Abstract
Vertebrate Bmp2 and Bmp4 diverged from a common ancestral gene and encode closely related proteins. Mice homozygous for null mutations in either gene show early embryonic lethality, thereby precluding analysis of shared functions. In the current studies, we present phenotypic analysis of compound mutant mice heterozygous for a null allele of Bmp2 in combination with null or hypomorphic alleles of Bmp4. Whereas mice lacking a single copy of Bmp2 or Bmp4 are viable and have subtle developmental defects, compound mutants show embryonic and postnatal lethality due to defects in multiple organ systems including the allantois, placental vasculature, ventral body wall, skeleton, eye and heart. Within the heart, BMP2 and BMP4 function coordinately to direct normal lengthening of the outflow tract, proper positioning of the outflow vessels, and septation of the atria, ventricle and atrioventricular canal. Our results identify numerous BMP4-dependent developmental processes that are also very sensitive to BMP2 dosage, thus revealing novel functions of Bmp2.
Keywords: Bone morphogenetic protein, BMP2, BMP4, Mouse mutant, BMP redundancy, Embryonic patterning, Heart development
1. Introduction
Bone Morphogenetic Proteins (BMPs) comprise a large subfamily of the TGF-β super family of secreted signaling molecules. BMPs are involved in many aspects of embryonic development, including cell proliferation and differentiation, apoptosis and cell fate determination (Hogan, 1996). Vertebrate BMP2 and BMP4 diverged from a single ancestral gene (McCauley and Bronner-Fraser, 2004) and form a distinct subclass of BMPs that share greater than 80% amino acid homology in the ligand domain. Both BMP2 and BMP4 preferentially bind to the type I BMP receptors, BMPR1A (Alk3) and BMPR1B (Alk6), but can also signal through ActRI (Alk2) (Miyazono et al., 2005). BMP2 and BMP4 elicit the same biological responses in numerous in vitro and in vivo assays. In addition, their expression patterns overlap extensively throughout embryonic and fetal development (Dudley and Robertson, 1997; Lyons et al., 1995).
Bmp2 and Bmp4 are widely expressed in embryos and adults; however, loss of function of either gene leads to early embryonic lethality (Winnier et al., 1995; Zhang and Bradley, 1996). BMP4 function in development has been studied more thoroughly to date. Most mice homozygous for a null allele of Bmp4 (Bmp4−/− mice) die at embryonic day (E) 6.5, with little or no mesoderm (Winnier et al., 1995). In the rare mutants that survive past this time, neither primordial germ cell (PGC) specification (Lawson et al., 1999) nor lens induction occurs (Furuta and Hogan, 1998) and hepatogenesis is defective (Rossi et al., 2001). Chimeric analysis has shown that BMP4 is required for chorioallantoic fusion, vascularization of the allantois, migration and survival of PGCs, and establishment of left-right asymmetry (Fujiwara et al.,2002, 2001). Tissue-specific inactivation of Bmp4 has revealed additional roles for BMP4 in later stages of heart morphogenesis, as it is required for proper septation of the ventricle, atrioventricular (AV) canal (Jiao et al., 2003) and outflow tract (OFT) (Liu et al., 2004). Deletion of Bmp4 in other regions of the embryo has revealed that Bmp4 regulates development of the limbs (Selever et al., 2004), craniofacial structures (Liu et al., 2005), lung (Eblaghie et al., 2006), and vestibular apparatus (Chang et al., 2008).
Analysis of mutants in which BMP4 levels are decreased, but not absent, has also revealed novel functionsofBMP4 during gestation and in the adult. Bmp4 null heterozygotes (Bmp4+/− mice) on a C57BL/6J background display several phenotypic abnormalities, including failure to maintain spermatogenesis and defects in the kidneys and eyes (Dunnet al., 1997; Huet al., 2004; Katagiri et al., 1998; Miyazaki et al., 2000). Moreover, our analysis of a BMP4 hypomorph revealed further requirements for BMP4 in fetal vascularization of the placenta, dorsal fusion of the vertebrae and ventral body wall closure (Goldman et al., 2006).
Bmp2−/− mice die at E8.5 as a result of disorganized and delayed cardiac development and defects in the amnion and chorion (Zhang and Bradley, 1996) and also display a failure of neural crest cell migration (Correia et al., 2007). Like Bmp4, Bmp2 is required for PGC specification and chorioallantoic fusion (Ying et al., 2001). Recent analyses of tissue-specific knock outs of Bmp2 in heart progenitors revealed that BMP2 plays an essential role in early stages of heart morphogenesis that is distinct from that of BMP4. Specifically, BMP2 regulates AV myocardial identity and is necessary for the initial formation of the endocardial cushions in the AV canal (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). In contrast, the rare Bmp4−/− embryos that survive to this stage form endocardial cushions (Jiao et al., 2003), suggesting a unique requirement for BMP2 in this morphogenetic process.
The similarity in BMP2 and BMP4 signaling activity and expression patterns begs the question of whether these genes function together in a redundant manner to regulate critical aspects of organogenesis that have not yet been interrogated by tissue-specific inactivation strategies. With the exception of tissue-specific deletion of both ligands from the limb bud mesenchyme, which revealed redundant roles in osteogenesis (Bandyopadhyay et al., 2006), this question has not been addressed in studies to date. Here, we present phenotypic analysis of compound mutant mice that are heterozygous for null alleles of both Bmp2 (Bmp2−/+, Zhang and Bradley, 1996) and Bmp4 (Bmp4lacZ/+, in which exon 3 is replaced with lacZ, Lawson et al., 1999) or that are heterozygous null for Bmp2 and homozygous for a hypomorphic allele of Bmp4 (Goldman et al., 2006). Our results identify numerous BMP4-dependent developmental processes that are also very sensitive to BMP2 dosage, thus revealing novel functions of Bmp2 in skeletal, placenta, heart, and eye development, as well as ventral body wall closure.
