Abstract
Estrogens play a pivotal role in the development and progression of prostate cancer (PCa). Their actions are mediated by estrogen receptors (ERs), particularly ERβ in the prostate epithelium. With the discovery of ERβ isoforms, data from previous studies that focused principally on the wild-type ERβ (ERβ1) may not be adequate in explaining the still controversial role of ERβ(s) in prostate carcinogenesis. In this study, using newly generated isoform-specific antibodies, immunohistochemistry (IHC) was performed on a tumor microarray comprised of 144 specimens. IHC results were correlated with pathological and clinical follow-up data to delineate the distinct roles of ERβ1, ERβ2, and ERβ5 in PCa. ERβ2 was commonly found in the cytoplasm and was the most abundant isoform followed by ERβ1 localized predominantly in the nucleus, and ERβ5 was primarily located in the cytoplasm. Logistic regression analyses demonstrated that nuclear ERβ2 (nERβ2) is an independent prognostic marker for prostate specific antigen (PSA) failure and postoperative metastasis (POM). In a Kaplan–Meier analysis, the combined expression of both nERβ2 and cytoplasmic ERβ5 identified a group of patients with the shortest POM-free survival. Cox proportional hazard models revealed that nERβ2 predicted shorter time to POM. In concordance with IHC data, stable, ectopic expression of ERβ2 or ERβ5 enhanced PCa cell invasiveness but only PCa cells expressing ERβ5 exhibited augmented cell migration. This is the first study to uncover a metastasis-promoting role of ERβ2 and ERβ5 in PCa, and show that the two isoforms, singularly and conjointly, have prognostic values for PCa progression. These findings may aid future clinical management of PCa.
Introduction
Prostate cancer (PCa) is the most common cancer and the second leading cause of death among American men (Jemal et al. 2009). Although androgen deprivation therapies (ADTs) remain as the mainstay treatment for advanced PCas, these therapies eventually fail, in part, due to the development of androgen hypersensitivity, ligand-independent androgen receptor (AR) transactivation, and AR gene mutations and/or amplification in PCas (Scher et al. 2004, Culig & Bartsch 2006). The recently discovered AR spliced variants in PCa further raise concerns on the efficacy of these therapies (Dehm et al. 2008, Guo et al. 2009). Thus, limitations revolving around ADTs have prompted exploration of other therapeutic options. Transdermal estrogen patches as a first-line therapy in patients with locally advanced or metastatic PCa (Langley et al. 2008) are now in phase II trials. However, despite significant research efforts, the role(s) of estrogen in the pathogenesis of PCa remains poorly understood.
The incidence of PCa rises dramatically in men older than 55. This phenomenon could be due to hormonal changes during aging. Among older men, androgen levels drop significantly, whereas their estrogen levels remain unchanged or increased, making the estrogen to androgen ratio elevated in the aging prostate (Ho et al. 2006). Epidemiological studies indicate a correlation between PCa and circulating estrogen levels, but not circulating testosterone levels, among different ethnic/racial groups with diverse PCa incidence (Rohrmann et al. 2007). In addition, estrogen is produced locally by aromatase, which converts androgen into estrogen in the prostate (Stone et al. 1986, Matzkin & Soloway 1992, Ellem et al. 2004, Takase et al. 2006). Estrogens are essential in supporting the normal functioning of the prostate, and yet have long been suspected as a risk factor for PCa. Long-term exposure to elevated levels of estrogen against a normal androgen background induced a high incidence of PCa in rodents (Leav et al. 1988, Prins et al. 2007, Ricke et al. 2008). Also, in utero exposure to higher levels of maternal estrogen in African–American men may be associated with higher PCa risk (Henderson et al. 1988), a thesis supported by animal studies (Prins 1997, Ho et al. 2006, Prins & Korach 2008).
Estrogen receptors (ER), ERα and ERβ, are the major mediators of estrogen signaling. Upon binding of estradiol-17β, ER in the nucleus forms a homo-/heterodimer and binds either directly to the classical estrogen-responsive element (ERE) or indirectly to an NFκB-, Ap1-, or Sp1-binding element via tethering with their respective transcription factors, thereby initiating downstream signaling cascades (Heldring et al. 2007). The roles of ERs in the prostate are not fully understood. ERα is found primarily in stromal cells of the prostate, and appears to regulate the growth and differentiation of prostatic epithelial cells in a paracrine fashion (Ellem & Risbridger 2009). In the prostatic epithelium, ERβ is the predominant ER subtype (Kuiper et al. 1996), but its function remains controversial. Hyperplasia and dysplasia have been found in the prostates of adult ERβ knockout mice (Krege et al. 1998, Weihua et al. 2001), arguing for an antiproliferative role of ERβ (Morani et al. 2008). However, other research groups did not observe such phenotypes in their ERβ knockout mice (Couse et al. 2000, Dupont et al. 2000, Antal et al. 2008). We and others have shown that the expression of ERβ in human prostate epithelial cells decreases as PCa develops and progresses to a higher grade (Leav et al. 2001, Zhu et al. 2004), but reappears in the lymph node and bone metastases (Lai et al. 2004, Zhu et al. 2004). The dynamic change in ERβ expression is epigenetically regulated by reversible cytosine methylation of an AP2 site in a CpG island located in the ERβ proximal promoter (Zhu et al. 2004, Zhang et al. 2007). These findings suggest that ERβ may play a protective role during early stages of prostate carcinogenesis, but either promote metastasis or support PCa cell survival at distant sites (Leav et al. 2001). Apropos to the postulate that ERβ promotes PCa metastasis, a few reports have demonstrated an association between ERβ immunopositivity in high-grade PCa and poor relapse-free survival time (Horvath et al. 2001, Nanni et al. 2009). However, further delineation of the role of ERβ (s) in PCa progression is needed.
The continuing controversy over the function of ERβs in prostate carcinogenesis could be due to variable expression levels of different ERβ isoforms in benign versus malignant tissues during different stages of the process. In humans, in addition to the wild-type ERβ or ERβ1, four spliced variants designated as ERβ2–5 have been identified (Moore et al. 1998). The ERβ2–5 isoforms share the first four functional domains with ERβ1, but each has a unique activation function 2 (AF2) domain. Since isoform-specific antibodies were unavailable until very recently, the use of various antibodies directed against different domains of the molecules might have generated immunohistochemistry (IHC) patterns that contradict each other.
No functions for ERβ2–5 isoforms in the prostate have yet been identified except in one study with 48 cases, suggesting that ERβ2 may be a poor prognostic marker for PCa (Fujimura et al. 2001). A breast cancer study, however, has implicated nuclear ERβ2 (nERβ2) and nuclear ERβ5 (nERβ5) to predict better prognosis (Shaaban et al. 2008). To clarify the role of ERβ1 and its isoforms ERβ2 and ERβ5 in PCa, we evaluated the expression patterns of these three ERβs in a set of tissue microarrays (TMAs) from a cohort of 144 patients with long follow-up using newly developed in-house isoform-specific antibodies. We found that nuclear expression of ERβ2 in PCa was an independent prognostic marker for prostate specific antigen (PSA) failure (PF), postoperative metastasis (POM), and time to POM. Furthermore, the coexpression of nERβ2 and cytoplasmic ERβ5 (cERβ5) was found associated with the worst prognosis in terms of POM-free survival time. We have further demonstrated that the stable, ectopic expression of ERβ5 in PCa cells increased cell migration and invasiveness without affecting cell growth, while the expression of ERβ2 augmented only cell invasion. In aggregate, our data suggest that ERβ isoforms have variable functions and prognostic values in PCa.
