, these data show that stimulation of endothelial cells with proinflammatory cytokines induces rapid activation of sphingomyelinases.
Figure 2.
Cytokine induced ASMase and NSMase activation in HRECs. HRECs cultured on 10-cm plates were stimulated with 5 ng/mL IL-1β (A, C) and 10 ng/mL TNFα (B, D) for 0 to 120 seconds. Each time point represents an individual plate. The cells were collected for ASMase (A, B) and NSMase (C, D) activity analysis using an assay kit. IL-1β significantly induced ASMase (A) activation after 15 seconds of stimulation, with a maximal activation after 30 seconds of stimulation. TNFα induced ASMase (B) activation after 45 seconds of stimulation. IL-1β (C) or TNFα (D) induced maximal NSMase activation after 45 seconds of stimulation. Results are presented as mean ± SD of six measurements. *P < 0.05 compared with basal expression level.
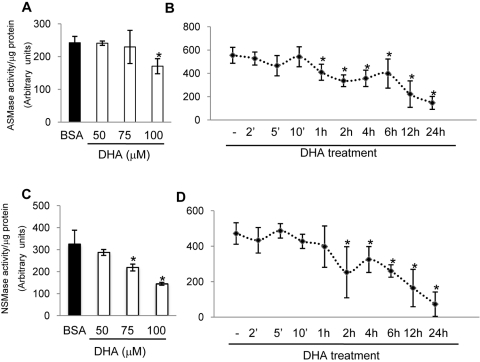
Inhibition of Cytokine-Induced ASMase and NSMase Activation by DHA in HRECs
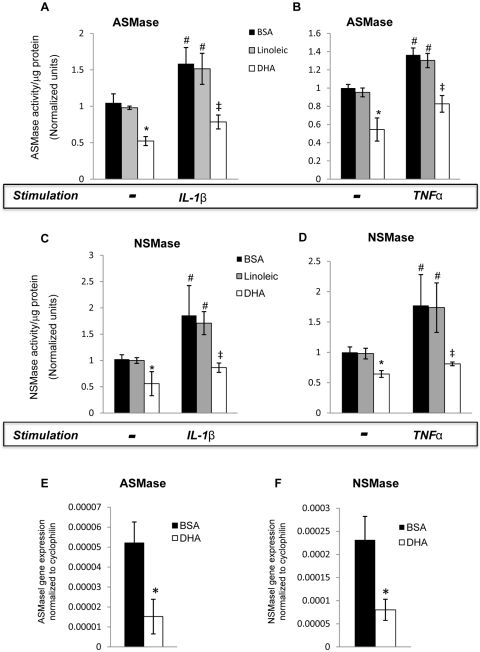
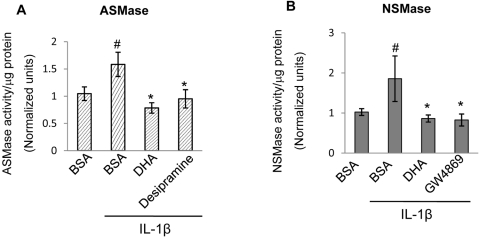
DHA, the most abundant ω3-PUFA in the retina, has been shown to have a pronounced anti-inflammatory effect, inhibiting cytokine-induced CAM expression in HRECs and endothelial cell activation.12 To determine the effect of DHA on basal ASMase and NSMase activity and expression level, HRECs were treated with DHA or linoleic acid (lipid control) for 24 hours. Pretreatment of HRECs with 100 μM BSA-bound DHA significantly decreased both ASMase (Figs. 3A, 3B, 3E) and NSMase (Figs. 3C, 3D, 3F) mRNA expression and activity level. Conversely, pretreatment of HRECs with 100 μM BSA-bound linoleic acid had no effect on ASMase or NSMase basal activity compared with vehicle control (BSA). We next determined the effect of DHA on TNFα- and IL-1β–induced ASMase and NSMase activity. As shown in Figure 3, DHA, but not linoleic acid, pretreatment of HRECs significantly downregulated TNFα- and IL-1β–induced ASMase (Figs. 3A, 3B) and NSMase (Figs. 3C, 3D) activity. To compare the effect of DHA on cytokine-induced ASMase and NSMase activation with the effects of specific inhibitors, HRECs were treated with 15 μM desipramine48,49 and 25 μM GW486950 before the addition of stimulatory agents. The inhibition of cytokine-induced ASMase and NSMase activity by DHA was as effective as it was by desipramine (Fig. 4A) and GW4869 (Fig. 4B), respectively.
