Abstract
Dust samples collected from Nebraska swine confinement facilities (hog dust extract [HDE]) are known to elicit proinflammatory cytokine release from human bronchial epithelial (HBE) cells in vitro. This response involves the activation of two protein kinase C (PKC) isoforms: PKCα and PKCɛ. Experiments were designed to investigate the relationship between the two isoenzymes and the degree to which each is responsible for cytokine release in HBE. Experiments also examined the contribution of TNF-α to IL-6 and IL-8 release. PKCα and PKCɛ activities were inhibited using isoform-specific pharmacologic inhibitors and genetically modified dominant-negative (DN) expressing cell lines. Release of the proinflammatory cytokines IL-6, IL-8, and TNF-α was measured and PKC isoform activities assessed. We found that HDE stimulates PKCα activity by 1 hour, and within 6 hours the activity returns to baseline. PKCα-specific inhibitor or PKCαDN cells abolish this HDE-mediated effect. Both IL-6 and IL-8 release are likewise diminished under these conditions compared with normal HBE, and treatment with TNF-α–neutralizing antibody does not further inhibit cytokine release. In contrast, PKCɛ activity was enhanced by 6 hours after HDE treatment. TNF-α blockade abrogated this effect. HDE-stimulated IL-6, but not IL-8 release in PKCɛDN cells. The concentration of TNF-α released by HDE-stimulated HBE is sufficient to have a potent cytokine-eliciting effect. A time course of TNF-α release suggests that TNF-α is produced after PKCα activation, but before PKCɛ. These results suggest a temporal ordering of events responsible for the release of cytokines, which initiate and exacerbate inflammatory events in the airways of people exposed to agricultural dust.
Keywords: protein kinase C, hog barn dust, cytokine release, lung, airway epithelium
CLINICAL RELEVANCE.
The regulation of TNF-α levels may represent a clinical target in addressing chronic inflammatory lung disease in response to inhaled dusts. In our study, neutralizing TNF-α, either by pretreatment of the cells with an antibody that binds to TNF-α or a recombinant fusion protein that functions as a false receptor, significantly diminishes the levels of TNF-α, IL-6, and IL-8 release in response to swine dust exposure to the bronchial epithelial cells. This raises the question as to whether or not anti–TNF-α drugs such as infliximab or etanercept might reduce lung inflammation in response to swine barn dust inhalation.
Clinical symptoms associated with long-term exposure to organic dusts and animal confinement facility gases are common in agricultural workers (1). Dusts from swine confinement barns contain swine fecal matter, dander, molds, bacteria, and other microbes (2). Inhalation of these dusts constitutes an important environmental risk. Swine confinement workers report respiratory and flu-like symptoms (3–5) and demonstrate diminished pulmonary function after prolonged exposure (6, 7). Elevated serum levels of both TNF-α and IL-6 were observed in previously nonexposed individuals who inhaled dusts from swine confinement facilities (8). In addition, dust exposure to alveolar macrophages and airway epithelial cells results in significant stimulated IL-8 production (9).
The intracellular signaling mechanisms of swine dust–induced lung inflammation have not been fully defined; however, we previously demonstrated that a soluble component of swine barn dust other than endotoxin activates protein kinase C (PKC), allowing for the release of IL-6 and IL-8 from bronchial epithelial cells (10). Downstream of this PKC activation, swine dust has been shown to up-regulate interleukin transcription and specifically activate nuclear factor (NF)-κB (11). Likewise, the expression of TNF-α and TNF receptor has been implicated in dust-stimulated interleukin release (12). PKCα activation is required for swine dust–induced inhibition of bronchial epithelial cell migration into a wound (13). However, the precise PKC isoform or isoenzymes that regulate swine dust–stimulated interleukin release have not been determined.
Previously, we demonstrated that cattle feedlot dust extracts stimulate the release of both IL-6 and IL-8 via the activation of PKCɛ in human bronchial epithelial cells (14). Because swine barn dusts have been shown to activate PKCα (13) and stimulate the release of TNF-α (12) in airway epithelium, we hypothesized that the swine barn dust–stimulated release of IL-6 and IL-8 is differentially regulated by the sequential actions of both PKCα and ɛ isoforms as well as TNF-α. To investigate the relationship between PKCα and PKCɛ and the degree to which each is responsible for cytokine release, experiments were designed to manipulate the activity of individual PKC isoforms, and to test the effects of these alterations on the HDE-stimulated release of IL-6 and IL-8 from human bronchial epithelial cells in vitro. Because preliminary experiments have shown that TNF-α may be involved in downstream cytokine signaling events, experiments were also conducted to examine the contribution of TNF-α to the activation of both isoenzymes and to the release of IL-6 and IL-8. These data suggest that airway epithelial cell exposure to organic dust results in the production and release of multiple proinflammatory cytokines that are in part dependent upon a defined sequential and temporal order as regulated by the action of PKC.
MATERIALS AND METHODS
Hog Barn Dust Extract Preparation
Settled dust from hog confinement facilities (hog dust extract [HDE]) was used for these experiments as previously described (10). Briefly, the HDE was prepared by placing 1 g of dust in 10 ml of HBSS, pH 7.4. The mixture was vortexed and mixed on a magnetic stir platform at room temperature for 1 hour. The mixture was serially centrifuged at 400 × g for 20 minutes at 4°C, and then at 10,000 × g for 10 minutes at 4°C. The final supernatant was filter-sterilized (0.22 μm) and either used immediately or frozen in aliquots at −20°C. The aqueous dust extract (subsequently referred to as “HDE”) was diluted to a final concentration of 5% (vol/vol) in growth medium for most of these experiments. The diluted HDE contains 2.2 mg/ml of total protein, and is not cytotoxic for the time periods tested (1–72 h). Endotoxin levels in HDE were assayed using the limulus amebocyte lysate assay commercially available (Sigma, St. Louis, MO) and shown to not be responsible for detectable levels of PKC activation or cytokine release (15).
