Abstract
The metabolic sensor AMP-activated kinase (AMPK) inhibits both the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) Cl− channel and epithelial Na+ channel (ENaC), and may inhibit secretion of proinflammatory cytokines in epithelia. Here we have tested in primary polarized CF and non-CF human bronchial epithelial (HBE) cells the effects of AMPK activators, metformin and 5-aminoimidazole-4-carboxamide-1-β-D-riboside (AICAR), on various parameters that contribute to CF lung disease: ENaC-dependent short-circuit currents (Isc), airway surface liquid (ASL) height, and proinflammatory cytokine secretion. AMPK activation after overnight treatment with either metformin (2–5 mM) or AICAR (1 mM) substantially inhibited ENaC-dependent Isc in both CF and non-CF airway cultures. Live-cell confocal images acquired 60 minutes after apical addition of Texas Red–dextran-containing fluid revealed significantly greater ASL heights after AICAR and metformin treatment relative to controls, suggesting that AMPK-dependent ENaC inhibition slows apical fluid reabsorption. Both metformin and AICAR decreased secretion of various proinflammatory cytokines, both with and without prior LPS stimulation. Finally, prolonged exposure to more physiologically relevant concentrations of metformin (0.03–1 mM) inhibited ENaC currents and decreased proinflammatory cytokine levels in CF HBE cells in a dose-dependent manner. These findings suggest that novel therapies to activate AMPK in the CF airway may be beneficial by blunting excessive sodium and ASL absorption and by reducing excessive airway inflammation, which are major contributors to CF lung disease.
Keywords: metformin, cystic fibrosis transmembrane conductance regulator, ENaC, airway surface liquid, inflammation
CLINICAL RELEVANCE.
Cystic fibrosis lung disease is characterized by excessive airway surface liquid reabsorption and inflammation, leading to recurrent infections, eventual destruction of lung parenchyma, and respiratory failure. This study, performed using primary human airway epithelial cells, suggests that novel therapies to activate the metabolic sensor AMP-activated kinase (AMPK) in the CF airway may be beneficial both by blunting excessive salt and fluid reabsorption and by reducing excessive airway inflammation.
The serious genetic disease cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) Cl− channel expressed at the apical membrane in a variety of epithelial tissues, including the lung, where CF lung disease causes considerable morbidity and mortality (1). Defective CFTR function prevents airway epithelium from adequately regulating the airway surface liquid (ASL) volume, which has an adverse effect on mucus clearance from the lung. The CF airway invariably becomes colonized by bacteria such as Pseudomonas aeruginosa and develops excessive inflammation, which can cause extensive damage to lung architecture and eventuate in respiratory failure (2). There is growing evidence that a lack of functional CFTR promotes an exaggerated or prolonged inflammatory response to bacterial and other insults, although the underlying pathophysiologic mechanisms are unclear (3–5). Both up-regulation of proinflammatory mediators (e.g., IL-8, IL-6, TNF-α, and GM-CSF) and down-regulation of anti-inflammatory mediators (e.g., IL-10 and inducible nitric oxide synthase) in the CF airway may play an important role in this process (6–8).
Excessive activity of the epithelial sodium channel (ENaC) on the luminal airway surface is a key factor involved in the development of the CF lung phenotype. This defect has been proposed to cause excessive reabsorption of the ASL, reduced airway clearance, and increased susceptibility to chronic inflammation, as demonstrated in a β-ENaC transgenic mouse model (9–11). Therapies to reduce chronic inflammation (steroids, nonsteroidal anti-inflammatory drugs, and statins), combat chronic bacterial colonization (inhaled antibiotics), and enhance airway clearance (hypertonic saline and inhaled recombinant human DNase) have improved outcomes in patients with CF lung disease (12–14). However, therapeutic trials aimed at reducing excessive airway ENaC activity (e.g., through treatment with aerosolized amiloride or its analogs) have proven disappointing, potentially due to poor access of the drug to the channels at the apical membrane, short activity half-life, and/or deleterious effects on airway permeability (14–16). Drugs that can be delivered through the blood that inhibit the channel without directly binding to it at the apical membrane may circumvent these problems and be promising choices for therapy. Furthermore, drugs that target multiple pathogenic factors in CF lung disease and combination therapeutic approaches that attack multiple cellular pathways or targets could have additive or synergistic beneficial effects.
AMP-activated kinase (AMPK) is a ubiquitous Ser/Thr kinase whose activity increases during conditions of metabolic and other cellular stresses. AMPK is activated via phosphorylation of the α subunit at Thr-172 in its “activation loop” by upstream AMPKKs (17), which include the LKB1 complex (18), the Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) (19), and TGF-β–activated kinase 1 (20, 21). As a cellular metabolic sensor, AMPK acts on a wide variety of substrates and cellular pathways, including the regulation of metabolic pathways (e.g., glycolysis, fatty acid synthesis and oxidation, cellular glucose uptake, and cholesterol synthesis), apoptosis and the cell cycle, nitric oxide production, transcriptional regulation, signaling pathways involved with inflammation, and membrane transport (22–24). Therefore, changes in cellular AMPK activity and function may have broad effects on overall cellular functioning.
