Abstract
Objective
To evaluate whether adolescent obesity is associated with difficulties for becoming pregnant later in life.
Design
Cross-sectional analysis of baseline data from a longitudinal cohort
Setting
Multiethnic, community-based observational study of US women
Patient(s)
3154 midlife women
Main Outcome Measure(s)
Lifetime nulliparity and lifetime nulligravidity
Result(s)
527 women (16.7%) women had never delivered a baby. Participants were categorized by self-reported high school body mass index (BMI): underweight (< 18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (> 30 kg/m2). The prevalence of lifetime nulliparity increased progressively across the high school BMI categories: 12.7%, 16.7%, 19.2%, and 30.9%, respectively (p<0.001). Multivariable logistic regression analysis confirmed that women who were obese adolescents had significantly higher odds of remaining childless as compared to normal weight women (odds ratio [OR] 2.84, 95% confidence intervals [CI] 1.59 to 5.10) after adjusting for adult BMI, history of non-gestational amenorrhea, marital status, ethnicity, study site, and measures of socioeconomic status. Furthermore, adolescent obesity was associated with lifetime nulligravidity (OR 3.93, 95% CI 2.12 to 7.26).
Conclusion(s)
Adolescent obesity is associated with lifetime nulliparity and nulligravidity in midlife U.S. women.
Keywords: adolescents, body mass index, gravidity, obesity, parity
Introduction
Obesity, previously primarily an adult condition, is increasing dramatically among the younger age groups (1, 2). In 2006, the prevalence of overweight in U. S. children and adolescents had been estimated at 17%, and is predicted to rise to 20% by 2010 (3). Excess weight in early life leads to adult obesity and increased cardiovascular morbidity (4, 5). A body mass index (BMI) greater than 25 kg/m2 at age 18 was associated with a 66% increased risk of premature death among women enrolled in the Nurses' Health Study cohort (6). Notably, this association of moderate adiposity at adolescence with premature death later in life was only partially explained by adult obesity. This finding implies that adolescent overweight status may be an independent predictor of lifetime health.
Non-obstetric reproductive effects of female adult obesity are largely attributed to increases in anovulation, amenorrhea and hyperandrogenism. Obesity prolongs the time to pregnancy and is associated with decreased fecundity in women with regular menstrual cycles (7-9). An increase in waist-hip ratio of as little as 0.1 unit was associated with a 30% decrease in the per-cycle probability of conception in presumably reproductively normal women undergoing donor sperm for insemination (10). A recent meta-analysis of 16 studies found that overweight women had a 67% increased risk of miscarriage when compared with women of normal weight (11).
Studies of reproductive sequelae of adolescent and childhood adiposity had been primarily focused on anovulation and its correlates. Obesity at age seven was associated with increased menstrual irregularities at age 33 in a large British cohort after adjustment for adult weight and other confounders (12). A BMI of greater than 25 kg/m2 at age 18 was a risk factor for subsequent anovulatory infertility in a subcohort from the Nurses' Health Study (13). Although anovulation is a useful marker of reproductive fitness, the ultimate yardstick of childbearing potential is ability to produce live offspring. We hypothesized that adolescent BMI would be associated with lifetime childlessness independent of other fertility-related factors. To address this question, we assessed the relationship between self-reported high school BMI and lifetime nulliparity in a large community based sample of midlife women, from the Study of Women's Health Across the Nation (SWAN).
Material and Methods
The study of women's health across the nation (SWAN)
SWAN is an ongoing multiethnic, multi-center longitudinal study of women as they transition from pre- to post menopause. The study began in 1994; the details about the sampling frame and sampling strategies have been published (14). Briefly, in order to obtain large numbers of women of diverse backgrounds from the general population, three sites (Los Angeles, Pittsburgh, New Jersey) used random digit dialing sampling from banks of telephone numbers. To obtain the sampling frame from the random digit dialing sites, a cross sectional screening survey was administered by telephone. The other four sites (Boston, Chicago, Detroit, Oakland) selected randomly from lists of names or of household addresses. These sites administered the cross sectional screening survey in person. Overall, 16,065 women participated in the cross-sectional interview, of whom approximately 40% were found to be eligible for the longitudinal cohort study. Of the eligible women, 3302 were recruited (51%). At each site, approximately half of the women were of one of the four purposely-sampled ethnic backgrounds (African American, Chinese, Japanese, or Hispanic), in addition to non-Caucasians who were recruited at each site.