Cell Culture
BEAS-2B cells (American Tissue Culture Collection, Manassas, VA), an SV40 transformed human bronchial epithelial cell line, were plated on type I collagen (Sigma)-coated dishes and maintained in culture in serum-free medium at 37°C in 5% CO2/95% air. Growth medium for maintenance of epithelial cell cultures was prepared by mixing equal volumes of growth factor–supplemented LHC basal medium with RPMI containing 1% penicillin/streptomycin and amphoterocin B (LHC-9/RPMI) as previously described (10). The transformed human bronchial epithelial cell line, 16HBE 14o-, was a gift from Dr. D. C. Gruenert of the Cardiovascular Research Institute of the University of California, San Francisco. The 16HBE cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum and Penicillin/Streptomycin. They were grown under the same conditions described for the BEAS-2B. Confluent monolayers of cells were pretreated for 1 hour with or without PKC inhibitors followed by 1 to 24 hours of exposure to 5% HDE at 37°C.
Cytokine Assays
After cell treatment conditions, media supernatants were collected and assayed for the concentration of interleukins released using a sandwich enzyme-linked immunosorbent assay (ELISA) (10). Flat-bottomed Immulon-II HB 96-well polystyrene ELISA plates (Thermo Electron Corporation, Milford, MA) were coated with 200 μl/well of purified (goat) anti-human IL-8 antibody diluted 1:500 (R&D Systems, Minneapolis, MN) or IL-6 antibody diluted 1:1,000 in Voller's buffer (pH 9.6) overnight at 4°C. After three washings in PBS-Tween 20, undiluted culture supernatants and human recombinant IL-8 or IL-6 standards (R&D Systems) were applied to the plates and incubated at room temperature for 2 hours. Plates were again washed three times with PBS-Tween and incubated with (rabbit) anti-human IL-8 antibody diluted 1:500 (Rockland, Gilberstville, PA) or IL-6 antibody (R&D Systems) diluted 1:1,000 in PBS-Tween/BLOTTO (0.2% instant nonfat milk in PBS-Tween) for 1 hour. After three washes, human serum-absorbed peroxidase conjugated (goat) anti-rabbit IgG (Rockland) was added at 1:2,000 (IL-6) or 1:1,000 (IL-8) in PBS-Tween/BLOTTO for 45 minutes. The plates were again washed three times, and 200 μl/well of peroxidase substrate (10 ng/ml orthophenylenediamine [Sigma]; 0.003% H2O2 in dH2O) was added. For the TNF-α–specific ELISA, assay conditions were as described above with the following exceptions: plates were coated with monoclonal anti-human TNFα at 2 μg/ml, and the secondary “bridge” antibody was biotinylated (rabbit) anti-human TNFα at 200 ng/ml, which was detected with steptavidin-HRP (1:200). The enzyme substrate was a two-part commercially available kit (H2O2 and tetramethylbenzidine; R&D Systems). For all ELISAs the reaction was terminated with 27.5 μl/well of 8 M sulfuric acid and plates were read at 490 nm or 450 nm in an automated ELISA reader (Dynex Technologies, Chantilly, VA). Values for cytokines measured in culture supernatants were normalized for total protein in the cell pellet for each condition using the Bradford protein assay. This correction controls for variation in the relative confluence of epithelial cell monolayers at the termination of each experiment. Results are expressed as pg of cytokine/ml/mg total protein.
Cell Viability Assay
Media supernatant (50 μl) from cell monolayers treated with HDE, PKC inhibitors, or media alone were assayed for cell viability using a commercially available kit (TOX-7; Sigma) to measure lactate dehydrogenase (LDH) release, according to the manufacturer's instructions. In addition, confluent 60-mm dishes were lysed as a positive control for LDH release.
PKC Isoform Assay
After media supernatants were removed from treated cells, the cell monolayers were flash-frozen in cell lysis buffer as described (16). The cells were scraped with a cell lifter, sonicated, and centrifuged at 10,000 × g for 30 minutes at 4°C. The supernatant was removed (cytosolic fraction) and the pellet was resuspended in cell lysis buffer containing 0.01% Triton X-100 and sonicated again (particulate fraction). PKC isoform activity was determined in crude whole cell cytosolic and particulate fractions of BEAS-2B similar to methods described previously (16, 17). Airway epithelial cells contain the α, β, δ, ɛ, and ζ PKC isoforms (18). To measure specific PKC isoform activity, 24 μg/ml PMA, 30 mM dithiotreitol, 150 μM ATP, 45 mM Mg-acetate, PKC isoform-specific substrate peptide, and 10 μCi/mL [γ-32P]-ATP were mixed in a Tris-HCl buffer (pH 7.5). Chilled (4°C) cell lysate (cytosolic or particulate) samples (20 μl) were added to 40 μl of the reaction mix and incubated for 15 minutes at 30°C. This mixture (60 μl) was then spotted onto P-81 phosphocellulose papers (Whatman, Clinton, NJ) to halt incubation, and papers were subsequently washed five times in 75 mM phosphoric acid for 5 minutes, washed once in 100% ethanol for 1 minute, dried, and counted in nonaqueous scintillant (National Diagnostics, Atlanta, GA). PKC activity was expressed in relation to the total amount of cellular protein assayed as picomoles of phosphate incorporated per minutes per milligram.
Cloning and Transfection of Human PKC Mutants in Airway Epithelial Cells
Mutant PKCα-expressing cells were generated in the parental BEAS-2B cells to produce stably transfected dominant-negative (DN) versions of PKCα using the tetracycline-responsive promoter (pTRE) expression system (Clontech, Palo Alto, CA), as previously described (19). BEAS-2B cells were transfected with the pTet-On expression vector encoding reverse tetracycline transactivator protein (rtTA) as well as a neomycin resistance gene using a cationic lipid technique (Lipofectamine 2000; Invitrogen, Carlsbad, CA). Cells were propagated and selected for neomycin resistance using G418 (400 μg/ml; Calbiochem, San Diego, CA). Antibiotic-resistant clones were then transfected by electroporation with a DN variant made by in vitro mutagenesis, substituting an alanine for lysine at position 368. Expression of PKCα from this vector is under the control of the tetracycline response element (TRE) (20). In addition, BEAS-2B cells were simultaneously co-transfected with a plasmid encoding the “humanized” green fluorescent protein (GFP) reporter to facilitate cell selection. Limiting dilution culture of these clones resulted in the “double stable” transgenic cell line pTO2PKCαDN (PKCαDN), which is G418 resistant and tetracycline (doxycycline) responsive. All PKCα plasmid vectors were generously provided by Dr. Dan Rosson (Lankenau Medical Research Center, Wynnewood, PA).