Figure 3.
Inhibition of cytokine-induced ASMase and NSMase activation and ASMase and NSMase expression by DHA in HRECs. HRECs treated with 100 μM fatty acid (DHA or linoleic acid) complexed to BSA or to BSA alone (vehicle control) overnight (24 hours) were stimulated with either 5 ng/mL IL-1β (A) for 30 seconds to assess the activation of ASMase and for 45 seconds to assess the activation of NSMase or with 10 ng/mL TNFα for 45 seconds to assess both ASMase and NSMase activation. The cells were then collected for ASMase (A, B) and NSMase (C, D) activity analysis using an assay kit. DHA decreased both ASMase (A, B) and NSMase (C, D) basal activity and downregulated cytokine-induced sphingomyelinases activity. RT-PCR analysis of ASMase (E) and NSMase (F) expression in HRECs, with or without DHA pretreatment. Results are presented as mean ± SD of nine measurements. *P < 0.05 compared with basal expression level in BSA and linoleic acid–treated HRECs. #P < 0.05 compared with nonstimulated HRECs. ‡P < 0.05 compared with cytokine-stimulated HRECs.
Figure 4.
Inhibition of ASMase and NSMase cytokine-induced activation by DHA and sphingomyelinases inhibitors. HRECs treated with 100 μM DHA complexed to BSA overnight and inhibitors for ASMase (desipramine, 15 μM for 1 hour) or NSMase (GW4869, 25 μM for 30 minutes) downregulated IL-1β–induced ASMase (A) and NSMase (B) activity below the basal level, respectively. Results are presented as mean ± SD of nine measurements. #P < 0.05 compared with basal expression level in BSA-treated HRECs. *P < 0.05 compared with IL-1β–stimulated HRECs.
Inhibition of ASMase and NSMase Activity by DHA
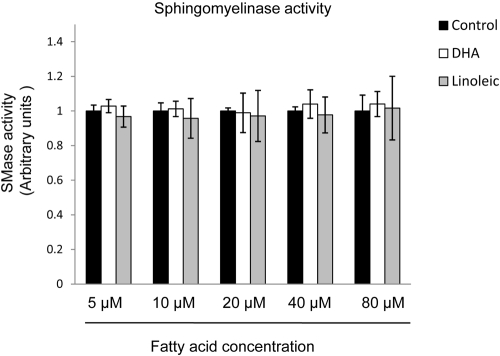
To determine the time course of the effect DHA on ASMase and NSMase activity, HRECs were treated with BSA-bound DHA or BSA alone. First, the dose of DHA to be used in this experiment was selected based on a dose-response experiment for ASMase (Fig. 5A) and NSMase (Fig. 5C) activity. The time course for the DHA effect on ASMase and NSMase activity in HRECs showed a gradual decrease in ASMase (Fig. 5B) and NSMase (Fig. 5D) activity with an early time point of 1 hour and 2 hours, respectively, and a maximum effect on the activity of both enzymes after 24 hours of DHA treatment. This gradual effect of DHA on sphingomyelinases activity corresponds with the gradual incorporation of DHA into the fatty acyl chains of phospholipids in plasma membrane microdomains, with a maximum incorporation after 24 hours of HREC treatment.51 To determine whether the effect of DHA on sphingomyelinases activity is attributed to a direct interaction with the enzymes, we performed the sphingomyelinase assay in a cell-free system in which 0.5 mU bacterial sphingomyelinase (homolog of NSMase) was incubated with 5 to 80 μM fatty acid (DHA, linoleic acid). In this cell-free system, fatty acids were used without a carrier molecule. In cell culture experiments in which fatty acids are complexed to BSA at 1:5 molar ratio, 100 μM DHA corresponds to 20 μM free fatty acid concentration without a carrier in a cell-free system. No fatty acids were added to the control samples. We found no direct effect of DHA or linoleic acid on SMase activity (Fig. 6), strongly suggesting that direct binding of DHA to sphingomyelinases does not play a role in the observed inhibitory effects.
Figure 5.
Inhibition of ASMase and NSMase activity by DHA. HRECs treated with 100 μM DHA complexed to BSA or BSA vehicle control overnight (24 hours) were collected for ASMase (A, B) and NSMase (C, D) activity analysis using an assay kit. The optimal dose of DHA to inhibit ASMase (A) and NSMase (C) activity in HRECs was 100 μM. The time course for DHA effect on ASMase and NSMase activity in HRECs showed a gradual decrease in ASMase (B) and NSMase (D) activity with an early time point of 1 hour and 2 hours, respectively, and a maximum effect on both enzymes activity after 24 hours of DHA treatment. Results are the mean ± SD of three independent experiments. *P < 0.05 compared with BSA vehicle control.