Recent work from our laboratory demonstrated that AMPK is more diffusely distributed and has greater activity in primary polarized human bronchial epithelial (HBE) cells derived from patients with CF as compared with HBE cells derived from patients with other non-CF lung diseases (5). Expression of exogenous wild-type CFTR in the CF cells reversed AMPK activation. These CFTR-dependent AMPK activity effects were confirmed in immortalized CF bronchial epithelial (CFBE) cells derived from a ΔF508 homozygote that were stably transfected to overexpress either wild-type or ΔF508 CFTR (5, 25). Moreover, compared with wild-type CFTR–expressing CFBE cells, ΔF508 CFTR–expressing CFBE cells had greater secretion of the proinflammatory cytokines TNF-α, IL-6, and IL-8. Further pharmacologic activation of AMPK in CFBE cells reduced proinflammatory cytokine production, suggesting that AMPK activation in CF airway cells is an adaptive response that reduces inflammation (5). In addition, we and others have previously found that AMPK activators inhibit both CFTR and ENaC channel activity in both the oocyte expression system and in polarized epithelial cells derived from lung and other tissues (24, 26–31). Membrane transport protein regulation by AMPK may provide a sensitive mechanism for the coupling of ion and solute transport to cellular metabolic status (24). Of note, excessive ENaC activity in the CF airway has been implicated in ASL volume depletion and subsequent manifestations of CF lung disease (9–11). Together, these findings led us to hypothesize that AMPK activators may be beneficial in the treatment of CF lung disease.
In contrast to primary airway cells, CFBE cells lack significant functional ENaC expression and thus may not be a suitable model system for the study of CF airway pathophysiology. The effects of AMPK activators on salt and fluid transport and the secretion of proinflammatory cytokines in primary polarized HBE cells are unknown. The goals of this study were to examine the effects of AMPK on the secretion of proinflammatory cytokines and salt and fluid transport in primary polarized HBE cells and to assess the potential utility of metformin as a novel therapeutic agent for CF lung disease using this model system. We first investigated the effects of the AMPK activators metformin and AICAR on ENaC and CFTR activity in HBE cells. We next investigated the effects of these drugs on real-time ASL reabsorption rates across polarized HBE cell cultures using live-cell confocal microscopy. We then used multiplex cytokine analysis to determine the effects of AMPK activation on proinflammatory cytokine secretion in both CF and non-CF primary polarized HBE cells. Finally, we tested the effects of lower, more therapeutically relevant concentrations of metformin, an FDA-approved drug for the treatment of diabetes mellitus, given over a 2-week period on ENaC activity and the secretion of proinflammatory cytokines. Our findings suggest that AMPK activation may be a beneficial therapeutic strategy for CF lung disease.
MATERIALS AND METHODS
Primary Human Bronchial Epithelial Cell Culture
HBE cells were cultured from excess pathological tissue following lung transplantation and organ donation under a protocol approved by the University of Pittsburgh Institutional Review Board. Cells were cultured on human placental collagen-coated Costar Transwell filters (Catalog #3470, 0.33 cm2 0.4-μm pore; Lowell, MA) in 2% Ultroser G medium as previously described and used for experimentation after at least 4 to 6 weeks of culture at an air–liquid interface (32). Non-CF HBE cells were obtained from donors with idiopathic pulmonary fibrosis (five patients), chronic obstructive pulmonary disease (three patients), primary pulmonary hypertension (three patients), or secondary pulmonary hypertension due to congenital heart disease (one patient). Qualitative differences due to disease state were not observed. CF HBE cells were obtained from 13 patients with CF and the following CFTR mutations: ΔF508, W1282X, 1717 G->A, 2789+5 G->A, N1303K, and four unknowns.
Immunoblotting and Quantitation of AMPK Activity
Immunoblotting of polarized HBE cell lysates from individual Transwell filters was performed as described previously (5). Briefly, the intensities of relevant bands were quantitated using a Versa-Doc Imager with Quantity One software (Bio-Rad, Hercules, CA). To measure AMPK activity in the cell lysates, the AMPK-α-pThr-172 band signal in each lane was corrected for local background intensity and normalized to the intensity of β-actin as a loading control.
Measurements of CFTR- and ENaC-Dependent Short-Circuit Current
Short-circuit current (Isc) measurements were performed using modified Ussing chambers designed to accept 0.33-cm2 Transwells with Ringer's solution in both the apical and basolateral chambers as previously described (5). After the filters were mounted and steady-state Isc was achieved, the ENaC-dependent Isc was calculated as the change in Isc following apical treatment with 10 μM amiloride. After amiloride addition, the CFTR-dependent Isc was defined as the difference between the peak Isc after addition of 10 μM forskolin and 1 mM isobutylmethylxanthine (IBMX) and the final Isc after basolateral treatment with 20 μM bumetanide to block Cl− secretion across the epithelium (32).
