Abstract
The growth factors that drive the division and differentiation of stem cells during early human embryogenesis are unknown. The secretion of endorphins, progesterone (P4), human chorionic gonadotropin, 17β-estradiol, and gonadotropin-releasing hormone by trophoblasts that lie adjacent to the embryoblast in the blastocyst suggests that these pregnancy-associated factors may directly signal the growth and development of the embryoblast. To test this hypothesis, we treated embryoblast-derived human embryonic stem cells (hESCs) with ICI 174,864, a δ-opioid receptor antagonist, and RU-486 (mifepristone), a P4 receptor competitive antagonist. Both antagonists potently inhibited the differentiation of hESC into embryoid bodies, an in vitro structure akin to the blastocyst containing all three germ layers. Furthermore, these agents prevented the differentiation of hESC aggregates into columnar neuroectodermal cells and their organization into neural tube-like rosettes as determined morphologically. Immunoblot analyses confirmed the obligatory role of these hormones; both antagonists inhibited nestin expression, an early marker of neural precursor cells normally detected during rosette formation. Conversely, addition of P4 to hESC aggregates induced nestin expression and the formation of neuroectodermal rosettes. These results demonstrate that trophoblast-associated hormones induce blastulation and neurulation during early human embryogenesis.
Introduction
Trophoblastic production of endorphins [1] suggests important, yet undefined, roles for this hormone during embryonic growth and development. Trophoblasts also have been reported to secrete an array of other pregnancy-associated hormones including progesterone (P4), human chorionic gonadotropin (hCG), 17β-estradiol, and gonadotropin-releasing hormone (GnRH) [1]. Given the close spatial localization of trophoblasts to the embryoblast, it is conceivable that these pregnancy-associated factors may directly signal the growth and development of the embryoblast. Evidence supporting this notion includes the presence of placental opioid-enhancing factor in amniotic fluid and placenta [2]. Likewise, trophoblastic and corpa luteal production of hCG/P4 is markedly elevated postconception and is obligatory for the maintenance of pregnancy [3]. This pilot study was therefore undertaken to determine if modulating opioid receptor and P4 receptor (PR) signaling would affect early embryonic growth and development. Our results indicate that both opioid and progesterone signaling are vital for normal blastulation and neurulation.
Materials and Methods
Human embryonic stem cell culture
Pluripotent H9 human embryonic stem cells (hESC; passage 29–33 XX karyotype; also known as WA09) obtained from WiCell Research Institute (Madison, WI, USA) were cultured on irradiated mouse embryonic fibroblast (MEF) feeder cells (BioVintage, CA, USA) plated on gelatin-coated wells and grown in Dulbecco's modified Eagle's medium (DMEM)-F12 supplemented with 20% Knockout™ Serum Replacement (KOSR), 1% nonessential amino acids (NEAAs), 1 mM l-glutamine, 4 ng/mL basic fibroblast growth factor (bFGF) (all from Invitrogen, Carlsbad, CA, USA) and 0.1 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). For experiments examining the effects of P4, hESC were cultured in TESR1 media (lacking Li) and treated with P4 [2 μM; stock P4 was solubilized in dimethyl sulfoxide (DMSO) and then diluted into media] or control (the equivalent volume of DMSO) for 9 days and cells collected for the measurement of nestin expression by immunoblot analysis as previously described [4,5].
Embryoid body formation
H9 hESC colonies were cultured for 4 days on an MEF feeder layer as described above. Intact colonies were enzymatically detached and cultured on an orbital shaker at 5% CO2 in medium containing Iscove's modified Dulbecco's medium and 20% fetal bovine serum (embryoid body media; both from Invitrogen) with or without ICI 174,864 (0.1 mM; Tocris Bioscience, Ellisville, MO, USA; stock ICI 174,864 was solubilized in water) and/or RU-486 (20 μM; Sigma Laboratories, St. Louis, MO, USA; stock RU-486 was solubilized in EtOH and then diluted into media) for another 10 days (when embryoid body formation occurs under normal conditions—day 14). Controls were treated with the equivalent volume of water or EtOH. Structures were then examined morphologically and the area of the structures quantitated using Image J Software (http://rsb.info.nih.gov/ij/).