Figure 6.
No direct effect of DHA on sphingomyelinase (SMase) activity in a cell-free system. To determine whether DHA decreases SMase activity through direct interaction with the enzyme, we measured SMase activity in a cell-free system in which 0.5 mU bacterial SMase (provided in an assay kit) were incubated with 5 to 80 μM fatty acid (DHA, linoleic acid). A concentration of 20 μM free fatty acid corresponds to a concentration of 100 μM fatty acid complexed to BSA in 5:1 molar ratio. No fatty acids were used for the control wells. No effect of DHA or linoleic acid on SMase activity was observed compared with control.
Effect of ASMase and NSMase Inhibition or Gene Silencing on TNFα- and IL-1β–Induced CAM Expression
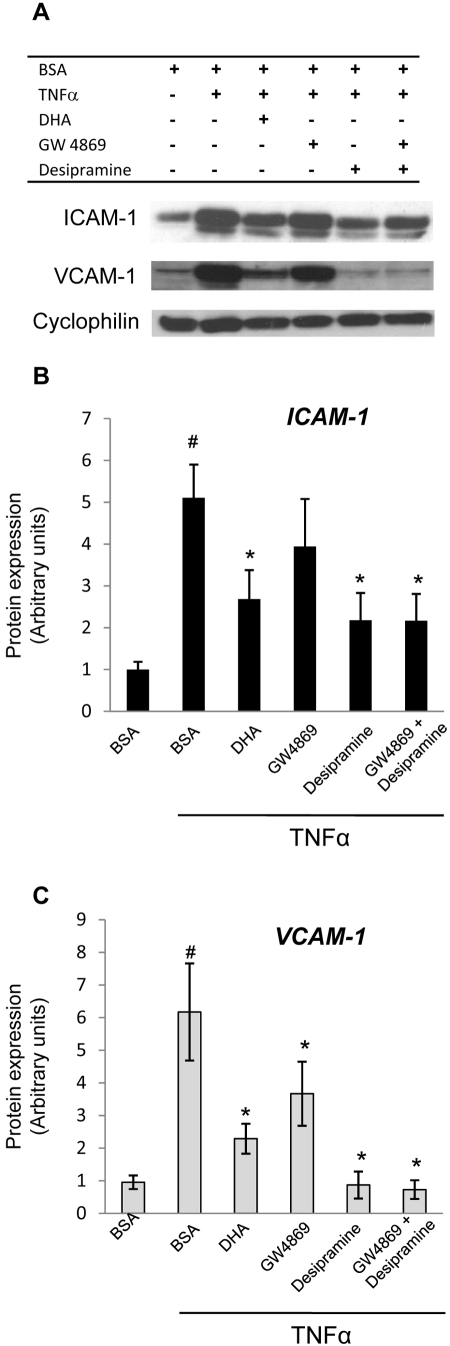
To establish the role of ASMase and NSMase in cytokine-induced inflammatory signaling in HRECs, ASMase and NSMase activity was inhibited using desipramine (Sigma) and GW4869 (Sigma), respectively. HRECs were treated with 15 μM desipramine48,49 for 1 hour and 25 μM GW486950 for 30 minutes before TNFα (10 ng/mL) stimulation. Six hours after cytokine stimulation, the activation of inflammatory pathways was analyzed using ICAM-1 and VCAM-1 protein expression levels as a measure. As shown in Figure 7, the inhibition of ASMase significantly reduced the expression of TNFα-induced adhesion molecules, similar to DHA. Inhibition of ASMase also reduced IL-1β–induced ICAM-1 expression (see Figs. 9A–C). Interestingly, the inhibition of NSMase did not have a significant effect on TNFα- and IL-1β–induced ICAM-1 expression, and, though the effect on TNFα-induced VCAM-1 expression was significant, it was less pronounced than the effect of ASMase inhibition or DHA.
Figure 7.
Decreases in TNFα-induced CAM expression by ASMase and NSMase inhibition in HRECs. HRECs were treated with 100 μM DHA complexed to BSA or BSA alone (vehicle control) for 24 hours or the inhibitors for ASMase (desipramine, 15 μM for 1 hour) or NSMase (GW4869, 25 μM for 30 minutes) before 10 ng/mL TNFα stimulation. The induction of ICAM-1 and VCAM-1 was assessed 6 hours later by immunoblot analysis (A). DHA and desipramine significantly reduced TNFα-induced CAM expression. GW4869 significantly inhibited TNFα-induced VCAM-1 expression. (B, C) Quantitative analysis of the data in (A) for ICAM-1 (B) and VCAM-1 (C) expression. Results are mean ± SD of four independent experiments. #P < 0.05 compared with control cells. *P < 0.05 compared with TNFα-stimulated cells.