2. Results and discussion
2.1. Bmp2 heterozygotes display numerous developmental defects on a C57BL/6J background
The effect of altering BMP dosage in mice is extremely strain dependent. For example, C57BL/6J mice that are heterozygous for a null allele of Bmp4 are not recovered in Mendelian ratios at weaning (Dunn et al., 1997) because of a low frequency of heart septation defects (Goldman et al., 2006), whereas mice heterozygous for the same Bmp4 null allele on other genetic backgrounds are recovered at normal ratios. Although it has previously been reported that Bmp2−/+ mice are viable on a mixed genetic background (Zhang and Bradley, 1996), we wished to determine whether the same is true in C57BL/6J mice. We observed a slightly lower frequency of Bmp2−/+ weanlings than expected in our colony (Table 1A, P = 0.005). Moreover, we noticed that prior to and immediately after the time of weaning, a number of Bmp2−/+ mice developed hydrocephalus and died (data not shown). These results suggest that C57BL/6J mice are also more sensitive to Bmp2 dosage than other strains. Thus, in order to maximize sensitivity to changes in BMP levels, all mice used in these studies were maintained on a C57BL/6J background.
Table 1.
Genotype analysis of live offspring from crosses with Bmp2−/+ mice.
| Age | Bmp2+/+ | Bmp2−/+ | Total |
|---|---|---|---|
| (A) Progeny from Bmp2+/+ × Bmp2−/+ | |||
| P21* | 176 (58%) | 128 (42%) | 304 |
| Age | Bmp2+/+; Bmp4+/+ | Bmp2−/+; Bmp4+/+ | Bmp2+/+; Bmp4lacZ/+ | Bmp2−/+; Bmp4lacZ/+ | Total |
|---|---|---|---|---|---|
| (B) Progeny from Bmp4lacZ/+ × Bmp2−/+ | |||||
| P21* | 43 (38%) | 38 (34%) | 21 (19%) | 10 (9%) 112 | |
| E12–E18 | 28 (33%) | 19 (22%) | 18 (21%) | 21 (24%) | 86 |
| Age | Bmp2+/+; Bmp4S2G/+ | Bmp2+/+; Bmp4S2G/S2G | Bmp2−/+; Bmp4S2G/+ | Bmp2−/+; Bmp4S2G/S2G | Total |
|---|---|---|---|---|---|
| (C) Progeny from Bmp4S2G/S2G × Bmp2−/+; Bmp4S2G/+ | |||||
| P21* | 41 (37%) | 37 (33%) | 30 (27%) | 4 (4%) | 112 |
| E12–E18* | 28 (21%) | 43 (33%) | 50 (38%) | 11 (8%) | 132 |
| E8.5–E10.5 | 46 (30%) | 40 (26%) | 40 (26%) | 28 (18%) | 154 |
Data are presented as number (percent). In (A), the expected frequency for each genotype is 50% of total progeny. In (B) and (C), the expected frequency for each genotype is 25% of total progeny.
The observed frequency is significantly different than the expected frequency by Chi-square analysis (P < 0.05).
Patterning of the axial skeleton is especially sensitive to BMP dosage. For example, Bmp4lacZ/+ mice on a C57BL/6J background display a number of developmental defects, including high frequencies of incompletely fused cervical vertebrae and small or missing 13th ribs (Goldman et al., 2006). We analyzed skeletons from Bmp2−/+ adults (>5 weeks old) for the presence of these defects. Notably, we detected identical defects in dorsal fusion of one or more cervical vertebrae (Fig. 1A and B) as well as defects in formation of the 13th rib (Fig. 1C and D) in 33% and 77%, respectively, of all Bmp2−/+ skeletons analyzed (n = 9).
Fig. 1.
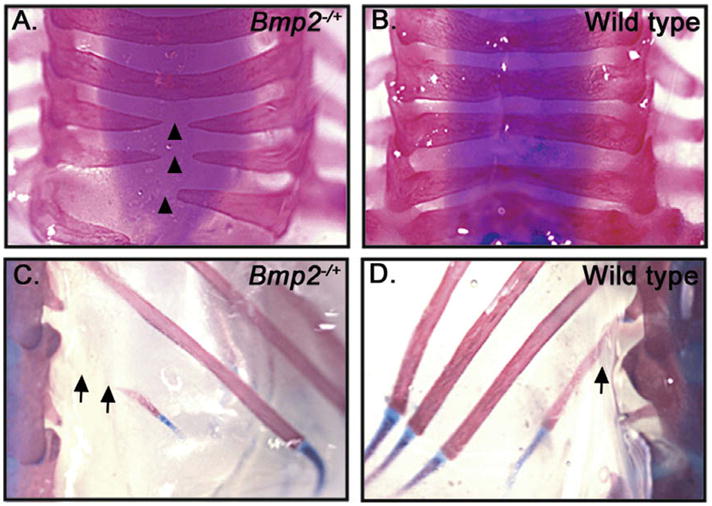
Skeletal defects in adult Bmp2−/+ mice. (A–D) Dorsal views of skeletal preparations of cervical (A and B) and thoracic (C and D) vertebrae from adults. Arrowheads indicate vertebrae that have not fused, arrows highlight the region missing from the 13th rib of mutant, but not wild type animals.
