Abstract
Aims
To determine the effect of exercise training on clinical events and health-related quality of life (HRQoL) of patients with systolic heart failure.
Methods and results
We searched electronic databases including Medline, EMBASE, and Cochrane Library up to January 2008 to identify randomized controlled trials (RCTs) comparing exercise training and usual care with a minimum follow-up of 6 months. Nineteen RCTs were included with a total of 3647 patients, the majority of whom were male, low-to-medium risk, and New York Heart Association class II–III with a left ventricular ejection fraction of <40%. There was no significant difference between exercise and control in short-term (≤12 months) or longer-term all-cause mortality or overall hospital admissions. Heart failure-related hospitalizations were lower [relative risk: 0.72, 95% confidence interval (CI): 0.52–0.99] and HRQoL improved (standardized mean difference: −0.63, 95% CI: −0.80 to −0.37) with exercise therapy. Any effect of cardiac exercise training on total mortality and HRQoL was independent of degree of left ventricular dysfunction, type of cardiac rehabilitation, dose of exercise intervention, length of follow-up, trial quality, and trial publication date.
Conclusion
Compared with usual care, in selected heart failure patients, exercise training reduces heart failure-related hospitalizations and results in clinically important improvements in HRQoL. High-quality RCT and cost-effectiveness evidence is needed for the effect of exercise training in community-based settings and in more severe heart failure patients, elderly people, and women.
Keywords: Exercise, Heart failure, Rehabilitation
Introduction
Patients with chronic heart failure (CHF) experience marked reductions in their exercise capacity which has detrimental effects on their activities of daily living, health-related quality of life (HRQoL), and ultimately their hospital admission rate and mortality.1
Exercise training is often a component of rehabilitation programmes offered to patients with CHF.1 In 2004, a Cochrane systematic review by Rees et al.2 on the effect of exercise-based interventions on people with heart failure demonstrated a clear improvement in short-term exercise capacity. Twenty-nine trials were included in that review, only one of which reported longer-term hospitalizations and mortality. The remaining trials were largely small scale and did not aim to assess clinical events. The ExTraMATCH Collaborative Group published, also in 2004, an individual patient data meta-analysis.3 This review reported a reduction in the mortality of CHF patients who received exercise-based intervention [hazard ratio: 0.65, 95% confidence interval (CI): 0.46–0.92]. However, the ExTraMATCH study was based on a limited bibliographic literature search (Medline plus hand-searching of selected leading cardiac journals) and included unpublished data. It has therefore been difficult to verify the data and comprehensiveness of this meta-analysis; several of the trials included in the Cochrane review were not included in the ExTraMATCH review. Reanalysis of the ExTraMATCH trial data using meta-analytic methods has shown that the effect of exercise training was not statistically significant when compared with control (relative risk: 0.88, 95% CI: 0.70–1.10).4 van Tol et al.,5 in 2006, published a meta-analysis supporting the improvements in exercise capacity as seen in the previous Cochrane review. They also saw an improvement in quality of life as measured by the Minnesota Living with Heart Failure (MLWHF) questionnaire. In 2007, Haykowsky et al.6 published a systematic review demonstrating the positive effect of exercise-based intervention on cardiac remodelling in patients with CHF. In summary, to date, there is consensus on the positive effect of exercise on exercise capacity but the effects on hospital admission rates, mortality, and HRQoL remains uncertain.
The aim of the present study was to determine the impact of exercise-based interventions on the mortality, hospitalization rate, HRQoL, and cost-effectiveness in patients with systolic CHF.
Methods
The review was undertaken in accord with the methods of The Cochrane Collaboration.7
Study selection
Previous meta-analyses and systematic reviews were searched for studies.2–5,8–10 The following electronic databases were searched from the searching end date of the previous Cochrane systematic review (2001) up to January 2008: Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, EMBASE, MEDLINE, CINAHL, PsycINFO, and the NHS Centre for Reviews and Dissemination (CRD) databases [Health Technology Assessment (HTA) and Databases of Abstracts of Reviews of Effects (DARE)]. Conference proceedings were searched on Web of Science: ISI Proceedings. Search filters were limited to randomized controlled trials (RCTs), systematic reviews, and meta-analyses and humans. The search was designed as part of a broader review that included the update of a number of cardiac rehabilitation reviews (see Supplementary material online, Appendix). No language or other limitations were imposed. Two reviewers independently scanned all the titles and abstracts and identified potentially relevant articles to be retrieved. Where there was uncertainty, full-text copies of papers were obtained.