ASL Height Measurements
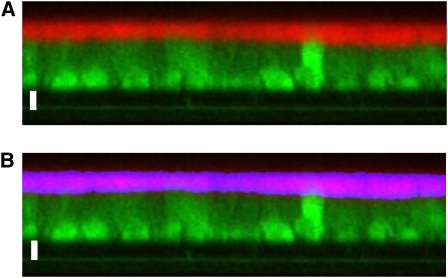
To measure the rate of ASL absorption in the presence and absence of AMPK activators, the height of fluorescently labeled fluid on the apical surface of well-differentiated HBE cell cultures was measured serially using confocal microscopy (33). The HBE cells were exposed on both the apical and basolateral sides to 1 mM AICAR, 5 mM metformin, or vehicle control overnight before ASL measurement. Ringer's solution (100 μl) was added to the apical side for this overnight treatment. Subsequently the apical fluid was aspirated with a Pasteur pipette and replaced with 2.5 μl of PBS containing 2 mg/ml Texas Red–dextran (10 kD; Molecular Probes; Eugene, OR). After a 1-minute equilibration period, 50 μl of perfluorocarbon (PFC, FC-77; Sigma, St. Louis, MO) was gently pipetted onto the apical surface to prevent evaporative losses while on the microscope stage. The cultures were imaged on a modified stage of an inverted confocal microscope (Zeiss LSM 510 META; Thornwood, NY) that was fitted with a 37°C serosal bath containing Ringer's solution. Z-scanning was performed with a ×40 oil immersion objective (1.3 NA), with the 543-nm laser at 20% maximal intensity. Three random images were acquired from each filter at 0, 30, 60, 120, 240, and 360 minutes and analyzed in a blinded fashion. The ASL height for each image was then calculated using an automated script in ImageJ software from the NIH (Bethesda, MD). As shown in Figure 1, Gaussian Blur filtering was applied, and the area containing Texas Red–dextran was divided by the image width to yield the average ASL height across the image. Due to irregularities in the HBE cell height, cellular debris, and inconsistent labeling of the ASL, the images were visually inspected to ensure that the automatic measurement represented the true ASL height. If the automatic measurement was not adequate, the ASL height was manually measured in three regions and averaged. In the representative images shown, the light intensity was adjusted to improve the clarity of the image (Figure 1).
Figure 1.
Method for airway surface liquid (ASL) height measurement. A quantity of 2.5 μl PBS containing Texas Red–dextran (10 kD) was applied to the apical surface of differentiated human bronchial epithelial (HBE) cultures and z-scanning confocal images were acquired 30 minutes later (A). The labeled ASL was selected using an automated thresholding routine as shown in blue (B), and the thresholded area was divided by the image width to derive the average ASL height for the entire image (12.33 μm in this example). The cells were loaded with CellTracker Green CMFDA (Molecular Probes) before the experiment to demonstrate the cell shapes and delineate the Transwell filter support. Bars = 10 μm.
Inflammatory Cytokine Expression Analysis
Either apical or basolateral supernatant samples (up to 200-μl aliquots) were used for cytokine expression analysis as indicated in the Figure legends. Samples were analyzed using the human cytokine Lincoplex kit (Linco Research, St. Charles, MO) run on a Bio-Plex suspension array system (Bio-Rad), as previously described (5, 34).
Metformin Dose–Response and Time Course Studies
Polarized, differentiated CF HBE cells were cultured at an air–liquid interface and the basolateral media were exchanged with fresh media containing 0, 0.03 mM, 0.1 mM, or 1 mM metformin every 2 days. To assure consistent drug delivery, the basolateral media were spiked with metformin on the interim days. Electrophysiologic measurement and basolateral fluid collection for cytokine expression were performed at 0, 2, 4, 7, 10, and 14 days. Transepithelial resistance (RT) and transepithelial potential difference (VT) were measured with an epithelial volt-ohmeter using Ag:AgCl electrodes (EVOM; World Precision Instruments, Sarasota, FL). Equivalent short-circuit current (Ieq) was calculated by Ohm's law (Ieq = VT/RT), corrected for the Transwell surface area, and normalized to the values obtained on Experiment Day 0. To establish an electrical circuit, 100 μl of media was temporarily applied for 1 hour to the apical surface of the HBE filters. After Ieq measurement, the basolateral medium was collected for cytokine analysis and fresh medium applied to the basolateral surface.
Statistics
For the confocal ASL height measurements, all experiments were performed on 12 HBE cell filters cultured from three independent tissue donors. For each individual experiment, the ASL height represents the average of three images/filter. Differences in the ASL height due to AMPK activation were determined by ANOVA with Holm-Sidak post hoc analysis at each time interval using Stat-View software (SAS, Cary, NC). All other statistics were performed using unpaired, two-tailed Student's t tests. The P values are indicated in the text, figures and/or legends.