2.2. Deletion of one copy of Bmp2 in Bmp4 mutant mice leads to embryonic and postnatal lethality
To examine potential functional redundancies between Bmp2 and Bmp4, we generated compound mutant mice heterozygous for a null allele of Bmp2 in combination with either a single null allele of Bmp4 (Lawson et al., 1999) or with two copies of a hypomorphic allele of Bmp4 (Bmp4S2G; Goldman et al., 2006). In these Bmp4 hypomorphs, a minimal furin cleavage motif within the prodomain is rendered non-cleavable by a point mutation. Mice homozygous for this mutation are viable but show a loss of PGCs and testicular degeneration. We have previously shown that in some tissues, Bmp4S2G/S2G mice display more severe phenotypic defects than do BmplacZ/+ mice, whereas in other tissues, the opposite is true (Goldman et al., 2006). Thus, the Bmp4S2G allele was included in our study in order to achieve a greater range of BMP4 dosage for analysis.
Intercrosses of Bmp4lacZ/+ and Bmp2−/+ mice were performed to generate compound double heterozygotes for phenotypic analysis. Bmp2−/+; Bmp4lacZ/+ pups were severely underrepresented at weaning (Table 1B, P < 0.00003). To determine when Bmp2−/+; Bmp4lacZ/+ mice die, we established timed matings and analyzed embryos at various developmental ages. Mutant and control embryos were age matched based on external morphological criteria such as somite number and limb morphology. Interestingly, double heterozygotes were recovered at a relatively normal frequency between E12 and E18 (P = 0.4), although a fraction of these were dead when recovered. These data suggest that developmental defects present in these mutants primarily affect late gestational and postnatal survival.
Removal of one copy of Bmp2 in mice homozygous for the Bmp4S2G hypomorphic allele led to lethality that occurred earlier in development than that observed in Bmp2−/+; Bmp4lacZ/+ compound mutants. As detailed in Table 1C, mice were recovered at only 16% of the expected frequency at weaning (P < 0.00005) and at ~30% of the expected frequency between E12 and E18 (P < 0.00005). Although Bmp2−/+; Bmp4S2G/S2G compound mutants were recovered at ~64% of the expected frequency between E8.5 and E10.5, this decreased recovery was not statistically significant (P = 0.2). Together, these results clearly demonstrate that Bmp2 and Bmp4 function redundantly to control processes that are essential for embryonic and postnatal survival.
2.3. Bmp2 is required for chorioallantoic development in a Bmp4 mutant background
To determine when and why Bmp2−/+; Bmp4S2G/S2G mutants were dying, we analyzed embryos between E8.5 and E10.5. BMP2 and BMP4 are known to be required individually for normal outgrowth and differentiation of the allantois. In Bmp2 null mutants (Ying et al., 2001), in embryos lacking Bmp4 expression in the extraembryonic mesoderm (Fujiwara et al., 2001) and in a fraction of Bmp4S2G/lacZ compound hypomorphs (Goldman et al., 2006) outgrowth of the allantois is severely impaired, resulting in a failure of chorioallantoic fusion. Consistent with these findings, the allantois had not successfully fused with the chorion in a significant number of Bmp2−/+; Bmp4S2G/S2G embryos recovered between E8.5 and E10.5 (n = 9/28). Interestingly, lack of chorioallantoic fusion was observed in some embryos despite extensive allantoic outgrowth (data not shown). These findings suggest that although lack of allantoic outgrowth accounts for failure of placentation in some mutant embryos, in other embryos the expanded allantois and chorion may not be competent to fuse to each other.
We previously demonstrated that BMP4 signaling is necessary for normal morphogenesis of placental blood vessels that are derived from the allantois (Goldman et al., 2006). To begin to ask whether BMP2 might also contribute to fetal vascularization of the placenta, we examined Bmp2 expression in the fetal components of the placenta. RT-PCR analysis of embryonic vessels manually dissected from the chorionic plate of E10.5 wild type embryos revealed that Bmp2 is expressed in the vasculature and/or associated smooth muscle (Fig. 2A). We were unable to detect Bmp2 or Bmp4 transcripts by in situ hybridization to either whole or sectioned placentas, and thus we were unable to analyze their spatial distribution. To determine which placental cell types express Bmp4, we examined expression of β-galactosidase protein, which is present in the nuclei of Bmp4 expressing cells in Bmp4lacZ/+ embryos (Lawson et al., 1999). Staining Bmp4lacZ/+ E10.5 placentas with an anti-β-galactosidase antibody together with an antibody to the endothelial cell marker CD31/PECAM-1 demonstrated that Bmp4 is expressed in fetal endothelial cells (Fig. 2B–D, arrowheads). An antibody directed against the active, phosphorylated forms of the BMP pathway specific Smads (Smad1, 5 and 8) was used to detect BMP-responsive cells in the placenta. Interestingly, pSmad1/5/8 expression was also localized to the endothelial cells in the fetal portion of the placenta at E10.5 (Fig. 2E–G, arrowheads).
Fig. 2.

Bmp2 and Bmp4 expression and function in the fetal vasculature of the placenta. (A) Bmp2 expression in E10.5 fetal vessels of the placenta and in the heart (positive control) detected by RT-PCR. The 723 bp Bmp2 band is not detected in the absence of reverse transcriptase (−RT, negative control). (B–G) Immunofluorescence analysis of placentas dissected from Bmp4lacZ/+ embryos at E10.5. PECAM-1 (green) detects endothelial cells (B,D,E and G), nuclear-localized β-galactosidase (red) detects Bmp4 expressing cells (B and C) and pSmad1/5/8 (red) detects BMP-responsive cells (E and F). Sections were counterstained with Hoechst (blue) to visualize nuclei (C,D,F and G). Nuclei in which β-galactosidase or pSmad1/5/8 are expressed appear pink (blue + red = pink). Arrowheads indicate fetal endothelial cells that stain for both PECAM-1 and Bmp4 expression (B–D) or pSmad1/5/8 (E–G). Fetal blood vessels contain nucleated erythrocytes and can thus be distinguished from maternal vessels that do not. (H–J) Wholemount PECAM-1 immunostaining of placentas from Bmp4S2G/S2G (H) Bmp2−/+; Bmp4lacZ/+ (I) and Bmp2−/+; Bmp4S2G/S2G (J) embryos at E10.5.
