Abstract
Context
Few studies directly compare amygdala function in depressive and anxiety disorders. Data from longitudinal research emphasize the need for such studies in adolescents.
Objective
To compare amygdala response to varying attention and emotion conditions among adolescents with major depressive disorder (MDD) or anxiety disorders, relative to adolescents with no psychopathology.
Design
Case-control study.
Setting
Government clinical research institute.
Participants
Eighty-seven adolescents matched on age, sex, intelligence, and social class: 26 with MDD (14 with and 12 without anxiety disorders), 16 with anxiety disorders but no depression, and 45 without psychopathology.
Main Outcome Measures
Blood oxygen level–dependent signal in the amygdala, measured by means of event-related functional magnetic resonance imaging. During imaging, participants viewed facial expressions (neutral, fearful, angry, and happy) while attention was constrained (afraid, hostility, and nose-width ratings) or unconstrained (passive viewing).
Results
Left and right amygdala activation differed as a function of diagnosis, facial expression, and attention condition both when patients with comorbid MDD and anxiety were included and when they were excluded (group × emotion × attention interactions, P≤.03). Focusing on fearful face–viewing events, patients with anxiety and those with MDD both differed in amygdala responses from healthy participants and from each other during passive viewing. However, both MDD and anxiety groups, relative to healthy participants, exhibited similar signs of amygdala hyperactivation to fearful faces when subjectively experienced fear was rated.
Conclusions
Adolescent MDD and anxiety disorders exhibit common and distinct functional neural correlates during face processing. Attention modulates the degree to which common or distinct amygdala perturbations manifest in these patient groups, relative to healthy peers.
Rates of anxiety and depression markedly increase in adolescence.1,2 Comorbidity data3–10 suggest that these conditions may share brain-based diatheses.11–13 However, noncomorbid cases of anxiety and depression2,10,14 raise questions about neural differences. In adults, biased amygdala engagement occurs in major depressive disorder (MDD)15–18 and anxiety disorders.19–24 For both conditions, increased amygdala activation has been reliably seen, suggesting shared neural-circuitry dysfunction. However, strong conclusions cannot be drawn because few studies directly contrast patient groups with each other and with healthy individuals.
Vital questions emerge on commonalities and distinctions between adolescent MDD and anxiety disorders. Work is important in this age group because most adult mood and anxiety disorders are preceded by adolescent disorders.5,6 Similar functional perturbations could present in adolescent and adult mood and anxiety disorders; alternatively, unique perturbations could present in adolescence that ultimately evolve into adult profiles. Studies of adolescents begin to consider these possibilities by charting early-emerging correlates of mood and anxiety disorders. Because anxiety disorders differ from MDD in several ways,1,2,10,25,26 specific neural correlates may be expected. Nevertheless, few neuroimaging studies compare adequately sized samples of patients with MDD and anxiety disorder at any age, and studies in adolescents appear to be especially rare. As in adults, initial findings in anxious adolescents27–30 and in individuals at risk for anxiety disorders31 show altered amygdala function relative to healthy subjects, with signs of enhanced activation to fearful faces.27,28,31
To our knowledge, only 2 studies have examined amygdala response to facial stimuli in adolescent MDD.28,29 Their results are inconsistent, with one study finding increased29 and the other decreased28 amygdala activity in MDD relative to healthy participants. Findings from 2 other studies32,33 suggest that biased amygdala function in individuals at risk for MDD occurs specifically when passively viewing emotional stimuli. Because neither study excluded subjects with anxiety disorders, the influence of anxiety on amygdala response remains unclear.
The primary goal of the present study is to compare amygdala engagement while varying attentional context and face-emotion stimuli among 3 groups of adolescents: patients with MDD, patients with anxiety disorder, and healthy subjects. Comparative analyses require “pure” groups, but previous research in adolescent MDD28,29,33,34 has included anxious individuals. Thus, we studied patients with MDD who did and did not have comorbid anxiety. Existing data support competing hypotheses. On the one hand, data in adults,16–21,24 together with the strong cross-sectional, longitudinal, and familial relationships among adolescent and adult anxiety and MDD,3–10,14 raise the expectation of overlapping amygdala perturbations, consistent with a “shared diathesis” perspective.11,12 On the basis of these data, one might expect similarly biased amygdala engagement in anxious and depressed adolescents relative to healthy peers. On the other hand, preliminary data suggest that amygdala engagement in anxious and depressed adolescents might vary with changing emotional state and attention,27,31–33 consistent with evidence of disorder-specific cognitive biases.13,35,36
METHODS
PARTICIPANTS
Eighty-seven adolescents were studied: 26 with MDD (14 with and 12 without anxiety disorders), 16 with anxiety disorders and no depression, and 45 without psychopathology. Patients with MDD were initially recruited; others were then selected from larger pools to form 3 groups, matched on age, sex, social class, and IQ (Table 1). While groups did not differ statistically on these variables, the anxiety group included somewhat younger subjects and more male subjects; we repeated all analyses covarying for age and sex. A previous report27 included 7 of the 16 patients with anxiety and 16 of the 45 healthy adolescents. In addition, another report examines genetic associations in some subjects from this sample but does not consider anxiety- or MDD-related differences in amygdala function.37
Table 1.
Demographic and Clinical Characteristics of Subjects With MDD, Anxiety Disorder, and No Psychopathology
| Measure | Healthy Controls (n=45) | MDD With and Without Anxiety Disorder (n=26) | MDD Without Anxiety Disorder (n=12) | Anxiety Disorder Without MDD (n=16) |
|---|---|---|---|---|
| Age, mean (SD), y | 13.93 (2.18) | 14.08 (2.23) | 14.20 (2.60) | 12.77 (1.85) |
| IQ, mean (SD) | 111.62 (13.57) | 110.38 (18.05) | 113.5 (21.82) | 112.14 (14.53) |
| SES,a mean (SD) | 52.00 (23.34) | 46.14 (19.34) | 42.1 (22.35) | 46.92 (24.62) |
| Female sex, No. (%) | 24 (53) | 15 (58) | 7 (58) | 5 (31) |
| DSM-IV diagnoses (current), No. (%) | ||||
| MDD | 0 | 26 (100) | 12 (100) | 0 |
| Any anxiety disorder | 0 | 14 (54) | 0 | 16 (100) |
| GAD | 0 | 10 (38) | 0 | 8 (50) |
| Social phobia | 0 | 8 (31) | 0 | 5 (31) |
| SAD | 0 | 7 (27) | 0 | 8 (50) |
| GAD alone | 0 | 3 (12) | 0 | 4 (25) |
| Social phobia alone | 0 | 1 (4) | 0 | 3 (19) |
| SAD alone | 0 | 2 (8) | 0 | 4 (25) |
| Pediatric Anxiety Rating Scale score, mean (SD) | NA | 15.32 (5.00) | 13.42 (4.76) | 16.44 (2.50) |
| Children’s Depression Rating Scale score, mean (SD) | 42.17 (8.43) | 59.12 (13.00) | 55.55 (13.40) | 46.86 (4.45) |
| Clinical Global Impressions Scale score, mean (SD) | NA | 4.73 (0.83) | 4.67 (0.89) | 4.19 (0.75) |
Abbreviations: GAD, generalized anxiety disorder; MDD, major depressive disorder; NA, not applicable; SAD, separation anxiety disorder; SES, socioeconomic status.