Eligibility criteria included: age 42-52, at least one menstrual period and no hormonal therapy within the prior three months, intact uterus and at least one ovary. Bilingual Chinese, Japanese and Spanish-speaking staff was available at the Oakland, Los Angeles, and New Jersey sites, respectively. All questionnaires were available in translation. Written informed consent was obtained from all participants. All appropriate institutional review boards had approved the study.
Demographic and lifestyle factors
Race/ethnicity was self-identified at the SWAN baseline visit. Marital status was dichotomized as ever married vs. never. Smoking was dichotomized as ever vs. never. Education was dichotomized based on completion of college. Socioeconomic status was roughly categorized into 3 levels based on ability to pay for basic family needs, such as food and shelter: very difficult/somewhat difficult/not at all difficult.
Definition of obesity
At baseline, weight and height were measured using standardized procedures. Reported high school BMI (HSBMI) was calculated using the self-reported high school weight and height at baseline. Both high school and adult BMI were categorized by using the World Health Organization cut-points of: underweight – less than 18.5 kg/m2; normal – 18.5-24.9 kg/m2; overweight – 25-29.9 kg/m2, and obese – greater than 30 kg/m2 (15).
Definitions of nulliparity and fertility parameters
Participants were asked to report the number of times they were pregnant and the outcome of each pregnancy. Number of pregnancies, livebirths, and spontaneous or induced abortions were evaluated as continuous and two-way categorical variables: nulliparity (any live birth vs. none), nulligravidity (any pregnancy vs. none), history of induced abortions (any vs. none), and history of miscarriage (any spontaneous abortion vs. none; two or more spontaneous abortions vs. else; and three or more spontaneous abortions vs. else). Nulliparity was used as a primary outcome as the most objective measure of lifetime childbearing that is unlikely to be affected by recall. Nulligravidity was used as a secondary outcome.
Data on history of any experienced infertility were available; however, due to inability to obtain medical records and verify infertility history and etiology, this information was used for secondary analyses. History of infertility was defined by the affirmative response to the question “Have you ever had a period of 12 months when you could not get pregnant although you were attempting to get pregnant or were letting yourself get pregnant?” If the respondent answered “yes”, she was classified as having experienced infertility, and further probed by the question “Did a doctor give you a reason why you were not getting pregnant?” Those who responded affirmatively were then asked to write in a reason. Two reviewers abstracted the responses independently (NS and AJP) by categorizing each answer into the known causes of infertility: unexplained, tubal, male, anovulation, diminished ovarian reserve, endometriosis and uterine factors. Disagreements were adjudicated (inter-observer agreement = 84.6%, kappa 0.81). In addition, the following dichotomous variables were created: use of fertility medications (self-report of ever taking fertility medications to help get pregnant), history of eating disorders (self-report of anorexia and/or bulimia), and history of salpingitis (self-report of any treatment of fallopian tube infection).
Menstrual regularity was ascertained by the answer to the question “Since the age of 18, have you ever experienced a time interval of 3 or more months when you did not have a menstrual period?” Participants who further stated that they were not “breastfeeding or pregnant every time it happened”, were classified as having history of amenorrhea. A dichotomous variable was created (any history of non-gestational amenorrhea ≥ 3 months vs. none). Continuous oral contraceptive (OCP) use was ascertained by the question: “During the interval from age 25 to 35, did you take birth control pills or other female hormones all the time without a break?” Sexual preferences were ascertained by asking “Who have you have generally had sex with over your adult lifetime?” All responses stating that a participant had generally had “sex with a woman” were used to create the corresponding dichotomous variable (any report of sexual preference for women vs. else [defined as preference for men or not sexually active]). To assess whether intention to remain voluntarily childless affected the association between body mass and parity, we analyzed participants who stated that they “never tried to get pregnant.”
Analytic sample
Of the total SWAN cohort of 3302 women, 148 women were excluded because of missing high school weight, height and/or missing pregnancy history from this analysis. Therefore, the final analytic sample included 3154 women.