RESULTS
Effects of AMPK Activators on CFTR and ENaC Currents in Primary HBE cells
AMPK has been shown to inhibit CFTR and ENaC activity in polarized epithelial cell lines derived from lung and other tissues (26, 27, 30, 31). To assess the effects of AMPK activators on these ion channels in the more physiologically relevant primary HBE cell model system, differentiated cultures were treated overnight (16 h) with either 1 mM AICAR, 5 mM metformin, or vehicle control in the basolateral medium (Figure 2). Significant AMPK activation with AICAR and metformin treatment occurred in both primary polarized non-CF and CF HBE cell monolayers, as assessed by immunoblotting for the activated form of AMPK (AMPK-α-pThr-172; Figures 2A and 2B). The treatment protocol used and typical responses of both CF and non-CF airway cells to amiloride, IBMX and forskolin, and bumetanide treatment are shown in Figure 2C. Of note, there was little or no cAMP agonist-stimulated Isc in the CF airway cells, consistent with our earlier findings (5). As found in other CFTR-expressing epithelial cells (26, 27), AMPK activation in non-CF cells through overnight AICAR or metformin treatment inhibited CFTR-dependent Isc, which was defined as the difference between the peak Isc measured after IBMX/forskolin treatment and the final Isc after bumetanide treatment (Figure 2D). Very similar effects on CFTR-dependent Isc were also found under these conditions by comparing the Isc measured before and after IBMX/forskolin stimulation (see Figure E1 in the online supplement). The AMPK activators AICAR and metformin also dramatically reduced amiloride-sensitive Isc in both non-CF and CF cells to a similar degree (∼ 60–70%; Figure 2E). Of note, amiloride-sensitive ENaC currents in untreated CF cells were significantly greater than in non-CF cells in this series of experiments (28.4 ± 7.0 μA/cm2 versus 11.5 ± 2.7 μA/cm2; P < 0.05, unpaired t test, n = 19–20 filters from six different cell lines for both CF and non-CF). Therefore, AMPK is activated in primary HBE cells by both AICAR and metformin, leading to a reduction in both CFTR-mediated Cl− secretion and ENaC-mediated Na+ absorption.
Figure 2.
Measurements of cystic fibrosis (CF) transmembrane conductance regulator (CFTR) and ENaC currents in primary airway cells. Differentiated HBE cultures were treated 16 hours before experimentation with either 1 mM AICAR, 5 mM metformin, or vehicle control on both the apical and basolateral sides with the addition of 100 μl of Ringer's solution to the apical side. (A) Sample immunoblot for the activated form of AMP-activated kinase (AMPK) (AMPK-α-pThr-172; upper panel) and β-actin as a loading control (lower panel) taken from lysates of non-CF airway cells. (B) AMPK activity of non-CF and CF airway cultures following AMPK activation therapy. Data shown are mean (±SE) AMPK-α-pThr-172 band intensities normalized to that of β-actin in the same lane, as described in Materials and Methods (*P < 0.05, unpaired t tests, n = 6 separate experiments/cell lines for both CF and non-CF cells). (C) Representative Ussing chamber short-circuit current (Isc) measurement traces obtained from CF (dashed line) and non-CF (solid line) airway cells treated with 10 μM amiloride, 1 mM IBMX + 10 μM forskolin, and 20 μM bumetanide at the indicated times. (D) Inhibition of CFTR-dependent Isc in non-CF airway cells by both AICAR and metformin as measured by Ussing chamber recordings as described in Materials and Methods (*P < 0.05, unpaired t tests, n = 5 filters for each condition). (E) Treatment with AICAR and metformin reduced amiloride-sensitive ENaC-dependent Isc in both non-CF and CF cells (*P < 0.01, unpaired t tests relative to untreated controls; n = 16–18 filters total for each condition obtained from four to five different CF and non-CF cell lines assayed in separate experiments). Results are normalized to the mean ENaC-dependent Isc of the untreated control filters for each cell line within each experiment.
Effects of AMPK Activators on ASL Reabsorption Rates in Primary HBE Cells
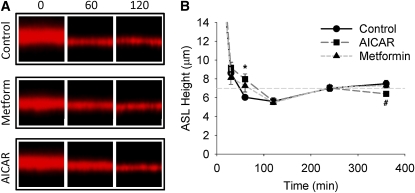
To determine whether the electrophysiologic alterations caused by AMPK activation are of sufficient magnitude to affect ASL volume and reabsorption rates, the ASL height of HBE cells with and without AMPK activators was assessed at various time points after an apical fluid challenge (cf. Figure 1 and Materials and Methods). In the first series of experiments, differentiated non-CF HBE cell cultures were exposed to AICAR and metformin overnight, and the ASL height was then serially measured using confocal microscopy (Figure 3). To label the ASL, 2.5 μl of PBS labeled with 2 mg/ml Texas Red–dextran was applied to the apical surface (Figure 3A). During the initial 0 to 30 minutes after the apical fluid challenge, the ASL height was variable, presumably do to unequal distribution of the dextran and the rapid formation of a fluid meniscus surrounding the Transwell perimeter (33). After this initial redistribution period when no significant differences were observed between control, AICAR-, or metformin-treated cultures, the ASL height measurements stabilized and became reproducible. At 60 minutes, the ASL height was increased in the AICAR (P < 0.0001) and metformin (P = 0.002) conditions compared with control non-CF HBE cells, indicating that the rates of ASL absorption were slowed in the AMPK-stimulated cultures (Figure 3B). Subsequently, there was no difference in the measured ASL height between the control and AMPK-stimulated cultures until 6 hours, when the ASL height was lower in the AICAR-treated HBE cells (P = 0.007 versus control and P = 0.023 versus metformin). Presumably, this decrease in ASL volume reflects a lack of Cl− secretion due to CFTR inhibition by AICAR (see Figure 2 and Ref. 27). These results suggest that the decreases in Na+ and Cl− transport caused by AMPK activation are of sufficient magnitude to alter ASL volume and reabsorption rates.
Figure 3.