Figure 9.
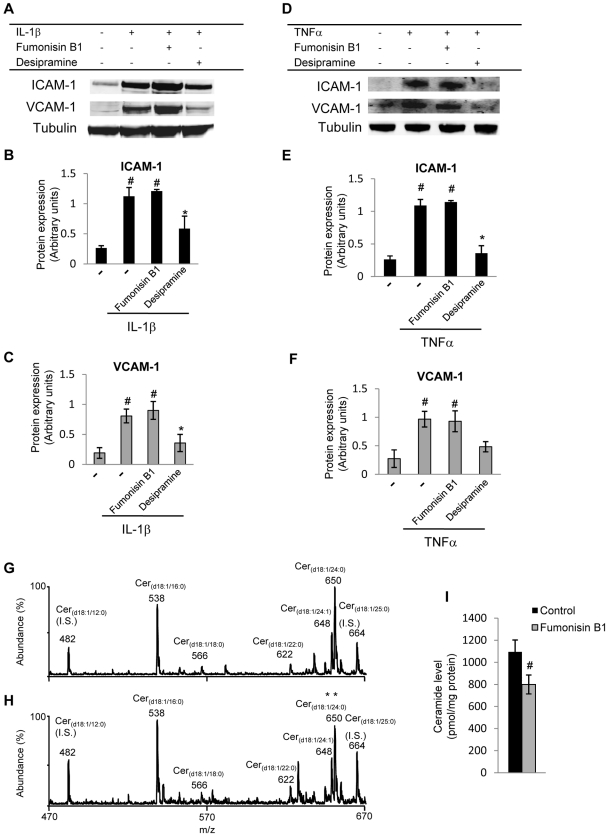
No effect of de novo ceramide production pathway inhibition on cytokine-induced CAM expression in HRECs. Fumonisin B1 was used to inhibit ceramide synthase, the enzyme that catalyzes the final step in the de novo pathway of ceramide synthesis. Cells were treated for 16 hours with 50 μM fumonisin B1 before TNFα (10 ng/mL) and IL-1β (5 ng/mL) stimulation. Expression levels of ICAM-1 and VCAM-1 were analyzed by Western blot. Representative blots are shown in (A) and (D), and quantitative analysis of four independent experiments is shown in (B, C, E, F). Fumonisin B1 pretreatment of HRECs did not affect TNFα- or IL-1β–induced adhesion molecule expression. In contrast, inhibition of ASMase significantly decreased TNFα- and IL-1β–induced ICAM-1 and VCAM-1 expression. Ceramide levels determined by tandem mass spectrometry precursor ion mode scanning for the characteristic ceramide product ion at m/z 264.4 in control and fumonisin B1–treated HRECs are presented in (G) and (H), respectively. Quantification of ceramide levels (I) demonstrates significant decreases in ceramide levels in fumonisin B1–treated compared with control HRECs. Results are presented as mean ± SD of three independent experiments. *P < 0.05 compared with cytokine-stimulated level. #P < 0.05 compared with control.
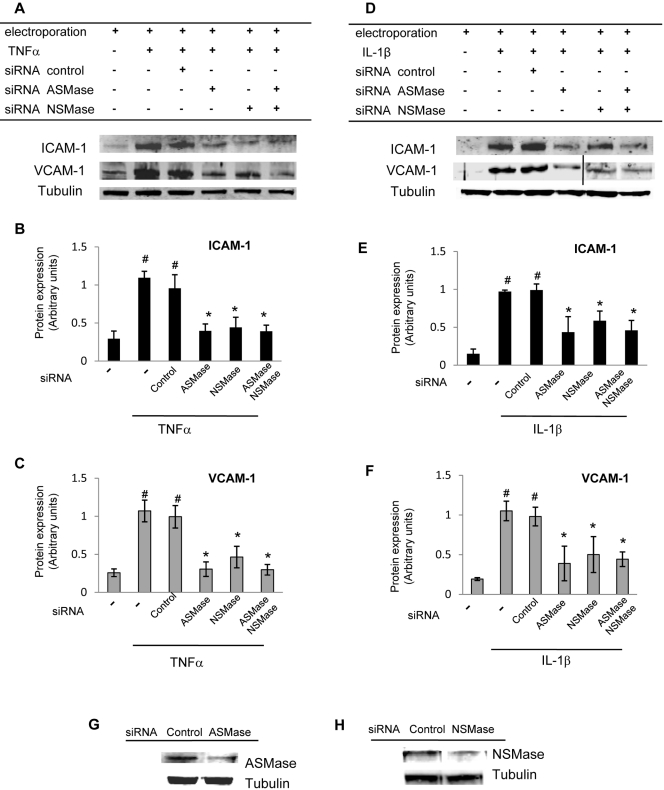
Gene silencing of ASMase and NSMase (100 nM siRNA for ASMase and NSMase) significantly decreased TNFα- and IL-1β–induced ICAM-1 and VCAM-1 expression in HRECs (Fig. 8) compared with untreated or control siRNA (100 nM)–treated cells. As in the inhibitor study, ASMase gene silencing had a more pronounced inhibitory effect than NSMase gene silencing. No additive effect on cytokine-induced CAM expression was observed in the samples in which both ASMase and NSMase gene silencing were attained.