Index generated from occupational and educational level of parents (theoretical range, 20–137); higher values indicate higher SES.
Diagnoses were assessed by means of the Schedule for Affective Disorders and Schizophrenia for School-Aged Children.38 As described previously,27,29 patients with MDD or anxiety were required (1) to show persistent, impairing anxiety or depressive symptoms, respectively, during 3 weeks of supportive therapy and (2) to meet previously reported exclusion criteria. All patients with anxiety were without lifetime history of depression; all nonanxious patients with MDD were without lifetime history of anxiety. All were medication free, and only 1 had had past exposure to any anxiolytic, such as a selective serotonin reuptake inhibitor. The study was approved by the institutional review board of the National Institute of Mental Health. All participants and their parents provided written informed consent or assent.
TASK
We used functional magnetic resonance imaging (fMRI) with a previously described paradigm.27,29,31,39 Briefly, participants viewed 32 faces (8 each of neutral, fearful, angry, and happy),40–42 each presented for 4000 milliseconds, 4 times in one 160-trial run, divided into four 40-trial epochs (32 faces, 8 fixation trials) and four 10-trial blocks (8 faces, 2 fixation trials). During 3 blocks, participants adopted different constrained attention conditions by rating the face stimuli on 5-point scales (1 [not at all] to 5 [very]): (1) “How hostile is this face?” (2) “How afraid are you of this face?” and (3) “How wide is the nose?” During the fourth block, participants passively viewed the faces (unconstrained attention). Order of face emotions and of attention conditions was randomized. Ratings and reaction times (RTs) were recorded.
fMRI PROCEDURES
Whole-brain blood oxygen level–dependent (BOLD) fMRI data were acquired on one of two 3-T imagers in groups matched with regard to imager (χ2=4.05, df=2, P=.13). T2*-weighted images were acquired in 23 axial sections parallel to the anterior commissure–posterior commissure line by means of an echo-planar single-shot gradient echo pulse sequence (matrix,64 × 64; repetition time, 2000 milliseconds; echo time, 40 milliseconds; field of view, 240 mm; voxels, 3.75 × 3.75 × 5.00 mm). As reported previously,27,29 high-resolution T1-weighted anatomic images were acquired.
Data from subjects who moved more than 2.5 mm in any plane were discarded. Subsequent analyses were conducted with SPM99 (Wellcome Department of Neurology, London, England) and Matlab 6.1 (MathWorks, Natick, Massachusetts) routines. Functional data were corrected for section timing and motion, anatomically coregistered, and spatially normalized to the SPM99 Montreal Neurological Institute T1-weighted template. We used SPM99 to maximize parallels with previous work.27,31,43 Nevertheless, group analyses conducted with SPSS 15.0 (SPSS Inc, Chicago, Illinois) remedied analytic problems created by outdated aspects of SPM99.
DATA ANALYSIS
Behavioral Data
Ratings and RTs confirmed participants’ task adherence. Because of equipment malfunction, data for 3 participants were not recorded. Behavioral group differences were examined with analyses of variance (ANOVAs), with diagnostic group as the between-subjects factor and face emotion and attention condition as within-subjects factors. To minimize type I errors, the Greenhouse-Geisser correction was applied.44
fMRI Data
We estimated event-related response amplitudes at the individual-subject level for each face emotion type in each attention condition by using the general linear model. The waveform for each event-related response was a rectangular pulse (4 seconds) convolved with the SPM99 hemodynamic response function. We generated contrast images with the use of pairwise comparisons across event types. We then divided each contrast image by subject-specific voxel time series means.45
Group-level analyses used random-effects models.46 Previous findings documented amygdala abnormalities on this task in pediatric anxiety27 and bipolar43 disorders. Hence, we used a region-of-interest (ROI) strategy focused on the amygdala, defined by standard criteria47 on the Montreal Neurological Institute template. All subjects had BOLD activity data in greater than 65% of ROI voxels. The BOLD signal changes for each event vs fixation baseline were averaged across all amygdala voxels and were subjected in SPSS 15.0 to multifactorial analyses of complex 2- and 3-way interactions.
Our primary hypothesis was that overall between-group amygdala differences vary as a function of both face-emotion type and attention. We tested this with omnibus 3-way group × face-emotion × attention-condition interactions, in repeated-measures ANOVAs for each amygdala, with one 3-level between-subject factor (group) and two 4-level within-subject factors (emotion, attention). With the use of Greenhouse-Geisser correction, 2 analyses were conducted, which varied composition of the MDD group: (1) including 14 patients with comorbid anxiety in the MDD group (n=26) and (2) including only noncomorbid MDD cases (n=12). Focused post hoc analyses decomposed significant 3-way interactions. These post hoc analyses, informed by previous findings, compared amygdala activation (1) to fearful faces viewed across different attention conditions and (2) across all face types presented in the passive-viewing condition.
Data from 3 studies in patients with anxiety,27 youths at risk for anxiety,31 and youths at risk for depression33 had led us to expect between-group differences to fearful faces, specifically, relative to other face types, viewed in particular attention conditions: we expected amygdala hyperactivation in anxiety when participants monitored their subjective fear, relative to passively viewing fearful faces. This prediction was first investigated by a 2-factor repeated-measures ANOVA testing the significance of group × attention-condition interactions for amygdala activation to fearful faces, relative to fixation, across all 4 attention conditions. This was followed by 3-group ANOVAs (Brown-Forsythe test when unequal variances existed) and 2-group t tests for the a priori–defined “fearful-afraid vs fearful-passive” contrast.
Previous research also suggested that specific anxiety-related and depression-related biases manifest during passive viewing.27,28,31,33 On the basis of findings by McClure et al,27 Pérez-Edgar et al,31 and Monk et al,33 we expected greater amygdala activation in patients with MDD than in anxious and healthy individuals when passively viewing fearful faces. However, previous studies have generated inconsistent data concerning amygdala response to other face emotion types, viewed passively. Thus, we performed a 2-factor repeated-measures ANOVA including all face-emotion classes to test the significance of a group × face-emotion interaction in passive viewing; post hoc tests contrasted fearful vs other emotions.
Finally, although the present study focused on the amygdala, secondary analyses examined the orbitofrontal cortex (OFC), guided by previous research.15,16,20,27,34,48–50 Procedures followed those for the amygdala by extracting values for entire ROIs,27,29 defined by means of standard, validated anatomic criteria, as delineated in previous research.47 Of note, the OFC ROI used herein encompasses both medial and lateral inferofrontal expanses of prefrontal cortex. Because of susceptibility-related signal loss, 2 individuals were excluded, yielding 26 adolescents with MDD, 15 adolescents with anxiety, and 44 healthy adolescents.
In addition to ROI analyses, supplementary voxel-based analyses generated coordinates of between-group peak-activation differences. Because our work was guided by regionally based a priori hypotheses and to minimize type II errors in this 3-group study, we treated results from our ROI-based analyses as primary. Nevertheless, findings from voxel-based analyses replicated those in whole-structure ROI approaches while also informing future work; they are accordingly summarized by means of Montreal Neurological Institute coordinates.