Inclusion and exclusion criteria
Studies were considered eligible if they were RCTs; included CHF patients (>18 years) with either ischaemic or non-ischaemic aetiology and specified criteria for the diagnosis of systolic heart failure such as an objective assessment of left ventricular ejection fraction or by clinical findings; received an exercise-based intervention either alone or as part of a comprehensive cardiac rehabilitation programme (defined as programmes also including components such as health education and psychological treatment); compared with standard medical care or attention placebo control group, and with a minimum follow-up of six months. Four categories of outcome were sought: mortality (all cause, death due to heart failure and sudden cardiac death), hospital admission/re-admission rates, HRQoL assessed by a validated outcome measure (e.g. MLWHF questionnaire or Short-Form 36 (SF-36)], and cost-effectiveness. Trials recruiting patients with heart failure associated with normal systolic function were excluded as were studies that included patients who had previously been offered cardiac rehabilitation. Full-text papers of all potentially eligible trials were independently assessed by two reviewers and disagreements were resolved by discussion.
Data extraction and risk of bias assessment
The following information categories were extracted: details of the study population and their baseline characteristics, details of the intervention (exercise training prescription and co-interventions) and control, length of follow-up, and details of individual outcome results. In order to assess the risk of bias, the following factors were considered: appropriate method of randomization (e.g. statement of computer-generated numbers) sequence, and an adequate concealment of randomization (e.g. randomization codes being concealed from those involved in running the trial), whether there was blinding (particularly blinding of outcome given the difficulties of blinding patients and carers given the nature of the intervention), whether all outcomes and losses to follow-up/drop-outs were reported, and whether intention-to-treat analysis was performed.7 Study authors were contacted to seek clarification on issues of reporting or to provide further outcome detail. Data extraction and risk of bias assessment were undertaken by a single reviewer using a standardized form and verified by a second reviewer.
Statistical analysis
Dichotomous outcomes were expressed as relative risks (RRs) and 95% CIs were calculated. Net changes in continuous variables were compared (i.e. exercise-based intervention minus control group differences) and a weighted mean difference or standardized mean difference (SMD) together with 95% CI was expressed for each study.7 Heterogeneity among included studies was explored qualitatively (with comparison of the characteristic of included studies) and quantitatively (using the χ2 test of heterogeneity and I2 statistic). A fixed-effects meta-analysis was used except when statistical heterogeneity was identified when the more conservative random-effects model was used.11 In studies reporting more than one HRQoL outcome, to prevent double counting in meta-analysis, we chose one of the reported HRQoL outcomes at random. The inference of meta-analysis did not change when selecting the alternative HRQoL measure score. Sensitivity analysis was undertaken to examine the effect of omission of the HF-ACTION trial.12 Meta-regression was used to examine the influence of a number of factors on all-cause mortality and HRQoL. The study level factors included: mean left ventricular ejection fraction; dose of exercise intervention (‘dose’ was calculated as the number of weeks multiplied by the number of sessions per week, multiplied by the duration of the session in hours); type of exercise (aerobic training alone or aerobic plus resistance training); type of cardiac rehabilitation (exercise-based cardiac rehabilitation vs. comprehensive cardiac rehabilitation); mean age; gender (% male); setting (hospital only, both hospital and home, or home only); and duration of follow-up. In order to assess the potential effect of a change in the standard of usual care over time, we added the year of publication as an additional study level factor (pre- vs. post-2000) to reflect when beta-blockers, angiotensin-receptor blockers, and angiotensin-converting enzyme inhibitors became established therapies for CHF.13 Funnel plots (i.e. scatter plots of the mean intervention effect vs. the inverse of variance of the intervention effect for each study) were used to explore the possibility of publication bias.14 All analyses were performed using RevMan, version 5.0, and STATA, version 10.0.
Results
Identification and selection of studies
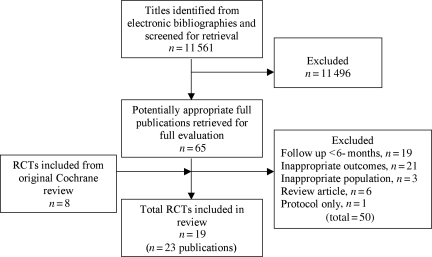
Our bibliographic search yielded 11 561 titles. After a review of the titles and abstracts of these, 65 full papers were retrieved and assessed. In total, 50 papers were excluded: 19 with follow-up <6 months, 21 with outcomes not relevant to this review (e.g. exercise capacity), 3 with an inappropriate study population, 6 were reviews, and 1 was a study protocol. Therefore, the total number of included trials was 19 (23 papers) (Figure 1). One trial was split into two substudies to reflect that the included patients were randomized to two different exercise interventions, both compared with usual care.15 The protocol of the HF-ACTION trial12,16 was identified by our search and so the trial was included, despite the results being published after January 2008.
Figure 1.
Summary of study selection.
Description of randomized controlled trials
The 19 trials included a total of 3647 patients, some 60% of which were contributed by the HF-ACTION trial12,16 (n = 2331) (Table 1). Recruited subjects were mainly uncomplicated CHF patients with NHYA class II and III and a left ventricular ejection fraction of <40%. Mean age ranged from 43 to 72 years and the majority were male (43–100%). With one exception, all trials were judged to be exercise-only interventions. In addition to exercise training, Austin et al.17,18 provided patients with education and psychological interventions (i.e. comprehensive cardiac rehabilitation). All studies used the modality of aerobic training with five also using resistance training. Exercise training programmes ranged widely across the studies: duration, 15–120 min per session; frequency, 2–7 sessions/week; intensity, 40% maximum heart rate to 85% of maximum oxygen uptake (VO2max); and overall duration, 24 weeks to 3 years. Exercise was centre delivered in 12 studies, entirely home based in one study and initially within centre and then at home in the remainder. Both intervention and control patients received usual care including medication and education advice, although controls received no formal exercise training. Four trials reported a follow-up longer than 12 months.18–21
Table 1.