Figure 8.
Inhibition in cytokine-induced CAM expression by ASMase and NSMase gene silencing in HRECs. Gene silencing of ASMase and NSMase (100 nM siRNA for ASMase and NSMase) significantly decreased TNFα- (A) and IL-1β– (D) induced ICAM-1 and VCAM-1 expression in HRECs. HRECs were treated with siRNA for 48 hours before cytokine stimulation for 6 hours to assess CAM expression. In contrast, control siRNA treatment (100 nM) had no effect. Quantitative analysis of the data presented in (A) and (D) is shown in (B, C) and (E, F), respectively. ASMase (G) and NSMase (H) protein levels in control siRNA–, ASMase siRNA–, and NSMase siRNA–treated HRECs were assessed by immunoblot analysis. Equal amounts of protein were added to each lane, as confirmed by tubulin levels. Results are presented as mean ± SD of five independent experiments. #P < 0.05 compared with control cells. *P < 0.05 compared with TNFα- and IL-1β–stimulated cells.
No Effect of Ceramide De Novo Synthesis Inhibition on TNFα- and IL-1β–Induced CAM Expression in HRECs
Ceramides can be synthesized in the cells at the membrane level from sphingomyelin hydrolysis by ASMase, NSMase, or both or in the endoplasmic reticulum by de novo synthesis. We next determined whether the ceramide de novo synthesis pathway is involved in mediating proinflammatory cytokine signaling in HRECs. Fumonisin B1was used to inhibit ceramide synthase, the enzyme that catalyzes the final step of the de novo pathway of ceramide synthesis. Cells were treated for 16 hours with 50 μM fumonisin B1 before TNFα (10 ng/mL) and IL-1β (5 ng/mL) stimulation. Expression levels of ICAM-1 and VCAM-1 were analyzed by Western blot (Figs 9A, 9D). Fumonisin B1 pretreatment of HRECs did not affect TNFα- and IL-1β–induced adhesion molecule expression (Fig. 9). In contrast, as shown above, the inhibition of ASMase significantly decreased TNFα- and IL-1β–induced ICAM-1 and VCAM-1 expression in HRECs. These data suggest that ceramide production at the membrane level, rather than the total cellular ceramide level, plays an essential role in modulating cytokine-induced inflammatory signaling in HRECs. Decreased ceramide levels in fumonisin B1-treated HRECs were confirmed by tandem mass spectrometry analysis. Inhibition of ceramide synthase by incubation of HRECs with fumonisin B1 resulted in a 26.55% ± 9.45% decrease in HREC total ceramide levels, as expected (Fig. 9I).