Neuroectodermal cell formation
hESC were differentiated into columnar neuroectodermal cells as described elsewhere [6] a protocol that mimics in vivo neuroectodermal development in terms of timing and morphology. In brief, hESC colonies were removed intact from the MEF layer after 4 days and were cultured in a special hESC growth medium (78.5% DMEM-F12, 20% KOSR, 1% NEAA, 1 mM l-glutamine, 0.1 mM 2-mercaptoethanol) for a further 4 days with daily replacement of media to form hESC aggregates. Colonies were then adhered to the culture surface where they form monolayer colonies in a chemically defined neural induction medium [32.6% F-12, 65.2% DMEM, 1% N2 supplement, 1% NEAA, 10 ng/mL bFGF (all from Invitrogen), 0.2% of 1 mg/mL Heparin (Sigma-Aldrich)]. Under this culture condition, columnar neuroectodermal cells appear in the center of each colony and organize into neural tube-like rosettes after a total of 9–10 d of differentiation culture. The neural induction media was replaced every other day. The neuroectodermal cells in the rosettes were selectively isolated through differential enzymatic treatment using dispase (0.5 mg/mL in DMEM-F12) and incubated for 2 h in neural induction medium to allow the nonneural cells to differentially attach to the flask. The floating cells (mostly aggregates of neuroectodermal cells) were transferred to new flasks where they rolled up to form round clusters. For treatments, cells were cultured in the neural induction media in the presence of RU-486 (20 μM) and/or ICI 174,864 (0.1 mM), and then examined morphologically and the cells collected for measurement of nestin expression by immunoblot analysis [4,5].
Results and Discussion
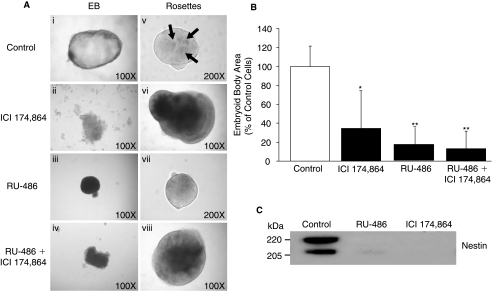
To investigate opioid signaling during early human embryogenesis, we utilized hESC derived from the inner cell mass of the blastocyst as a model of early embryogenesis [4,5]. Treatment of hESC colonies with the δ-opioid receptor–selective antagonist ICI 174,864 [7,8] for 10 days inhibited the formation of the embryoid body cystic structure (cavitation), and instead formed nonspherical structures that were ∼40% the size of normal spheroidal embryoid bodies (Fig. 1A and B). ICI 174,864 also inhibited normal neuroectodermal rosette formation from hESC, inducing a more dense morphology compared with control rosettes (Fig. 1A). To confirm that blocking opioid signaling inhibits hESC differentiation into neuroectodermal cells, the treated hESC were collected for immunoblot analysis and probed with a monoclonal antibody against human nestin, an early marker of neural precursor cell formation. Two bands representative of nestin (205 and 220 kDa) were detected in control rosettes, but neither band was detected in cell aggregates treated with ICI 174,864 (Fig. 1C). These results indicate that ICI 174,864, in addition to disrupting the normal development of hESC into embryoid bodies, also inhibits neuroectodermal rosette formation demonstrating that δ-opioid receptors are required for normal human blastulation and neurulation.
FIG. 1.