RESULTS
SAMPLE CHARACTERISTICS AND BEHAVIOR
Table 1 provides sample demographic and clinical characteristics; Table 2 provides behavioral performance during imaging. Behavioral data showed the expected face-emotion × attention-condition interactions for ratings (F4.6,368.6=63.6, P < .001) and RTs (F5.5,446.9=15.4, P < .001). Both ratings and RTs for the “afraid” and “hostile” questions were highest for angry and fearful faces and lowest for happy faces; “nose” ratings and RTs were highest for happy and angry faces and lowest for neutral faces. No 2- or 3-way interactions with group were found for either ratings or RTs (P=.15 to .76). No significant main effects of group emerged on ratings (F2,81=1.2, P =.32) or RTs (F2,81=1.6, P =.20). Similar findings were shown when patients with comorbid MDD and anxiety were excluded (available upon request). Absence of group effects indicates that all groups similarly altered their behavior across emotion and attention conditions.
Table 2.
Task Performance by Group
| Behavioral Measures | Healthy Controls (n=45) | MDD With and Without Anxiety Disordera (n=25) | Anxiety Disorder Without MDDb (n=14) |
|---|---|---|---|
| Ratings, mean (SD) | |||
| How hostile–neutral faces | 1.74 (0.61) | 1.82 (0.56) | 1.86 (0.88) |
| How hostile–fearful faces | 2.04 (0.83) | 2.31 (0.89) | 2.27 (1.08) |
| How hostile–angry faces | 3.17 (1.01) | 3.42 (0.86) | 3.34 (0.96) |
| How hostile–happy faces | 1.10 (0.18) | 1.33 (0.42) | 1.53 (0.71) |
| How afraid–neutral faces | 1.49 (0.64) | 1.68 (0.69) | 1.69 (0.83) |
| How afraid–fearful faces | 1.83 (0.77) | 2.14 (0.99) | 1.93 (1.01) |
| How afraid–angry faces | 2.38 (0.99) | 2.52 (0.93) | 2.76 (1.23) |
| How afraid–happy faces | 1.14 (0.24) | 1.35 (0.49) | 1.41 (0.64) |
| How wide is the nose–neutral faces | 2.19 (0.58) | 2.12 (0.45) | 2.16 (0.40) |
| How wide is the nose–fearful faces | 2.17 (0.54) | 2.31 (0.62) | 2.15 (0.49) |
| How wide is the nose–angry faces | 2.59 (0.65) | 2.59 (0.60) | 2.77 (0.50) |
| How wide is the nose–happy faces | 2.59 (0.53) | 2.69 (0.53) | 2.52 (0.48) |
| Reaction times, mean (SD), ms | |||
| How hostile–neutral faces | 1820.19 (438.00) | 1986.28 (377.51) | 1894.08 (469.09) |
| How hostile–fearful faces | 2031.00 (495.40) | 2104.97 (398.01) | 1923.39 (373.43) |
| How hostile–angry faces | 1964.88 (400.65) | 2000.80 (384.47) | 2159.22 (445.21) |
| How hostile–happy faces | 1534.44 (351.09) | 1656.25 (428.98) | 1715.38 (278.31) |
| How afraid–neutral faces | 1692.53 (432.68) | 1925.43 (435.90) | 1713.75 (390.73) |
| How afraid–fearful faces | 1828.02 (421.88) | 1968.81 (370.44) | 1853.62 (414.40) |
| How afraid–angry faces | 1983.81 (443.39) | 2057.27 (495.37) | 2093.40 (564.12) |
| How afraid–happy faces | 1459.04 (370.12) | 1732.17 (421.51) | 1634.82 (368.52) |
| How wide is the nose–neutral faces | 1823.26 (363.40) | 1918.47 (310.54) | 2048.53 (308.56) |
| How wide is the nose–fearful faces | 1912.18 (345.08) | 1991.08 (316.78) | 1955.11 (260.15) |
| How wide is the nose–angry faces | 1971.25 (415.22) | 2082.00 (365.71) | 2145.36 (308.08) |
| How wide is the nose–happy faces | 1982.59 (394.67) | 2111.49 (340.06) | 2022.38 (271.16) |
Abbreviation: MDD, major depressive disorder.
Fourteen with and 11 without anxiety disorder. Because of equipment malfunction, data for 1 participant were not recorded.
Because of equipment malfunction, data for 2 participants were not recorded.
IMAGING
Amygdala Activation
We tested our primary hypothesis by using repeated-measures ANOVAs for BOLD responses in each amygdala. These analyses showed the expected 3-way group × face-emotion × attention-condition interactions in left and in right amygdalas (Table 3).
Table 3.
Statistical Analyses of Regions of Interest (Omnibus Repeated-Measures ANOVA)
| Effecta | Patients With Comorbid MDD/Anxiety Includedb |
Patients With Comorbid MDD/Anxiety Excludedc |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left Amygdala |
Right Amygdala |
Left Amygdala |
Right Amygdala |
|||||||||
| F Value | df | P Value | F Value | df | P Value | F Value | df | P Value | F Value | df | P Value | |
| Main effect | ||||||||||||
| Group (between-subject effect) | 0.98 | 2, 84 | .38 | 1.25 | 2, 84 | .29 | 0.52 | 2, 70 | .60 | 1.40 | 2, 70 | .25 |
| Emotion (within-subject effect) | 6.49 | 2.9, 240.1 | <.001d | 3.34 | 2.6, 219.2 | .03d | 4.45 | 2.8, 194.2 | .006d | 1.70 | 2.5, 177.4 | .18 |
| Attention (within-subject effect) | 7.53 | 2.8, 238.5 | <.001d | 0.16 | 2.8, 236.2 | .91 | 5.66 | 2.8, 196.1 | .001d | 0.15 | 2.6, 185.1 | .91 |
| 2-Way interaction | ||||||||||||
| Group × emotion | 2.16 | 5.7, 240.1 | .05 | 1.80 | 5.2, 219.2 | .11 | 2.02 | 5.6, 194.2 | .07 | 1.94 | 5.1, 177.4 | .09 |
| Group × attention | 3.00 | 5.7, 238.5 | .009d | 0.88 | 5.6, 236.2 | .50 | 2.74 | 5.6, 196.1 | .02d | 0.63 | 5.3, 185.1 | .69 |
| Emotion × attention | 1.21 | 7.4, 621.1 | .30 | 1.69 | 6.6, 553.6 | .12 | 1.45 | 7.2, 505.0 | .18 | 1.31 | 6.0, 416.6 | .25 |
| 3-Way interaction | ||||||||||||
| Group × emotion × attention | 2.68 | 14.8, 621.1 | .001d | 2.01 | 13.2, 553.6 | .02d | 2.71 | 14.4, 505.0 | .001d | 1.90 | 11.9, 416.6 | .03d |
Abbreviations: ANOVA, analysis of variance; MDD, major depressive disorder.
Results of omnibus repeated-measures analyses of variance (Greenhouse-Geisser corrected). “Group” includes MDD, anxiety, and control; “emotion,” neutral, fearful, angry, and happy; “attention,” hostile, afraid, nose, and passive.
Patients with MDD with and without comorbid anxiety disorder (n=26), patients with anxiety (n=16), and healthy controls (n=45).
Patients with MDD without comorbid anxiety disorder (n=12), patients with anxiety (n=16), and healthy controls (n=45).
Significant at P < .05.