In Bmp4lacZ/S2G compound hypomorphs, fetal blood vessels in the placenta do not expand across the chorionic plate and fail to undergo branching morphogenesis (Goldman et al., 2006). To determine whether Bmp2 also contributes to this process, we examined fetal blood vessel morphology by wholemount PECAM staining of placentas isolated from E10.5 double mutants that had successfully undergone chorioallantoic fusion. Whereas placental vasculature in Bmp4S2G/S2G (Fig. 2H and Goldman et al., 2006) and Bmp2−/+; Bmp4S2G/+ embryos (n = 14, not shown) appeared identical to that of wild type embryos, a fraction of the Bmp2−/+; Bmp4lacZ/+ (3 out of 9) and Bmp2−/+; Bmp4S2G/S2G (1 out of 7) embryos that were examined displayed abnormalities in the placental vasculature (Fig. 2I and J). Specifically, the fetal blood vessels in mutant placentas were thinner, appeared atretic and underwent excessive branching relative to those in control placentas (Fig. 2H–J). We also examined embryonic and yolk sac vasculature in double mutants as it has previously been shown that endothelial cell-specific loss of BmpR1a (Alk3) severely disrupts angiogenesis in the yolk sac and embryo (Park et al., 2006). Blood vessel morphology in the embryo and yolk sac was normal in all mutants examined (data not shown, n = 7), suggesting that adequate levels of BMP2/4 signaling are present to direct fetal blood vessel development in these mutants and/or that distinct members of the BMP family are responsible for signaling to vascular endothelial cells outside of the placenta.
Our data demonstrate that BMP2 and BMP4 signal to fetal endothelial cells in the placenta, and that these signals are required for normal blood vessel development to occur. The striking differences in vascular morphogenesis observed when Bmp2 levels are decreased in a Bmp4-deficient background (excessive branching) compared to that seen when only Bmp4 dosage is reduced (lack of branching), raise some intriguing possibilities about how BMP2 and BMP4 function in this process. These disparate phenotypes might indicate that each ligand has a distinct role in directing vascular morphogenesis: for example, BMP4 may be necessary for endothelial branching whereas BMP2 might inhibit endothelial branching. Alternatively, endothelial cells may respond differently to variations in total BMP dosage. These possibilities remain to be explored.
2.4. BMP2 regulates ventral body wall closure, eye and limb development together with BMP4
Ventral body wall closure is critically dependent on BMP4 dosage (Goldman et al., 2006; Katagiri et al., 1998). To determine whether BMP2 function is also required for this process, we analyzed skeletal preparations of Bmp2−/+ adults to look for incomplete sternal fusion, which is the least severe form of ventral body wall closure defect (Brewer and Williams, 2004). Defective fusion of the sternum, leading to a split xiphoid process, was observed in two-thirds of all Bmp2−/+ mice that were examined (Table 2 and Fig. 3A and B). A split xiphoid process was also observed in Bmp4lacZ/+ adults at low frequency (Table 2), consistent with previous reports (Katagiri et al., 1998). Two-thirds of all Bmp2−/+; Bmp4lacZ/+ embryos displayed more severe forms of VBD (Table 2), including umbilical hernia and omphalocoele, or complete failure of ventral body wall fusion leading to the externalization of all of the viscera (thoracoabdominoschisis) as shown in Fig. 3C and D. Thus, ventral body wall closure is exquisitely sensitive to total BMP2 and BMP4 dosage.
Table 2.
Ventral body wall closure and eye defects in Bmp2 and Bmp4 single and double mutants.
| Genotype | Ventral body wall defect (VBD) | Small/missing eyesa |
|---|---|---|
| Bmp2−/+ | Split xiphoid processb 6/9 | 0/20 |
| Bmp4lacZ/+ | Split xiphoid processb 3/10 | 4/20d |
| Bmp2−/+; Bmp4lacZ/+ | VBDc: 6/9 Omphalocele 5/9 Thoracoabdominoschisis 1/9 |
14/21 |
Data are presented as number defective/total analyzed.
Assessed in E12.5–E18 embryos.
Assessed in adults >5 weeks old.
Assessed in E15.5–E18.5 embryos.
Data are from (Goldman et al., 2006).
Fig. 3.
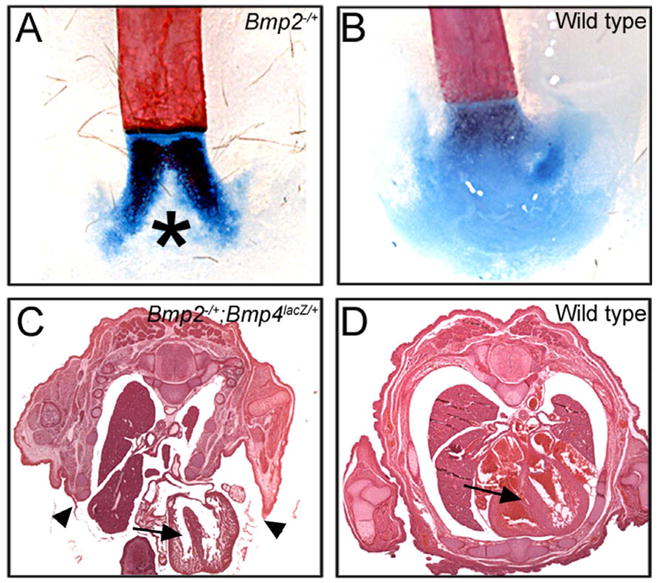
Body wall closure defects in Bmp2 heterozygotes and Bmp2−/+; Bmp4lacZ/+ compound mutants. (A and B) Alcian Blue and Alizarin Red-stained skeletons from adult mice show split xiphoid processes in Bmp2−/+ (asterisk), but not wild type mice. (C and D) Hematoxylin and eosin stained cross sections through the thoracic cavity of E15.5 embryos. Arrowheads indicate lateral edges of the ventral body wall of a Bmp2−/+; Bmp4lacZ/+ mutant which have failed to fuse, leading to externalization of the heart (arrow) and other organs.