Statistical analysis
Associations between demographic and clinical characteristics of the cohort by reported HSBMI categories were assessed using ANOVA or Kruskal-Wallis tests for continuous variables and chi square or Fisher's exact tests for categorical variables, as appropriate. Correlation between continuous data (association between reported HSBMI and current BMI) was assessed by Pearson correlation analysis. Logistic regression was used to test for trend across the HSBMI categories. To evaluate the independent association of the reported HSBMI on nulliparity, multivariable logistic models were specified with nulliparity as the outcome and HSBMI categories as the independent variables of interest. Logistic regression analysis was conducted using model-building strategies suggested by Hosmer and Lemeshow (16). Appropriate regression diagnostics were performed to examine whether the model fit was supported over the entire set of covariate patterns. Separate sensitivity analyses were conducted by excluding those who reported tubal factor infertility, male infertility, “never having had sex”, preference for sex with women, history of continuous OCP use without a break, and those who reported that they “never tried to get pregnant”. Lastly, a combined sensitivity analysis was performed, excluding all of the above-mentioned groups considered to have a potential reason for nulliparity. A secondary analysis was performed to assess the association between the reported HSBMI and nulligravidity. All statistical tests used a two-tailed alpha of 0.05. All analyses were performed using STATA 9.2 (StataCorp LP, College Station, TX).
Results
527 women were childless in our study population of 3154, yielding an overall lifetime nulliparity percentage of 16.7. Table 1 illustrates socio-demographic characteristics of the study population. In agreement with other studies (4), the reported HSBMI was significantly correlated with adult BMI (r=0.55, p=0.001). Of note, women with a higher reported HSBMI were less likely to marry and graduate from college (p<0.01 for both).
Table 1. Socio-demographic characteristics of the study sample by high school BMIa.
| < 18.5 kg/m2 underweight (n=535) | 18.5-24.9 kg/m2 normal (n=2246) | 25.0-29.9 kg/m2 overweight (n=276) | ≥ 30.0 kg/m2 obese (n=97) | P value | |
|---|---|---|---|---|---|
| Median high school | 17.6 | 20.7 | 26.6 | 32.9 | |
| BMI, kg/m2 | |||||
| Age at baseline, years | 45.8 (2.7) | 45.9 (2.7) | 45.8 (2.7) | 45.5 (2.4) | 0.52 |
| Ethnicityb | |||||
| Caucasian | 193 (12.9) | 1118 (74.8) | 142 (9.5) | 41 (2.7) | <0.01 |
| African-American | 149 (16.9) | 587 (66.5) | 97 (11.0) | 50 (5.7) | <0.01 |
| Chinese | 79 (32.2) | 158 (64.5) | 7 (2.9) | 1 (0.4) | <0.01 |
| Hispanic | 55 (21.5) | 175 (68.4) | 23 (9.0) | 3 (1.2) | 0.07 |
| Japanese | 59 (21.4) | 208 (75.4) | 7 (2.5) | 2 (0.7) | <0.01 |
| Education, n (%) of college graduates | 208 (39.3) | 1009 (45.1) | 98 (35.6) | 34.7 | <0.01 |
| Ever-married | 471 (88.4) | 1966 (87.6) | 222 (80.4) | 71 (73.2) | <0.01 |
| History of smoking | 205 (38.5) | 946 (42.5) | 137 (50.0) | 53 (55.2) | <0.01 |
| Lowest SES categoryc | 47 (8.8) | 186 (8.3) | 38 (13.8) | 14 (14.4) | <0.01 |
Unless otherwise indicated, continuous variables presented as mean (SD) with P values calculated by Kruskal- Wallis tests. Categorical variables presented as n (percent) with P values calculated by χ2.