Three-way interactions indicated that between-group differences in amygdala response varied with both face emotion and attention condition. Interactions were decomposed by post hoc tests focusing on hypothesized group differences. Specifically, differences were expected (1) in select attention conditions in fearful face–viewing events and (2) when fearful faces vs other face emotions were passively viewed.
Fearful Face Viewing
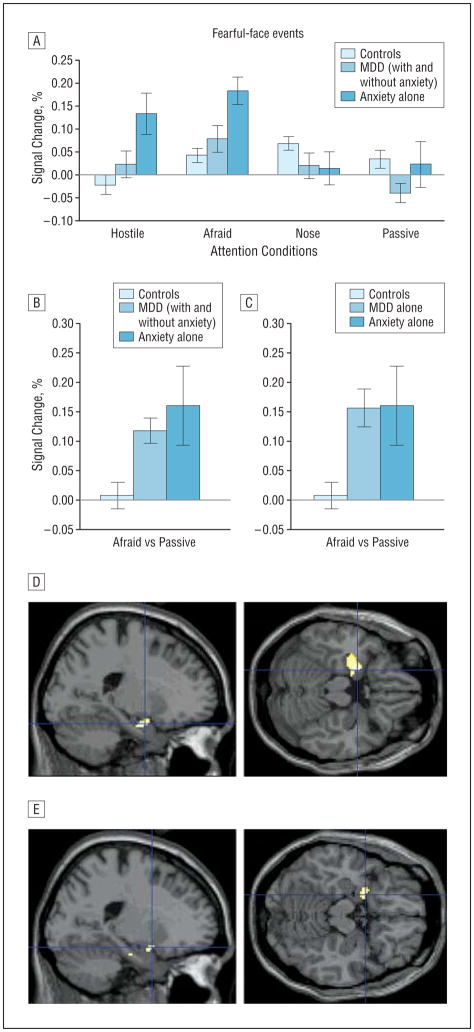
On the basis of previous research,27,31,33 we predicted between-group differences in amygdala response to fearful faces with hyperactivation in patients with anxiety during afraid ratings. As expected, significant bilateral group × attention-condition interactions emerged when patients with comorbid MDD and anxiety were included (left: F5.8,244.0=6.4, P < .001, Figure 1A; right: F5.5,231.0 =2.5, P =.03) or excluded (left: F5.6,197.8 =6.1, P < .001; right: F5.3,185.9=2.3, P =.05). Patients with anxiety showed the predicted amygdala hyperactivation when rating subjectively experienced fear to fearful faces but not when passively viewing these faces.
Figure 1.
Amygdala activation to fearful faces in patients with anxiety and major depressive disorder (MDD) relative to healthy controls for select attention conditions. A, Left amygdala activation to fearful faces relative to fixation (error bars reflect standard errors) displaying the group (healthy controls, MDD with and without anxiety disorder, anxiety disorder alone) × attention condition interaction. A similar activation pattern was found for the right amygdala and when patients with comorbid MDD/anxiety were excluded (not shown). B and C, Left amygdala activation to fearful faces during afraid ratings vs passive viewing (“fearful-afraid vs fearful-passive” contrast) showing significantly enhanced activation among both anxiety and MDD groups compared with healthy controls, with no difference between patients with anxiety and patients with MDD. D and E, The fearful-afraid vs fearful-passive contrast evidences significantly greater left amygdala activation in patients with anxiety alone than in controls (D) (Montreal Neurological Institute [MNI] coordinates: −20, −2, −20, P=.001 (shown in figure); −10, −4, −16, P=.002; MNI coordinates are small-volume corrected) and in patients with MDD alone than in controls (E) (MNI coordinates: −20, 4, −16, P=.007; small-volume corrected). Highlighted areas indicate regions where the differences in blood oxygen level–dependent activation between groups were significant (for display purposes, uncorrected threshold was set at P=.0005 [D] and P=.005 [E]).
In the a priori–defined fearful-afraid vs fearful-passive contrast, significant between-group differences were evident only in the left amygdala (patients with comorbid MDD and anxiety included: F2,25.8=4.7, P =.02, Figure 1B; excluded: F2,25.6=5.3, P =.01, Figure 1C). Patients in both the anxiety (t18.5 =2.2, P =.04; see also Figure 1D) and MDD (with and without anxiety: t69=3.2, P = .002; without anxiety: t55 = 3.2, P = .002; see also Figure 1E) groups showed greater amygdala activation than did healthy peers, with no significant differences between patient groups, thus supporting the “shared-diathesis” perspective.
We also compared groups on other fearful face contrasts (eg, afraid-nose, hostile-nose; compare Figure 1A). This demonstrated consistent evidence of increased activation in anxious subjects relative to healthy subjects and somewhat less consistent evidence of enhanced activation in depressed subjects relative to healthy subjects and in patients with anxiety relative to those with MDD (results available on request). Further analyses showed between-group differences in amygdala activation during afraid ratings to show some degree of emotion specificity: no between-group differences emerged for afraid ratings of neutral or happy faces (P > .35).
Passive Viewing
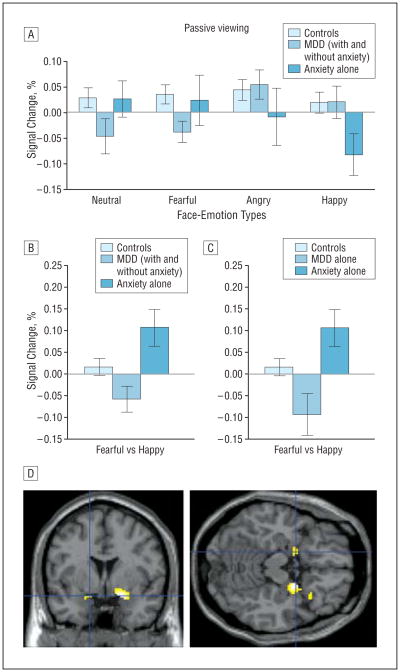
As noted previously, prior studies most consistently suggest disorder-specific biases under unconstrained attention conditions.27,28,31,33 Thus, we were particularly interested in between-group comparisons for passive-viewing events. Significant group × face-emotion interactions emerged in the left (F5.5,230.3=3.2, P =.006; Figure 2A) and right (F5.4,226.8=3.2, P =.04) amygdala. Similar results occurred when comorbid MDD/anxiety cases were excluded (left: F5.4,188.1=3.4, P =.005; right: F5.3,186.1=2.2, P=.05).
Figure 2.
Differential amygdala activation in patients with major depressive disorder (MDD) and patients with anxiety during passive viewing of fearful vs other face-emotion types. A, Left amygdala activation to passively viewed facial expressions relative to fixation (error bars reflect standard errors) among patients with MDD (with and without comorbid anxiety disorder), patients with anxiety disorder, and healthy controls displaying the group × face-emotion interaction in the passive viewing condition. A similar activation pattern was found for the right amygdala and when patients with comorbid MDD/anxiety were excluded (not shown). B and C, Patients with anxiety and those with MDD showed opposite and significantly different left (shown in figure) and right amygdala responses to fearful faces vs happy faces passively viewed (“fearful-passive vs happy-passive” contrast). Patients with MDD and those with anxiety also differed from healthy controls in left amygdala activation in this contrast. D, The fearful-passive vs happy-passive contrast evidences significantly greater left and right amygdala activation in patients with anxiety than in patients with MDD even when those with MDD with comorbid anxiety were excluded (Montreal Neurological Institute coordinates: left, −16, 2, −16, P=.01, small-volume corrected; right, 22, 0, −14, P=.001, small-volume corrected). Highlighted areas indicate regions where the differences in blood oxygen level–dependent activation between groups were significant (for display purposes, uncorrected threshold was set at P=.005).