Materials and methods
Patient population
With Institutional Review Board (IRB) approval, we included 144 patients with PCa who underwent radical prostatectomy at the Massachusetts General Hospital (Boston, MA, USA) from September 1993 to March 1995 in this study. All hematoxyolin–eosin (H&E)-stained sections from each case were reviewed, and the Gleason score was reassigned on the basis of the current grading recommendation provided by the International Society of Urological Pathology (Epstein et al. 2005). The tissue blocks containing the index PCa (tumor focus with the highest Gleason score) were selected for inclusion in the TMA. The relevant clinicopathological data collected included age; pre-operative PSA; Gleason score; American Joint Committee on Cancer (AJCC) T stage; surgical margin; and POM, follow-up of PSA recurrence, and overall survival. PF was any detectable PSA after PSA nadir post-surgery. The value was 0.5 ng/ml before 1999 and 0.2 ng/ml since 1999. POM comprised bone and lymph node metastases.
TMA construction
The TMAs were constructed using a manual tissue array instrument (Beecher Instruments, Silver Spring, MD, USA). Briefly, the H&E-stained slides were reviewed for accuracy of Gleason scores and for the availability of adequate areas of each component Gleason grade pattern; the index tumor, defined as the largest and/or highest Gleason score, was identified on the slide; and the representative highest Gleason score areas were marked. Three tissue cylinders with a diameter of 0.6 mm were punched from selected areas of each donor block and brought into a recipient paraffin block. Each block contained normal prostate tissue derived from normal peripheral zone away from the tumor and benign prostatic hyperplasia. The normal tissue serves as an internal control and a reference of staining intensity for adjacent cancer foci. After construction of the TMA blocks, H&E-stained sections were made for histological evaluation.
Antibodies
ERβ1-specific antibody (GC-17, Biogenex, San Ramon, CA, USA) recognizing amino acid residue 502–518 of the AF2 domain was developed in our laboratory, and shown to be highly specific in both western blot and IHC assays (Leav et al. 2001). Polyclonal monospecific antibodies specific for C-terminal peptides within ERβ2 (482-MKMETLLPEATMEQ-495) and ERβ5 (459-LMLLSHVRHARYAP-472) were prepared by immunizing rabbits with the targeted peptide conjugated to BSA according to the affinity-purified package offered by New England Peptide (Gardner, MA, USA). Because we found purified IgGs of ERβ2 and ERβ5 to be unstable, we used antisera for all subsequent analyses. The expression of all isoforms in western analyses was confirmed with an N-terminal-specific antibody (H150, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Western blot analyses
To evaluate the specificity of our ERβ2 and ERβ5 antisera, we expressed various ERβ isoforms (ERβ1, ERβ2, and ERβ5) in HEK293 cells and used these as reagents for testing the specificity of an antiserum. Methods for cell culture maintenance, DNA transfection, and western blot analyses were similar to those previously reported (Leung et al. 2006). The dilution ratio for the primary antibodies used in this study was maintained at 1:1000.
IHC analyses
The detection of ERβ1, ERβ2, or ERβ5 expression on human prostate paraffin-embedded sections was carried out according to our published protocols for ERβ1 (Leav et al. 2001). The optimal dilution ratio for each primary antiserum was determined empirically, and found to be 1:100 for ERβ1 and ERβ5 and 1:500 for ERβ2. These dilution ratios were used throughout our study. The specificity of ERβ2 and ERβ5 antisera was determined by neutralizing each antiserum with a 10× excess (by weight) of its respective targeted peptide. Peptides derived from ERβ1 (GCKSSITGSECSPAEDS), ERβ3 (CSWRLFMLREAS), ERβ4 (CVRHARWGEKQFIHLK), and ERβ5 (CSHVRHARYAP) were also applied to the IHC studies to further evaluate the antibody specificity. The antibody–peptide precipitate was removed by centrifugation at 12 000 g for 10 min after overnight incubation at 4 °C. The supernatant (pre-absorbed serum) was used in parallel with the primary antiserum as a negative control to establish specificity of its respective antiserum.
In TMA studies, sections were stained according to the same IHC protocol. IHC results were scored, evaluated, and graded independently in a blinded fashion by two investigators (C-L W and S W), including one experienced urological pathologist (C-L W). Nuclear and cytoplasmic signals of each ERβ isoform were examined separately. Signal intensity and percentage of signal coverage of each region were scored according to the Allred scoring system (Allred et al. 1993). The intensity signal was graded from 0 to 3 (0, none; 1, weak; 2, intermediate; 3, strong), and the percentage of positive tumor cells was scored from 1 to 5 (1, <1%; 2, 1–10%, 3, 10–30%; 4, 30–60%; 5, >60%). For example, if the same specimen was not stained by either of the antibodies, it will be scored as 0+1 (Allred score (Ascore)=1, i.e. negative).
Subcellular localization studies
PC3 cells (ATCC, Manassas, VA, USA) were maintained under standard conditions and cultured on coverslips. A full-length sequence of ERβ isoforms 1, 2, and 5 was subcloned into pEF-yellow fluorescent protein (YFP)-C1 (Clontech) and expressed as a fusion protein with an N-terminal YFP tag in PC3 cancer cells following the Lipofectamine 2000 (Invitrogen) transfection protocol. At 48 h after transfection, cells were incubated with 250 nM Mito-tracker Red CM-H2XRos (Invitrogen) for 45 min and fixed with 4% formaldehyde for 15 min. Cells were then permeabilized with 0.2% Triton X-100 before their nuclei were counterstained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and preserved in Prolong Antifade Gold reagent (Invitrogen). Fluorescent signals were captured and evaluated with an Axiovert 200M fluorescent microscope equipped with an AxioCam MRm camera and Axiovision 4.7 software (Carl Zeiss, Thornwood, NY, USA). Optical sections were generated using ApoTome (Carl Zeiss).
Construction of PC3 cells stably expressing ERβ isoforms
Full-length sequences of ERβ1, ERβ2, and ERβ5 were subcloned into a pLenti6 lentiviral vector recombined with an ubiquitin promoter by Multisite Gateway recombination reactions (Invitrogen) and transfected into 293FT cells for production of lentivirus according to the manufacturer's protocol. Lentivirus carrying the LacZ gene was used as a control. The use of the ubiquitin promoter, which is a constitutive but relatively weak promoter, minimized artifacts introduced by other virus-based promoters. The titer of each lentivirus was measured, and the multiplicity of infection of PC3 cells was 0.7. Lentivirus-infected PC3 cells were cultured in medium supplemented with blasticidin at 8 μg/ml and selected for 3 weeks. PC3 cells stably expressing LacZ, ERβ1, ERβ2, and ERβ5 were designated as PC3–LacZ, PC3–ERβ1, PC3–ERβ2, and PC3–ERβ5 respectively. Expression of the transgenes was confirmed by real-time PCR assays.
Cell migration and invasion assays
Cellular mobility of PC3–LacZ, PC3–ERβ1, PC3–ERβ2, and PC3–ERβ5 cells was evaluated with a wound-healing assay (Rodriguez et al. 2005), and their invasiveness through the extracellular matrix was determined by the BD BioCoat Matrigel Invasion Chamber method (BD Biosciences, San Jose, CA, USA) following the manufacturer's protocol.
Statistical analysis
Descriptive statistics were calculated for demographic and clinical measures. Preliminary associations between Ascores measuring the expression level of cytoplasmic and nERβ1, 2, and 5 were analyzed using Mantel–Haenszel χ2 test statistics to evaluate associations with PF and POM. A significant relationship with at least one outcome was found for one isoform of each type (P<0.05). These Ascores were included in logistic regression analyses, adjusted for Gleason score and age. Ascores were dichotomized at cutpoints where slopes of Ascores changed, determined by statistical evaluations of curves fitted to the data, and visual assessment. Two cutpoints were chosen for each outcome and each Ascore. The best model was chosen by evaluating goodness-of-fit statistics. Cox proportional hazard models were used to analyze time to PF and time to POM. Kaplan–Meier plots of PF and POM survival were analyzed, stratified by dichotomized cytoplasmic and nuclear levels. An α-level of 0.05 was used to determine significance, unless otherwise stated. Analyses were performed with SAS for Windows, Version 9.1.2 (SAS Institute, Cary, NC, USA) and GraphPadPrism4 (La Jolla, CA, USA).