Summary of trial characteristics
| Study | Study population |
Outcomes |
||
|---|---|---|---|---|
| Author (ref.), country | Sample size, mean age, gender | NYHA class, mean LVEF | Measuresa | Follow-up |
| Austin et al.,17,18 UK, single centre | n= 200, 71.9 years, 43% male | II–III, not reported | HRQoL, mortality | 24 weeks and 5 years |
| Belardinelli et al.,32 Italy, single centre | n = 99, 55 years, 89% male | II–IV, 28.2% | HRQoL, mortality, hospitalization and cost-effectiveness | 14 and 26 months |
| Dracup et al.,19 USA, multicentre | n = 173, 54 years, 72% male | II–IV, 26.4% | HRQoL, mortality, hospitalization | 6 and 12 months |
| Giannuzzi et al.,24 Italy, multicentre | n = 90, 60.5 years, not reported | II–III, <35% | Mortality, hospitalization | 6 months |
| Gielen et al.,34 Switzerland, single centre | n = 20, 54 years, 100% male | II–III, 25.8% | Mortality | 6 months |
| Gottlieb et al.,28 USA, single centre | n = 33, 66 years, 86% male | II–III, <40% | Mortality, HRQoL | 6 months |
| Hambrecht et al.,35 Germany, single centre | n = 22, 51 years, 100% male | II–III, 26.5% | Mortality, hospitalization | 6 months |
| Hambrecht et al.,33 Germany, single centre | n = 20, 55 years, 100% male | II–III, 23.5% | Mortality | 6 months |
| Hambrecht et al.,22 Germany, single centre | n = 73, 54 years, 100% male | I–IV, 29% | Mortality | 6 months |
| HF-ACTION (2009), USA/Canada, multicentre | n = 2331, 59 years, 72% male | II–III, 25% | Mortality, hospitalization, HRQoL | 6, 9, 12, 24, and 36 months |
| Keteyian et al.,25 USA, single centre | n = 40, 56 years, 100% male | II–III, 21% | Mortality, hospitalization | 24 weeks |
| Klecha et al.,39 Poland, single centre | n = 50, 60 years, 76% male | II–III, 27.9% | Mortality | 6 months |
| Klocek et al.15 (A), Poland, single centre | n = 28, 54 years, 100% | II–III, 33.4% | HRQoL | 26 weeks |
| Klocek et al.15 (B), Poland, single centre | n = 28, 56 years, 100% | II–III, 33.7% | HRQoL | 26 weeks |
| Koukouvou et al.,26 Greece, single centre | n = 26, 53 years, 100% | II–III, <40% | HRQoL | 26 weeks |
| McKelvie et al.,23 Canada, multicentre | n = 181, 65 years, 81% | I–II, <40% | HRQoL, mortality | 12 months |
| Mueller et al.,20 Switzerland, single centre | n = 50, 55 years, 100% | Not reported, <40% | Mortality, hospitalization | 5 years |
| Passino et al.,36 Italy, single centre | n = 95, 61 years, 87% | I–III, 34.1% | HRQoL, hospitalization | 9 months |
| Pozehl et al.,40 USA, single centre | n = 21, 66 years, 90% | II–IV, 28.7% | Mortality | 24 weeks |
| Willenheimer et al.,27 Sweden, single centre | n = 54, 64 years, 72% | I–III, 45.5% | HRQoL, mortality | 10 months |
aOutcomes relevant to this review.
Risk of bias
A number of studies failed to give sufficient detail to assess their potential risk of bias (Table 2). Details of generation and concealment of random allocation sequence and intention-to-treat analysis were particularly poorly reported. Only the studies of Austin et al.,17 Hambrecht et al.,22 McKelvie et al.,23 and the HF-ACTION trial12 provided an adequate description of the randomization process. Nevertheless, in none of the studies was there objective evidence of imbalance in baseline characteristics. Four trials stated that they performed intention-to-treat analysis.17,21,24,25 However, although often not stated, many studies appeared to compare exercise and control group outcomes according to initial random allocation. Where reported, losses to follow-up varied considerably across studies and the impact of losses to follow-up or drop out was only examined in a few trials. Only the studies of Koukouvou et al.,26 McKelvie et al.,23 and Willenheimer et al.27 reported blinding of outcome assessment.
Table 2.