Post hoc tests focused on fearful vs other face emotions because previous research did not generate more specific hypotheses. Interactions with group reflected amygdala activation differences for the fearful-passive vs happy-passive contrast, both when patients with comorbid MDD and anxiety were included (left: F2,84 =6.6, P=.002, Figure 2B; right: F2,84=5.1, P =.008) and when they were excluded (left: F2,70=6.5, P =.003, Figure 2C; right: F2,70=4.6, P =.01). Consistent with the “disorder-specificity” perspective, opposite patterns emerged in patient groups: anxious patients showed activation whereas depressed patients showed deactivation of amygdala response to fearful vs happy faces. This difference was significant whether patients with comorbid MDD and anxiety were included (left: t40=3.3, P =.002, Figure 2B; right: t40 =2.8, P =.008) or excluded (left: t26 =3.1, P =.004, Figure 2C; right: t26=2.4, P =.02). Both patient groups also showed significantly different responses from healthy controls, with amygdala hyperactivation in anxiety (left: t59=2.2, P =.03; right: t59=2.6, P =.01) and hypoactivation in MDD (left only; with or without comorbid anxiety disorder: t69=−2.2, P =.03; without comorbid anxiety disorder: t55=−2.4, P =.02).
Of note, post hoc results also showed that between-group differences reflected responses to passive-happy events, independent of the response to fearful faces. Comparing groups on the neutral-passive vs happy-passive contrast showed amygdala hyperactivation in anxiety, relative to both healthy controls and patients with MDD, similar to the fearful-passive vs happy-passive contrast. However, healthy controls and patients with MDD did not differ (P=.09). Further analyses demonstrated the between-group differences for happy faces to be specific to passive viewing: no between-group differences emerged during afraid or hostility ratings (P≥.37). Finally, we repeated all amygdala ROI analyses to covary age and sex. No differences in results occurred (available on request).
OFC Activation
Secondary analyses examined group differences in OFC in the a priori–defined fearful-afraid vs fearful-passive contrast. Results were largely consistent with those emerging in the amygdala-based analyses, both when patients with comorbid MDD and anxiety were included (left OFC: F2,82=3.2, P =.05, Figure 3A) and when they were excluded (left OFC: F2,68=2.7, P =.08, Figure 3B). Patients with anxiety showed significantly enhanced left OFC activation relative to healthy subjects (t57 =2.2, P =.04, Figure 3C); a nonsignificant trend emerged for the MDD vs control comparison, but only when patients with comorbid MDD and anxiety were included (t68=1.8, P =.07). No significant differences emerged between the anxiety and MDD groups.
Figure 3.
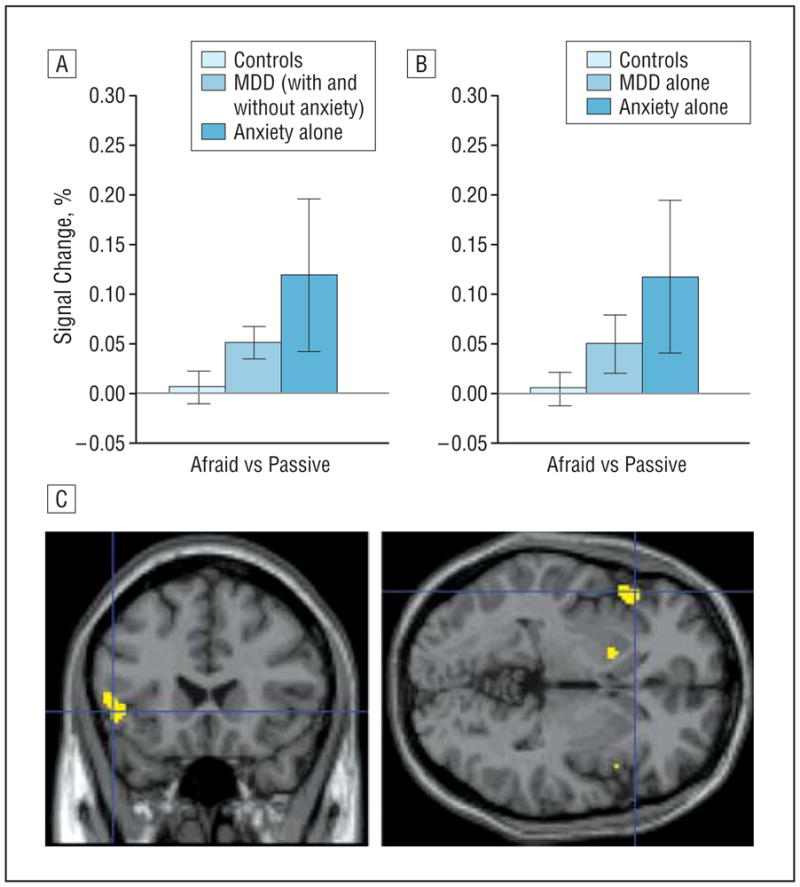
Orbitofrontal cortex (OFC) activation in the “fearful-afraid vs fearful-passive” contrast in patients with major depressive disorder (MDD) and anxiety. A and B, Left OFC activation to fearful faces during afraid ratings vs passive viewing (fearful-afraid vs fearful-passive contrast) (error bars reflect standard errors) showing significantly enhanced activation among patients with anxiety compared with healthy controls. C, The fearful-afraid vs fearful-passive contrast evidences significantly greater left lateral OFC activation in patients with anxiety than in controls (Montreal Neurological Institute coordinates: left, −50, 22, −2, P=.046 (shown in figure); −14, 18, −10, P=.05, small-volume corrected). Highlighted areas indicate regions where the differences in blood oxygen level–dependent activation between groups were significant (for display purposes, uncorrected threshold was set at P=.005).
We also examined group differences in the fearful-passive vs happy-passive contrast that evidenced disorder specificity in amygdala response. Between-group differences were also found in the right OFC, both when patients with comorbid MDD and anxiety were included (F2,82=4.2, P =.02, Figure 4A) and when they were excluded (F2,68=5.3, P =.007, Figure 4B). Patients with anxiety showed significantly greater activation than did patients with MDD (with and without comorbid anxiety: t39=2.1, P =.04; without comorbid anxiety: t25=2.5, P =.02) and than did healthy controls (t57=3.2, P =.002). Patients with MDD, however, did not differ from healthy controls.
Figure 4.