To generate DN PKCɛ, a mutation within the ATP-binding site of PKCɛ was created at amino acid position 437 using site-direct mutagenesis, rendering the kinase inactive (21–24). The PKC DN cDNA was similarly cloned into the Clontech pEGFP-N1 vector (gift of Dr. Christer Larsson, Lund University, Sweden). This construct was transfected using Lipofectamine 2000 into live BEAS-2B cells. Stable transfectants were selected for 3 weeks with media containing 200 μg/ml G418, and isolated stable clones were extracted using Corning cloning cylinders (Corning, Acton, MA); GFP-positive clones were sorted by fluorescence-activated cell sorting to enrich the population of transfected cells.
Reagents
LHC basal medium and growth factor supplements were purchased from Lonza (Walkersville, MD). The PKCα isoform-specific inhibitor Gö6976 and PKCɛ-specific inhibitor Ro-31–8220 were purchased from Calbiochem/EMD (Gibbstown, NJ). Monoclonal anti-human TNF-α–neutralizing antibody and anti-human IL-6 and IL-8 ELISA capture antibodies, biotin-conjugated anti-TNF, streptavidin-HRP, recombinant human IL-6, IL-8, and TNF-α and ELISA substrate kit were all obtained from R&D Systems. ELISA bridge and detection antibodies were from Rockland (Gilbertsville, PA). The pharmaceutical fusion protein TNF-α inhibitor etanercept was obtained from Amgen/Wyeth. All other reagents not specified were purchased from Sigma.
Statistical Analysis
All quantitative experiments were performed in parallel three or more times, and each data point represents the mean of at least three independent measurements. Therefore, each data point graphically presented represents at least nine measurements used to generate the standard error of the mean. All data were analyzed using GraphPad Prism (version 4.00 for Windows; GraphPad Software, San Diego CA) and represented as mean ± SE. Data were analyzed for statistical significance using a two-way ANOVA employing Bonferroni or Tukey's multiple comparison post-test corrections depending on equality of sample sizes between repeated experiments. Significance was accepted at the 95% confidence interval.
RESULTS
PKC Isoform–Specific Inhibitors Differentially Block IL-6 and IL-8 Release
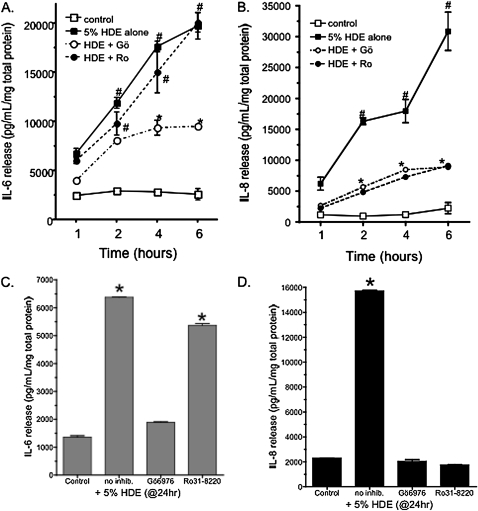
Previously, we reported that HDE stimulates both IL-6 and IL-8 in a PKC-dependent manner (10). To identify which PKC isoenzyme is activated in response to HDE, BEAS-2B were pretreated for 1 hour with either PKCα- or PKCɛ-specific inhibitors followed by stimulation with 5% HDE for up to 6 hours. IL-6 and IL-8 were then assayed by ELISA. HDE alone stimulated a significant release of both IL-6 and IL-8 by 6 hours of exposure (Figure 1). Pretreatment with the PKCα-specific inhibitor Gö 6976 (1 μM) resulted in a significant decrease in HDE-stimulated IL-6 release (Figure 1A). However, no decrease in HDE-stimulated release of IL-6 was detected when the cells were pretreated with the PKCɛ-selective inhibitor, Ro 31-8220 (1 μM). Similar to IL-6 release, Gö 6976 also inhibited HDE-stimulated IL-8 release, but unlike IL-6, Ro 31-8220 was very effective (P < 0.05 versus HDE alone) at blocking HDE-induced elevations in IL-8 (Figure 1B). PKCδ and ζ inhibitors (Rottlerin and myristolated PKCζ inhibitor peptide) failed to block HDE-stimulated release of IL-6 and IL-8 (data not shown). Identical results were obtained when a cell line that forms tight junctions (16HBE14o-) was used to confirm the observations made in the BEAS-2B cell line (Figures 1C and 1D). Treatment with PKC isoform inhibitors did not result in a decrease in cell viability (data not shown). These data suggest that HDE-induced IL-6 release is dependent upon PKCα, but not PKCɛ; conversely, both PKCα and PKCɛ inhibitors blocked HDE-stimulated IL-8 release.
Figure 1.
Inhibition of protein kinase C (PKC)α, but not PKCɛ, blocks IL-6 release. Treatment of BEAS-2B cells with 5% hog barn dust extract (HDE) results in a steady increase in both (A) IL-6 and (B) IL-8 release from 1 to 6 hours in culture. Pretreatment of the cells with the PKCα-specific inhibitor Gö6976 (Gö; 1 μM) decreases the release of IL-8 at all time points and IL-6 at 4 to 6 hours. Treatment with the PKCɛ-specific inhibitor Ro-31–8220 (Ro; 1μM), only decreases IL-8 release and does not significantly affect the IL-6 release. Similar results were observed using the bronchial epithelial cell line 16HBE14o- (panels C [IL-6] and D [IL-8]). Data shown represent the means ± SEM of the pooled results from four parallel experiments. (#P < 0.01 versus Control media; *P < 0.01 versus HDE alone, two-way ANOVA, Bonferroni post-test).