Results
Evaluation of ERβ2 and ERβ5 antisera for isoform specificity
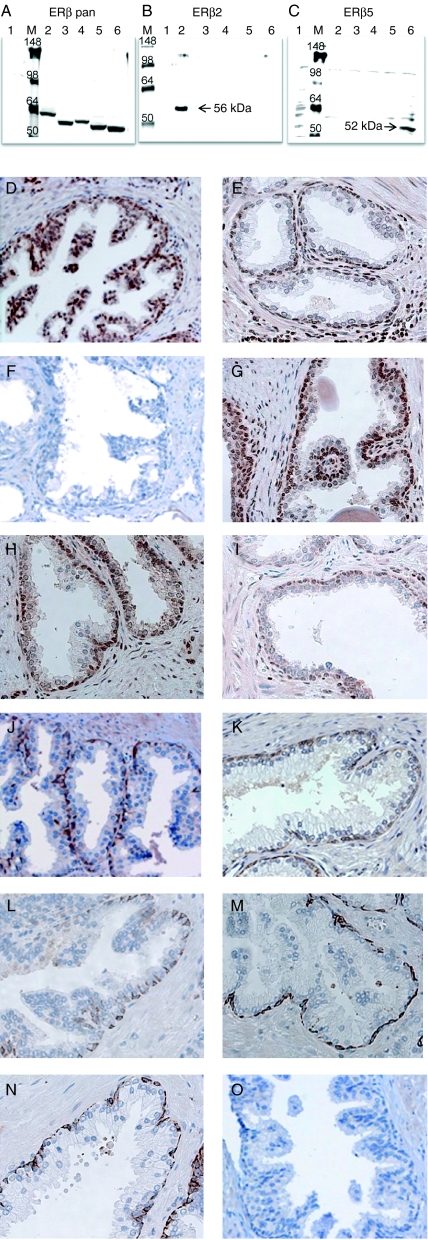
A specific antibody targeting each isoform is necessary for studying the expression of ERβ isoforms. An ERβ1-specific antibody (GC17) that we had raised previously has been widely used in PCa research (Leav et al. 2001, Adams et al. 2002, Pais et al. 2003, Zhu et al. 2004). We recently also developed polyclonal monospecific antisera to ERβ2 and ERβ5 based on isoform-specific C-terminal sequences. We first overexpressed full-length ERβ1, 2, 3, 4, or 5 expression plasmids (Leung et al. 2006) in HEK293 cells and used the cell lysates to test the specificity of ERβ2 and ERβ5 antiserum by western blot analysis. We used a pan-ERβ antibody to confirm the expression of each ERβ isoform protein in HEK293 cells (Fig. 1A). We then reblotted the same lysates with ERβ2-specific (Fig. 1B) or ERβ5-specific (Fig. 1C) antiserum. ERβ2-specific antiserum recognized a single 56-kDa band in lane 3, which was loaded with a lysate from HEK293 cells expressing the ERβ2 transgene. No cross-reactivity with other isoforms (lanes 2, 4, 5, and 6) was detected in this ERβ2 western blot. Similarly, the ERβ5 antiserum strongly recognized a 53-kDa band in lane 6 that was loaded with a lysate from HEK293 cells harboring the ERβ5 transgene.
Figure 1.
Specificity of ERβ isoform-specific antibodies. Western blot analyses: HEK293 cells expressing ERβ1–5 lysate were tested against ERβ pan antibody ((A) Santa Cruz H150), ERβ2-specific antibody (B), and ERβ5-specific antibody (C). Lane 1 was loaded with control cell lysate (HEK293 cells only), whereas lanes 2–6 were loaded with HEK293 cell lysate expressing ERβ1–5 respectively. M stands for protein marker (Invitrogen SeeBlue Plus2 protein ladder). The expected protein band of the ERβ isoforms 2 and 5 was labeled by an arrow in the middle and right panel respectively. Immunohistochemical (IHC) analyses: IHC analyses of benign prostatic tissue were carried out using ERβ2- (D–I) and β5-specific antisera (J–O). ERβ1-, ERβ2-, ERβ3-, ERβ4-, and ERβ5-specific peptides were applied to the IHC analyses. Figures showing IHC analyses with or without ERβ isoform-specific peptide are arranged as follow: D and J (no peptide); E and K (+ERβ1 peptide); F and L (+ERβ2 peptide); G and M (+ERβ3 peptide); H and N (+ERβ4 peptide); I and O (+ERβ5 peptide).
We then investigated whether we could use the new antisera for IHC. To validate the specificity of an antiserum, we immunostained serial prostate archival sections with or without ERβ isoform-specific peptides. At low magnification (×100), the ERβ2 antiserum primarily stained the cytoplasm of the basal and luminal epithelial cells of benign glands (Fig. 1D) and also stained the nuclei of some stromal cells. The immunopositive signals were not blocked by other ERβ isoform-specific peptides (Fig. 1E, G, H, and I) but totally abolished by the ERβ2 peptide (Fig. 1F). These results showed that the specificity of ERβ2 antiserum for ERβ2 was excellent. Similarly, ERβ5 antibody strongly stained the cytoplasm of basal epithelial cells but not the luminal epithelial cells (×100) and weakly stained some stromal cells (Fig. 1J). Such immunopositive signals of ERβ5 were totally eliminated by its blocking peptide (Fig. 1O) but not by other isoform-specific peptides (Fig. 1K, L, M, and N), verifying the specificity of ERβ5 antiserum for IHC analyses.
Immunocytochemistry analyses of ERβ isoforms in PCa specimens
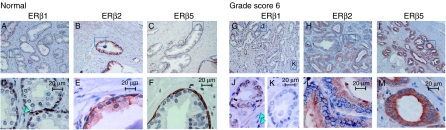
Although we showed the antisera to be specific to their respective antigen, we also found that they may crossreact with other ERβ isoforms. We therefore further examined our ERβ-stained sections at high magnification (×630) to determine whether the staining pattern was unique for each antibody/antiserum. Immunostained benign glands (Fig. 2A–F) and cancer foci, consisting of Gleason grade 3 cancer glands (Fig. 2G–M), were compared in parallel. In benign prostate glands, ERβ1 was localized principally in the nuclei of basal epithelial cells (Fig. 2A and D) as previously reported for GC17 staining (Leav et al. 2001). ERβ1 was also localized in the nuclei of a few luminal epithelial cells but was found quite frequently in the perinuclear zone in luminal epithelial cells. The nuclei of some stromal cells were also strongly stained by the ERβ1 antibody (GC17). In contrast, the ERβ2 was localized predominantly to the cytoplasm of both basal and luminal epithelial cells, with clear localization in the supranuclear zone of the luminal cells (Fig. 2B and E); nuclear localization was uncommon. ERβ5, however, was localized almost exclusively in basal epithelial cells in benign prostate glands (Fig. 2C and F); intracellular localization was primarily cytoplasmic. In PCa, such as in Gleason score 6 (3+3) cancer foci (Fig. 2K), some foci of ERβ1 positivity were lost in both nuclear and cytoplasmic compartments, but some remained (Fig. 2J). ERβ2 (Fig. 2H and L) and ERβ5 (Fig. 2I and M), however, displayed a diffused pattern of cytoplasmic staining, with occasional nuclear positivity. In summary, the three antibodies/antirsera produced distinct staining patterns in both benign glands and cancerous prostatic foci, thereby providing support for the suggestion that each of them is highly specific and probably recognizes only one ERβ isoform in IHC studies.