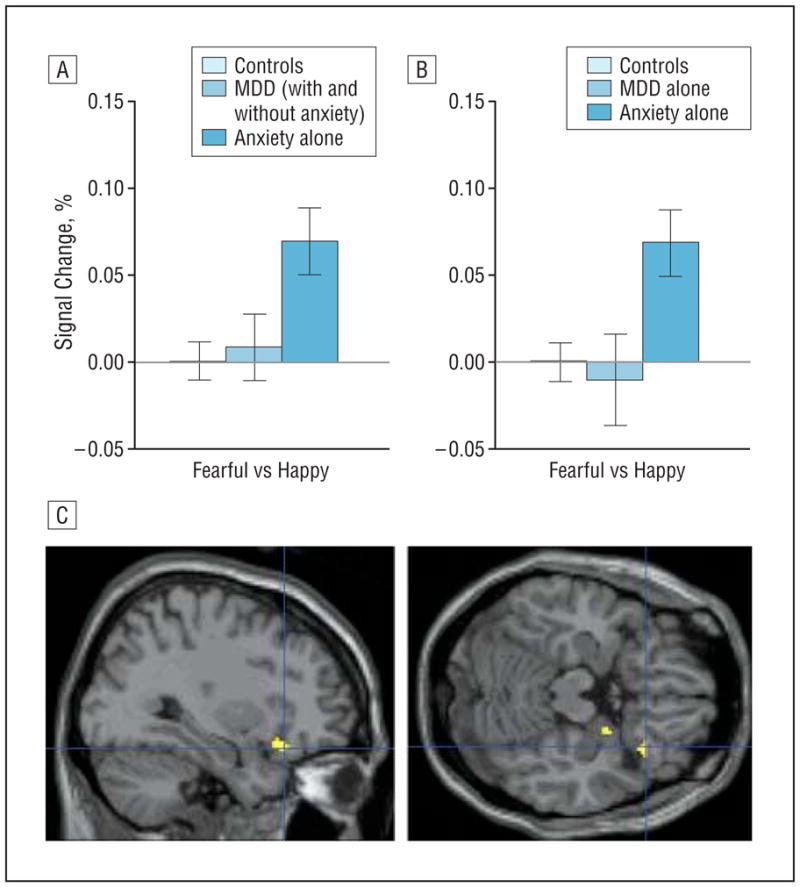
Orbitofrontal cortex (OFC) activation in the “fearful-passive vs happy-passive” contrast in patients with major depressive disorder (MDD) and anxiety. A and B, Right OFC activation to fearful faces during passive viewing of fearful vs happy faces (fearful-passive vs happy-passive contrast) (error bars reflect standard errors) showing significantly enhanced activation among patients with anxiety compared with those with MDD and healthy controls. C, The fearful-passive vs happy-passive contrast evidences significantly greater right lateral OFC activation in patients with anxiety than in those with MDD (with and without comorbid anxiety) (Montreal Neurological Institute coordinates: 32, 24, −18, P=.005, small-volume corrected; no suprathreshold voxels emerge for the anxiety vs MDD-alone comparison). Highlighted areas indicate regions where the differences in blood oxygen level–dependent activation between groups were significant (for display purposes, uncorrected threshold was set at P=.0005).
Repeating the OFC-related analyses covarying for age and sex did not change the results, with 1 exception. The significance of the difference between the anxiety and MDD groups in the fearful-passive vs happy-passive contrast was diminished when patients with comorbid MDD and anxiety were included (F1,37=2.8, P =.10) but not when MDD alone was considered (F1,23=5.1, P =.03).
COMMENT
The present study generated 2 key findings. First, when adolescents viewed faces expressing fear and focused their attention on internally experienced fear, relative to passive viewing, both patients with anxiety and those with MDD exhibited greater amygdala activation than did healthy peers. Second, distinct emotion-specific amygdala responses in MDD and anxiety disorders occurred during passive viewing, where patients also significantly differed from healthy peers.
The degree to which MDD and anxiety disorders represent nosologically distinct conditions remains unclear. Particularly intense debate occurs regarding youth. This arises in light of longitudinal data demonstrating strong but relatively nonspecific associations over time among MDD and anxiety disorders in adolescents and in adults.5,6,51,52 The present data suggest that adolescent anxiety disorders and MDD exhibit neural commonalities but also demonstrable differences, depending on the specific attention and emotion states engaged during fMRI. From a theoretical perspective, this suggests that adolescent anxiety disorders and MDD involve complex, overlapping, yet distinguishable patterns of amygdala-related biases. For some biases, related to subjective-state monitoring, similar perturbation of amygdala engagement and associated psychological processes may occur in MDD and anxiety. For other psychological processes spontaneously engaged during unconstrained, passive viewing of faces, disorder-specific biasing may occur. Viewed broadly, these data support the view of neural distinctions between MDD and anxiety as complex and nuanced but clearly demonstrable.
DISORDER SPECIFICITY
Our study finds evidence of specifically perturbed amygdala engagement in adolescent MDD and anxiety disorders, manifest in select attention states for specific face emotions. This conclusion emerges from our omnibus approach to between-group contrasts. Such a statistical approach is necessarily complex: it rests on tests of 3-way, group × emotion × attention interactions. Significant interactions emerge because between-group differences in anxious and depressed adolescents occur only when viewing fearful vs happy faces passively but not when viewing other emotions or when viewing these same emotions in other attention states.
Disorder specificity was expected during passive viewing, given previous research.27,28,31,33 However, differences between the present and these previous studies complicate cross-study comparisons. These differences encompass clinical features of samples, task-stimulus features, and task-related cognitive processes. Nevertheless, the finding that disorder specificity emerges during passive viewing is consistent with other work.27,28,31,33 This suggests that disorder-specific findings emerge when subjects use information processing strategies elicited naturally, during passive viewing, an instance where task instructions do not constrain attention. Further work is needed to specify the precise psychological nature of these disorder-specific processes that may emerge spontaneously.
Despite consistency across the present and previous studies, questions remain. For example, both Monk et al33 and the present study showed MDD-related between-group differences in amygdala response during passive viewing; however, Monk et al found amygdala hyperactivation in at-risk adolescents viewing morphed faces showing varying blends of fear; the present study found amygdala hypoactivation in MDD-affected subjects viewing faces showing full displays of fear. Thus, these inconsistencies may be due to methodologic differences.
Other questions emerge related to developmental perspectives. Owing to strong longitudinal and family-based aggregation among MDD and anxiety disorders manifest in adolescents and adults,3–10,14 one might expect brain imaging findings in adult MDD and anxiety16–21,24 to parallel the findings observed in this study in adolescents. Nevertheless, few imaging studies contrast anxious and depressed adults with any paradigm; none use paradigms similar to the one used herein, which shows that different conclusions emerge concerning between-group comparisons as a function of relatively subtle task-related features. As with inconsistencies in work with adolescents, the dearth of studies directly comparing anxious and depressed adults emphasizes the need for more research on the nature of perturbed amygdala engagement in risk and expression of MDD and anxiety. In pursuing such work, the present findings highlight the need to consider the sensitivity of group differences to variations in attention conditions across fMRI paradigms.
One finding calls for particular attention. The MDD-related deactivation specifically to passively viewed happy faces represents a major contributor to the disorder-specific between-group differences in the fearful-passive vs happy-passive contrast. Given the tendency in previous research to focus on hyperactivation, this finding for deactivation may appear intuitively surprising and in need of replication. Nevertheless, previous research consistently finds that between-group differences during passive viewing observed with this paradigm at least partially reflect anomalous patterns of amygdala deactivation in one or another unique subgroup.27,31 Moreover, previous work demonstrates the importance of happy faces, specifically, as an optimal comparison condition, while also suggesting that happy faces index reward-related processes uniquely perturbed in MDD but not in anxiety disorders.27,33 Finally, despite some divergence between the present findings and associated hypotheses emerging from previous studies,27,31,33 our findings documenting disorder-related specificity during passive viewing extend other work. For example, Thomas et al28 also used passive viewing, although no other attention manipulation, and found amygdala hyperactivation in anxious children and amygdala deactivation in children with MDD.