Effect of AMPK activation on the ASL height of non-CF airway cells. A quantity of 2.5 μl of PBS containing 2 mg/ml Texas Red–dextran was applied to the apical surface of differentiated non-CF airway cells that were pretreated overnight with 1 mM AICAR or 5 mM metformin. (A) Representative Z-scanning confocal microscopy images at 0, 60, and 120 minutes after the addition of the fluorescently labeled PBS. (B) Mean ASL height ± SE at various time points up to 360 minutes after apical fluid application (n = 12 filters from three different non-CF tissue donors). *AICAR- and metformin-treated cultures were significantly different from control cultures; #significant difference between AICAR and control cultures (P values are indicated in text).
In a second series of experiments, the effects of AMPK activation on ASL volume were assessed in HBE cells cultured from patients with CF (Figure 4). Because HBE cells cultured from CF donors display minimal CFTR-mediated Cl− secretion, we reasoned that the effects of AMPK activation on ASL volume would primarily reflect decreased Na+ absorption. As anticipated based on the potent inhibition of ENaC activity by AMPK activation, the ASL height was increased by AICAR at 60 minutes (P = 0.001) and 120 minutes (P = 0.002) after the apical volume challenge. The ASL height was also increased by metformin at 60 minutes compared with untreated CF HBE cells (P = 0.009). In contrast to the wild-type CFTR–expressing cultures, there was no difference in the ASL height at the later time points in CF HBE cells with AMPK activation. These results suggest that AMPK activation slows the rate of ASL absorption in CF HBE cells and as such may augment mucus transport in the CF airway.
Figure 4.
Effect of AMPK activation on the ASL height of CF airway cells. A quantity of 2.5 μl of PBS containing 2 mg/ml Texas Red–dextran was applied to the apical surface of differentiated CF primary airway cells that were pretreated overnight with AICAR or metformin. (A) Representative Z-scanning confocal microscopy images at 0, 60, and 120 minutes after the addition of the fluorescently labeled PBS. (B) Mean ASL height ± SE at various time points up to 360 minutes after apical fluid application (n = 12 filters from three different CF tissue donors). *AICAR- and metformin-treated cultures were significantly different from control cultures; #significant difference between AICAR and control cultures (P values are indicated in text).
Effects of AMPK Activators on the Secretion of Inflammatory Mediators from Primary HBE Cells
In another series of experiments we investigated the effects of AMPK activator treatment on the secretion of various proinflammatory cytokines by HBE cells cultured in the presence or absence of LPS. After overnight treatment with metformin (2 mM) or AICAR (1 mM), AMPK activation was again observed in both non-CF and CF cells (Figures 5A and 5B). Overnight LPS treatment did not have a significant effect on basal AMPK activity or on the ability of AICAR and metformin to induce AMPK activation. Consistent with our data obtained in immortalized CFBE cells (5), primary CF cells had much greater production of TNF-α under baseline conditions than non-CF cells, which was greatly reduced by pharmacologic AMPK activation in both cell types (Figure 5C). Indeed, it appears that an inverse relationship exists between the degree of AMPK activation and TNF-α suppression. As IL-6 (Figure 5D), IL-8 (Figure 5E), and GM-CSF (Figure 5F) are additional important proinflammatory products of the NF-κB and peroxisome proliferator–activated receptor (PPAR) pathways that have been shown to be up-regulated in the CF airway (35), we also investigated the effects of AMPK activators on these mediators. In general, similar significant inhibitory effects or trends were also observed in these inflammatory mediators with AMPK activators.
Figure 5.
Effects of overnight metformin and AICAR treatment in the presence or absence of LPS on AMPK activity and inflammatory cytokine levels in non-CF and CF airway cells. Differentiated primary airway cultures were treated 16 hours before experimentation with either 2 mM metformin, 1 mM AICAR, or vehicle in the presence or absence of LPS (1 μg/ml). Cytokine concentrations were measured from apical samples collected in 100 μl Ringer's solution, as described in Materials and Methods. The cells were then lysed to immunoblot for the activated form of AMPK (AMPK-α-pThr-172) and β-actin. (A) Representative immunoblots of CF and non-CF cultures treated with vehicle control (C), metformin (M), or AICAR (A) in the presence or absence of LPS. (B) Summary of relative AMPK activities of non-CF and CF cell monolayers treated with AMPK activators ± LPS. Data shown are mean ± SE AMPK activities relative to the untreated condition in the absence of LPS (#P < 0.05, unpaired t test relative to control in the absence of LPS). Relative TNF-α (C), IL-6 (D), IL-8 (E), and GM-CSF (F) concentrations from non-CF and CF cell cultures treated with AMPK activators ± LPS. Data shown are mean ± SE cytokine concentrations relative to untreated non-CF conditions in the absence of LPS. *P < 0.05 relative to control within the same group; §P < 0.05 relative to non-CF control.
In summary, these results demonstrate that, although AMPK is already modestly activated in CF airway cells (5), further pharmacologic activation over a relatively short time frame may have beneficial effects by inhibiting excessive ENaC currents, ASL reabsorption, and inflammation, which are major culprits in the pathogenesis of CF lung injury (2). However, the effects and potential utility of AMPK activators at more physiologically relevant doses that may be achieved in vivo with these drugs given over longer time periods are not clear based on the preceding studies. We thus performed further studies to determine the specific dose–response relationships and time dependence of the inhibitory effects of metformin on Na+ absorption and inflammation in CF airway cultures.