Distribution of women by BMI categories within ethnicity
SES, socioeconomic status
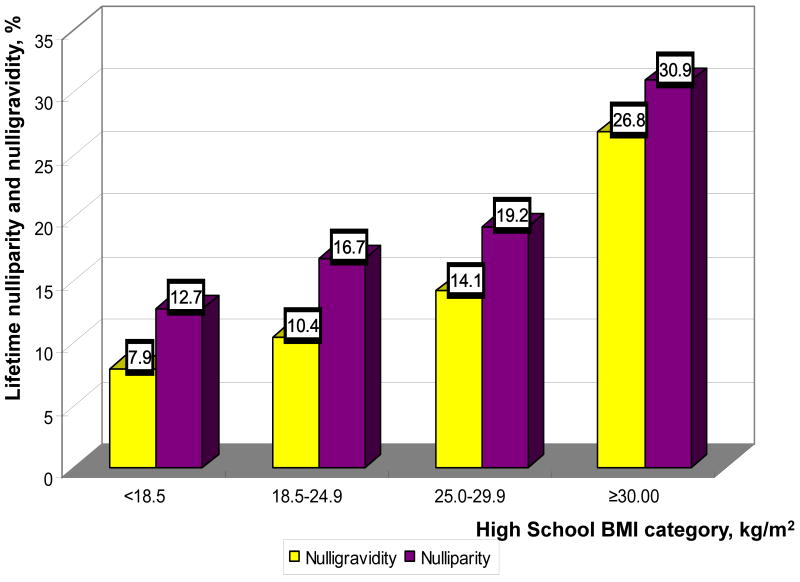
Increasing reported HSBMI was associated with higher rates of lifetime nulliparity and nulligravidity, p for trend <0.01 (Table 2, Figure 1). There was no difference in frequency of spontaneous or induced abortion by adolescent adiposity. Among the medical factors known to affect fertility, the percentages of self-reported infertility, treatment for salpingitis and history of eating disorders were not significantly different by HSBMI categories. Of note, the proportion of participants reporting amenorrhea was significantly higher among those in the higher HSBMI categories (p<0.01).
Table 2. Childbearing parameters of the study sample by high school BMI a.
| < 18.5 kg/m2 underweight (n=535) | 18.5-24.9 kg/m2 normal (n=2246) | 25.0-29.9 kg/m2 overweight (n=276) | ≥ 30.0 kg/m2 obese (n=97) | P value | |
|---|---|---|---|---|---|
| Number of children | 2.2 (1.4) | 2.0 (1.4) | 2.1 (1.5) | 1.6 (1.5) | <0.01 |
| Nulliparity | 68 (12.7) | 376 (16.7) | 53 (19.2) | 30 (30.9) | <0.01 |
| Number of pregnancies | 3.1 (2.0) | 2.9 (1.9) | 2.9 (2.1) | 2.3 (2.1) | <0.01 |
| Nulligravidity | 42 (7.9) | 233 (10.4) | 39 (14.1) | 26 (26.8) | <0.01 |
| Spontaneous abortion(s) | |||||
| One or more | 149 (27.8) | 596 (26.5) | 83 (30.1) | 27 (27.8) | 0.62 |
| Two or more | 51 (9.5) | 167 (7.4) | 25 (9.1) | 6 (6.2) | 0.32 |
| Three or more | 21 (3.9) | 53 (2.4) | 9 (3.30 | 3 (3.1) | 0.19 |
| Induced abortion(s) | 154 (28.8) | 670 (29.8) | 67 (24.3) | 21 (21.7) | 0.09 |
| Medical factors potentially affecting fertility | |||||
| Amenorrhea b | 70 (13.2) | 345 (15.4) | 59 (21.5) | 30 (30.9) | <0.01 |
| Continuous OCP use c | 36 (6.8) | 186 (8.3) | 20 (7.3) | 9 (9.4) | 0.61 |
| Report of infertility d | 131 (25.2) | 513 (23.5) | 64 (24.0) | 24 (25.3) | 0.85 |
| Salpingitis | 19 (3.6) | 106 (4.8) | 11 (4.0) | 5 (5.3) | 0.65 |
| Eating disorders | 10 (1.9) | 26 (1.2) | 2 (0.7) | 1 (1.0) | 0.48 |
| Fertility medications e | 22 (4.2) | 124 (5.5) | 11 (4.0) | 4 (4.1) | 0.50 |
| Social factors potentially affecting fertility | |||||
| Preference for sex with women | 5 (1.0) | 28 (1.3) | 8 (3.0) | 2 (2.1) | 0.12 |
| Did not try to get pregnant | 1 (0.2) | 14 (0.6) | 7 (2.6) | 2 (2.1) | 0.02 |
Unless otherwise indicated, continuous variables presented as mean (SD) with P values calculated by Kruskal- Wallis tests. Categorical variables presented as n (percent) with P values calculated by χ2 or Fisher's exact test as appropriate.