Numerous studies have revealed that BMP signaling is critical for eye development during embryogenesis (Wordinger and Clark, 2007), and to date Bmp4 and Bmp7 have been shown to be the major BMP ligands involved in eye morphogenesis (Dudley et al., 1995; Furuta and Hogan, 1998; Wawersik et al., 1999). Interestingly, we noted a substantial increase in the frequency of missing or small eyes in Bmp2−/+; Bmp4lacZ/+ compound mutants relative to that observed in Bmp4lacZ/+ embryos (Dunn et al., 1997) (Table 2). Bmp2 and Bmp4 are both expressed in the developing eye, but their expression domains are non-overlapping (Dudley and Robertson, 1997; Furuta and Hogan, 1998), raising the possibility that these two ligands signal to different cell populations. However, the fact that lowering Bmp2 dosage increases the penetrance of missing/small eyes in a Bmp4lacZ/+ background, suggests that overall levels of these two proteins coordinately regulate eye development. Indeed, existing data support the idea that thresholds of BMP signaling regulate distinct morphogenetic and patterning events in the developing retina (Murali et al., 2005).
BMP4 is a known regulator of limb development, as Bmp4lacZ/+ mice and mice lacking Bmp4 expression in the limb display variable penentrance pre- and post-axial polydactyly (Dunn et al., 1997; Goldman et al., 2006; Selever et al., 2004). Recently, an allelic series of limb-mesenchyme specific knockouts of Bmp2 and Bmp4 demonstrated that these ligands function redundantly to control osteogenesis in the limb (Bandyopadhyay et al., 2006). We observed a greater frequency of forelimb post-axial digit duplications in Bmp2−/+; Bmp4lacZ/+ forelimbs (n = 13/14) than we previously observed in Bmp4lacZ/+ forelimbs (n = 32/58); (Goldman et al., 2006), but additional limb defects were not observed in these compound heterozygotes. Defects in skeletal patterning or differentiation were not observed in Bmp2−/+; Bmp4S2G/S2G mice (n = 10), consistent with our previous findings that the Bmp4S2G mutation is silent in the limb.
2.5. Bmp2 is required for OFT lengthening and for septation of the ventricle, atrium and AV canal in a Bmp4- deficient background
BMP signaling is critical for many aspects of heart development. Ablation of Bmpr1a/Alk3 in all early mesodermal progenitors that contribute to the heart leads to failure to specify the first heart field (FHF), which normally forms the cardiac crescent and contributes primarily to the left ventricle (Klaus et al., 2007). By contrast, at least a subset of cells from the second heart field (SHF), which contribute primarily to the future right ventricle and the inflow and outflow tracts, differentiate as cardiomyoctes in these mutants. Tissue-specific deletion of Bmpr1a/Alk3 (Gaussin et al., 2005, 2002; Song et al., 2007; Stottmann et al., 2004; Yang et al., 2006) or ActRI/Alk2 (Kaartinen et al., 2004; Wang et al., 2005) in myocardial, endocardial or neural crest cells has shown that BMPs are required for lengthening and septation of the OFT, development of the AV and semilunar valves, epithelial-mesenchymal transition (EMT) of endocardial cells that form the AV cushions, and later stages of septation of the atrium, AV canal, and ventricle. Bmp2 and Bmp4 are expressed in overlapping and/or adjacent domains throughout cardiac development (Abdelwahid et al., 2001; Jiao et al., 2003; Kaartinen et al., 2004; Liu et al., 2004; Ma et al., 2005), and are required individually for many of the above morphogenetic processes. Deletion of Bmp2 in myocardial progenitors, for example, has revealed an essential and non-redundant role for this ligand in inducing endocardial cells to undergo EMT and form the AV cushions, and in specifying AV myocardial fate (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). By contrast, loss of Bmp4 expression in the myocardium (Jiao et al., 2003) and/or splanchnic and branchial arch mesoderm of the SHF (Liu et al., 2004) causes defects in later stages of AV canal morphogenesis, after the endocardial cushions are formed, as well as defects in remodeling of the branchial arch arteries and septation of the distal OFT and ventricle.
The above studies reveal unique roles for BMP2 and BMP4 in specific aspects of heart development, but do not rule out the possibility that both ligands contribute redundantly to these same, or to distinct processes. To test this possibility, we performed histological analysis of mice recovered from Bmp2−/+ and Bmp4lacZ/+ or Bmp4S2G/S2G intercrosses at E15.5–E18 (Fig. 4, Table 3). Bmp2−/+; Bmp4lacZ/+ embryos showed frequent and severe heart defects, which are detailed below. We saw a less frequent but similar spectrum of defects in Bmp2−/+; Bmp4S2G/S2G mice (data not shown). Serial sectioning through the entire heart did not reveal any evidence of defects in septation of the OFT in compound mutants but we did observe a high frequency of double outlet right ventricle (DORV), in which the aortic orifice is mispositioned in the right ventricle (Fig. 4E, arrow, Table 3). The majority of Bmp2−/+; Bmp4lacZ/+ embryos also displayed membranous ventricular septal defects (VSD) (Fig. 4F, arrow), and atrial septal defects (ASD) (Fig. 4F, arrowhead). DORV with VSD was observed in one Bmp4lacZ/+ single mutant out of the 12 that were examined, whereas no cardiac abnormalities were detected in Bmp2−/+ single mutants (Table 3).