Risk of bias assessment
| Author (ref.) | Adequate sequence generation | Allocation concealment | Outcome blinding | Intention-to-treat analysis | Groups balanced at baseline | Complete outcome reported |
|---|---|---|---|---|---|---|
| Austin et al.17,18 | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| Belardinelli et al.32 | ? | ? | ? | ? | ✓ | ✗ |
| Dracup et al.19 | ? | ? | ? | ? | ✓ | ✗ |
| Giannuzzi et al.24 | ? | ? | ? | ✓ | ✓ | ✗ |
| Gielen et al.34 | ? | ? | ? | ? | ✓ | ✗ |
| Gottlieb et al.28 | ? | ? | ? | ? | ✓ | ✗ |
| Hambrecht et al.35 | ? | ? | ? | ? | ✓ | ✗ |
| Hambrecht et al.33 | ? | ? | ? | ? | ✓ | ✗ |
| Hambrecht et al.22 | ✓ | ? | ? | ✓ | ✓ | ✗ |
| HF ACTION (2009) | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| Keteyian et al.25 | ? | ? | ? | ✓ | ✓ | ✗ |
| Klecha et al.39 | ? | ? | ? | ? | ✓ | ✗ |
| Klocek et al.15 | ? | ? | ? | ? | ✓ | ? |
| Koukouvou et al.26 | ? | ? | ✓ | ? | ✓ | ? |
| McKelvie et al.23 | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Mueller et al.20 | ? | ? | ? | ? | ✓ | ✗ |
| Passino et al.36 | ? | ? | ? | ? | ✓ | ✗ |
| Pozehl et al.40 | ? | ? | ? | ? | ✓ | ✗ |
| Willenheimer et al.27 | ? | ? | ✓ | ? | ✓ | ✓ |
✓, risk of bias criteria met; ✗, risk of bias criteria not met; ?, inadequate reporting to assess risk of bias criteria.
Outcomes
Pooled outcome findings are summarized in Table 3.
Table 3.
Meta-analysis results
| Outcome | n studies | Number of patients | Statistical method | Effect estimate, mean (95% CI) | Statistical heterogeneity, I2 and P-value |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| <12-month follow-up | 13 | 962 | Relative risk (fixed effects) | 1.03 (0.70–1.53) | 0%, 0.95 |
| >12-month follow-up | 4 | 328 | Relative risk (fixed effects) | 0.91 (0.78–1.06) | 41%, 0.17 |
| All hospital admissions | |||||
| <12-month follow-up | 8 | 659 | Relative risk (fixed effects) | 0.79 (0.58–1.07) | 0%, 0.54 |
| >12-month follow-up | 4 | 2658 | Relative risk (fixed effects) | 0.96 (0.90–1.02) | 37%, 0.19 |
| Hospital admission due to heart failure | |||||
| <12-month follow-up | 7 | 569 | Relative risk (fixed effects) | 0.72 (0.52–0.99) | 16%, 0.31 |
| HRQoL | |||||
| MLWHF | 6 | 700 | Weighted mean difference (random effects) | −10.33 (−15.89 to −4.77) | 71%, 0.004 |
| All scales | 9 | 779 | Standardized mean difference (random effects) | −0.63 (−0.8 to −0.37) | 79%, <0.0001 |
MLWHF, Minnesota Living with Heart Failure questionnaire.
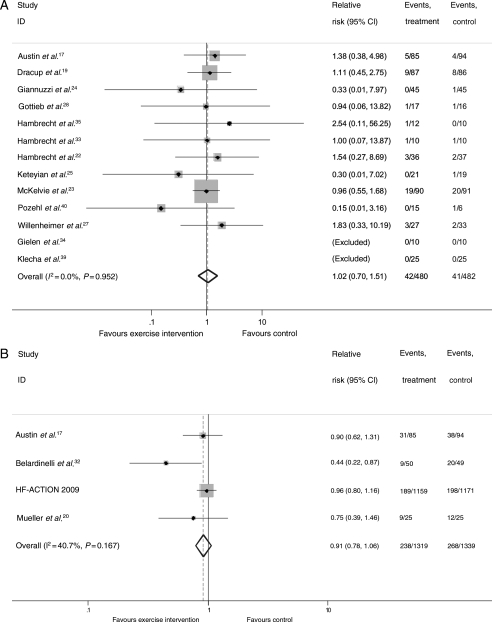
Mortality
There was no difference in pooled all-cause mortality between the exercise-based intervention and control groups up to 12-month follow-up (RR: 1.02, 95% CI: 0.70–1.51, heterogeneity χ2 = 3.89, P = 0.952, Figure 2A) or when pooling the four trials with follow-up of longer than 12 months (RR: 0.91, 95% CI: 0.78–1.06, heterogeneity χ2 = 5.06, P = 0.167, Figure 2B). A significant reduction in longer-term mortality was seen with exclusion of HF-ACTION trial (RR: 0.62, 95% CI: 0.39–0.98). There was a lack of consistency in reporting deaths due to heart failure or sudden cardiac death.
Figure 2.
Meta-analysis: all-cause mortality <12-month follow-up (A) and >12-month follow-up (B).