Preclinical Studies to Evaluate the Potential Utility of Metformin in the Treatment of CF Lung Disease
In evaluating AMPK activators to pursue for further preclinical studies, we considered drugs that are safe, already FDA-approved for other indications, and available in an oral formulation to be attractive candidates for initial Phase II pilot clinical studies. As AICAR (acadesine) is currently only available in intravenous formulation and has only been tested clinically for brief time periods (hours to days) in the setting of acute myocardial ischemia and reperfusion (36), we decided to pursue additional preclinical studies with metformin, one of the most widely prescribed drugs in the world for the treatment of type 2 diabetes mellitus. Unlike other oral diabetes drugs like sulfonylureas, metformin does not have significant hypoglycemic effects on nondiabetic subjects, and it also does not cause significant weight loss in nonobese subjects with CF (37, 38). These considerations make metformin a potentially attractive choice for use in patients with CF lung disease.
A dose–response and time-course experiment was performed to examine the effects of metformin on ENaC currents and proinflammatory mediators in polarized, well-differentiated CF primary airway cells. The cultures were treated with 0, 0.03, 0.1, and 1 mM metformin, and the equivalent Isc (Ieq) and inflammatory profiles were examined at Days 0, 2, 4, 7, 10, and 14. These concentrations were chosen because 0.03 mM is in the range of what may be reached with a typical metformin dosing regimen of 1,000 mg twice daily in an adult patient (38). Both a time- and dose-dependent inhibition of Ieq by metformin was observed (Figure 6). Because the majority of the basal Ieq in CF HBE cells is amiloride-sensitive, the decreased Ieq values observed after metformin treatment most likely correlate with ENaC inhibition. As shown in the online supplement (Figure E2), the metformin-dependent inhibition of Ieq at 0.03 and 0.1 mM occurred largely via an increase in the transepithelial resistance (RT) (or a decrease in the transepithelial conductance [= 1/Rt]), rather than an effect on the transepithelial voltage (VT). At the highest concentration (1 mM), both RT and VT were affected. In addition, metformin treatment generally inhibited the basolateral secretion of the various pro-inflammatory mediators previously studied (Figure 7). The lowest concentration of metformin (0.03 mM) caused significant or marginally significant reductions in both TNF-α (Figure 7A) and IL-6 (Figure 7B) starting within 2 days of treatment; this persisted throughout the 14-day study period. Higher metformin concentrations generally caused greater inhibition at most time points. The dose-dependent effects of metformin on IL-8 (Figure 7C) were generally marginal in these experiments, possibly because the IL-8 concentration was above the upper limit of the assay for a number of the samples. GM-CSF levels (Figure 7D) were significantly reduced by all metformin concentrations at 2 days, but then there was recovery back toward untreated control levels by the later time points for the cells treated with 0.03 and 0.1 mM metformin. In summary, these results demonstrate that lower, more pharmacologically relevant concentrations of metformin have an inhibitory effect on both ENaC and proinflammatory mediators in polarized primary CF airway cells.
Figure 6.
Dose–response and time dependence of the metformin effect on Ieq across differentiated CF airway cell cultures. The Ieq across polarized CF airway cultures was serially measured after the addition of 0, 0.03, 0.1, and 1 mM metformin. Data shown are mean Ieq ± SE normalized to the values obtained on Day 0; n = 3–4 cultures. *P < 0.01, §P < 0.05, and #0.05 < P < 0.10 versus control conditions at the respective time point.
Figure 7.
Dose–response and time dependence of the metformin effect on secretion of cytokines from differentiated CF airway cell cultures. After the addition of 0, 0.03, 0.1, and 1 mM metformin, serial 48-hour samples of the basolateral media were collected and subsequently used for multiplex cytokine analysis. Data shown are mean (± SE) TNF-α (A), IL-6 (B), IL-8 (C), and GM-CSF (D) concentrations normalized to untreated control conditions at the respective time point; n = 3–4 cultures. *P < 0.05, #0.05 < P < 0.10.
DISCUSSION
Because AMPK has previously been shown to modulate transepithelial ion transport and inflammation, we tested the hypothesis that therapies to activate AMPK in the CF airway would have a beneficial effect in primary human airway epithelia. We found that treatment with AICAR or metformin, which activate AMPK via different mechanisms, caused a dose-dependent decrease in sodium absorption and inflammatory cytokine secretion from primary CF airway cells. Furthermore, AMPK activation decreased the ASL absorption rate after apical fluid administration. As excessive salt and fluid absorption and exuberant inflammation are characteristic pathological features of CF lung disease, these results provide hope that therapeutic strategies to augment AMPK activity will have a beneficial effect in the CF airway.
The ability of AMPK-activating drugs to inhibit both ENaC and CFTR activity are consistent with previous results from our and other laboratories. Because these drugs inhibit chloride secretion in addition to sodium absorption, ASL height measurements were performed to clarify whether AMPK activation would have the net result of increasing or decreasing the ASL volume. After the apical fluid challenge, when HBE cells assume a predominantly absorptive function (10), both AICAR and metformin augmented the ASL height, suggesting that under these conditions the predominant electrophysiologic effect of AMPK activation was that of ENaC inhibition. Subsequently, when the apical fluid bolus had been absorbed, there was a trend toward a lower ASL height after AMPK activation in non-CF airway cell cultures. Presumably at this later time point, when HBE cells assume a predominantly secretory function (10), the decreased ASL volume by AMPK activation reflects CFTR inhibition. Given the paucity of functional CFTR in the CF airway, it is unlikely that CFTR inhibition by AMPK activators will cause a significant reduction in chloride secretion in vivo.