Fig. 4.
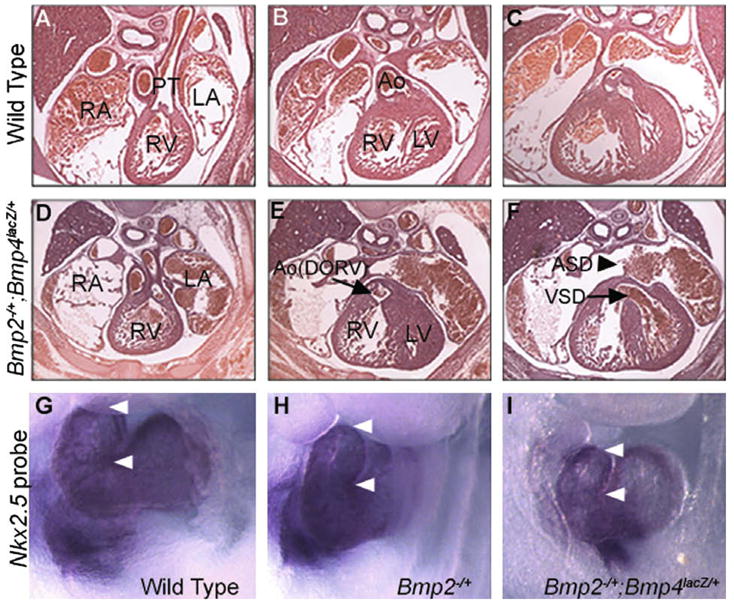
Defects in heart septation and OFT lengthening in Bmp2−/+; Bmp4lacZ/+ compound mutants. (A–F) Hematoxylin and eosin stained cross sections through the thoracic region of an E15.5 wild type (A–C) or Bmp2−/+; Bmp4lacZ/+ mutant embryo (D–F). The mutant heart shows inappropriate communication between the aorta (Ao) and the right ventricle (RV) (DORV, arrow in E) as well as ventricular (VSD, arrow in F) and atrial septal defects (ASD, arrow head in F). PT, pulmonary trunk; RA, right atrium; LA, left atrium; LV, left ventricle. (G–I) Expression of Nkx2.5 in E9.5 embryos, analyzed by whole mount in situ hybridization to highlight heart structures. The OFT of Bmp2−/+; Bmp4lacZ/+ mutants is shortened relative to that of stage-matched controls. White arrowheads mark the distal and proximal ends of the OFT. Variable expression of Nkx2.5 was observed regardless of genotype (data not shown).
Table 3.
Heart defects in Bmp2 and Bmp4 single and double mutants.
| Defect | Bmp4lacZ/+ | Bmp2−/+ | Bmp2−/+; Bmp4lacZ/+ |
|---|---|---|---|
| DORV | 1/12 | 0/7 | 6/13 |
| VSD | 1/12 | 0/7 | 10/15 |
| Complete AVCD | 0/12 | 0/7 | 3/16 |
| ASD | 0/12 | 0/7 | 7/11 |
Data are presented as number defective/total analyzed. E15.5–E18.5 embryos were analyzed.
Proper positioning of the great arteries requires sufficient OFT lengthening and rotation (Bajolle et al., 2006). To ask whether the defect in positioning of the outflow vessels (DORV) that we observed in Bmp2−/+; Bmp4lacZ/+ embryos is predated by defects in OFT morphogenesis in earlier embryos, we examined OFT morphology in stage-matched control and Bmp2−/+; Bmp4lacZ/+ embryos at E9.5. Expression of Nkx2.5 was analyzed by whole mount in situ hybridization to visualize the heart. We did not detect a consistent decrease in Nkx2.5 expression in Bmp2−/+; Bmp4lacZ/+ mutants relative to controls but shortening of the OFT was observed in 50% of Bmp2−/+; Bmp4lacZ/+ embryos (Fig. 4I, n = 4) relative to Bmp4lacZ/+ (data not shown), Bmp2−/+ (Fig. 4H) or wild type littermates (Fig. 4G). These data suggest that decreased BMP signaling prevents the earliest stages of OFT elongation, accounting at least in part for the OFT defects observed at later stages.
Bmp2 and Bmp4 are required individually for initial formation of the AV cushions (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006) and for later stages of AV canal septation (Jiao et al., 2003), respectively, and we found that they also function in combination during septation of the AV canal. A subset of Bmp2−/+; Bmp4lacZ/+ mutant embryos showed complete AV canal defects (AVCDs), in which a single AV canal was present that contained a common valve (arrowheads) instead of distinct mitral and tricuspid valves (Fig. 5B). By contrast, AVCDs were never detected in Bmp2−/+ or Bmp4lacZ/+ single mutants (Table 3).
Fig. 5.