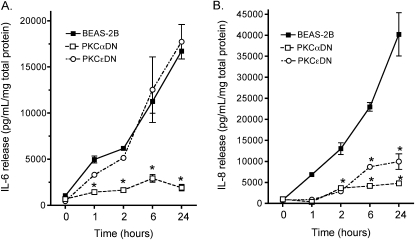
To confirm that our results were the product of pharmacologic PKC isoform inhibition and not a nonspecific chemical artifact, we used BEAS-2B cell lines that expressed a DN form of either PKCα or PKCɛ. IL-6 release was abrogated only in those BEAS-2B expressing a DN PKCα when treated with 5% HDE for up to 24 hours (Figure 2). Cells expressing DN PKCɛ demonstrated an identical IL-6 release in response to HDE as control wild-type BEAS-2B (Figure 2A). In contrast, the HDE-stimulated release of IL-8 was blocked in both PKCα and PKCɛ DN-expressing cell lines (Figure 2B). These findings support the chemical isoform inhibitor data and reveal that HDE-stimulated IL-6 release requires PKCα activation, while HDE-stimulated IL-8 release requires both activatable PKCα and PKCɛ.
Figure 2.
PKCα and PKCɛ are required for HDE-stimulated interleukin release. Parental BEAS-2B cells exposed to 5% HDE for up to 24 hours respond by releasing more IL-6 (A) and IL-8 (B) than untreated cells. Genetically transformed cells lacking catalytically active PKCα (PKCαDN) fail to release IL-6 and IL-8 in response to HDE over time. In contrast, PKCɛ-deficient cells (PKCɛDN) behave similarly to wild-type cells in terms of IL-6 release, but show an attenuated IL-8 response after HDE challenge. Data points represent means ± SEM of data pooled from four identically designed experiments (*P < 0.01 versus parental BEAS-2B, two-way ANOVA, Bonferroni post-test).
PKC Isoform Activation Exists in a Temporal Sequence in HDE-Stimulated BEAS-2B
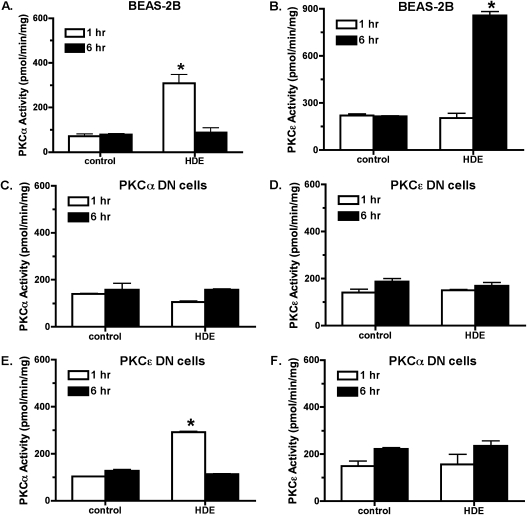
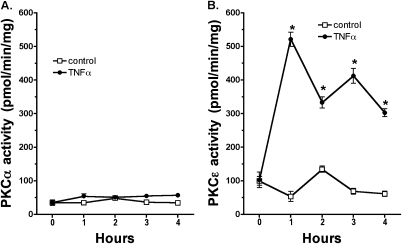
To identify specific isoform activity in response to HDE challenge and to determine the time and duration of this kinase activity, specific PKC isoenzyme activity was assayed. HDE (5%) stimulates a rapid increase in PKCα activity, reaching a maximal activation by 1 hour (Figure 3A). This PKCα activation is no longer observed 6 hours after HDE treatment. In contrast, PKCɛ isoform activity is significantly (P < 0.05 versus control media) elevated 6 hours after HDE exposure with no increase in activity observed at the earlier time point (Figure 3B). Likewise, PKCɛ activity diminishes after 6 hours, with a return to baseline levels by 24 hours (25). These data demonstrate that the PKCα activation in response to HDE stimulation precedes the activation of PKCɛ. Similar to the interleukin release data, HDE failed to stimulate PKCα activity in PKCα DN cells (Figure 3C) and failed to stimulate PKCɛ activity in PKCɛ DN cells (Figure 3D). As expected, HDE did significantly stimulate PKCα activity at 1 hour of exposure in PKCɛ DN cells (Figure 3E). When PKCα DN cells were treated with HDE, however, no activation of PKCɛ was observed, although we have previously shown PKCɛ to be functional in PKCα DN cells (13, 26). These data demonstrate that activatable PKCα must be present in airway epithelium before HDE is capable of stimulating PKCɛ and sequentially place the action of PKCα upstream of PKCɛ.
Figure 3.
PKC α activity is required for HDE-stimulated PKCɛ activation. (A) Parental BEAS-2B cells demonstrated a rapid increase in PKCα activity within 1 hour of 5% HDE challenge. PKCα activity returns to baseline levels by 6 hours of HDE treatment. (B) PKCɛ activity is not increased at 1 hours after HDE exposure, but is significantly enhanced by 6 hours. (C) As expected, HDE failed to stimulate PKCα activity in PKCαDN cells, while HDE also failed to activate PKCɛ in PKCɛDN cells (D). (E) In PKCɛDN cells, HDE can still activate PKCα at 1 hour similar to parental BEAS-2B. (F) In PKCαDN cells, HDE fails to activate PKCɛ at 6 hours of treatment (*P < 0.01 versus media controls at matched time points).
HDE Stimulates the Release of TNF-α
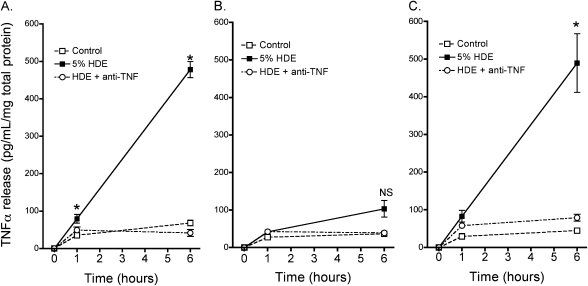
Because it has previously been established that swine confinement dusts stimulate the release of TNF-α in airway epithelium (12), we stimulated BEAS-2B and PKC isoform DN cell lines with 5% HDE and assayed for TNF-α release. In the parental BEAS-2B cells, only slight increases in TNF-α were observed 1 hour after HDE exposure, but by 6 hours after HDE stimulation BEAS-2B released nearly 500 pg/ml/mg TNF-α (Figure 4). When we examined this question in the DN cell lines, HDE failed to stimulate TNF-α release in PKCα DN cells at all time points examined. In contrast, HDE treatment stimulated TNF-α release in PKCɛ DN cells in a manner closely resembling the trend observed in the parental BEAS-2B cell line; TNF-α release was significantly enhanced at both the 1-hour and 6-hour time points. In the BEAS-2B and PKCɛDN cell lines, pretreatment with a TNF-α–neutralizing antibody (1 μg/ml) dramatically inhibited the HDE-mediated increases, confirming the specificity of the assay for TNF. These data provide evidence that PKCα, but not PKCɛ, is required for the HDE-stimulated release of TNF-α in the airway epithelial cell.