Figure 2.
Immunohistochemical analyses of ERβ isoforms in benign prostate glands (A–F) and prostate adenocarcinoma in PCa specimen (G–M). PCa specimens from cancers with Gleason score 6 (G–M) and its adjacent normal region (A–F) were immunostained with ERβ1-, ERβ2- and ERβ5-specific antibodies/antisera. See Materials and methods for the experimental conditions. Figures D–F and J–M are the magnified view (×630) of a region (marked by a rectangle) in Figure A–C and G–I (×100) respectively. In ERβ1 immunostaining, positive signals were found mostly in the nuclei of basal epithelial cells in benign foci (A and D) and in the nuclei of PCa cells (G and J) despite sparse nuclear staining in stromal cells (indicated by light blue arrow heads in figures D and J). ERβ1 staining was lost in some PCa foci (K). ERβ2 staining is highly cytoplasmic in both benign (basal and luminal epithelial cells, B and E) and PCa foci (H and L). Immunopositive signals of ERβ5 were observed in the cytoplasmic region of the epithelial cells in adjacent normal foci (C and F) as well as in PCa cells in Gleason score 6 cancer (I and M).
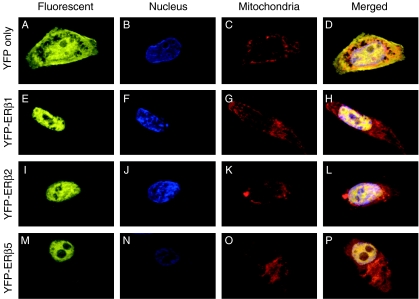
Subcellular localization of ERβ isoforms in PCa cells
To elucidate the subcellular localization of ERβ isoforms in PCa cells, we ectopically expressed ERβ1, ERβ2, or ERβ5 in PC3 cells in the form of an YFP fusion protein, with YFP serving as a control (Fig. 3A–D). YFP–ERβ1 was found principally in the nucleus, with very weak signals in the cytoplasm (Fig. 3E). The cytoplasmic signal did not overlap the Mito-tracker red signal (Fig. 3G), suggesting that ERβ1 does not reside in mitochondria in PC3 cells. Similarly, the YFP signal of ERβ2 (Fig. 3I) and ERβ5 (Fig. 3M) was found in both the nuclei and cytoplasm of the PC3 cells, and no overlapping signal with the Mito-tracker red signals was detected in both cases (Fig. 3K and O). Of interest was the stronger cytoplasmic signal of YFP–ERβ5 among the ERβ isoforms.
Figure 3.
Subcellular localization of ERβ isoforms in PC3 cells. Vectors carrying ERβ1 (E–H), ERβ2 (I–L), and ERβ5 (M–P) were transiently transfected into PC3 cells, and expressed in the form of fusion protein with an N-terminal YFP tag. Cells transfected with YFP only serve as a control (A–D). Cells were counterstained with nucleus-specific (DAPI, B, F, J, and N) and mitochondria-specific fluorescent dyes (Mito-tracker red, C, G, K, and O). Subcellular localization of each isoform was determined by fluorescence microscopy. Extended focus was applied to average and combine three optical sections of each signal. Merged images of YFP, DAPI, and Mito-tracker red signals are shown in D, H, I, and P.
Characteristics of the patients whose prostates were used to construct the TMA
The majority of the patients (n=133) were Caucasian–Americans (92.4%; Table 1). Only 11 were non-Caucasian: African–American (3.5%), Hispanic (2.8%), or Asian (1.4%). Ages ranged from 46 to 77 years (mean=62). Approximately 44% of the patients had low-grade cancer (Gleason score <7), 40% had intermediate-grade PCa (Gleason score =7), and 16% had higher-grade cancer (Gleason score >7). The postoperative PSA levels of patients were measured for an average of 6.2 years. PF was detected in 37.5% of the patients (n=54). POM was found in 8.3% of the patients (n=12). The average time to PF and POM was 6.2 and 8.6 years respectively.
Table 1.
Characteristics of 144 patients
| Variables | n (% total patients) |
|---|---|
| Age (years) | |
| 46–50 | 4 (2.8) |
| 50–59 | 49 (34.0) |
| 60–69 | 81 (56.3) |
| 70–77 | 10 (6.9) |
| Race | |
| Caucasian–American | 133 (92.4) |
| African–American | 5 (3.5) |
| Hispanics | 4 (2.8) |
| Asian | 2 (1.4) |
| Pathology stage | |
| T2 | 115 (79.9) |
| T3 | 29 (20.1) |
| Gleason score | |
| <7 | 63 (43.8) |
| =7 | 58 (40.2) |
| >7 | 23 (16.0) |
| Surgical margin | |
| Positive | 58 (40.3) |
| Negative | 86 (59.7) |
| PSA failure | |
| Yes | 54 (37.5) |
| No | 90 (62.5) |
| Metastasis | |
| Detected | 12 (8.3) |
| Not detected | 132 (91.7) |
TMA analyses
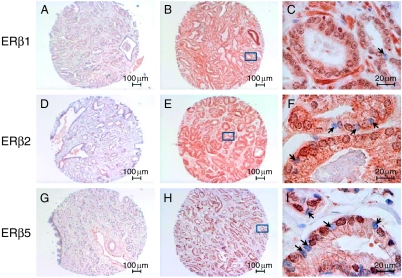
To elucidate the clinical relevance of the expression of ERβ1, ERβ2, and ERβ5 in PCa, we used our newly validated antisera and ERβ1-specific antibody (GC17) in an IHC analysis of a set of TMAs comprising 144 specimens with a well-documented clinical history. Figure 4 shows typical results of IHC staining with ERβ1, 2, or 5 antibody/antisera. Typical TMA sections showing low to moderate staining of ERβ isoforms are shown in Fig. 4A, D, and G. In a few rare instances, both nuclear and cytoplasmic staining of ERβ isoforms was observed in some of the TMA sections (Fig. 4B, E, and H). With higher magnification (×630), nuclei in PCa cells were differentially stained by the isoforms – antibody/antisera (Fig. 4C, F, and I). We noted different staining patterns in some specimens of similar cancer grade even with the same antibody/antiserum and IHC conditions. Variations were greater in the specimens positive for ERβ1 and ERβ5. The pattern for ERβ2 was less variable.
Figure 4.
Typical IHC results of ERβ immunostaining on TMAs. TMAs with 144 specimens were immunostained with ERβ1 (A–C), ERβ2 (D–E), and ERβ5 (G–I) antibodies/antisera. The Allred scoring system (Allred score=signal score+intensity score) was used to grade the immunostaining signals. Typical low Allred-scored sections (Allred=0+1 for nuclear positivity; Allred=1+2 to 1+3 for cytoplasmic positivity) are shown in A, D, and G. Higher Allred-scored sections (Allred=3+5 for both nuclear and cytoplasmic positivity) with strong nuclear and cytoplasmic staining are shown in B, E, and H. C, F, and I represent a magnified view (×630) of a region (marked by a rectangle) in B, E, and H respectively. Negative nuclear staining in C, F, and I is marked by solid arrows.