Hospital admissions
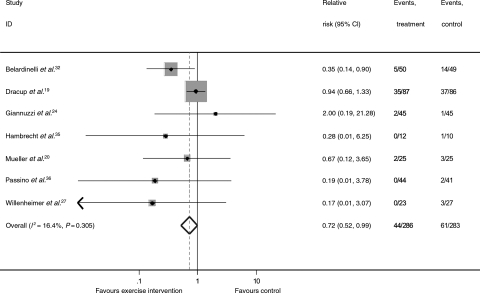
Although there was weak evidence of a trend towards a reduction in the overall hospital admission rate up to 12 months (RR: 0.79, 95% CI: 0.58–1.07, heterogeneity χ2 = 5.07, P = 0.535; see Supplementary material online, Figure S1A), there was no evidence of an effect beyond 12 months (RR: 0.96, 95% CI: 0.90–1.02, heterogeneity χ2 = 4.74, P = 0.192; see Supplementary material online, Figure S1B). This longer-term result was consistent when HF-ACTION was excluded (RR: 0.75, 95% CI: 0.52–1.08). There was a significant reduction in heart failure-specific hospital admissions with exercise-based interventions (RR: 0.72, 95% CI: 0.52–0.99, heterogeneity χ2 = 7.17, P = 0.305, Figure 3).
Figure 3.
Meta-analysis: hospitalizations due to heart failure.
Health-related quality of life
Ten studies assessed HRQoL using a validated scale. Most used the disease-specific MLWHF scale; other included scales were EuroQoL (EQ-5D), Psychological Wellbeing Index (PGWB), Patients Global Assessment of Quality of Life (PGAQoL), Spritzer's Quality of Life Index (QLI), and the recently developed Kansas City Cardiomyopathy Questionnaire (KCCQ). The study by Gottlieb et al.28 only reported HRQoL values at follow-up in the exercise group but not the controls (see Supplementary material online, Table S1). Across the five studies that reported total MLWHF score, there was strong evidence of improvement with exercise (mean difference: −10.3, 95% CI: −15.9 to −4.8, heterogeneity χ2 = 17.49, P = 0.004; see Supplementary material online, Figure S2). Pooling across all studies regardless of the HRQoL measure used, there was also strong evidence of improvement with exercise (SMD: −0.57, 95% CI: −0.83 to −0.31, heterogeneity χ2 = 45.03, P < 0.0001; see Supplementary material online, Figure S3); a finding that remained on exclusion of HF-ACTION (SMD: −0.63, 95% CI: −0.89 to −0.37).
Cost-effectiveness
Only the Belardinelli trial reported a cost-effectiveness analysis.29 Fourteen-month survival and healthcare costs were extrapolated to 15.5 years and incremental cost per life-year-gained ratios for exercise-based intervention vs. controls were compared. The estimated incremental cost for the exercise-based intervention group, $US 3227/patient, was calculated by subtracting the averted hospitalization cost, $US 1336/patient, from the cost of exercise training and wage lost due to exercise training estimated at $US 4563/patient. For patients receiving exercise training, the estimated increase in life expectancy was 1.82 years/person in a time period of 15.5 years, compared with patients in the control group. The cost-effectiveness ratio for long-term exercise in patients was determined at $US 1773/life-year saved, at a 3% discount rate at 1999 costs.
Meta-regression analysis
Univariate meta-regression analyses showed no evidence of a relationship between the effect of exercise training and all-cause mortality and any of the covariates. There was a significantly (P = 0.04) larger improvement in HRQoL when exercise training was undertaken in a centre-setting compared with a home-setting. No other covariates were related to the effect of exercise training on HRQoL (Table 4).
Table 4.
Univariate meta-regression results
|
P-value |
||
|---|---|---|
| All-cause mortality | HRQoL | |
| Mean left ventricular ejection fraction (%) | 0.54 | 0.19 |
| Mean age (years) | 0.76 | 0.62 |
| Sex (% male) | 0.56 | 0.40 |
| Type of rehabilitation (exercise only vs. comprehensive) | 0.65 | 0.59 |
| Type of exercise (aerobic training alone vs. aerobic plus resistance training) | 0.75 | 0.50 |
| Exercise dose (number of weeks × number of sessions/week × average duration of session in hours) | 0.66 | 0.14 |
| Exercise setting (hospital only, home only, both hospital and home) | 0.65 | 0.04 |
| Duration of follow-up (months) | 0.93 | 0.11 |
| Publication date (pre-2000 vs. 2000 or later) | 0.89 | 0.47 |
| Risk of bias | ||
| Random code generation | 0.90 | 0.11 |
| Random code concealment | 0.93 | 0.27 |
| Outcome blinding | 0.96 | 0.74 |
| Intention-to-treat analysis | 0.88 | 0.53 |
Small study bias
Whereas there was no evidence of funnel plot asymmetry for either all-cause mortality (see Supplementary material online, Figure S4) or overall hospitalizations (see Supplementary material online, Figure S5), the funnel plot for HRQoL outcomes did demonstrate asymmetry (see Supplementary material online, Figure S6).14 Given the statistically significant small study bias seen with HRQoL, regression-based adjustment was applied and it was found that the improvement in HRQoL with exercise training remained (SMD: −0.16, 95% CI: −0.02 to −0.29).30
Discussion
This systematic review shows that in systolic CHF patients, exercise-based intervention reduces the level of hospitalizations due to heart failure and improves HRQoL. We identified trials which consistently reported higher levels of HRQoL. In those using the MLWHF questionnaire, exercise intervention groups were on average 10 points higher than controls. A difference of four points has been shown to represent a clinically important and meaningful difference for the patient.31 The HRQoL effects of exercise therapy appear to be consistent across a number of CHF groups (i.e. age, gender, and left ventricular ejection fraction) as well as a range of exercise intervention delivery strategies (exercise dose, aerobic only exercise vs. aerobic plus resistance exercise, and exercise only vs. comprehensive cardiac rehabilitation). We found no evidence that exercise training either increases or decreases all-cause mortality.