Figure 4.
HDE-stimulated TNF-α release by epithelial cells requires functional PKCα, but is independent of PKCɛ activity. In BEAS-2B cells, no significant stimulation of TNF-α release is observed by 1 hour of treatment with 5% HDE, but by 6 hours, significant release of TNF-α has occurred (A). In contrast, no release of TNF-α is detected in PKCαDN cells exposed to HDE at any time point (B). Unlike PKCα, PKCɛDN cells remain responsive to HDE and may even generate an enhanced release of TNF-α (C). Co-incubation of the cells with a TNF-α–neutralizing antibody before and during HDE challenge confirms the specificity of the assay for TNF-α (open circles). Results shown are means ± SEM for data pooled from three separate experiments. *P < 0.01 versus media controls at matched time points, one-way ANOVA, multiple pair-wise comparisons using Tukey's test. NS, not significant.
TNF-α Directly Stimulates PKCɛ, but Not PKCα, Activity
To confirm the temporal sequence of PKC isoform activation in response to HDE stimulation in airway epithelial cells, we exposed BEAS-2B directly to purified recombinant TNF-α and assayed both PKCα and PKCɛ activities in the same cells. We observed that 100 pg/ml TNF-α increased PKCɛ activity up to 5-fold by 1 hour of exposure (Figure 5B). PKCɛ remained elevated up to 4 hours after TNF-α exposure, but returned to baseline activity levels by 6 hours. However, TNF-α (100 pg/ml) failed to stimulate PKCα at any time point measured (Figure 5A). These data provide evidence that PKCɛ activation, but not PKCα activation, can occur in response to TNF-α. The data also show that the timeline of TNF-α–stimulated PKCɛ activation is consistent with the time of HDE-stimulated PKCɛ activation.
Figure 5.
TNF-α stimulates PKCɛ, but not PKCα activity in bronchial epithelial cells. Treatment of BEAS-2B cells with recombinant human TNF-α (100 pg/ml) results in a rapid and substantial stimulation of PKCɛ activity within 1 hour of exposure (B). By 4 hours, the level of PKCɛ activity subsides. PKCα activity, however, is unaffected by TNF-α stimulation at all time points tested (A) (*P < 0.01 versus control media at each matched time point).
TNF-α Directly Stimulates both IL-6 and IL-8 Release
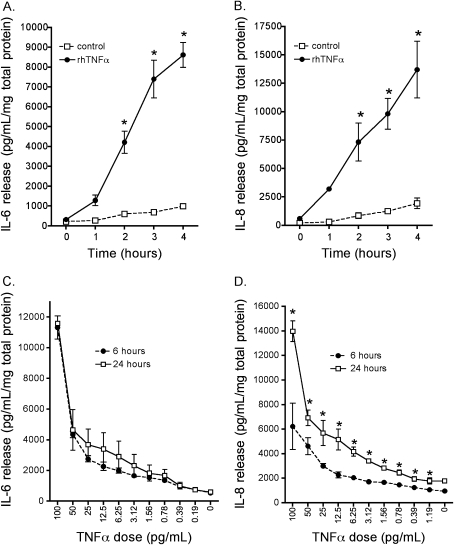
Two events must occur for HDE to stimulate interleukin release via a TNF-α–dependent mechanism: (1) the time course of TNF-α production must occur sequentially between the activation times of PKCα and PKCɛ, and (2) the concentration of TNF-α elicited by HDE must be sufficient to stimulate interleukin release in airway epithelial cells. To directly measure this sequence, we exposed BEAS-2B with a concentration of TNF-α (100 pg/ml) that we previously detected in response to HDE exposure (Figure 4) and measured the time course of IL-6 and IL-8 release into the media supernatants. Both IL-6 and IL-8 were significantly elevated in response to 100 pg/ml TNF-α between 2 and 4 hours of exposure (Figures 6A and 6B). Concentrations as low as 25 pg/ml significantly elevated both IL-6 and IL-8 in BEAS-2B (Figures 6C and 6D). Of interest is the finding that TNF-α stimulated the maximal release of IL-6 by 6 hours of exposure to BEAS-2B (Figure 6C). This contrasts with TNF-α–stimulated IL-8 release, which was detected at 24 hours of exposure (Figure 6B). These results demonstrate that the small concentrations of TNF-α elicited by HDE are capable of stimulating significant IL-6 release.
Figure 6.
TNF-α stimulates a rapid maximal release of IL-6, while the optimal IL-8 release is delayed. BEAS-2B cells respond vigorously to treatment with 100 pg/ml recombinant human TNF-α by releasing substantially more IL-6 (A) and IL-8 (B) than untreated control cells. Release of both cytokines is detectably increased within 1 hour of TNF treatment, and accumulation of cytokine continues for up to 24 hours thereafter. Cells release both (C) IL-6 and (D) IL-8 dose-responsively when stimulated with TNF-α. The similarity of the slopes of the dose–response curves for the 6- and 24-hour time points suggests that IL-6 release occurs early, whereas the additional increase in IL-8 release at 24 hours indicates a delayed response to TNF-α. Means ± SEM were calculated from pooled data derived from two parallel experiments. *P < 0.01 versus control medium at matched time points (A, B) and versus 6-hour values for each dose (C, D), two-way ANOVA, Bonferroni post-test.