Distinct subcellular localization patterns of ERβ isoforms in PCa specimens
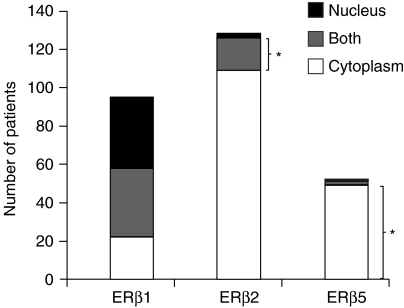
ERβ1, 2, and 5 showed distinctly different expression patterns in this set of PCa TMAs. ERβ2 was found to be the dominant ERβ isoform in PCa (Fig. 5). Over 80% of the samples (n=127) showed a positive ERβ2 signal. Most of the ERβ2 (n=108) was localized in the cytoplasm of the PCa cells, but ∼10% resided in the nucleus. ERβ1 was the second most common isoform in this set of TMAs. Since 84% of the patients (n=121) had intermediate-grade PCa (Gleason 5–7) at prostatectomy, in agreement with previous studies, most of their prostate specimens still retained ERβ1 staining (Fig. 2; Leav et al. 2001). In contrast to the exclusive nuclear localization of ERβ1 in basal cells of benign glands, ERβ1 was found more often in both the nuclear and cytoplasmic compartments or in only the cytoplasmic compartment of PCa cells. ERβ5 is the least common isoform; only 36% of patients (n=52) showed ERβ5 positivity, which was predominantly expressed in the cytoplasm of the cancer cells (>90% of the cases, n=49).
Figure 5.
Distribution of ERβ isoforms in this prostate cancer TMA. The immunopositivity signal of each isoform was graded according to the Allred scoring system. Only an Allred score greater than cutpoint (i.e. Allred>3) was considered positive. The number of patients showing positive immunostaining signals in different cellular compartments was determined and analyzed. The correlations between two ERβ isoform expression were determined based on Spearman correlation test. P<0.05 was considered to be statistically significant. An asterisk represents a specific group of patients with a higher possibility of showing the worst clinical outcome.
Since we had previously shown that the various ERβ isoforms formed heterodimers with ERβ1 in cell culture under different conditions of ligand stimulation (Leung et al. 2006), we calculated Spearman correlation analyses to determine the possibility of finding two of the three ERβs in the same cellular compartment in the PCa specimens (Table 2). Despite low-to-moderate correlations, a significant difference from zero was found for the correlation between ERβ1 and ERβ2 in the nucleus (r=0.45) and the cytoplasm (r=0.39; P<0.01), suggesting that ERβ1 and ERβ2 may be coexpressed in the nuclei or cytoplasm of PCa cells. ERβ5, which is predominantly cytoplasmic, therefore showed a better correlation only with cERβ1 (r=0.37) and cERβ2 (r=0.26; P<0.01), implying that some specimens may coexpress ERβ5 with at least one other ERβ.
Table 2.
Spearman correlation S(r) between expression of estrogen receptor β1 (ERβ1), ERβ2, and ERβ5 isoform
| Nucleus | Cytoplasm | ||||
|---|---|---|---|---|---|
| na | r | P value | r | P value | |
| ERβ1 versus ERβ2 | 134 | 0.45 | <0.01 | 0.39 | <0.01 |
| ERβ1 versus ERβ5 | 134 | 0.10 | 0.23 | 0.38 | <0.01 |
| ERβ2 versus ERβ5 | 134 | 0.06 | 0.48 | 0.24 | <0.01 |
P values testing r=0.
Patient numbers are <144 due to missing data.
Statistical results of TMA studies
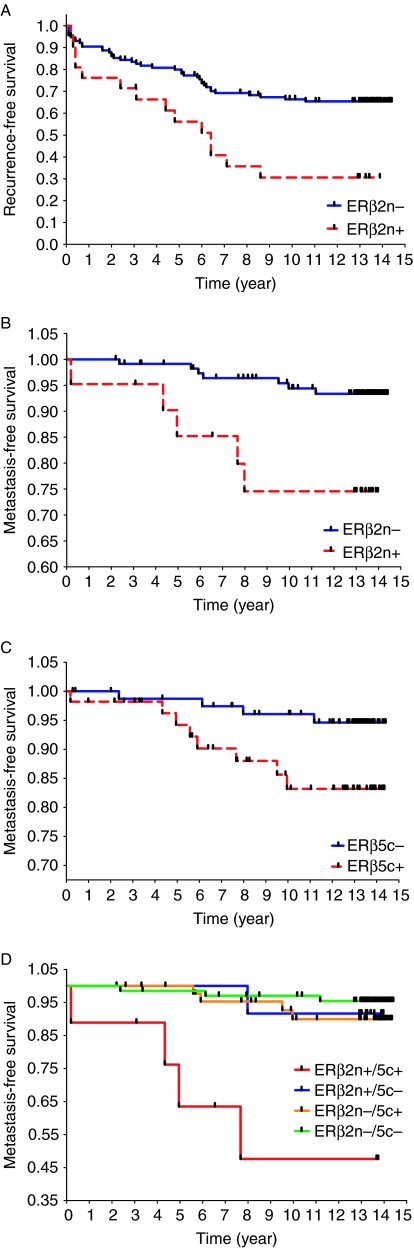
nERβ2 and cERβ5 Ascores were significantly related to PF and/or POM. Ascores were dichotomized as negative (≤3) and positive (>3) based on statistical assessment and results of a previously published study (Shaaban et al. 2008). Descriptive statistics evaluating differences between dichotomized Ascores and clinicopathological features of PCa showed that age, preoperative PSA, and Gleason score were significantly different (P<0.10) for at least one isoform (nERβ2 and cERβ5; Table 3). Positive expression of nERβ2 was significantly associated with PF (adjusted odds ratio (adjOR)=3.8, P=0.04) and POM (adjOR=3.1, P=0.02), while cERβ5 positivity did not show a significant association with either PF (adjOR=0.8, P=0.58) or POM (adjOR=3.1, P=0.09; Table 3). Significantly poorer survival (time to POM), independent of Gleason score, age, and preoperative PSA, was associated with nERβ2 positivity (adjusted hazard ratio adjHR=4.6, P=0.02). These results suggest that nERβ2 is an independent prognostic marker for predicting time to POM. Time to PF was not significantly associated with positivity of nERβ2 or cERβ5 (P>0.05). Kaplan–Meier plots and the log-rank test statistics showed that time to PF and time to POM were significantly shorter for patients with nERβ2 positivity (P<0.01; Fig. 6A and B). cERβ5 positivity, on the other hand, was significantly associated only with a reduced survival time to POM (P=0.03; Fig. 6C) but not time to PF (P>0.05, data not shown). In a combined analysis, patients whose prostate specimen was positive for both nERβ2 and cERβ5 had the shortest POM-free survival (P<0.01, degrees of freedom=3), i.e. worst clinical outcome (Fig. 6D). Comparisons among pairs of groups revealed that the group with a double positive for nERβ2 and cERβ5 was significantly different from the group with a double negative (P<0.01) and with the group with only nERβ2 positive (P=0.04).
Table 3.