SHARED DIATHESIS
The present study also provides evidence of amygdala perturbations common to both adolescent MDD and anxiety disorders. These data suggest that at least some adolescent anxiety disorders share an underlying neural diathesis with adolescent MDD. Importantly, as with disorder specificity, disorder common manifestations occurred to particular face-emotion types, when viewed in specific attention states. Support for this conclusion again emerges from our focus on necessarily complex tests of 3-way interactions. Thus, both patient groups had greater amygdala activation than healthy peers only when viewing fearful faces specifically. These differences occurred particularly when focusing on subjectively experienced fear, relative to passively viewing the same fearful faces or relative to viewing happy or neutral faces in various attention states. Previous research27,31 had led us to expect amygdala perturbations in anxious patients specifically when viewing fearful faces and rating fear; the present study extends this observation to MDD, with or without anxiety.
Findings from our secondary analyses in the lateral OFC also provide some support for both the disorder-specificity and the shared-diathesis perspectives. This pattern is consistent with previous work implicating a distributed neural circuitry devoted to emotional modulation of perception and behavior.27,53–56 Taken together, findings suggest that adolescent anxiety disorders and MDD can exhibit neural commonalities but also distinctions, depending on the specific attention and emotion states engaged.
DEVELOPMENT
Common and unique neural perturbations were not affected by sex and age. However, the present study was not specifically designed to examine questions of sex and age specificity across adolescence and adulthood, questions that require large samples of adolescents and adults. Previous research does indicate differences in patterns of neural responses under varying emotion/attention conditions between healthy adolescents and adults, although no previous work, to our knowledge, has directly compared samples of depressed or anxious and healthy adolescents and adults.39,57–59 The present work now sets the stage for such large, comparative studies among adolescents and adults with anxiety and mood disorders. Studies directly comparing these groups are needed, given the demonstrated effects of subtle task variations on between-group differences. Such studies, which may disclose similar or unique functional perturbations across pathological conditions and age groups, are particularly important in light of improved etiologic/pathogenic models and treatment options.60
BEHAVIORAL DATA
In addition to the fMRI results, we found expected variations in task performance as a function of attention condition and face emotion type, as shown previously.27,31,32,39 However, groups did not differ on task performance. Thus, the present report, when combined with others on amygdala function in both adults and adolescents,27,31,33,61 firmly establishes that between-group differences in amygdala function emerge even in the absence of between-group differences in task performance. The observed amygdala differences in the present study specifically were independent of rated anxiety and are not epiphenomena of between-group differences in experienced anxiety or other task performance differences. Some research, however, suggests that differences in task performance facilitate interpretation of differences in neural activation.62 From this perspective, the failure of a task to elicit expected between-group differences in behavior might suggest that the underlying psychological process engaged by the task is not directly relevant for the condition being studied.
In the present report, the failure to observe between-group differences in behavior, in the context of between-group differences in neural response, emerges for a task that is clearly disorder-relevant. Disorder relevance reflects the definition of clinical anxiety as a condition characterized by excessive subjectively reported anxiety. Comparable results emerged in another study of anxious adolescents,30 using another disorder-relevant paradigm that engages threat-attention interactions during orienting, another process previously linked to clinical anxiety. This study also found between-group differences in the amygdala in the context of no between-group differences in behavior. Moreover, the study used stimuli presented too rapidly to be perceived, in terms of their capacity to be rated as elicitors of subjectively experienced anxiety.
Taken together, these 2 studies dissociate individual differences in amygdala function and individual differences in the subjective experience of anxiety during imaging. Importantly, though, both studies demonstrate adolescent between-group differences in amygdala function by using tasks previously linked to clinical anxiety. The present report shows that between-group differences occur specifically during monitoring of subjective fear, the most clinically relevant attention state engaged in the present study, but not in other attention states.
LIMITATIONS
Our findings must be viewed in light of 4 limitations. First, results are based on small sample sizes. Because anxiety and MDD frequently co-occur, it is difficult to gather large, noncomorbid samples. As a result, true-positive effects might have been obscured. Given that type II error is more likely than type I error with small sample sizes, negative findings should be interpreted with more caution than positive findings.
Second, additional aspects of our sample complicate interpretations. For example, findings emerging from analyses that included patients with comorbid MDD and anxiety raise the question of the degree to which anxiety-comorbidity influences or changes biased neural engagement in MDD, and whether findings can be attributed to MDD per se. It was not feasible to recruit sufficiently large samples of subjects in 4 mutually exclusive groups (MDD alone, anxiety alone, comorbid MDD and anxiety, and healthy controls). Similar concerns prevented us from recruiting sufficiently large samples of adolescents with specific anxiety disorders. However, we repeated all analyses with comorbid patients excluded from the MDD group; these analyses supported conclusions emerging from other analyses. Nonetheless, some unanswered questions remain because our adolescent participants with “pure” anxiety or “pure” depression may develop heterotypic comorbidity in the future. Longitudinal studies conducting serial fMRI assessments might provide more definitive insights on the developmental trajectories of emerging comorbidity patterns. Similarly, because comorbidity among the anxiety disorders also complicates interpretations, future studies should examine brain imaging data in “pure” anxiety groups. However, such studies will face the problem that few cases with anxiety occur in the absence of comorbidity and that such samples may be unrepresentative, particularly of cases typically seen in clinical settings.
Third, our analysis is limited to the amygdala and OFC regions, which may be perceived as a restricted view of (neural) dysfunction in anxiety and depressive disorders.
Fourth, the cognitive task used has advantages and disadvantages. Regarding advantages, previous work suggests that the task elicits disorder-specific behavioral response profiles.27,31,33,43 Moreover, the task explicitly assesses neural activity engaged when participants report distress (ie, experience internal fear), a defining feature of anxiety disorders. On the other hand, ratings of distress engage a series of complex, incompletely specified psychological processes that require introspection and can be directed toward various environmental features. Because fearful faces signal threat but are not directly threatening, a task focusing attention on more general aspects of threat might generate unique findings. Furthermore, in the passive-viewing condition, no information is generated concerning the cognitive processes engaged in each group. The use of only 8 specific emotion events in each attention condition is also a limitation because tasks with more replicates possess greater statistical power.63 However, because the present analyses attempted to disclose between-group differences as a function of different emotion and attention conditions, we needed considerable variation on both factors. In an adolescent sample, for practical reasons, this resulted in relatively few specific emotion events in each attention condition, to minimize task duration. Finally, this concern probably relates more to instances where studies fail to detect hypothesized between-group differences than to studies such as ours that confirm hypothesized differences. Thus, although this paradigm appears to be sensitive to both commonalities and differences in the neural correlates of adolescent MDD and anxiety disorders, further refined tasks may generate more precise conclusions concerning the nature of these commonalities and differences.
Acknowledgments
Funding/Support: This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland.
Footnotes
This report was prepared while Dr Beesdo was a visiting scientist at the National Institute of Mental Health.
Author Contributions: Dr Beesdo takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all of the data in the study.