Neutralizing TNF-α Blocks HDE-Stimulated IL-6 and IL-8
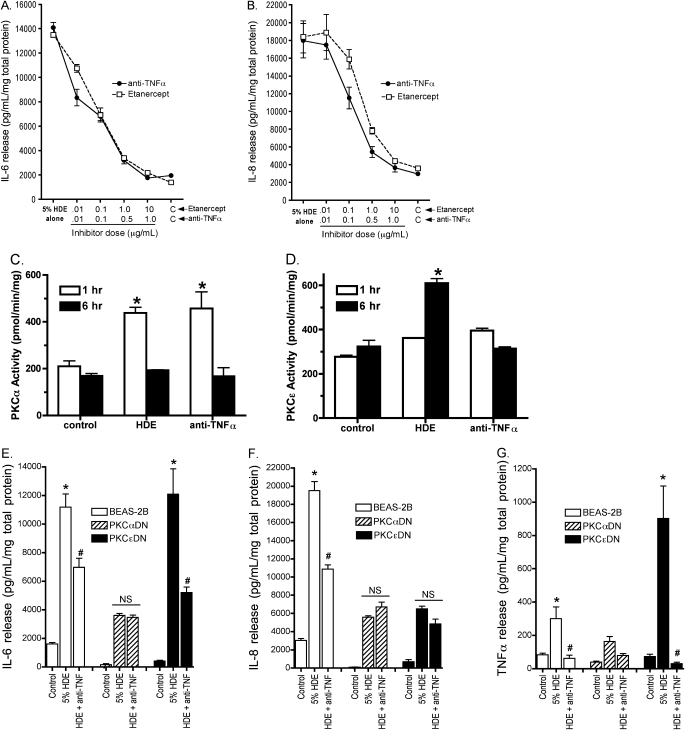
To determine the requirement of HDE-stimulated TNF-α on interleukin release, we sought to neutralize TNF-α using both anti–TNF-α neutralizing antibodies and the pharmaceutical TNF receptor fusion protein (etanercept) in cells stimulated with 5% HDE and assay subsequent interleukin production. Both anti–TNF-α (1 μg/ml) and etanercept (10 μg/ml) dose-dependently decreased IL-6 (Figure 7A) and IL-8 (Figure 7B)–stimulated release. Likewise, anti–TNF-α antibodies blocked PKCɛ activation in response to HDE, but not PKCα activation (Figures 7C and 7D). This suggests that TNF-α release is precedent to and required for HDE-stimulated interleukin release from airway epithelium.
Figure 7.
Both the anti-human TNF-α–neutralizing antibody and the TNF-α blocker etanercept dose-responsively inhibit the release of IL-6 (A) and IL-8 (B) in BEAS-2B cells within 24 hours. Both inhibitors reduced the 5% HDE-stimulated release of IL-6 to near baseline levels, and of IL-8 by 176% (highest dose). PKCα activity in response to HDE challenge was not affected by 1 μg/ml anti–TNF-α neutralizing antibodies (C), while preincubation with anti–TNF-α antibodies blocked HDE-stimulated PKCɛ activity (D). Likewise, anti–TNF-α antibodies significantly decreased HDE-stimulated IL-6 release in BEAS-2B and PKCɛDN cells, but did not further reduce the diminished response to HDE-stimulated IL-6 observed in PKCαDN cells (E). Anti–TNF-α (1 μg/ml) significantly decrease IL-8 release in BEAS-2B, but had no additional effect on the already reduced levels of IL-8 observed in both PKCαDN and PKCɛDN cells (F). As expected, anti–TNF-α (1 μg/ml) neutralized all TNF-α release from all three cell lines, even though the PKCɛDN cell line demonstrated an enhanced response to HDE stimulation of TNF-α (G). Data are pooled from three parallel experiments. *P < 0.001 versus media control; #P < 0.01 versus 5%HDE treatment, two-way ANOVA, Bonferroni post-test. NS, not significant.
The impact of anti–TNF-α neutralizing antibodies on cytokine release was also determined using PKC DN cell lines. HDE-stimulated IL-6 release was significantly decreased by anti–TNF-α in parental and PKCɛDN cells (Figure 7E), but no such decrease was observed in the already diminished IL-6 levels stimulated by HDE in PKCαDN cells. TNF-α antibody blockade did not affect the HDE stimulated IL-8 release in either PKCαDN or PKCɛDN cell lines (Figure 7F). As a control, anti–TNF-α antibody pretreatment completely neutralized TNF-α release under all assay conditions, even in the PKCɛDN cell line, where TNF-α release was surprisingly enhanced (Figure 7G). These data collectively indicate that TNF-α is an essential intermediary, whose release occurs after the activation of PKCα and before the activation of PKCɛ, required for the HDE-stimulated release of IL-6 and IL-8 (Figure 8).
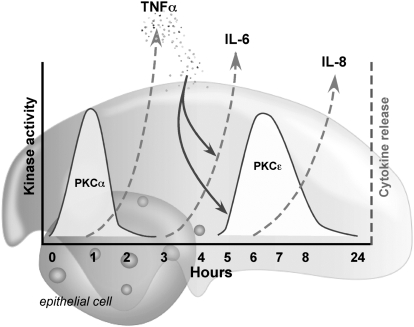
Figure 8.
Proposed model for HDE-induced sequential PKC action in bronchial epithelial cell release of IL-6 and IL-8. The early activation of PKCα (t = 1 h) in response to hog barn dust stimulates the production and release of TNF-α (t = 2–4 h). TNF-α then binds to TNF receptors on the bronchial epithelial cell and stimulates the release of IL-6 and the activation of PKCɛ (t = 6 h). PKCɛ activation results in the complete release of IL-8 (t = 24 h).
DISCUSSION
Our results demonstrate that there is a temporal ordering to the PKC signaling events that occur before cytokine release as measured in vitro in human bronchial epithelial cells exposed to swine confinement facility dust. It has been established by others that dusts from swine confinement facilities induce lung inflammation associated with the elevation of TNF-α, IL-6, and IL-8 (27, 28). In fact, an important source of these cytokines has been identified as the airway epithelial cells (9), and PKC activation is an identified regulator of interleukin release (10). However, the subcellular signaling pathways for this complex mixture of proinflammatory cytokines has not been closely examined with respect to which PKC isoenzymes control swine dust–stimulated cytokine release and how this might be orchestrated temporally.