Descriptive statistics and results of regression analyses
| Nuclear ERβ2 (n=136a) | Cytoplasmic ERβ5 (n=137a) | |||||
|---|---|---|---|---|---|---|
| Positiveb (%) | Negativeb (%) | P value | Positiveb (%) | Negativeb (%) | P value | |
| Clinicopathological features of PCa | ||||||
| Number of patients (n) | 21 | 115 | 56 | 81 | ||
| Age (years) | ||||||
| Mean±s.d. | 59.5±5.9 | 62.3±6.1 | 0.06 | 62.3±6.1 | 61.7±6.3 | 0.58 |
| Preoperative PSA (ng/ml) | ||||||
| Median (min, max) | 5.0 (3.0, 20.1) | 6.8 (0.05, 32.3) | 0.03 | 6.6 (0.3, 32.3) | 6.2 (0.05, 23.2) | 0.71 |
| Gleason score | ||||||
| <7 | 4 (19.1) | 53 (46.1) | 22 (39.3) | 36 (44.4) | ||
| =7 | 13 (61.9) | 44 (38.3) | 0.06 | 22 (39.3) | 35 (43.2) | 0.36 |
| >7 | 4 (19.1) | 18 (15.7) | (df=2) | 12 (21.4) | 10 (12.4) | (df=2) |
| Pathology stagec | ||||||
| T2 | 19 (90.5) | 89 (77.4) | 43 (76.8) | 66 (81.5) | ||
| T3 | 2 (9.5) | 26 (22.6) | 0.14 | 13 (23.2) | 15 (18.5) | 0.50 |
| Surgical margins | ||||||
| Positive | 7 (33.3) | 50 (43.5) | 25 (44.6) | 32 (39.5) | ||
| Negative | 14 (66.7) | 65 (56.5) | 0.47 | 31 (55.4) | 49 (60.5) | 0.60 |
| PSA failure | ||||||
| Yes | 14 (66.7) | 39 (33.9) | 22 (39.3) | 31 (38.3) | ||
| No | 7 (33.3) | 76 (66.1) | <0.01 | 34 (60.7) | 50 (61.7) | 0.90 |
| Post-operative metastasis | ||||||
| Yes | 5 (23.8) | 7 (6.1) | 8 (14.3) | 4 (4.9) | ||
| No | 16 (76.2) | 108 (93.9) | <0.01 | 48 (85.7) | 77 (95.1) | 0.06 |
| Outcome | adjOR | adjHR | P value | adjOR | adjHR | P value |
|---|---|---|---|---|---|---|
| Adjusted odds ratio (adjORd) and hazard ratio (adjHRd) | ||||||
| PSA failure | 3.8 | 0.04 | 0.8 | 0.58 | ||
| Time to PSA failure | 1.8 | 0.10 | 0.8 | 0.47 | ||
| Post-operative metastasis | 3.1 | 0.02 | 3.1 | 0.09 | ||
| Time to post-operative metastasis | 4.6 | 0.02 | 3.1 | 0.07 | ||
P values test i) differences in mean age and median preoperative PSA between positive and negative ERβ isoforms; ii) differences in Allred positivity rates among categories of Gleason score, pathology stage, and surgical margins.
Table entries for count data indicate number of patients (%column total). Column totals are <144 due to missing data.
Cutpoint for positive: Allred score >3; cutpoint for negative: Allred score ≤3.
No stages 1 and 4 were detected.
AdjOR measures the odds of a positive isoform from logistic regression, adjusted for age, Gleason score, and preoperative PSA; AdjHR measures the hazard (or risk) of a positive isoform from a Cox proportional hazards model with the same adjustments.
Figure 6.
Evaluation of ERβ isoforms as a predictor for time to PSA failure and time to metastasis by the Kaplan–Meier (KM) plot with the log-rank test. The Allred cutpoint (>3 or ≤3) for each ERβ isoform was used to determine ‘positive’ and ‘negative’ expression. (A) KM plot of nuclear ERβ2 versus recurrence/PSA-free survival (P<0.01). (B) KM plot of nuclear ERβ2 versus metastasis-free survival (P<0.01). (C) KM plot of cytoplasmic ERβ5 in metastasis-free survival (P=0.03). (D) KM plot of nuclear ERβ2 and cytoplasmic ERβ5 versus metastasis-free survival (P<0.01). Patients were further stratified into four groups according to status of ERβ isoform expression: i) both nuclear ERβ2 and cytoplasmic ERβ5 positive (n=9); ii) nuclear ERβ2 positive and cytoplasmic ERβ5 negative (n=12); iii) nuclear ERβ2 negative and cytoplasmic ERβ5 positive (n=46); and iv) both nuclear ERβ2 and cytoplasmic ERβ5 negative (n=68). P<0.05 was considered to be statistically significant.
Migration and invasion of PCa cells expressing ERβ isoforms
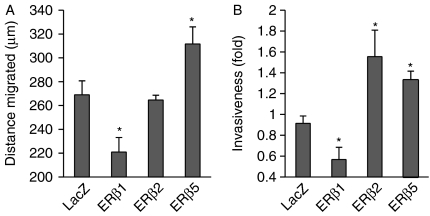
On the basis of the results from the TMA study suggesting that both ERβ2 and ERβ5 could be the markers for predicting time to POM, we further investigated the role of these two isoforms in PCa metastasis. We stably infected LacZ, ERβ1, ERβ2, and ERβ5 in PC3 cells with lentivirus, and measured cell mobility and invasion ability of the cells expressing different isoforms with wound-healing and invasion assays respectively. Stable ectopic expression of each ERβ isoform in PC3 cells was confirmed with established real-time PCR assays (Leung et al. 2006). A greater than 200-fold increase in ERβ transcript was detected in each stable-transfected cell line. In wound-healing assays, PC3–ERβ5 cells migrated significantly faster than the others after 24 h of wound introduction (Fig. 7A). PC3–ERβ2 cells migrated as fast as the PC3–LacZ control cells, but PC3–ERβ1 cells migrated significantly more slowly than control cells. In invasion assays, the efficiency of invasion across the matrix gel membrane of PC3–ERβ2 and PC3–ERβ5 cells was 64 and 42% respectively greater than that of PC3–LacZ cells within 24 h (Fig. 7B). Interestingly, ectopic expression of ERβ1 in PC3 cells significantly reduced PCa cell invasiveness by 34% as compared with PC3–LacZ.
Figure 7.
Tumor metastasis of PC3 expressing ERβ isoforms. (A) Results of wound-healing assay. Migration distance of PC3 expressing LacZ (as a control), ERβ1, ERβ2, and ERβ5 was recorded and calculated after 24 h of wound introduction. Experiments were carried out in triplicate and were repeated with three independent sets. Student's t-test was used to compare the mean distance migrated of each ERβ isoform-expressing cell line versus LacZ control cells (average±s.e.m., *P<0.05). (B) Results of Matrigel-based invasion assay. Fixed number (5×104) of cells was set up in a 24-well plate according to the manufacturer's recommendation. The cells that crossed the matrigel membrane were stained and counted under a microscope. Cell numbers were normalized by the MTS method. The fold invasiveness was calculated relative to the number of cells invaded in LacZ control. Experiments were carried out in triplicate and were repeated with three independent sets. Student's t-test was used to compare the fold invasiveness of each cell line versus LacZ control cells (average±s.e.m., *P<0.05).
Discussion
This is the first study comparing the protein expression of ERβ1 with that of its isoforms ERβ2 and ERβ5 in specimens from a cohort of patients with PCa (n=144) with long clinical follow-up. The immunohistochemical expression of the three ERβs was analyzed in a set of TMAs derived from the cohort using an ERβ1-specific antibody and two newly characterized antisera for ERβ2 and ERβ5. nERβ2 was found to be an independent predictor for PF (adjOR=3.8, P=0.04), POM (adjOR=3.1, P=0.02), and POM-free survival (adjHR=4.6, P=0.02), whereas cERβ5 was a predictor for POM-free survival in Kaplan–Meier analysis. More importantly, patients positive for both ERβ isoforms (nERβ2+ and cERβ5+) were found to have the shortest POM-free survival (P<0.01). To provide mechanistic insights into the clinical relevance and prognostic significance of both isoforms, we ectopically expressed ERβ isoforms in PC3 cells and found that stable expression of these isoforms in this cell line increased the efficiency of cell migration (for PC3–ERβ5 cells) and cell invasiveness (for both PC3–ERβ2 and PC3–ERβ5 cells) but did not alter cell proliferation (unpublished data). Taken together, these findings established that ERβ2 and ERβ5 are strongly associated with PCa metastasis.