AMPK activation has long been known to inhibit HMG-CoA reductase (39), the rate-limiting enzyme in sterol biosynthesis and the production of isoprenoids, which are increased in the CF airway and may contribute to excessive inflammation there (40, 41). Moreover, pharmacologic activation of AMPK has been reported to attenuate LPS-induced expression of proinflammatory cytokines through down-regulation of IκK activity and inhibition of NF-κB (42). These findings led us to hypothesize that therapies to activate AMPK in the CF airway may be beneficial in reducing the expression of proinflammatory mediators that contribute to excessive CF airway inflammation (5). AMPK activity was generally inversely related to the elaboration of TNF-α, IL-6, IL-8, and GM-CSF by primary differentiated non-CF airway cells. While the secretion of these cytokines was increased in airway cells cultured from CF donors and after LPS exposure, AMPK activation continued to mitigate inflammatory cytokine secretion under these conditions. Furthermore, the time-course and dose–response experiments with metformin strengthen the notion that AMPK activation decreases inflammation in CF airway epithelia at concentrations similar to those achieved with oral dosing of the drug in vivo. Overall, these results indicate that metformin may have beneficial effects on inflammation in patients with CF lung disease.
In addition to the potential beneficial effects of metformin on airway epithelium, metformin may have additional benefits in the treatment of patients with CF. The incidence of CF-related diabetes mellitus (CFRD) has been increasing with the increased lifespan of individuals with CF, currently occurring in approximately 30% of adult patients with CF as per the CF patient registry. While CFRD is traditionally believed to be caused by insulinopenia due to pancreatic β cell fibrosis, recent studies have suggested that impaired insulin sensitivity contributes to CFRD and that insulin resistance frequently develops during infections (37, 43). Small observational studies of patients with CFRD have demonstrated that metformin is not associated with adverse events such as hypoglycemia or lactic acidosis (37). Furthermore, the efficacy of metformin was comparable to that of insulin, in regard to HbA1C reduction and weight gain. Therefore, the available data suggest that metformin therapy is not only safe in patients with CF, but it may potentially improve CFRD, mitigate airway inflammation, and augment ASL volume and mucociliary clearance.
AMPK activation has a relatively modest effect on ASL hydration and inflammation at metformin concentrations that are likely achieved after typical dosing regimens. However, it is possible that small changes in airway physiology have significant effects on mucus clearance and inflammation. Therapies such as azithromycin slow the rate of lung function decline and lower the risk of exacerbation in patients with CF, yet have relatively modest effects on cultured cells (44). Furthermore, our studies do not account for the anti-inflammatory effect of AMPK activation on leukocytes (45, 46), which may further mitigate airway inflammation in vivo.
AMPK activation in primary CF airway epithelial cultures caused a dose-dependent reduction in Na+ absorption, ASL absorption, and inflammatory cytokine production. Previous studies conducted in primary human airway cultures have proven to adequately predict in vivo nasal potential difference and/or clinical response. For example, the in vitro response of cultured HBE cells to amiloride, UTP, chlorzoxazone, denufosol, and 17 β-estradiol have all subsequently been confirmed in the human respiratory tract in vivo (9, 47–50). Thus, the beneficial effects of AMPK activation on primary CF cultures provide ample rationale for further studies to determine its clinical utility in the treatment of CF airways disease. In summary, these preclinical experiments using metformin and AICAR on airway epithelia provide promise that AMPK activation represents a novel therapeutic strategy to both augment airway surface hydration and mitigate inflammation.
Supplementary Material
Acknowledgments
The authors thank the Lung Transplantation Program at the University of Pittsburgh Medical Center for facilitating tissue acquisition and Joseph Latoche, Erin McKenna, and Jenny Karlsson for technical assistance.