The temporal sequence of our findings is supported by the study of Burvall and coworkers, with dust-induced IL-6 release being precedent to that of IL-8 release (29). This is also consistent with early serum responses of TNF-α and IL-6 reported by Wang and colleagues (8). However, in a subsequent study using the alveolar epithelial cell line A549, Burvall and coworkers reported that the production of mRNA for IL-6 was precedent to mRNA production of TNF-α (12). This discrepancy may be simply due to known functional differences between these two cell lines, or perhaps due to the different functions that alveolar and bronchial epithelial cells play in innate defense, as the regulation of IL-6 release differs in the proximal versus distal airways. Indeed, we have shown that HDE is capable of blunting the cilia stimulatory pathways of the bronchial epithelium in an interleukin-dependent manner (30). Therefore it is plausible that dust regulation of IL-6 in the alveolar epithelium initially impacts inflammatory cell recruitment while altering mucociliary clearance in the bronchial epithelium.
In addition to the possibility that swine dust may elicit a different initial response in different epithelial cells of the lung, our present study defines that different PKC isoforms control the release of different cytokines in the same cell stimulated with the same agent. Such a cellular orchestration in response to a complex inhaled substance adds specific isoenzyme control to compartmentalized enzyme action as points of regulation when multiple proinflammatory cytokines are released in response to an inhaled injurious environmental agent. Airway epithelial cells contain the α, β, δ, ɛ, and ζ isoforms of PKC (31). Because PKC inhibitors such as Gö6976 and Ro-31-8220 do not exert absolute specificity for PKCα and PKCɛ, respectively, and may even exert PKC-independent effects, we have chosen to confirm our inhibitor findings through the use of DN PKC isoform–expressing cell lines.
We have previously determined that TNF-α activates PKC in bovine bronchial epithelium (31), and the current study identifies PKCɛ as the isoform responsible for this activation. This finding differs from those of Page and colleagues (32), who report that TNF-α stimulates IL-8 release in bronchial epithelial cells via a PKCδ-dependent mechanism. We observed no inhibition of HDE-stimulated IL-6 or IL-8 after pretreatment with the PKCδ inhibitor rottlerin, even though rottlerin effectively inhibited PKCδ activity in BEAS-2B (data not shown). The inhibition of PKCδ also failed to block stimulated IL-8 release in response to cattle feedlot dust (26), cigarette smoke (33), aldehyde adducted proteins (34), and TNF-α (data not shown). In contrast, lysophosphatidic acid stimulates airway epithelial IL-8 via a PKCδ-dependent mechanism (35). This suggests that different agents are capable of stimulating IL-8 production through more than one PKC isoenzyme.
Another aspect of PKC regulation of TNF-α that requires further study is the surprising observation that TNF-α release is significantly enhanced in the PKCɛDN cells in response to HDE exposure. In this cell line, the levels of PKCα activation are not altered at baseline or under conditions of HDE stimulation as compared with normal BEAS-2B cells (Figure 3). We have previously characterized the PKC isoform expression in the DN cell lines used in this study, and observed no differences in the levels of PKCδ or PKCζ in the PKCɛDN cell line (13). This finding rules out that an enhanced PKCα protein level or catalytic activity in “compensation” to the expression of dominantly inactive epsilon is responsible for the observed augmentation of IL-8 release in response to HDE. However, this does underscore the essential nature of PKCɛ in IL-8 release, since IL-8 is diminished but TNF-α is enhanced in response to HDE in the PKCɛDN cells. If the activation of NF-κB by TNF-α is indeed principally mediated by PKCɛ, perhaps a negative feedback signal to turn off TNF-α production after the activation of NF-κB is not evoked in the PKCɛDN cell, thus resulting in the generation of higher than usual TNF-α levels. Further studies are required to investigate such a feedback regulation pathway.
The regulation of TNF-α levels may represent a clinical target in addressing chronic inflammatory lung disease in response to inhaled dusts. In our study, neutralizing TNF-α, either by pretreatment of the cells with an antibody that binds to TNF-α or a recombinant fusion protein that functions as a false receptor, significantly diminishes the levels of TNF-α, IL-6, and IL-8 release in response to swine dust exposure to the bronchial epithelial cells. This raises the question as to whether or not anti–TNF-α drugs such as infliximab or etanercept might reduce lung inflammation in response to swine barn dust inhalation. Preclinical animal models are currently underway to address the efficacy of this approach using an in vivo assay of swine dust inhalation exposure and lung inflammation.
In summary, the results of these experiments are evidence that a cascade of events initiated by HDE exposure and mediated by two PKC isoforms ultimately result in the release of proinflammatory cytokines. Initial exposure to HDE causes early increase in PKCα activity, the subsequent release of TNF-α, and the delayed activation of PKCɛ. IL-6 release in response to HDE requires prior activation of PKCα, but appears to be independent of PKCɛ activation. TNF-α appears necessary for the HDE-stimulated release of both IL-6 and IL-8, and the increase in PKCɛ activity, although PKCα activity is unaffected by TNF-α treatment. Hog dust–stimulated IL-8 release requires increased activation of both PKC isoforms. Depending upon the nature of the exposure with regard to the composition of the dust, our findings suggest a potential mechanistic pathway for the production of proinflammatory mediators in response to agricultural dust exposures.
Acknowledgments
The authors acknowledge expert editorial assistance from Lisa Chudomelka, M.A., and thank Anthony Floreani, M.D., for providing the PKCαDN cell line.