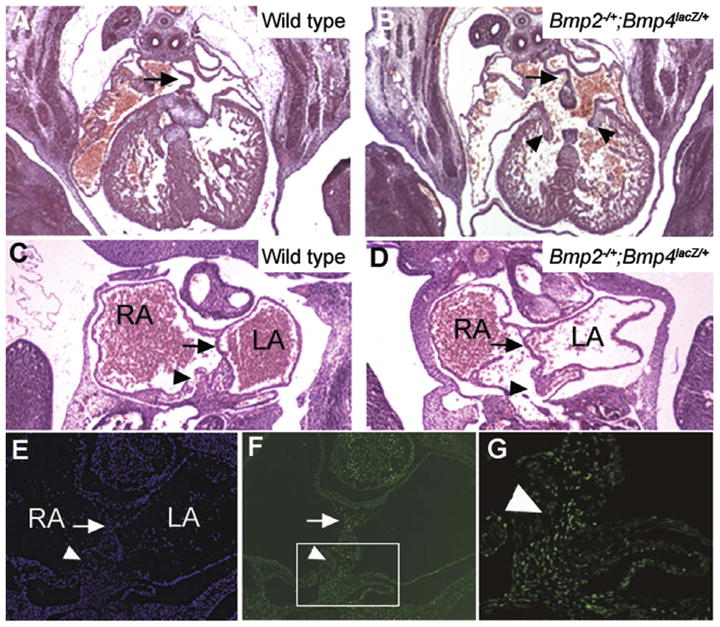
AV canal defects in Bmp2−/+; Bmp4lacZ/+ compound mutants. (A and B) Hematoxylin and eosin stained cross sections through the thoracic region of an E12.5 wild type (A) or Bmp2−/+; Bmp4lacZ/+ mutant embryo (B) reveals the complete absence of AV canal septation and the presence of a common set of AV valves (arrowheads) in the mutant heart. Arrows indicate an intact ASP. (C and D) Frontal sections through an E11.5 wildtype (C) or Bmp2−/+; Bmp4lacZ/+ compound mutant embryo (D) reveals reduction of tissue in the AV mesenchymal mass (arrowheads) whereas the ASP is intact (arrows). (E–G) Hoeschst (E) and pSmad1/5/8 immunofluorescent staining (F, low power and G, high power of boxed region in F) of E11.5 frontal section through the heart of a wild type embryo. Extensive pSmad1/5/8 staining is seen in the ASP (arrow) and in the mesenchymal mass (arrowhead) that septates the AV canal.
To further understand the ontogeny of these defects, we examined the morphology of the AV canal in frontal sections of the heart in E11.5 (45 somites) embryos. Tissues that contribute to AV septation include the muscular atrial septum primum (ASP), which is derived from the FHF, the mesenchymal cap carried on its leading edge and the AV cushions, both of which are derived from endocardial cells that have undergone EMT, and a distinct mesenchymal cell population known as the atrial spine or the dorsal mesenchymal protrusion, that is derived from the SHF (Lamers and Moorman, 2002; Mommersteeg et al., 2006; Snarr et al., 2007a, 2007b; Webb et al., 1998). These three mesenchymal cell populations must fuse to form the AV mesenchymal complex (Fig. 5C, arrow) in order for normal AV canal septation to occur. In addition to the previously mentioned defects in formation of the ASP (Fig. 4F), which lead to partial AVCD, we detected holes in the AV mesenchymal mass in a subset of Bmp2−/+; Bmp4lacZ/+ embryos (Fig. 5C and D), causing complete AVCD.
To determine which of the cell populations that contribute to formation of the AV septum are normally responsive to BMP signaling, we analyzed expression of pSmad1/5/8 by immunostaining frontal sections through the heart of E10 and E11.5 WT embryos. As shown in Fig. 5E–G, cells in the ASP (arrow), and in the AV mesenchymal complex (F, boxed region and G), including those in the atrial spine, express high levels of pSmad1/5/8.
Previous studies have shown that BMP signals are required for multiple aspects of OFT morphogenesis including elongation, septation and valve formation (Delot, 2003; Kaartinen et al., 2004; Liu et al., 2004; Stottmann et al., 2004; Yang et al., 2006). The current studies show that the requirement of BMPs for elongation of the OFT and positioning of great vessels can be uncoupled from that required for septation of the distal OFT and valve formation. This may reflect differences in BMP dosage sensitivity, ligand specificity, or the mechanisms underlying these distinct processes. Bmp4 expressed in the splanchnic and branchial arch mesoderm, the aorticsac and OFT myocardium has been shown to be uniquely required for OFT septation and valve formation (Liu et al., 2004) and the current studies suggest that BMP2 may not contribute to this process. By contrast, BMP2 and BMP4 (these studies), together with BMP7 (Liu et al., 2004), have overlapping functions in promoting elongation of the OFT. BMPs have been proposed to play multiple roles in this process. For example, BMPs are required to signal to neural crest cells to enable them to fully populate the OFT. When these signals are blocked by deletion of BmpR1a in neural crest cells in mouse (Stottmann et al., 2004) or by physical ablation of the BMP-dependent cardiac neural crest in chick (Yelbuz et al., 2002) the OFT fails to elongate normally due to defective migration of myocardial cells from the SHF into the OFT. Bmp2, Bmp4, and Bmp7 are expressed in, or adjacent to, the developing neural crest (Dudley and Robertson, 1997) and may be the ligands that enable these cells to properly colonize the OFT. Alternatively, BMP2, BMP4, and/or BMP7 may signal directly to the OFT myocardium or its progenitors, as suggested by analysis of Bmp7 null mutants that also have reduced levels of Bmp4 specifically in the OFT (Liu et al., 2004). In the chick, BMP2 has been proposed to be required for myocardial differentiation of the splanchic mesoderm that populates the OFT (Waldo et al., 2001), and it is possible that BMP2, BMP4 and/or BMP7 have assumed this function in mouse. Curiously, failure to downregulate expression of Bmp2 during heart development leads to truncation of the OFT due to decreased proliferation of SHF cells, and these defects can be rescued by deletion of one copy of Smad1 (Prall et al., 2007). BMP4 has also been shown to negatively regulate proliferation of myocardial cells within the OFT (Liu et al., 2004). Thus, these ligands play both positive and negative roles in outgrowth of the OFT.