Discussion
Omega 3 PUFAs have long been known to modulate inflammatory processes and are now widely used in clinics as an adjuvant immunosuppressant in the treatment of various diseases with an inflammatory component.52 Recently, Connor et al.5 showed that increasing omega 3 PUFA retinal levels by dietary or genetic means may be of benefit in preventing retinopathy and that the DHA-protective effect is mediated, in part, by the suppression of retinal TNFα gene expression and protein level in a mouse model of oxygen-induced retinopathy. We have previously shown that DHA suppresses cytokine-induced adhesion molecule expression in endothelial cells through cholesterol depletion and displacement of important signaling molecules from caveolae/lipid rafts.51 We now demonstrate that DHA-treated HRECs exhibit decreased expression levels and decreased basal and TNFα- and IL-1β–induced activity of ASMase and NSMase enzymes. The time course showed maximum effect of DHA on sphingomyelinases expression level and activity after more than hours of treatment. The time course of this effect can be explained by our previously published observation that DHA incorporates into caveolae/lipid raft phospholipids at a rate of 10% in 1.5 hours and 90% in 24 hours and dramatically alters the lipid environment of these specialized microdomains, decreasing their cholesterol content by approximately 70%.51 Proinflammatory cytokines TNFα and IL-1β have been shown to activate ASMase in various cell types,22,53–55 and our results are in agreement with the results of these studies. We have found that IL-1β treatment of HRECs rapidly induced the activation of ASMase after only 15 seconds of stimulation, whereas TNFα increased the activity of both ASMase and NSMase after 45 seconds of stimulation. This rapid and transient time course of the effect is in agreement with the fact that induction of inflammatory response is always transient and that the extent of the initial event is always small because it is later amplified several-fold with each signal transduction step. For instance, as demonstrated in numerous studies of the NFκB pathway, IκBα is phosphorylated within 1 minute from cytokine stimulation and is dephosphorylated back to the control state by 5 minutes; in GPCR signaling, ERK phosphorylation occurs within 30 seconds of receptor activation and is back to a normal level by 2 minutes. Indeed, a recent study56 described the importance of transient versus sustained ERK phosphorylation in inducing different signaling pathways. Moreover, the sphingomyelinase literature is replete with demonstrations of the very fast (within 1 minute) and transient nature of sphingomyelinase activation. This rapidity is often used as proof that sphingomyelinase is an important first responder.53,54,57 Although the induction of sphingomyelinases is transient, the effect of the sphingomyelinases activation (i.e., conversion of sphingomyelin to ceramide and further activation of the NFκB pathway and inflammatory genes, such as ICAM-1 and VCAM-1) is long-lasting. Indeed, in this study, the inhibition or gene silencing of ASMase and NSMase decreased proinflammatory cytokine TNFα- and IL-1β–induced adhesion molecule expression in HRECs. Stimulation of the sphingomyelin pathway by TNFα with sphingomyelinases-induced membrane ceramide was shown to lead to the activation of nuclear factor κB (NF-κB) and to a marked increase in nuclear NF-κB binding in human leukemia (HL-60) cells.54 Similarly, the activation of sphingomyelinases is shown to be an important signaling system for IL-1β in murine T-helper EL-4 cells,53 and IL-1β action in these cells is mediated through NF-κB activation.58 NF-κB is a major transcription factor controlling the expression of an array of inflammatory response genes, including adhesion molecules.59
Ample experimental evidence suggests that plasma membrane microdomains are definite sites for ceramide production in response to different agonists and stress signals. Liu and Anderson22 showed elevated ceramide levels with consequent decreases in the sphingomyelin content of the caveolae compartments of human fibroblasts in response to the proinflammatory cytokine IL-1β. Bilderback et al.20 showed that sphingomyelin hydrolysis takes place in caveolae-enriched domains in NIH 3T3 fibroblasts in response to nerve growth factor treatment. The release of ceramides alters the dynamics of these rafts and may drive signal transduction processes by allowing the oligomerization of specific cell surface molecules, such as ligated receptors. TNFα receptor60,61 and IL-1 receptor53 are among the lipid microdomain-associated receptors that are affected by sphingolipid composition of the microdomains.
There are two different pathways of ceramide generation in the cells. The first occurs at the membrane level by the hydrolysis of sphingomyelin by sphingomyelinases. The second is localized to the endoplasmic reticulum by de novo synthesis involving the enzyme ceramide synthase. Results of this study demonstrate that plasma membrane ceramide production by sphingomyelinases rather than de novo synthesis is important for inflammatory signaling in HRECs.
Several recent studies demonstrated that oxidized products of DHA formed either through lipoxygenase,5,62,63 cyclooxygenase,63,64 or nonenzymatic pathways65 have potent anti-inflammatory properties. Although anti-inflammatory mediators formed by DHA oxidation were not analyzed in this study, they could represent an important component of the anti-inflammatory action of DHA and sphingomyelinase inhibition. Endothelial cells are rich in caveolae, and ASMase is preferentially expressed in endothelia.66 Thus our findings could represent the first identification of a unique, endothelial-specific anti-inflammatory mechanism of DHA action.