Comparison with previous systematic reviews
Most previous systematic reviews of exercise training for heart failure have identified an insufficient number of deaths and hospitalizations to reliably comment on these outcomes.2–5,8–10 However, our finding of a statistically non-significant difference in all-cause mortality with exercise training vs. control is consistent with the reanalysis of individual patient data meta-analysis of the ExTraMATCH Collaborative.4 More recent trials have been conducted in the context of optimal medical therapy. For example, at entry to the HF-ACTION trial, 94% of the patients were receiving beta-blockers and angiotensin-receptor blockers or angiotensin-converting enzyme inhibitors,16 and 45% had an implantable cardioverter-defibrillator or biventricular pacemaker implanted at the time of enrolment. Given the proven survival advantage of these medical treatments,13 any incremental all-cause mortality benefit with exercise is therefore likely to be small. On the basis of the observed levels of mortality seen in four trials with long-term follow-up,12,18,20,32 a total of some 12 000 patients would need to be randomized to exercise-based cardiac rehabilitation or usual care to demonstrate a statistically significant benefit of exercise (at 5% α and 80% power).
The improvements seen in HRQoL with exercise training are in accordance with the previous systematic review of van Tol et al.5 The recent systematic review of Chien et al.10 concluded that home-based exercise training does not improve the HRQoL of heart failure patients. Eight of the trials included in this review combined an initial period of supervised hospital-based exercise training followed by a home-based programme.17,21–23,33–36 Only one of the included studies assessed an entirely home-based programme.28 Although we found a larger improvement in HRQoL with exercise training in those studies based solely in a hospital setting, there was a significant improvement in HRQoL compared with control in the home-based exercise intervention studies.
Mechanism of action
The precise mechanism through which exercise-based interventions benefit CHF patients remains unclear. One explanation, applicable to patients with ischaemic cardiomyopathy, is that exercise improves myocardial perfusion by alleviating endothelial dysfunction and therefore dilating coronary vessels and by stimulating angiogenesis by way of intermittent ischaemia.3 Ventricular remodelling has been shown to be attenuated by exercise training.6 Indeed, Belardinelli et al.37 demonstrated that aerobic training improved myocardial contractility and diastolic filling. Regardless of the cause, there are important neurohormonal and musculoskeletal abnormalities in heart failure.3 Exercise training may reduce adrenergic tone and increase vagal tone, as suggested by an assessment of variability of heart rate. Skeletal muscle dysfunction and wasting may also respond to exercise training. Hambrecht et al.33 have demonstrated that regular physical activity in CHF patients stimulates vasodilatation in the skeletal muscle vasculature.
Study limitations
Although we believe this to be the most comprehensive systematic review of RCT-based evidence for the impact of exercise-based intervention on patients with heart failure to date, we acknowledge that this review has a number of limitations. The general lack of reporting methods in the included RCTs made it difficult to assess their methodological quality and thereby judge their risk of bias and potential to overestimate the effect of exercise-based interventions. However, they do not appear to be sensitive to risk of bias criteria such as intention-to-treat analysis and outcome bias. Although a specific goal of this review was to clarify the impact of exercise-based interventions on clinical events, many included trials were relatively small and of short-term follow-up and there were low numbers of deaths and hospitalizations reported by the majority of the trials. In many of the studies, we identified event data in the trial descriptions of losses to follow-up and exclusions rather that reported outcomes per se. Most included studies were in low-to-moderate risk males and included predominately (43–100%) patients with NYHA class II–III and LVEF <40%, with a mean age of participants across studies ranging from 43 to 72 years. The generalizability of our findings may therefore be limited. Although the majority of evidence in this review comes from the recently reported HF-ACTION study,16 the findings of previous trials appear consistent with this important trial.