Estrogen has been shown to be involved in normal and malignant functions of the prostate (Ho et al. 2006). Four decades ago, the oral estrogen diethylstilbestrol was the treatment of choice for PCa (Huggins & Hodges 2002). It was ultimately abandoned because of its serious cardiovascular and thromboembolic toxicity (Hanash et al. 1970, Eisen et al. 1975) and the emergence of gonadotropin agonists/antagonists (Schally et al. 1983). However, other forms of estrogen, including various ER selective modulators, recently have emerged as effective and economical therapies, largely because of clinical data showing minimal hepatic toxicity of these agents if they are administrated parentally via an intramuscular or transdermal route (Ockrim et al. 2006). These therapies also have significant efficacy in protecting against osteoporotic fracture, hot flashes, asthenia, and cognitive dysfunction in patients with PCa (Ockrim et al. 2006). With this renewed interest in using estrogens as single or combination therapy for PCa, the need to understand how ERs affect PCa is pressing. Results from this study serve as the first exploration to open a new line of investigation.
The action of estrogen in human normal and malignant prostatic tissue is believed to be mediated partly by ERβ because it is the predominant ER subtype in the prostate epithelium (Kuiper et al. 1996, Leav et al. 2001). However, the role of ERβ in prostate and breast cancers is still debatable, even after 10 years of extensive research (Ho et al. 2006). Data from different research groups conflict (Speirs et al. 2004, Skliris et al. 2006), in part because of insufficient understanding of ERβ isoforms that were discovered relatively recently (Leung et al. 2006) and the lack of antibodies to distinguish them in vitro and in clinical specimens. Unlike breast cancer research, in which the discovery of these molecules has stimulated new research directions (Speirs & Walker 2007, Powell & Xu 2008), similar developments in PCa research have been limited. The use of N-terminal-specific ERβ antibodies in IHC experiments is always problematic because they recognize all ERβ isoforms, and mRNA expression data clearly showed that each ERβ isoform has a unique, tissue- and cell type-specific expression pattern (Leung et al. 2006). This study is the first to provide data at the protein level illustrating this uniqueness in PCa. Our IHC staining revealed unique tissue-/cell type- and cellular compartment-specific distribution for each isoform in the normal and malignant prostate.
The functions of each isoform in the normal prostate epithelium are unclear. Thus, in this study, ERβ1 positivity was not associated with PCa progression, but the PC3 cell model suggested that ERβ1 inhibits both PCa cell migration and invasion, which is consistent with the current belief that ERβ is antiproliferative, tumor suppressive (Morani et al. 2008) and impedes epithelial–mesenchymal transition (Mak et al. 2010). However, others have suggested that ERβ is involved in an aggressive PCa phenotype (Horvath et al. 2001, Nanni et al. 2009). We speculate that this kind of discrepancy will be resolved in the future by including ERβ isoforms during data analysis. In this TMA study, ERβ2, the most commonly expressed isoform (>80%) in PCa cells, is primarily cytoplasmic, and its nuclear positivity correlates with PF, POM, and a shorter time to POM. These results are in agreement with a study with a smaller cohort analyzed with different clinical outcomes (Fujimura et al. 2001). However, this study was the first to investigate ERβ5 and its prognostic value in PCa. Both ERβ2 and ERβ5 elevated PCa cell invasion, but only the cells expressing ERβ5 migrated more rapidly. This suggests that ERβ2 and ERβ5 could regulate different tumor metastasis pathways in PCa cells, perhaps explaining why co-existence of ERβ2 and ERβ5 in PCa tissue could predict the worst clinical outcome, probably due to synergistic actions of ERβ2 and ERβ5 in PCa progression. Through the use of isoform-specific antibodies, we have gained new insights into the prognostic values of ERβ isoforms that will undoubtedly continue to increase our understanding of ERβs in the development and progression of PCa. The utility of ERβ2 and ERβ5 as PCa prognostic markers is obviously worthy of further investigation.
Both isoforms have been found in various tissues (Pedersen et al. 2001, Scobie et al. 2002, Poola 2003, Cammarata et al. 2005, Wong et al. 2005), including the prostate (Leung et al. 2006). The functions of these isoforms are not yet clear, but they are known to be involved in ERα (Peng et al. 2003, Poola et al. 2005) and ERβ1 signaling (Leung et al. 2006). At the molecular level, either ERβ2 or ERβ5 by itself does not transactivate ERE-driven promoter but when heterodimerized with ERβ1 can alter its transactivation activity (Leung et al. 2006). The roles of ERβ2 and ERβ5 in cancer were not studied until recently. Their nuclear expression in breast cancer tissue was significantly correlated with better overall survival (Shaaban et al. 2008), a finding that differs from our data showing a significant correlation between ERβ2/ERβ5 positivity in PCa cells and poor POM-free survival. This discrepancy suggests a fundamental difference in the role played by estrogens/estrogen signaling in the pathogenesis of PCa versus that of breast cancer, which has introduced an intriguing question regarding the clinical management of these cancers.
We recognize that our cohort is small (144 patients) compared with that of the breast cancer study (Shaaban et al. 2008) and thus includes relatively few patients with PF (n=54; ≥2 ng/ml) and POM (n=12). However, the possibility of using ERβ2 and ERβ5 for PCa prognosis shows great promise, partly because of the long lead-time before postoperative PF (6.2 years) and time to POM (8.6 years). Therefore, the confirmation of ERβ2, in conjunction ERβ5 or other established markers, as an independent prognostic marker in future cohort studies, could help to identify candidates for clinical trials of new interventions designed to curb PCa progression based on information gained at the time of radical prostatectomy from IHC analysis for ERβs. Moreover, more active surveillance and/or adjuvant therapies, such as external beam radiotherapy and ADTs (Michaelson et al. 2008, Wirth et al. 2008), may be indicated for the subset of patients at higher risk for metastases because of their positivity for ERβ2 and ERβ5.
Mechanistic studies aimed at elucidating the biological roles of ERβs require relevant cell model systems. Cammarata et al. (2005) were the first to report different subcellular localizations of ERβ isoforms in breast cancer cells. In a PCa cell line (PC3), ERβ1 resides mostly in the nucleus. Although very few ERβ1 molecules were detected in cytoplasm, they were not localized in the mitochondria in this PCa cell line, a finding different from other published cell data (Chen et al. 2004). Both ERβ2 and ERβ5 were localized in both cytoplasmic and nuclear compartments in PC3 cells. It is not clear whether such unique patterns of subcellular localizations of ERβ isoforms in PCa cells are related to their metastasis-promoting functions or simply to differential protein trafficking and/or processing. Those are the questions worthy of further investigation. As a caveat, it is almost certain that there are no perfect cell model systems that could fully recapitulate the in vivo situation, whereby epithelial cells are surrounded by different types of stromal cells in a three-dimensional architecture. Thus, interpretations of data from cell model systems would best be supported by parallel correlative studies of human tissues.
In conclusion, in a group of PCa patients with relatively long follow-up, our data demonstrate that expression of ERβ2 in the nucleus of PCa cells is an independent prognostic marker of PF, POM, and time to POM, and cERβ5 positivity predicts shorter POM-free survival. Additionally, the expression of both nERβ2 and cERβ5 identified a group of patients with the worst clinical outcome. Finally, forced expression of ERβ2 or ERβ5 in PC3 cells uncovered that these isoforms have metastasis-promoting action, supporting our IHC findings. Given the long duration of time to PF/POM, prognostic markers such as these should help in stratifying patients in clinical trials and in devising more effective customized therapies for advanced PCa.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grants from NIH (ES006096, CA15776, and CA112532 to S-M Ho), the Bertucci Foundation of Prostate Cancer Research (to C-L Wu), and the US Army Prostate Cancer Program (81XWH-04-1-0165 to S-M Ho), and an internal funding source from the University of Cincinnati Medical Center.
Acknowledgements
We thank Dr Neville Tam, Dr Olivia Lee, and Mr Prashant Angiah Srikanthan in the Department of Environmental Health, University of Cincinnati Medical Center for their excellent technical support and Nancy Voynow for her professional editing of this manuscript.