Non-gestational amenorrhea was defined as experiencing a time interval of 3 months or greater without a menstrual period that was not due to pregnancy or breast-feeding since the age 18
Participants who took oral contraceptive pills all the time without a break from age 25 to 35
Infertility was defined by the question “Have you ever had a period of 12 months when you could not get pregnant although you were attempting to get pregnant or were letting yourself get pregnant?”
Use of fertility medications such as clomiphene citrate
Figure 1.
Prevalence of lifetime nulliparity and nulligravidity by high school BMI categories. (yellow bars – nulligravidity, purple bars – nulliparity).
Twenty-three percent (732) of all participants reported history of a period of infertility. There was no significant difference in the cause of infertility by HSBMI category. Preference for the same-sex sexual relationship did not differ by HSBMI categories. There was a statistically significant increase in the number of woman who “never tried to get pregnant” with the higher reported HSBMI (p=0.02); yet, the magnitude of this difference was small (ranging from 0.2% to 2.6%).
Multivariable logistic regression models were used to estimate the effect of the reported HSBMI on lifetime nulliparity while adjusting for adult BMI, history of non-gestational amenorrhea, marital status, education, socioeconomic status, ethnicity, and study site (Table 3). Notably, there was an increasing trend in the risk of nulliparity with greater HSBMI category. Out of the variables with significant association in bivariate analysis, smoking was not included in the final model as it was neither a confounder in the association between HSBMI and infertility nor a significant contributor to model. Sensitivity analyses were performed to determine if several potential reasons for childlessness (see Table 4) might have biased the observed association between the reported HSBMI and nulliparity. For all tested subsets, the association of the reported HSBMI ≥30 kg/m2 and nulliparity remained statistically significant and the point estimate remained similar to that of the full cohort (Table 4).
Table 3. Association of reported high school BMI with lifetime nulliparity and nulligravidity, multivariable-adjusted odds ratios (95% confidence interval).
| Model Outcomea | < 18.5 kg/m2 underweight | 18.5-24.9 kg/m2 normal | 25.0-29.9 kg/m2 overweight | ≥ 30.0 kg/m2 obese |
|---|---|---|---|---|
| Nulliparity | ||||
| Unadjusted | 0.72 (0.55-0.96) | (reference) | 1.18 (0.86-1.63) | 2.23 (1.43-3.47) b |
| Fully adjusted | 0.71 (0.51-1.00) | (reference) | 1.22 (0.81-1.84) | 2.84 (1.59-5.10) b |
| Nulligravidity | ||||
| Unadjusted | 0.74 (0.52-1.04) | (reference) | 1.42 (0.98-2.04) | 3.16 (1.97-5.05) b |
| Fully adjusted | 0.77 (0.51-1.15) | (reference) | 1.51 (0.96-2.39) | 3.93 (2.12-7.26) b |
Logistic regression models adjusted for adult BMI, history of non-gestational amenorrhea, marital status, ethnicity, study site, education, and socioeconomic status
Denotes statistical significance
Table 4. Sensitivity analyses of the association of high school BMI ≥30 kg/m2 and lifetime nulliparity.
| Analytic sample a | n | Odds ratio (95% CI) b |
|---|---|---|
| Full analytic sample | 3098 | 2.84(1.59-5.10) |
| Excluding women who reported tubal factor infertility | 3032 | 2.82 (1.57–5.09) |
| Excluding women who reported male factor infertility | 3044 | 2.94 (2.62–5.34) |
| Excluding users of fertility medications | 2940 | 3.21(1.77–5.83) |
| Excluding continuous users of birth control pills during mid-reproductive years, | 2852 | 3.10 (1.65–5.82) |
| Excluding women who stated that they “never tried to get pregnant” | 3074 | 2.59 (1.42–4.71) |
| Excluding women who reported preference for same-sex relationship | 3055 | 2.84 (1.57–5.15) |
| Excluding all of the above-mentioned groups considered to have a potential reason for nulliparity | 2437 | 3.41 (1.66–7.00) |
Logistic regression models with lifetime nulliparity as the model outcome, adjusted for adult BMI at baseline, history of non-gestational amenorrhea, marital status, ethnicity, study site, education, and socioeconomic status
Odds ratio for the association of the reported HSBMI ≥30 kg/m2 with the model outcome after adjustment for multiple covariates
The fully adjusted logistic regression model was stratified by ethnicity to verify whether the point estimates are consistent. For all ethnic groups, the odds ratio remained greater than unity. Due to the small numbers of Hispanic, Asian and Chinese participants in the highest HSBMI category (see Table 1), only the Caucasian and African-American subsets retained a statistically significant association between the reported HSBMI of greater than 30 kg/m2 and nulliparity (OR=2.83; 95% CI: 1.23-6.54 and OR= 2.42; 95% CI: 1.04-5.62, respectively).