To improve generalizability, future exercise intervention trials should include more severe CHF patients, elderly people, and women and be sufficiently large and of long enough duration to accrue meaningful numbers of clinical outcomes and report these outcomes by key patient subgroups (e.g. atrial fibrillation and diabetes mellitus). There is a need to examine more community or home-based exercise intervention programmes and how such programmes can be most clinically and cost effectively integrated alongside current models of service delivery. Few of the included studies reported the actual level of exercise training undertaken by participants. Notably, the HF-ACTION study where only ∼30% of patients randomized to exercise training exercised at or above their exercise prescription.16 Future studies therefore need to consider interventions to enhance the long-term adherence to exercise training.38
Conclusions
Compared with usual care, in low-to-moderate risk NYHA class II and III systolic heart failure patients, exercise-based intervention reduces heart failure-related hospitalizations and results in clinically important improvements in HRQoL. Exercise training did not reduce or increase all-cause mortality. Any effect of cardiac exercise training on total mortality and HRQoL was independent of degree of left ventricular dysfunction, type of cardiac rehabilitation, dose of exercise intervention, length of follow-up, trial quality, and trial publication date. High-quality RCT and cost-effectiveness evidence is needed to assess the effect of exercise training in community-based settings and in more severe heart failure patients, elderly people, and women.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The project was supported by National Institute of Health Research Cochrane Programme Grant.
Conflict of interest: none declared.
Declaration
A more detailed review has been published and will be updated in the Cochrane Database of Systematic Reviews [Davies EJ, Moxham T, Rees K, Singh S, Coats AJS, Ebrahim S, Lough F, Taylor RS. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No.: CD003331. DOI: 10.1002/14651858.CD003331.pub3]. This is a version of a Cochrane review, which is available in The Cochrane Library. Cochrane systematic reviews are regularly updated to include new research, and in response to feedback from readers. The results of a Cochrane review can be interpreted differently, depending on people's perspectives and circumstances. Please consider the conclusions presented carefully. They are the opinions of review authors, and are not necessarily shared by The Cochrane Collaboration.
Supplementary Material
Acknowledgements
We wish to thank Dr Jackie Austin for providing additional information on her trial. Also wish to acknowledge Dr Philippa Davies who assisted in the selection and exclusion of studies and Ms. Lianne Perry for her administrative support.
References
- 1.Working Group on Cardiac Rehabilitation and Exercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Recommendations for exercise training in chronic heart failure patients. Eur Heart J. 2001;22:125–135. doi: 10.1053/euhj.2000.2440. doi:10.1053/euhj.2000.2440. [DOI] [PubMed] [Google Scholar]
- 2.Rees K, Taylor RS, Singh S, Coats AJS, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2004:CD003331. doi: 10.1002/14651858.CD003331.pub2. doi:10.1002/14651858.CD003331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piepoli MF, Davos C, Francis DP, Coats AJ Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotzsche P. Does exercise training lower mortality in patients with chronic heart failure? [letter] BMJ. 2005 Available at http://www.bmj.com/cgi/eletters/328/7433/189#91496. [Google Scholar]
- 5.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841–850. doi: 10.1016/j.ejheart.2006.02.013. doi:10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodelling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;19:2329–2336. doi: 10.1016/j.jacc.2007.02.055. doi:10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. Version 500 [updated February 2008]. www.cochrane-handbook.org. (20 March 2009) [Google Scholar]
- 8.Lloyd-Williams F, Mair FS, Leitner M. Exercise training and heart failure: a systematic review of current evidence. Br J Gen Pract. 2002;52:47–55. [PMC free article] [PubMed] [Google Scholar]
- 9.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. doi:10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Chien CL, Lee CM, Wu YW, Chen TA, Wu YT. Home-based exercise increases exercise capacity but not quality of life in people with chronic heart failure: a systematic review. Aust J Physiother. 2008;54:87–93. doi: 10.1016/s0004-9514(08)70041-2. [DOI] [PubMed] [Google Scholar]
- 11.Der Simonsen R, Laird N. Meta analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. doi:10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Piña IL HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. doi:10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Shekelle P, Morton S, Atkinson S, Suttorp M, Tu W, Heidenreich P, Gubens M, Maglione M, Jungvig L, Roth E, Newberry S. Pharmacologic Management of Heart Failure and Left Ventricular Systolic Dysfunction: Effect in Female, Black, and Diabetic Patients, and Cost-Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Evidence Report/Technology Assessment No. 82 (Prepared by the Southern California-RAND Evidence-based Practice Center under Contract No 290-97-0001). AHRQ Publication No. 03-E045. [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta analysis detected by a simple graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klocek M, Kubinyi A, Bacior B, Kawecka-Jaszcz K. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. Int J Cardiol. 2005;103:323–329. doi: 10.1016/j.ijcard.2004.10.021. doi:10.1016/j.ijcard.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;153:1451–1459. doi: 10.1001/jama.2009.457. doi:10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004. doi:10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Austin J, Williams WR, Ross L, Hutchison S. Five-year follow-up findings from a randomized controlled trial of cardiac rehabilitation for heart failure. Eur J Cardiovasc Prev Rehabil. 2008;15:162–167. doi: 10.1097/HJR.0b013e3282f10e87. doi:10.1097/HJR.0b013e3282f10e87. [DOI] [PubMed] [Google Scholar]