Taken together, our data demonstrate that BMP2 and BMP4 function redundantly to regulate critical aspects of AV canal septation. Previous studies have indicated that BMP2 signals from the myocardium are uniquely required for initial formation of the AV cushions, while BMP4 is required after the cushions have formed, to stimulate proliferation of cushion cells. Although BMP2 is not sufficient to promote normal AV canal septation in the absence of BMP4 (Jiao et al., 2003), both ligands clearly contribute to this process since a combined reduction in Bmp2 and Bmp4 gene dosage leads to AVCDs that are not observed in either single mutant. Further studies will be required to determine the exact cell populations that are affected in these mutants, although our preliminary analysis suggests that BMP2 and BMP4 are required for normal formation, proliferation and/or survival of both the ASP and of AV mesenchymal cells, including the atrial spine.
3. Experimental procedures
3.1. Mouse embryo collection
Bmp4lacZ/+ mice were obtained from Dr. B. Hogan and genotyped as described (Lawson et al., 1999). Bmp4S2G/S2G mice were genotyped as described (Goldman et al., 2006). Bmp4lacZ/+ and Bmp4S2G/S2G mice were backcrossed for a minimum of six generations to C57BL/6J prior to mating with Bmp2−/+ mice (Zhang and Bradley, 1996) obtained from Dr. Y. Mishina on a pure C57BL/6J background. Mice carrying the Bmp2 mutation were identified by the presence of a ~550 bp PCR product using the primers 5′-CAAACAGATGCAAGAGGAT TATGA-3′ and 5′-TGAGCCCAGAAAGCGAAGGA-3′ with an annealing temperature of 60 °C.
3.2. Histology, immunostaining and in situ hybridization
Embryos and placentas were fixed and processed for histology, wholemount and section immunostaining, and in situ hybridization as previously described (Goldman et al., 2006). For phosphoSmad staining, embryos and placentas were fixed for 45 min to 1 h on ice, whereas for all other staining procedures, embryos were fixed overnight at 4 °C. For immunostaining, anti-β-galactosidase (Immunology Consultants Laboratory, 1:500), anti-pSmad (gift of Dr. Ed Laufer, 1:1000) and anti-PECAM MEC 13.3 (BD Pharmingen, 1:400) were used. An Nkx2.5 probe was generated by subcloning a BamHI/XhoI fragment from Pgk:Nkx2.5 obtained from I. Skerjanc.
3.3. Skeletal preparations
Embryonic and adult skeletons were processed and stained with Alcian Blue and Alizarin Red as previously described (Goldman et al., 2006).
3.4. RT-PCR
Fetal vessels atop the chorionic plate were manually dissected from E10.5 placentas. As a positive control, E10.5 hearts were collected separately. Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. cDNA was reverse transcribed with an oligo d(T) primer using an AMV Reverse Transcriptase Kit (Life Sciences) and following the manufacturer’s instructions. To amplify a 723 bp Bmp2 cDNA fragment, the primers 5′-AGACGTC CTCAGCGAATTG-3′ (exon 2) and 5′-CGCTGTTTGTGTTTG GCTTC-3′ (exon 3) were employed. These primers span an intron >6 kb.
3.5. Statistical analysis
Chi-square analysis was used to evaluate intercross data. A P value of <0.05 was considered statistically significant.
Acknowledgments
We thank Dr. B.L.M. Hogan for the Bmp4lacZ/+ mice, Dr. E.N. Meyers for help with data interpretation, Y. Mishini for helpful comments on the manuscript, and J. Cheney for technical assistance. This work was supported in part by a postodoctoral fellowship from the American Heart Association to D.C.G. and by a grant from the NIH to JLC (RO1 HD42598).
References
- Abdelwahid E, Rice D, Pelliniemi LJ, Jokinen E. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. [DOI] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res. 2006;98:421–428. doi: 10.1161/01.RES.0000202800.85341.6e. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4 and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S, Williams T. Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays. 2004;26:1307–1321. doi: 10.1002/bies.20137. [DOI] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, Wu DK. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;236:2493–2501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- Delot EC. Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways. Mol Genet Metab. 2003;80:27–35. doi: 10.1016/j.ymgme.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Dehart DB, Sulik KK, Hogan BL. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development. 2002;129:4685–4696. doi: 10.1242/dev.129.20.4685. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Dunn NR, Hogan BL. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc Natl Acad Sci USA. 2001;98:13739–13744. doi: 10.1073/pnas.241508898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97:219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, Christian JL. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 2006;133:1933–1942. doi: 10.1242/dev.02368. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, Mishina Y, Garbers DL, Zhao GQ. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004;276:158–171. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Boorla S, Frendo JL, Hogan BL, Karsenty G. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet. 1998;22:340–348. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers WH, Moorman AF. Cardiac septation: a late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res. 2002;91:93–103. doi: 10.1161/01.res.0000027135.63141.89. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial–mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M. Conservation and divergence of BMP2/4 genes in the lamprey: expression and phylogenetic analysis suggest a single ancestral vertebrate gene. Evol Dev. 2004;6:411–422. doi: 10.1111/j.1525-142X.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Mommersteeg MT, Soufan AT, de Lange FJ, van den Hoff MJ, Anderson RH, Christoffels VM, Moorman AF. Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res. 2006;99:351–353. doi: 10.1161/01.RES.0000238360.33284.a0. [DOI] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selever J, Liu W, Lu MF, Behringer RR, Martin JF. Bmp4 in limb bud mesoderm regulates digit pattern by controlling AER development. Dev Biol. 2004;276:268–279. doi: 10.1016/j.ydbio.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Snarr BS, O’Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007a;101:971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007b;236:1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–2218. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Webb S, Brown NA, Anderson RH. Formation of the atrioventricular septal structures in the normal mouse. Circ Res. 1998;82:645–656. doi: 10.1161/01.res.82.6.645. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood) 2007;232:979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, Kirby ML. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.cir.0000023044.44974.8a. [DOI] [PubMed] [Google Scholar]
- Ying Y, Qi X, Zhao GQ. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci USA. 2001;98:7858–7862. doi: 10.1073/pnas.151242798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]