Using the lifetime nulligravidity as the outcome, a secondary analysis was performed. The reported HSBMI of greater than 30 kg/m2 was confirmed as an independently associated variable with nulligravidity (OR 3.93; 95% CI: 2.12–7.26) while adjusting for adult BMI, history of non-gestational amenorrhea, education, marital status, race, study site, and financial strain. There was no statistically significant interaction between the reported HSBMI and any other variable in all models tested.
Discussion
The strengths of our analysis of 3154 middle-aged U.S. women include the large sample size, the multiethnic composition, and the ability to analyze the women with history of menstrual regularity. SWAN participants were required to have a menstrual period within the three months prior to the study entry, thus effectively eliminating subjects with long-term amenorrhea. Therefore, our data point to influences on fertility that are weight-related, yet are not necessarily linked with anovulation. Another strength of our report is the multi-ethnic composition of the study population with significant inclusion of minorities that are often under-represented in clinical research.
The main finding of this cross-sectional study is the association of BMI ≥30 kg/m2 in adolescence with increased likelihood of lifelong childlessness as compared with women with normal adolescent BMI. Adolescent obesity (reported HSBMI ≥30kg/m2) remained independently associated with lifetime nulliparity and nulligravidity after adjustment for adult BMI at study entry, history of non-gestational amenorrhea, marital status, ethnicity, study site, education, and socioeconomic status. The findings were not affected by tubal or male factor infertility, use of fertility treatments, decisions to remain voluntarily childless, or preferences for same sex sexual relationship. We did not see an association between adolescent obesity and spontaneous miscarriage, which is in contradistinction to data generated in several studies and a recent meta-analysis. A potential explanation is that SWAN by design excluded women who did not have a menstrual period in the three months preceding the study entry. Thus, women with polycystic ovary syndrome were likely under-represented in SWAN as compared to general population and this may contribute to the observed lack of association between obesity and spontaneous miscarriage.
As we were unable to verify the etiology of infertility, we used nulliparity as the primary outcome as it is least likely to be affected by recall. However, there was no difference in self-report of infertility by the HSBMI category. The study questionnaire did not distinguish between primary or secondary infertility (i.e., difficulties in conceiving after a prior conception). It is possible that the adverse effect of elevated BMI on fertility varies with age. In support of this notion, a recent report by Sneed et al of 1273 women aged 22 to 44 years examined the influence of age on IVF success rates (17). In this study, BMI was observed to be an effect modifier of the relationship between age and conception. A higher BMI was a negative predictor of pregnancy only in the younger age group, diminishing considerably in the late 30's. It is possible that our inability to assess the temporal sequence between the history of infertility and body mass index contributed to the finding of no difference in reporting of infertility by HSBMI category.
Limitations of the study include the cross-sectional nature of the data that, in general, should be viewed as hypothesis-generating and descriptive rather than establishing a cause-and-effect relationship. In particular, certain variables are subject to prevalence/incidence bias, because we could not be certain that a given factor precedes the outcome. However, our exposure (reported HSBMI) and outcome (lifetime parity and gravidity) variables have been chosen to represent events that are unlikely to have changed recently for most participants. In addition, it should be pointed out while smoking did not appear to have an influence on the outcomes of interest, this effect is likely only pertinent to this study and not the general population.