- 19.Dracup K, Evangelista LS, Hamilton MA, Erickson V, Hage A, Moriguchi J, Canary C, MacLellan WR, Fonarow GC. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J. 2007;154:877–883. doi: 10.1016/j.ahj.2007.07.019. doi:10.1016/j.ahj.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Mueller L, Myers J, Kottman W, Oswald U, Boesch C, Arbrol N, Dubach P. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil. 2007;21:923–931. doi: 10.1177/0269215507079097. doi:10.1177/0269215507079097. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. doi:10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. doi:10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 23.McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, Hendrican K, Yusef S. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144:23–30. doi: 10.1067/mhj.2002.123310. doi:10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 24.Giannuzzi P, Temporelli PL, Corra U, Tavazzi L. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 2003;108:554–559. doi: 10.1161/01.CIR.0000081780.38477.FA. doi:10.1161/01.CIR.0000081780.38477.FA. [DOI] [PubMed] [Google Scholar]
- 25.Keteyian SJ, Levine AB, Brawner CA, Kataoka T, Rogers FJ, Schairer JR, Stein PD, Levine TB, Goldstein S. Exercise training in patients with heart failure. A randomized, controlled trial. Ann Intern Med. 1996;124:1051–1057. doi: 10.7326/0003-4819-124-12-199606150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Koukouvou G, Kouidi E, Iacovides A, Konstantinidou E, Kaprinis G, Deligiannis A. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J Rehabil Med. 2004;36:36–41. doi: 10.1080/11026480310015549. doi:10.1080/11026480310015549. [DOI] [PubMed] [Google Scholar]
- 27.Willenheimer R, Rydberg E, Cline C, Broms K, Hillberger B, Oberg L, Erhardt L. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int J Cardiol. 2001;77:25–31. doi: 10.1016/s0167-5273(00)00383-1. doi:10.1016/S0167-5273(00)00383-1. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb SS, Fisher ML, Freudenberger R, Robinson S, Zietowski G, Alves L, Krichten C, Vaitkevicus P, McCarter R. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. J Cardiac Fail. 1999;5:188–194. doi: 10.1016/s1071-9164(99)90002-7. doi:10.1016/S1071-9164(99)90002-7. [DOI] [PubMed] [Google Scholar]
- 29.Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, Glied S. Cost-effectiveness analysis of long-term moderate exercise training in chronic heart failure. Am J Cardiol. 2001;87:984–988. doi: 10.1016/s0002-9149(01)01434-5. doi:10.1016/S0002-9149(01)01434-5. [DOI] [PubMed] [Google Scholar]
- 30.Moreno SG, Sutton AJ, Turner EH, Abrams KA, Cooper NJ, Palmer TM, Ades AE. Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ. 2009;339:b2981. doi: 10.1136/bmj.b2981. doi:10.1136/bmj.b2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAlister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E. Cardiac Resynchronization Therapy for Congestive Heart Failure. Rockville, MD, USA: Agency for Healthcare Research and Quality (AHRQ); 2004. [PMC free article] [PubMed] [Google Scholar]
- 32.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 33.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 34.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. doi:10.1016/S0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 35.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, Riede U, Schlierf G, Kübler W, Schuler G. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25:1239–1249. doi: 10.1016/0735-1097(94)00568-B. doi:10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 36.Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol. 2006;47:1835–1839. doi: 10.1016/j.jacc.2005.12.050. doi:10.1016/j.jacc.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 37.Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation. 1998;97:553–561. doi: 10.1161/01.cir.97.6.553. [DOI] [PubMed] [Google Scholar]
- 38.Beswick AD, Rees K, West RR, Taylor FC, Burke M, Griebsch I, Taylor RS, Victory J, Brown J, Ebrahim S. Improving uptake and adherence in cardiac rehabilitation: literature review. J Adv Nurs. 2005;49:538–555. doi: 10.1111/j.1365-2648.2004.03327.x. doi:10.1111/j.1365-2648.2004.03327.x. [DOI] [PubMed] [Google Scholar]
- 39.Klecha A, Kawecka-Jaszcz K, Bacior B, Kubinyi A, Pasowicz M, Klimeczek P, Banyś R. Physical training in patients with chronic heart failure of ischemic origin: effect on exercise capacity and left ventricular remodeling. Eur J Cardiovasc Prev Rehabil. 2007;14:85–91. doi: 10.1097/HJR.0b013e3280114f12. doi:10.1097/HJR.0b013e3280114f12. [DOI] [PubMed] [Google Scholar]
- 40.Pozehl B, Duncan K, Hertzog M. The effects of exercise training on fatigue and dyspnea in heart failure. Eur J Cardiovasc Nurs. 2008;7:127–132. doi: 10.1016/j.ejcnurse.2007.08.002. doi:10.1016/j.ejcnurse.2007.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.