References
- Adams JY, Leav I, Lau KM, Ho SM, Pflueger SM. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52:69–81. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, Osborne CK, McGuire WL. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. Journal of the National Cancer Institute. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. PNAS. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata PR, Flynn J, Gottipati S, Chu S, Dimitrijevich S, Younes M, Skliris G, Murphy LC. Differential expression and comparative subcellular localization of estrogen receptor beta isoforms in virally transformed and normal cultured human lens epithelial cells. Experimental Eye Research. 2005;81:165–175. doi: 10.1016/j.exer.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. American Journal of Physiology. Endocrinology and Metabolism. 2004;286:E1011–E1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- Couse JF, Curtis HS, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. Journal of Steroid Biochemistry and Molecular Biology. 2000;74:287–296. doi: 10.1016/s0960-0760(00)00105-9. [DOI] [PubMed] [Google Scholar]
- Culig Z, Bartsch G. Androgen axis in prostate cancer. Journal of Cellular Biochemistry. 2006;99:373–381. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Research. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Eisen M, Napp HE, Vock R. Inhibition of platelet aggregation caused by estrogen treatment in patients with carcinoma of the prostate. Journal of Urology. 1975;114:93–97. doi: 10.1016/s0022-5347(17)66952-0. [DOI] [PubMed] [Google Scholar]
- Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Annals of the New York Academy of Sciences. 2009;1155:174–186. doi: 10.1111/j.1749-6632.2009.04360.x. [DOI] [PubMed] [Google Scholar]
- Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. Journal of Clinical Endocrinology and Metabolism. 2004;89:2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. American Journal of Surgical Pathology. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Takahashi S, Urano T, Ogawa S, Ouchi Y, Kitamura T, Muramatsu M, Inoue S. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochemical and Biophysical Research Communications. 2001;289:692–699. doi: 10.1006/bbrc.2001.6038. [DOI] [PubMed] [Google Scholar]
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Research. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash KA, Taylor WF, Greene LF, Kottke BA, Titus JL. Relationship of estrogen therapy for carcinoma of the prostate to atherosclerotic cardiovascular disease: a clinicopathologic study. Journal of Urology. 1970;103:467–470. doi: 10.1016/s0022-5347(17)61984-0. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiological Reviews. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. British Journal of Cancer. 1988;57:216–218. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Leung YK, Chung I. Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Annals of the New York Academy of Sciences. 2006;1089:177–193. doi: 10.1196/annals.1386.005. [DOI] [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G, et al. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Research. 2001;61:5331–5335. [PubMed] [Google Scholar]
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. Journal of Urology. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA: A Cancer Journal for Clinicians. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. PNAS. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS, Brown LG, True LD, Hawley SJ, Etzioni RB, Higano CS, Ho SM, Vessella RL, Corey E. Metastases of prostate cancer express estrogen receptor-beta. Urology. 2004;64:814–820. doi: 10.1016/j.urology.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani el-N, Dearnaley D, et al. Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer. BJU International. 2008;102:442–445. doi: 10.1111/j.1464-410X.2008.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. Journal of the National Cancer Institute. 1988;80:1045–1053. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. American Journal of Pathology. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. PNAS. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin H, Soloway MS. Immunohistochemical evidence of the existence and localization of aromatase in human prostatic tissues. Prostate. 1992;21:309–314. doi: 10.1002/pros.2990210407. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA: A Cancer Journal for Clinicians. 2008;58:196–213. doi: 10.3322/CA.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochemical and Biophysical Research Communications. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. Journal of Internal Medicine. 2008;264:128–142. doi: 10.1111/j.1365-2796.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V, Illi B, D'Eletto M, et al. Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. Journal of Clinical Investigation. 2009;119:1093–1108. doi: 10.1172/JCI35079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockrim J, Lalani el-N, Abel P. Therapy insight: parenteral estrogen treatment for prostate cancer – a new dawn for an old therapy. Nature Clinical Practice & Oncology. 2006;3:552–563. doi: 10.1038/ncponc0602. [DOI] [PubMed] [Google Scholar]
- Pais V, Leav I, Lau KM, Jiang Z, Ho SM. Estrogen receptor-beta expression in human testicular germ cell tumors. Clinical Cancer Research. 2003;9:4475–4482. [PubMed] [Google Scholar]
- Pedersen SB, Bruun JM, Hube F, Kristensen K, Hauner H, Richelsen B. Demonstration of estrogen receptor subtypes alpha and beta in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Molecular and Cellular Endocrinology. 2001;182:27–37. doi: 10.1016/s0303-7207(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-beta isoforms. Journal of Molecular Endocrinology. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- Poola I. Molecular assays to profile 10 estrogen receptor beta isoform mRNA copy numbers in ovary, breast, uterus, and bone tissues. Endocrine. 2003;22:101–112. doi: 10.1385/ENDO:22:2:101. [DOI] [PubMed] [Google Scholar]
- Poola I, Abraham J, Baldwin K, Saunders A, Bhatnagar R. Estrogen receptors beta4 and beta5 are full length functionally distinct ERbeta isoforms: cloning from human ovary and functional characterization. Endocrine. 2005;27:227–238. doi: 10.1385/ENDO:27:3:227. [DOI] [PubMed] [Google Scholar]
- Powell E, Xu W. Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. PNAS. 2008;105:19012–19017. doi: 10.1073/pnas.0807274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS 1997 Developmental estrogenization of the prostate gland. In Prostate: Basic and Clinical Aspects, ch 10, pp 247–263. Ed. KN Rajesh. Boca Raton, FL: CRC Press Inc.
- Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reproductive Toxicology. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB Journal. 2008;22:1512–1520. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods in Molecular Biology. 2005;294:23–29. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. Journal of Clinical Endocrinology and Metabolism. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- Schally AV, Redding TW, Comaru-Schally AM. Inhibition of prostate tumors by agonistic and antagonistic analogs of LH-RH. Prostate. 1983;4:545–552. doi: 10.1002/pros.2990040602. [DOI] [PubMed] [Google Scholar]
- Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocrine-Related Cancer. 2004;11:459–476. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PT. Human oestrogen receptors: differential expression of ER alpha and beta and the identification of ER beta variants. Steroids. 2002;67:985–992. doi: 10.1016/s0039-128x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clinical Cancer Research. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. British Journal of Cancer. 2006;95:616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs V, Walker RA. New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. Journal of Pathology. 2007;211:499–506. doi: 10.1002/path.2130. [DOI] [PubMed] [Google Scholar]
- Speirs V, Carder PJ, Lane S, Dodwell D, Lansdown MR, Hanby AM. Oestrogen receptor beta: what it means for patients with breast cancer. Lancet Oncology. 2004;5:174–181. doi: 10.1016/S1470-2045(04)01413-5. [DOI] [PubMed] [Google Scholar]
- Stone NN, Fair WR, Fishman J. Estrogen formation in human prostatic tissue from patients with and without benign prostatic hyperplasia. Prostate. 1986;9:311–318. doi: 10.1002/pros.2990090402. [DOI] [PubMed] [Google Scholar]
- Takase Y, Levesque MH, Luu-The V, El Alfy M, Labrie F, Pelletier G. Expression of enzymes involved in estrogen metabolism in human prostate. Journal of Histochemistry and Cytochemistry. 2006;54:911–921. doi: 10.1369/jhc.6A6927.2006. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. PNAS. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MP, Hakenberg OW, Froehner M. Adjuvant hormonal treatment – the bicalutamide early prostate cancer program. Frontiers of Radiation Therapy and Oncology. 2008;41:39–48. doi: 10.1159/000139877. [DOI] [PubMed] [Google Scholar]
- Wong NA, Malcomson RD, Jodrell DI, Groome NP, Harrison DJ, Saunders PT. ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. Journal of Pathology. 2005;207:53–60. doi: 10.1002/path.1807. [DOI] [PubMed] [Google Scholar]
- Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26:7346–7354. doi: 10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. American Journal of Pathology. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]