Another limitation is the fact that determination of high school weight was made by self-report and recall. While there was not a validation study in SWAN, the literature suggests that agreement between self-report and measured weight is reasonable, with one study reporting a correlation coefficient of 0.84 between recalled and measured high school BMI (18). Data from the Newton Girls Study provided similar results with a correlation of 0.61 (p<0.001) between the BMI percentile at menarche and recalled body size at menarche (19). We used the WHO categories for adult BMI cut-points in our assessments. However, CDC recommends using the percentile BMI for age and gender to define overweight status in childhood (4). Study participants were asked to recall their weight when they left high school and hence were approximately 17-18 years of age. In adolescence, the 85th percentile roughly approximates BMI of 25 kg/m2 and thus adult cutoffs are appropriate (20). Although we have evaluated several social factors that may influence childbearing, we did not have an ability to consider other possible societal issues (i.e. discrimination and prejudices against obese individuals or simply fewer sexual partners) that might account for the observed relation. Lastly, the distribution of BMI varies by ethnicity and is different in Asian women relative to other groups (21). The use of the same cut-points for defining obesity regardless of ethnicity might result in misclassification of some women and, consequently, underestimate the impact of obesity.
Our study suggests a detrimental impact of adolescent adiposity on parity later in life. In 1952, Rogers and Mitchell observed a higher incidence of obesity in a group of young women ages 16-29 with amenorrhea (22). In a subcohort analysis from the Nurses' Health Study (NHS), women with a history of higher adolescent BMI had an increased risk of anovulatory infertility over 17 years of follow-up (13). In NHS, the relative risks for all categories of BMI above 23.9 were statistically significant, suggesting that even moderate adiposity played a role in disturbing menstrual function. Similarly, a 1958 British birth cohort study reported that obesity at age 7, defined as approximately 98th percentile based on a population life table, conferred a 78% higher risk of menstrual irregularity at age 33 (12). While the data are overwhelming that obesity influences fertility, it should be noted the precise mechanism remains to be elucidated. Bolumar et al reported a harmful effect of obesity on reproduction only in smokers (9); whereas Sneed et al found a detrimental effect of obesity on IVF pregnancy rates only in young patients and not in women over 35 (17). On the contrary, Gesink et al (7) and Jensen et al (8) found that neither age nor smoking made an impact on the association of obesity with decreased fertility as manifested by monthly pregnancy rates. However, the uncertain mechanism notwithstanding, we herein report an independent effect of adolescent obesity on lifetime nulliparity apart from other covariates.
There is a considerable body of evidence to support the biological plausibility of the negative impact of adiposity on fertility, mainly due to attenuation of the central hypothalamic drive in overweight and obese women (23, 24). In a detailed evaluation of daily hormone patterns from a subcohort of the same sample as the current study, a progressive decrease in urinary luteinizing hormone, follicle-stimulating hormone, and luteal pregnanediol glucuronide was observed with increasing BMI (25). Selective impairment in LH pulse amplitude but not frequency has been observed in obese ovulatory women (26). An alternate theory was suggested based on peripheral impairment of the hypothalamo-pituitary-ovarian (HPO) axis, possibly via impaired endometrial receptivity (27). However, recent data from the donor oocyte model did not observe a decrease in implantation in the obese donor egg recipients (28), supporting the hypothesis of the hypothesis that the adverse effects of obesity on fertility are exerted at the hypothalamic-pituitary-ovarian axis levels of the reproductive system and not through an effect on uterine receptivity.
In summary, we found that self-reported adolescent overweight status is independently associated with reduced lifetime parity and gravidity in a multi-ethnic cross-sectional sample of middle-aged U.S. women. The magnitude of the effect was higher with the increasing reported HSBMI. The cross-sectional nature of our study implies that it should be regarded as hypothesis-generating: does adolescent obesity result in diminished fertility? Prospective studies are needed to corroborate our results.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women's Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495). This manuscript was supported by T32 HD040135 (AJP) and K24 HD 41978 (NS).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. Supplemental funding from The National Institute on Child and Human Development is also gratefully acknowledged.
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999-present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark –Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair, Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Presented at the 54th Annual Meeting of the Society for Gynecologic Investigation, Reno, NV, March 14-17, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alex J. Polotsky, Department of Obstetrics & Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx.
Susan M. Hailpern, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx.
Joan H. Skurnick, Department of Preventive Medicine and Community Health, New Jersey Medical School–University of Medicine and Dentistry of New Jersey, Newark, NJ.