RU-486 and ICI 174,864 prevent embryoid body and neuroectodermal rosette formation. (A) Embryoid body formation: H9 hESC colonies (Day 4) were cultured in the presence of (i) embryoid body media containing (ii) ICI 174,864 (0.1 mM), (iii) RU-486 (20 μM), or (iv) RU-486 (20 μM) + ICI 174,864 (0.1 mM). Colonies were followed for 10 days (when embryoid body formation occurs under normal conditions; Day 14) and then their structure examined morphologically. Rosette formation: H9 hESC colonies (Day 4) were cultured in hESC growth media for 4 days before being placed in (v) neural induction media containing (vi) ICI 174,864 (0.1 mM), (vii) RU-486 (20 μM), or (viii) RU-486 (20 μM) + ICI 174,864 (0.1 mM). Colonies were assessed morphologically at 17+ days (when rosette formation occurs). Control structures typically display a minimum of three rosettes within the neuroectodermal aggregate (arrows). Figures are representative examples of each treatment and have been converted to grayscale. (B) Embryoid body size was quantified using Image J Software. Results are expressed as mean ± SEM, n = 6 (*P < 0.05, **P < 0.01 compared to control). (C) Structures in (Av-viii) were collected and an equal amount of protein run on SDS-PAGE and the immunoblot probed with the 10C2 monoclonal antibody against human nestin (Chemicon, Temecula, CA, USA).
δ-Opioid antagonists may function to inhibit embryogenesis by regulating hCG release [9] required for P4 production. Trophoblastic and corpa luteal production of hCG/P4 is markedly elevated postconception and is obligatory for the maintenance of pregnancy [3]. Inhibition of P4 signaling using the PR antagonist RU-486 is known to result in endometrial decidual degeneration, trophoblast detachment and decreased hCG production from the syncytiotrophoblast, and P4 production from the corpus luteum [10]. In addition, RU-486 induces cervical softening and dilatation, release of endogenous prostaglandins and an increase in myometrial sensitivity to the contractile effects of prostaglandins leading to the expulsion of the embryo/fetus. Although these abortive actions of RU-486 are well demonstrated, the effect of suppressing P4 production with RU-486 on the development of the early human embryo has not been studied.
Based on the above observations that opioid signaling is important for embryogenesis and the relationship between opioid signaling and P4 production [9], we tested the relevance of P4 signaling in early embryogenesis using RU-486. Treatment of hESC colonies with RU-486, like ICI 174,864, prevented the development of embryoid bodies (Fig. 1A); hESC colonies failed to form normal cystic structures after 10 days in culture, and instead formed solid irregular spheres that were ∼20% the size of normal spheroidal embryoid bodies (Fig. 1B). Since P4 also is a known neurotrophic factor in the adult brain [11], we examined whether PR signaling is required for neurogenesis. RU-486 treatment of hESC colonies blocked the normal differentiation of hESC into neuroectodermal rosettes cultured in neural induction media containing P4 (Fig. 1A; RU-486 is a competitive receptor antagonist in the presence of P4). This was confirmed by the suppression of nestin expression in RU-486–compared to P–treated hESC aggregates (Fig. 1C). These results indicate the obligatory role of PR signaling in normal embryoid body and neuroectodermal rosette formation during early embryogenesis. To test whether P4 is directly required for neuroectodermal rosette formation, we treated hESC aggregates with P4 and measured nestin expression. P4 induced the expression of the 205- and 220-kDa forms of nestin (Fig. 2) confirming the requirement for P4 signaling in the formation of neuroectodermal rosettes. Together, these results suggest that the upregulation of trophoblastic P4 production following conception [3] is required not only for trophoblast attachment, but also for the normal development of the embryo. These results also reveal for the first time the basis for why neural induction media induces neural precursor cell formation; the N2 supplement used in the neural induction media contains P4.
FIG. 2.
P4 induces hESC differentiation into neural precursor cells. H9 hESC colonies (Day 4) were cultured in TESR1 media with P4 (2 μM) or control (DMSO) for 9 days. Equal amounts of protein from cell lysates were analyzed by immunoblot using a monoclonal antibodies against nestin (clone 10C2; Chemicon). DMSO did not increase nestin expression (data not shown).
The relative binding affinity of RU-486 for PR is twice that of P4 [12], and is used at a dose of 200–600 mg for the termination of pregnancies (this equates to ∼6–19 μM, equivalent to that used in our study (20 μM; ref. 13)). Thus, blocking PR signaling with RU-486 during early pregnancy will block blastulation and neurulation.