In conclusion, this study provides a novel mechanism for the anti-inflammatory effect of DHA in the primary tissue affected by retinopathy: HRECs. We demonstrated that DHA pretreatment of HRECs leads to the inhibition of ASMase and NSMase expression and activity level. Inhibition or gene silencing of sphingomyelinases recapitulates the effect of DHA on cytokine-induced CAM expression in HRECs. Inhibition of ASMase and NSMase activity in the endothelial cells by DHA intake or by specific inhibitors may be of benefit in preventing conditions associated with vascular inflammation, such as diabetic retinopathy.
Acknowledgments
The authors thank Svetlana Bozack for technical assistance.
Footnotes
Supported by Juvenile Diabetes Research Foundation Grant 2–2005-97 (JVB) and by National Institutes of Health Grant EY-016077 (JVB), RR 025386 (GER, JVB), and Michigan Agricultural Experiment Station MICL02163 (JVB).
Disclosure: M. Opreanu, None; T.A. Lydic, None; G.E. Reid, None; K.M. McSorley, None; W.J. Esselman, None; J.V. Busik, None
References
- 1.Lecomte M, Paget C, Ruggiero D, Wiernsperger N, Lagarde M. Docosahexaenoic acid is a major n-3 polyunsaturated fatty acid in bovine retinal microvessels. J Neurochem 1996;66:2160–2167 [DOI] [PubMed] [Google Scholar]
- 2.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20. Arch Ophthalmol 2007;125:671–679 [DOI] [PubMed] [Google Scholar]
- 3.Decsi T, Minda H, Hermann R, et al. Polyunsaturated fatty acids in plasma and erythrocyte membrane lipids of diabetic children. Prostaglandins Leukot Essent Fatty Acids 2002;67:203–210 [DOI] [PubMed] [Google Scholar]
- 4.Futterman S, Kupfer C. The fatty acid composition of the retinal vasculature of normal and diabetic human eyes. Invest Ophthalmol 1968;7:105–108 [PubMed] [Google Scholar]
- 5.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 2007;13:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol 1995;147:642–653 [PMC free article] [PubMed] [Google Scholar]
- 7.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 2001;158:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol 2002;86:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limb GA, Chignell AH, Green W, LeRoy F, Dumonde DC. Distribution of TNF alpha and its reactive vascular adhesion molecules in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol 1996;80:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 11.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994;118:445–450 [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Esselman WJ, Jump DB, Busik JV. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci 2005;46:4342–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol 2006;290:L405–L413 [DOI] [PubMed] [Google Scholar]
- 14.Li S, Song KS, Lisanti MP. Expression and characterization of recombinant caveolin: purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J Biol Chem 1996;271:568–573 [PubMed] [Google Scholar]
- 15.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A 1995;92:10339–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A 1993;90:10909–10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M. Caveolae, transmembrane signalling and cellular transformation. Mol Membr Biol 1995;12:121–124 [DOI] [PubMed] [Google Scholar]
- 18.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct 2003;32:257–283 [DOI] [PubMed] [Google Scholar]
- 19.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J 1998;335(pt 3):465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilderback TR, Gazula VR, Lisanti MP, Dobrowsky RT. Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways. J Biol Chem 1999;274:257–263 [DOI] [PubMed] [Google Scholar]
- 21.Cremesti A, Paris F, Grassme H, et al. Ceramide enables fas to cap and kill. J Biol Chem 2001;276:23954–23961 [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Anderson RG. Compartmentalized production of ceramide at the cell surface. J Biol Chem 1995;270:27179–27185 [DOI] [PubMed] [Google Scholar]
- 23.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 2004;82:27–44 [DOI] [PubMed] [Google Scholar]
- 24.Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues: role of palmitoylation in subcellular localization. J Biol Chem 2007;282:10047–10056 [DOI] [PubMed] [Google Scholar]
- 25.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal 2007;19:229–237 [DOI] [PubMed] [Google Scholar]
- 26.Grassme H, Schwarz H, Gulbins E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem Biophys Res Commun 2001;284:1016–1030 [DOI] [PubMed] [Google Scholar]
- 27.Schissel SL, Jiang X, Tweedie-Hardman J, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH: implications for atherosclerotic lesion development. J Biol Chem 1998;273:2738–2746 [DOI] [PubMed] [Google Scholar]
- 28.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J 2008;22:3419–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 2002;110:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierini LM, Maxfield FR. Flotillas of lipid rafts fore and aft. Proc Natl Acad Sci U S A 2001;98:9471–9473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol 1998;275:L843–L851 [DOI] [PubMed] [Google Scholar]