This work was supported by grants from the National Institutes of Health (NIH) (K08 HL087932 to M.M.M. and R01 DK075048 to K.R.H.) and Cystic Fibrosis Foundation (MYERBU07Q0 to M.M.M., HALLOW06P0 to K.R.H., and the University of Pittsburgh Cystic Fibrosis Research Development Center to J.M.P. and K.R.H.). This work was also supported by NIH Center Grants to the University of Pittsburgh (P30 DK072506 [Basic and Clinical Studies of Cystic Fibrosis] and P30 DK079307 [Pittsburgh Center for Kidney Research]).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0147OC on July 17, 2009
Conflict of Interest Statement: K.R.H. holds stock in Bristol-Myers Squibb (up to $1,000), and has received sponsored research grants from the National Institutes of Health ($100,000 or more), Cystic Fibrosis Foundation ($100,000 or more), and American society of Nerphrology ($50,000–$100,000). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Welsh MJ, Smith AE. Cystic fibrosis. Sci Am 1995;273:52–59. [DOI] [PubMed] [Google Scholar]
- 2.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev 1999; 79(1, Suppl)S215–S255. [DOI] [PubMed] [Google Scholar]
- 3.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997;100:2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med 2004;169:645–653. [DOI] [PubMed] [Google Scholar]
- 5.Hallows KR, Fitch AC, Richardson CA, Reynolds PR, Clancy JP, Dagher PC, Witters LA, Kolls JK, Pilewski JM. Up-regulation of AMP-activated kinase by dysfunctional cystic fibrosis transmembrane conductance regulator in cystic fibrosis airway epithelial cells mitigates excessive inflammation. J Biol Chem 2006;281:4231–4241. [DOI] [PubMed] [Google Scholar]
- 6.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-κB and altered IκB-b processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 2000;23:396–403. [DOI] [PubMed] [Google Scholar]
- 7.Soltys J, Bonfield T, Chmiel J, Berger M. Functional IL-10 deficiency in the lung of cystic fibrosis (cftr(−/−)) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J Immunol 2002;168:1903–1910. [DOI] [PubMed] [Google Scholar]
- 8.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest 1998;102:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med 1981;305:1489–1495. [DOI] [PubMed] [Google Scholar]
- 10.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol 2001;118:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 2004;10:487–493. [DOI] [PubMed] [Google Scholar]
- 12.Boyle MP. Adult cystic fibrosis. JAMA 2007;298:1787–1793. [DOI] [PubMed] [Google Scholar]
- 13.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006;354:229–240. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 2006;354:241–250. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh AJ, Sabater JR, Zamurs A, Smith RT, Paradiso AM, Hopkins S, Abraham WM, Boucher RC. Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease. J Pharmacol Exp Ther 2004;311:929–938. [DOI] [PubMed] [Google Scholar]
- 16.Burrows E, Southern KW, Noone P. Sodium channel blockers for cystic fibrosis. Cochrane Database Syst Rev 2006;3:CD005087. [DOI] [PubMed] [Google Scholar]
- 17.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 2005;280:29060–29066. [DOI] [PubMed] [Google Scholar]
- 20.Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 2006;281:25336–25343. [DOI] [PubMed] [Google Scholar]
- 21.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 2006;103:17378–17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 2004;29:18–24. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG. The AMP-activated protein kinase pathway: new players upstream and downstream. J Cell Sci 2004;117:5479–5487. [DOI] [PubMed] [Google Scholar]
- 24.Hallows KR. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 2005;14:464–471. [DOI] [PubMed] [Google Scholar]
- 25.Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, Sorscher EJ, Clancy JP. Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers. J Physiol 2005;569:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 2003;284:C1297–C1308. [DOI] [PubMed] [Google Scholar]
- 27.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 2003;278:998–1004. [DOI] [PubMed] [Google Scholar]
- 28.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 2000;105:1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker J, Jijon HB, Churchill T, Kulka M, Madsen KL. Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol Gastrointest Liver Physiol 2003;285:G850–G860. [DOI] [PubMed] [Google Scholar]
- 30.Woollhead AM, Scott JW, Hardie DG, Baines DL. Phenformin and 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J Physiol 2005;566:781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 2005;280:17608–17616. [DOI] [PubMed] [Google Scholar]
- 32.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 2000;279:C461–C479. [DOI] [PubMed] [Google Scholar]
- 33.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95:1005–1015. [DOI] [PubMed] [Google Scholar]
- 34.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 2005;175:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 2008;34:146–162. [DOI] [PubMed] [Google Scholar]
- 36.Mangano DT, Miao Y, Tudor IC, Dietzel C. Post-reperfusion myocardial infarction: long-term survival improvement using adenosine regulation with acadesine. J Am Coll Cardiol 2006;48:206–214. [DOI] [PubMed] [Google Scholar]
- 37.Onady GM, Langdon LJ. Insulin versus oral agents in the management of Cystic Fibrosis Related Diabetes: a case based study. BMC Endocr Disord 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Physicians' Desk Reference. New York: Thomson Reuters; 2009.
- 39.Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase: copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem 1989;186:129–136. [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Elmer HL, Ross KR, Kelley TJ. Isoprenoid-mediated control of SMAD3 expression in a cultured model of cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 2004;31:234–240. [DOI] [PubMed] [Google Scholar]
- 41.Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L1286–L1295. [DOI] [PubMed] [Google Scholar]
- 42.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci 2004;24:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardin DS, Leblanc A, Marshall G, Seilheimer DK. Mechanisms of insulin resistance in cystic fibrosis. Am J Physiol Endocrinol Metab 2001;281:E1022–E1028. [DOI] [PubMed] [Google Scholar]
- 44.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW III. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290:1749–1756. [DOI] [PubMed] [Google Scholar]
- 45.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 2008;181:8633–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L497–L504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol 1999;276:C827–C837. [DOI] [PubMed] [Google Scholar]
- 48.Singh AK, Devor DC, Gerlach AC, Gondor M, Pilewski JM, Bridges RJ. Stimulation of Cl(-) secretion by chlorzoxazone. J Pharmacol Exp Ther 2000;292:778–787. [PubMed] [Google Scholar]
- 49.Deterding RR, Lavange LM, Engels JM, Mathews DW, Coquillette SJ, Brody AS, Millard SP, Ramsey BW. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am J Respir Crit Care Med 2007;176:362–369. [DOI] [PubMed] [Google Scholar]
- 50.Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest 2008;118:4025–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.