Previous data has implicated P4 as acting in the arcuate nucleus and anteroventral periventricular nucleus through β-endorphin and dynorphin B neurons to affect preoptic area GnRH neurons and gonadotropin secretion [14,15]. Our results also suggest that there may be signaling crosstalk between P4 and opioids during early embryogenesis in the regulation of GnRH secretion, although the role of GnRH and gonadotropins during embryogenesis remains to be elucidated.
Conclusion
These data indicate for the first time that pluripotent hESC have an absolute requirement for steroid and opioid signaling during blastulation and neurulation. Furthermore, our data support a critical molecular signaling link between trophoblastic and/or maternal hormone production and early embryonic growth and development. The abortifacient effects of RU-486 therefore also extend to blocking normal embryonic growth and development.
Author Disclosure Statement
The authors declare no conflict of interest. This is Geriatrics Research, Education, and Clinical Center VA article number 2008.08.
References
- 1.Zhuang LZ. Li RH. Study on reproductive endocrinology of human placenta (II)—hormone secreting activity of cytotrophoblast cells. Sci China B. 1991;34:1092–1097. [PubMed] [Google Scholar]
- 2.DiPirro JM. Kristal MB. Placenta ingestion by rats enhances delta- and kappa-opioid antinociception, but suppresses mu-opioid antinociception. Brain Res. 2004;1014:22–33. doi: 10.1016/j.brainres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Larson P. Kronenberg H. Melmed S. Polonsky K. Williams Textbook of Endocrinology. Saunders; Philadelphia, PA: 2002. [Google Scholar]
- 4.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Porayette P. Gallego MJ. Kaltcheva MM. Meethal SV. Atwood CS. Amyloid-beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin. Biochem Biophys Res Commun. 2007;364:522–527. doi: 10.1016/j.bbrc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Li X. Zhang S. In vitro differentiation of neural precursors from human embryonic stem cells. Methods Mol Biol. 2006;331:169–177. doi: 10.1385/1-59745-046-4:168. [DOI] [PubMed] [Google Scholar]
- 7.Corbett AD. Gillan MG. Kosterlitz HW. McKnight AT. Paterson SJ. Robson LE. Selectivities of opioid peptide analogues as agonists and antagonists at the delta-receptor. Br J Pharmacol. 1984;83:271–279. doi: 10.1111/j.1476-5381.1984.tb10143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson SJ. Corbett AD. Gillan MG. Kosterlitz HW. McKnight AT. Robson LE. Radioligands for probing opioid receptors. J Recept Res. 1984;4:143–154. doi: 10.3109/10799898409042545. [DOI] [PubMed] [Google Scholar]
- 9.Cemerikic B. Schabbing R. Ahmed MS. Selectivity and potency of opioid peptides in regulating human chorionic gonadotropin release from term trophoblast tissue. Peptides. 1992;13:897–903. doi: 10.1016/0196-9781(92)90047-7. [DOI] [PubMed] [Google Scholar]
- 10.Fiala C. Gemzel-Danielsson K. Review of medical abortion using mifepristone in combination with a prostaglandin analogue. Contraception. 2006;74:66–86. doi: 10.1016/j.contraception.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Stein DG. Wright DW. Kellermann AL. Does progesterone have neuroprotective properties? Ann Emerg Med. 2008;51:164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Heikinheimo O. Kekkonen R. Lahteenmaki P. The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action. Contraception. 2003;68:421–426. doi: 10.1016/s0010-7824(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 13.Exelgyn and Laboratories (February 2006) “Mifegyne UK Summary of Product Characteristics (SPC)” Retrieved on September 9, 2008. http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid=617 http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid=617
- 14.Gu G. Simerly RB. Hormonal regulation of opioid peptide neurons in the anteroventral periventricular nucleus. Horm Behav. 1994;28:503–511. doi: 10.1006/hbeh.1994.1048. [DOI] [PubMed] [Google Scholar]
- 15.Dufourny L. Caraty A. Clarke IJ. Robinson JE. Skinner DC. Progesterone-receptive beta-endorphin and dynorphin B neurons in the arcuate nucleus project to regions of high gonadotropin-releasing hormone neuron density in the ovine preoptic area. Neuroendocrinology. 2005;81:139–149. doi: 10.1159/000086527. [DOI] [PubMed] [Google Scholar]