This material is the result of work supported with resources and the use of facilities at the Omaha VA Medical Center, Omaha, NE (Department of Veterans Affairs [VA Merit Review] to T.A.W.) This work was supported by NIH-NIOSH (R01OH008539) to D.J.R. and NIH-NIAAA (R01AA017993) to T.A.W.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0065OC on July 27, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kirkhorn SR, Garry VF. Agricultural lung diseases. Environ Health Perspect 2000;108:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Essen SG, Auvermann BW. Health effects from breathing air near CAFOs for feeder cattle or hogs. J Agromed 2005;10:55–64. [DOI] [PubMed] [Google Scholar]
- 3.Bongers P, Houthuijs D, Remijn B, Brouwer R, Biersteker K. Lung function and respiratory symptoms in pig farmers. Br J Ind Med 1987;44:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haglind P, Rylander R. Occupational exposure and lung function measurements among workers in swine confinement buildings. J Occup Med 1987;29:904–907. [PubMed] [Google Scholar]
- 5.Holness DL, O'Blenis EL, Sass-Kortsak A, Pilger C, Nethercott JR. Respiratory effects and dust exposures in hog confinement farming. Am J Ind Med 1987;11:571–580. [DOI] [PubMed] [Google Scholar]
- 6.Donham KJ, Reynolds SJ, Whitten P, Merchant JA, Burmeister L, Popendorf WJ. Respiratory dysfunction in swine production facility workers: dose–response relationships of environmental exposures and pulmonary function. Am J Ind Med 1995;27:405–418. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, Merchant JA. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am J Respir Crit Care Med 1995;151:47–53. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Malmberg P, Larsson P, Larsson BM, Larsson K. Time course of interleukin-6 and tumor necrosis factor-alpha increase in serum following inhalation of swine dust. Am J Respir Crit Care Med 1996;153:147–152. [DOI] [PubMed] [Google Scholar]
- 9.Palmberg L, Larsson BM, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 1998;53:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 2002;93:289–296. [DOI] [PubMed] [Google Scholar]
- 11.Liden J, Ek A, Palmberg L, Okret S, Larsson K. Organic dust activates NF-kappaB in lung epithelial cells. Respir Med 2003;97:882–892. [DOI] [PubMed] [Google Scholar]
- 12.Burvall K, Palmberg L, Larsson K. Expression of TNFalpha and its receptors R1 and R2 in human alveolar epithelial cells exposed to organic dust and the effects of 8-bromo-cAMP and protein kinase A modulation. Inflamm Res 2005;54:281–288. [DOI] [PubMed] [Google Scholar]
- 13.Slager RE, Allen-Gipson DS, Sammut A, Heires A, Devasure J, Von Essen SG, Romberger DJ, Wyatt TA. Hog barn dust slows airway epithelial cell migration in vitro through a PKC{alpha}-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 2007;293:L1469–1474. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cepsilon in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L1163–L1170. [DOI] [PubMed] [Google Scholar]
- 15.Rojas-Corona RR, Skarnes R, Tamakuma S, Fine J. The Limulus coagulation test for endotoxin: a comparison with other assay methods. Proc Soc Exp Biol Med 1969;132:599–601. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt TA, Schmidt SC, Rennard SI, Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc Soc Exp Biol Med 2000;225:91–97. [DOI] [PubMed] [Google Scholar]
- 17.Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 2003;163:1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alpert SE, Walenga RW, Mandal A, Bourbon N, Kester M. 15-HETE-substituted diglycerides selectively regulate PKC isotypes in human tracheal epithelial cells. Am J Physiol 1999;277:L457–L464. [DOI] [PubMed] [Google Scholar]
- 19.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992;89:5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosson D, O'Brien TG, Kampherstein JA, Szallasi Z, Bogi K, Blumberg PM, Mullin JM. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J Biol Chem 1997;272:14950–14953. [DOI] [PubMed] [Google Scholar]
- 21.Hong DH, Petrovics G, Anderson WB, Forstner J, Forstner G. Induction of mucin gene expression in human colonic cell lines by PMA is dependent on PKC-epsilon. Am J Physiol 1999;277:G1041–G1047. [DOI] [PubMed] [Google Scholar]
- 22.Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol 2000;279:H1679–H1689. [DOI] [PubMed] [Google Scholar]
- 23.Pass JM, Zheng Y, Wead WB, Zhang J, Li RC, Bolli R, Ping P. PKCepsilon activation induces dichotomous cardiac phenotypes and modulates PKCepsilon-RACK interactions and RACK expression. Am J Physiol Heart Circ Physiol 2001;280:H946–H955. [DOI] [PubMed] [Google Scholar]
- 24.Wu JM, Xiao L, Cheng XK, Cui LX, Wu NH, Shen YF. PKC epsilon is a unique regulator for hsp90 beta gene in heat shock response. J Biol Chem 2003;278:51143–51149. [DOI] [PubMed] [Google Scholar]
- 25.Slager RE, Sisson JH, Pavlik JA, Johnson JK, Nicolarsen JR, Jerrells TR, Wyatt TA. Inhibition of protein kinase C epsilon causes ciliated bovine bronchial cell detachment. Exp Lung Res 2006;32:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen SG, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and 8 requires protein kinase C-epsilon human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L1163–L1170. [DOI] [PubMed] [Google Scholar]
- 27.Larsson BM, Palmberg L, Malmberg PO, Larsson K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax 1997;52:638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J 1997;10:381–387. [DOI] [PubMed] [Google Scholar]
- 29.Burvall K, Palmberg L, Larsson K. Influence of 8-bromo-cyclicAMP on interleukin -6 and -8 mRNA levels in A549 human lung epithelial cells exposed to organic dust: a time-kinetic study. Life Sci 2004;75:2733–2749. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt TA, Sisson JH, Von Essen SG, Poole JA, Romberger DJ. Exposure to hog barn dust alters airway epithelial ciliary beating. Eur Respir J 2008;31:1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt TA, Ito H, Veys TJ, Spurzem JR. Stimulation of protein kinase C activity by tumor necrosis factor-alpha in bovine bronchial epithelial cells. Am J Physiol (Lung Cell. Mol. Physiol 1997;273:L1007–L1012. [DOI] [PubMed] [Google Scholar]
- 32.Page K, Li J, Zhou L, Iasvoyskaia S, Corbit KC, Soh JW, Weinstein IB, Brasier AR, Lin A, Hershenson MB. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J Immunol 2003;170:5681–5689. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt TA, Heires AJ, Sanderson SD, Floreani AA. Protein kinase C activation is required for cigarette smoke-enhanced C5a-mediated release of interleukin-8 in human bronchial epithelial cells. Am J Respir Cell Mol Biol 1999;21:283–288. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH. Malondialdehyde-acetaldehyde-adducted bovine serum albumin activates protein kinase C and stimulates interleukin-8 release in bovine bronchial epithelial cells. Alcohol 2001;25:159–166. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem 2006;281:19501–19511. [DOI] [PMC free article] [PubMed] [Google Scholar]