- 32.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997;387:569–572 [DOI] [PubMed] [Google Scholar]
- 33.Smart EJ, Graf GA, McNiven MA, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 1999;19:7289–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grassme H, Riethmuller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res 2007;46:161–170 [DOI] [PubMed] [Google Scholar]
- 35.Holopainen JM, Subramanian M, Kinnunen PK. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry 1998;37:17562–17570 [DOI] [PubMed] [Google Scholar]
- 36.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol 2000;184:285–300 [DOI] [PubMed] [Google Scholar]
- 37.Nurminen TA, Holopainen JM, Zhao H, Kinnunen PK. Observation of topical catalysis by sphingomyelinase coupled to microspheres. J Am Chem Soc 2002;124:12129–12134 [DOI] [PubMed] [Google Scholar]
- 38.Bertrand D. [Mental health and cultural issues: the return of Khmers from France to Cambodia for healing purposes]. Sante 1997;7:330–334 [PubMed] [Google Scholar]
- 39.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998;395:82–86 [DOI] [PubMed] [Google Scholar]
- 40.Ko YG, Lee JS, Kang YS, Ahn JH, Seo JS. TNF-alpha-mediated apoptosis is initiated in caveolae-like domains. J Immunol 1999;162:7217–7223 [PubMed] [Google Scholar]
- 41.Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta 2002;1592:265–280 [DOI] [PubMed] [Google Scholar]
- 42.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 2008;57:1952–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jump DB, Clarke SD, MacDougald O, Thelen A. Polyunsaturated fatty acids inhibit S14 gene transcription in rat liver and cultured hepatocytes. Proc Natl Acad Sci U S A 1993;90:8454–8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botolin D, Jump DB. Selective proteolytic processing of rat hepatic sterol regulatory element binding protein-1 (SREBP-1) and SREBP-2 during postnatal development. J Biol Chem 2003;278:6959–6962 [DOI] [PubMed] [Google Scholar]
- 45.Agardh E, Gustavsson C, Hagert P, Nilsson M, Agardh CD. Modifying a standard method allows simultaneous extraction of RNA and protein, enabling detection of enzymes in the rat retina with low expressions and protein levels. Metabolism 2006;55:168–174 [DOI] [PubMed] [Google Scholar]
- 46.Busik JV, Reid GE, Lydic TA. Global analysis of retina lipids by complementary precursor ion and neutral loss mode tandem mass spectrometry. Methods Mol Biol 2009;579:33–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kielczewski JL, Jarajapu Y, McFarland EL, et al. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circ Res 2009;105:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinrich M, Wickel M, Schneider-Brachert W, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J 1999;18:5252–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolzer M, Werth N, Sandhoff K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett 2004;559:96–98 [DOI] [PubMed] [Google Scholar]
- 50.Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem 2003;278:13775–13783 [DOI] [PubMed] [Google Scholar]
- 51.Chen W, Jump DB, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest Ophthalmol Vis Sci 2007;48:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83:1505S–1519S [DOI] [PubMed] [Google Scholar]
- 53.Mathias S, Younes A, Kan CC, Orlow I, Joseph C, Kolesnick RN. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science 1993;259:519–522 [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Costanzo M, Golde DW, Kolesnick RN. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor kappa B translocation in intact HL-60 cells. J Biol Chem 1993;268:20520–20523 [PubMed] [Google Scholar]
- 55.Marathe S, Schissel SL, Yellin MJ, et al. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase: implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem 1998;273:4081–4088 [DOI] [PubMed] [Google Scholar]
- 56.York RD, Yao H, Dillon T, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 1998;392:622–626 [DOI] [PubMed] [Google Scholar]
- 57.Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science 1992;255:1715–1718 [DOI] [PubMed] [Google Scholar]
- 58.Stylianou E, O'Neill LA, Rawlinson L, Edbrooke MR, Woo P, Saklatvala J. Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J Biol Chem 1992;267:15836–15841 [PubMed] [Google Scholar]
- 59.Monaco C, Paleolog E. Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res 2004;61:671–682 [DOI] [PubMed] [Google Scholar]
- 60.Natoli G, Costanzo A, Guido F, Moretti F, Levrero M. Apoptotic, non-apoptotic, and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem Pharmacol 1998;56:915–920 [DOI] [PubMed] [Google Scholar]
- 61.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell 1992;71:765–776 [DOI] [PubMed] [Google Scholar]
- 62.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and proinflammatory gene expression. J Biol Chem 2003;278:43807–43817 [DOI] [PubMed] [Google Scholar]
- 63.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Musiek ES, Brooks JD, Joo M, et al. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. J Biol Chem 2008;283:19927–19935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003;22:5897–5906 [DOI] [PubMed] [Google Scholar]