Joan C. LO, Division of Research, Kaiser Permanente Northern California, Oakland, CA.
Barbara Sternfeld, Division of Research, Kaiser Permanente Northern California, Oakland, CA.
Nanette Santoro, Department of Obstetrics & Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Overweight trends among children and adolescents. [December 1, 2008]; Available at www.cdc.gov/nccdphp/dnpa/obesity/trend/
- 3.Koplan JP, Liverman CT, Kraak VI, Wisham SL. Institute of Medicine of the National Academies. National Academy Press; Washington, DC: 2006. Progress in Preventing Childhood Obesity: How Do We Measure Up? [Google Scholar]
- 4.Dietz WH, Robinson TN. Overweight Children and Adolescents. New England Journal of Medicine. 2005;352:2100–9. doi: 10.1056/NEJMcp043052. [DOI] [PubMed] [Google Scholar]
- 5.Strauss RS, Pollack HA. Epidemic Increase in Childhood Overweight, 1986-1998. JAMA. 2001;286:2845–8. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- 6.van Dam RM, Willett WC, Manson JE, Hu FB. The Relationship between Overweight in Adolescence and Premature Death in Women. Annals of Internal Medicine. 2006;145:91–7. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Human Reproduction. 2007;22:414–20. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–8. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. American Journal of Epidemiology. 2000;151:1072–9. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- 10.Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306:484–7. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertility & Sterility. 2008;90:714–26. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- 12.Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1997;21:432–8. doi: 10.1038/sj.ijo.0800424. [DOI] [PubMed] [Google Scholar]
- 13.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. American Journal of Obstetrics & Gynecology. 1994;171:171–7. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 14.Sowers MF, Crawford S, Sternfeld B, Morgenstein D, Gold E, Greendale G, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, K J, Marcus R, editors. Menopause: biology and pathobiology. San Diego: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 15.WHO Technical report series 894. Geneva: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 2000. [Google Scholar]
- 17.Sneed ML, Uhler ML, Grotjan HE, Rapisarda JJ, Lederer KJ, Beltsos AN. Body mass index: impact on IVF success appears age-related. Human reproduction. 2008;23:1835–1839. doi: 10.1093/humrep/den188. [DOI] [PubMed] [Google Scholar]
- 18.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1995;19:570–2. [PubMed] [Google Scholar]
- 19.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? American Journal of Epidemiology. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 20.Baker S, Barlow S, Cochran W, Fuchs G, Klish W, Krebs N, et al. Overweight children and adolescents: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Journal of Pediatric Gastroenterology & Nutrition. 2005;40:533–43. doi: 10.1097/01.mpg.0000161147.16590.12. [DOI] [PubMed] [Google Scholar]
- 21.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 22.Rogers J, Mitchell GW. The relation of obesity to menstrual disturbances. New England Journal of Medicine. 1952;247:53–5. doi: 10.1056/NEJM195207102470204. [DOI] [PubMed] [Google Scholar]
- 23.Grenman S, Ronnemaa T, Irjala K, Kaihola HL, Gronroos M. Sex steroid, gonadotropin, cortisol, and prolactin levels in healthy, massively obese women: correlation with abdominal fat cell size and effect of weight reduction. Journal of Clinical Endocrinology & Metabolism. 1986;63:1257–61. doi: 10.1210/jcem-63-6-1257. [DOI] [PubMed] [Google Scholar]
- 24.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. Journal of Clinical Investigation. 1975;55:699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. Journal of Clinical Endocrinology & Metabolism. 2004;89:2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 26.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, et al. Pulsatile Luteinizing Hormone Amplitude and Progesterone Metabolite Excretion Are Reduced in Obese Women. Journal of Clinical Endocrinology & Metabolism. 2007;92:2468–73. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- 27.Wang JX, Davies M, Norman RJ. Body mass and probability of pregnancy during assisted reproduction treatment: retrospective study. BMJ. 2000;321:1320–1. doi: 10.1136/bmj.321.7272.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Styne-Gross A, Elkind-Hirsch K, Scott RT., Jr Obesity does not impact implantation rates or pregnancy outcome in women attempting conception through oocyte donation. Fertil Steril. 2005;83:1629–34. doi: 10.1016/j.fertnstert.2005.01.099. [DOI] [PubMed] [Google Scholar]