Additional Contributions: Kenneth Towbin, MD, Jennifer Cameron, PhD, and Alan Zametkin, MD, provided medical oversight; Harvey Iwamoto, MA, performed programming; and Nina Shiffrin, BS, Darcy Mandell, BS, and John S. Rich, MD, assisted in data processing. We thank the families who participated.
Financial Disclosure: Dr Beesdo has received speaking honoraria from Pfizer Corp and travel support from Eli Lilly and Company. Dr Wittchen has received research support from Eli Lilly and Company, Novartis, Pfizer Inc, and Schering-Plough; has been a consultant to Eli Lilly and Company, Hoffman-La Roche Pharmaceuticals, Novartis, Pfizer Inc, Wyeth, and Sanofi-Aventis; and has received speaking honoraria from Novartis, Schering-Plough, Pfizer Inc, and Wyeth.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication [published correction appears in Arch Gen Psychiatry. 2005;62(7):768] Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Wittchen H-U, Lieb R, Schuster P, Oldehinkel AJ. When is onset? investigations into early developmental stages of anxiety and depressive disorders. In: Rapoport JL, editor. Childhood Onset of “Adult” Psychopathology, Clinical and Research Advances. Washington, DC: American Psychiatric Press; 1999. pp. 259–302. [Google Scholar]
- 3.Lieb R, Isensee B, Höfler M, Pfister H, Wittchen H-U. Parental major depression and the risk of depressive and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2002;59(4):365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163 (6):1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- 5.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, Wittchen H-U. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 7.Bittner A, Goodwin RD, Wittchen H-U, Beesdo K, Höfler M, Lieb R. What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J Clin Psychiatry. 2004;65(5):618–626. doi: 10.4088/jcp.v65n0505. [DOI] [PubMed] [Google Scholar]
- 8.Wittchen H-U, Beesdo K, Bittner A, Goodwin RD. Depressive episodes—evidence for a causal role of primary anxiety disorders? Eur Psychiatry. 2003;18(8):384–393. doi: 10.1016/j.eurpsy.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64(6):651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 10.Wittchen H-U, Kessler RC, Pfister H, Lieb R. Why do people with anxiety disorders become depressed? a prospective-longitudinal community study. Acta Psychiatr Scand Suppl. 2000;(406):14–23. [PubMed] [Google Scholar]
- 11.Goldberg D. A dimensional model for common mental disorders. Br J Psychiatry Suppl. 1996 June;(30):44–49. [PubMed] [Google Scholar]
- 12.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56(10):921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 13.Pine DS. A neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 14.de Graaf R, Bijl R, Spijker J, Beekman ATF, Vollebergh WAM. Temporal sequencing of lifetime mood disorders in relation to comorbid anxiety and substance use disorders—findings from the Netherlands Mental Health Survey and Incidence Study. Soc Psychiatry Psychiatr Epidemiol. 2003;38(1):1–11. doi: 10.1007/s00127-003-0597-4. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Fu CH, Williams S, Cleare A, Brammer M, Walsh KJ, Andrew C, Pich E, Williams P, Reed L, Mitterschiffthaler M, Suckling J, Bullmore E. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 17.Sheline YI, Barch D, Donnelly J, Ollinger J, Snyder A, Mintun M. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 18.Surguladze S, Brammer M, Keedwell P, Giampietro V, Young A, Travis M, Williams S, Phillips M. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47 (9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 20.Stein MB, Goldin P, Sareen J, Zorrilla L, Brown G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 21.Straube T, Mentzel HJ, Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59(2):162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ, Nemeroff CB. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31(10):2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 24.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Pine DS, Cohen P, Johnson JG, Brook JS. Adolescent life events as predictors of adult depression. J Affect Disord. 2002;68(1):49–57. doi: 10.1016/s0165-0327(00)00331-1. [DOI] [PubMed] [Google Scholar]
- 26.Moffitt TE, Caspi A, Harrington H, Milne BJ, Melchior M, Goldberg D, Poulton R. Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychol Med. 2007;37(3):441–452. doi: 10.1017/S0033291706009640. [DOI] [PubMed] [Google Scholar]
- 27.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 28.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 29.Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biol Psychiatry. 2006;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-Edgar K, Roberson-Nay R, Hardin M, Poeth K, Guyer A, Nelson E, McClure E, Henderson H, Fox N, Pine D, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35 (4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pine DS, Klein RG, Mannuzza S, Moulton JL, III, Lissek S, Guardino M, Woldehawariat G. Face-emotion processing in offspring at risk for panic disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(7):664–672. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- 33.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, III, Guardino M, Masten CL, McClure EB, Fromm S, Blair JR, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 34.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 36.Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognit Ther Res. 2005;29(1):29–45. [Google Scholar]
- 37.Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen P-H, Pine DS, Ernst M. Amygdala function and 5HTT gene variants in adolescent anxiety and major depressive disorder [published online ahead of print October 23, 2008] Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Monk CS, McClure E, Nelson E, Zarahn E, Bilder R, Leibenluft E, Charney D, Ernst M, Pine D. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 40.Ekman P, Friesen W. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychology Press; 1976. [Google Scholar]
- 41.Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning, I: methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 42.Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: establishing a larger stimulus set. Paper presented at: Cognitive Neuroscience Society Annual Meeting; April 15, 2002; San Francisco, CA. [Google Scholar]
- 43.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geisser S, Greenhouse S. An extension of Box’s results on the use of the F distribution in multivariate analysis. Ann Math Stat. 1958;29(3):885–891. [Google Scholar]
- 45.Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6(2):122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 46.Holmes AP, Friston KJ. Generalisability, random effects and population inference [abstract] Neuroimage. 1998;7(suppl):s754. [Google Scholar]
- 47.Szeszko PR, Robinson D, Alvir J, Bilder R, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56(10):913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- 48.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59 (7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 49.Straube T, Kolassa I-T, Glauer M, Mentzel H-J, Miltner WHR. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56(12):921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJR, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 51.Krueger RF, Caspi A, Moffitt TE, Silva PA. The structure and stability of common mental disorders (DSM-III-R): a longitudinal-epidemiological study. J Abnorm Psychol. 1998;107(2):216–227. doi: 10.1037//0021-843x.107.2.216. [DOI] [PubMed] [Google Scholar]
- 52.Shear MK, Bjelland I, Beesdo K, Gloster AT, Wittchen H-U. Supplementary dimensional assessment in anxiety disorders. Int J Methods Psychiatr Res. 2007;16(suppl 1):S52–S64. doi: 10.1002/mpr.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips ML, Drevets W, Rauch SL, Lane R. Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 54.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 55.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 56.LeDoux J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, NY: Simon & Schuster Ltd; 1996. [Google Scholar]
- 57.McClure EB, Monk C, Nelson E, Zarahn E, Leibenluft E, Bilder R, Charney D, Ernst M, Pine D. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55(11):1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Thomas KM, Drevets W, Whalen PJ, Eccard C, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 59.Guyer AE, Monk CS, McClure EB, Nelson EE, Roberson-Nay R, Adler A, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety [published online ahead of print August 27, 2008] Neuropsychopharmacology. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5(1):67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- 63.Murphy K, Garavan H. Deriving the optimal number of events for an event-related fMRI study based on the spatial extent of activation. Neuroimage. 2005;27(4):771–777. doi: 10.1016/j.neuroimage.2005.05.007. [DOI] [PubMed] [Google Scholar]