Abstract
This manuscript describes how the permeability of pulmonary artery microvascular endothelial cell (RPMEC) monolayer is elevated by hypoxia and the role played by HSP27 phosphorylation. p38 MAP kinase activation leading to HSP27 phosphorylation was previously shown by our laboratory to alter the actin cytoskeleton and tethering properties of RPMEC. This effect was independent of hypoxia-induced contractility which was ROCK-dependent rather than HSP27-dependent. Results described here show that increased HSP27 phosphorylation not only does not underlie hypoxia-induced permeability, but may actually augment the endothelial barrier. Hypoxia causes gap formation between RPMEC and increases MLC2 phosphorylation. The phosphorylation of MYPT1, which inhibits MLC2 phosphatase, is also increased in hypoxia. In addition, FAK phosphorylation, which alters focal adhesion signaling, is increased in hypoxia. Overexpressing phospho-mimicking HSP27 (pmHSP27), which induces significant actin stress fiber formation, surprisingly renders RPMEC resistant to hypoxia- or TGFβ-induced permeability. siRNA against pmHSP27 reverses the increased actin stress fiber formation in pmHSP27-overexpressing cells, and disrupting actin stress fibers in pmHSP27-overexpressing RPMEC renders them more susceptible to hypoxia. Finally, hypoxia-induced gap formation, as well as phosphorylation of MLC2, MYPT1 and FAK are almost abolished by overexpressing pmHSP27 in RPMEC. These effects of pmHSP27 overexpression might represent decreased cytoskeletal plasticity and increased tethering which counteracts permeability-inducing contractility. Thus hypoxia activates two pathways one leading to contractility and increased permeability, the other leading to actin stress fibers, stronger adhesion, and reduced permeability. Altering HSP27 phosphorylation, which tips the balance towards decreased permeability, might be targeted in managing endothelial barrier dysfunction.
Keywords: MLC2, Cell Contraction, Pulmonary Edema, p38
INTRODUCTION
Hypoxia is a common stimulus which can trigger several signaling pathways that alter cell physiology and cause injury in a variety of organs. High altitude pulmonary edema and pulmonary arterial hypertension (Wojciak-Stothard et al., 2006) are examples of lung diseases that have been linked to hypoxia-induced dysfunction of pulmonary endothelial cells. These cells form a tightly regulated permeability barrier the compromise of which in response to different stimuli causes pulmonary edema (Dudek et al., 2004; Garcia et al., 1995). The mechanism through which hypoxia induces edema is still not completely understood. We have recently described an important role for p38 MAP kinase and downstream signaling components in mediating the effects of hypoxia on pulmonary microvascular endothelial cells. p38 MAP Kinase is implicated in stress response, and when activated by hypoxia, it phosphorylates and activates MK2, which then phosphorylates HSP27 leading to actin filament formation in endothelial cells (An et al., 2005; Kayyali et al., 2002). HSP27 binds to actin when it is unphosphorylated, however, once phosphorylated, HSP27 disassociates from actin, allowing it to polymerize to form stress fibers. The latter effect has been associated with increased cell contractility and increased gap formation between cells (Schneider et al., 1998). In this manuscript we characterize the effect of HSP27 phosphorylation on endothelial permeability and related signaling events.
Although we have recently demonstrated that hypoxia increases the contractility of RPMEC, our results suggested that increased contractility was dissociated from p38-MK2-HSP27 mediated stress fiber formation (An et al., 2005). The increased contractility of cells is generally believed to be due to interaction of actin filaments with myosin. This process has been shown to be regulated by MLC2 phosphorylation, which in turn is regulated by kinases, such as MLCK and phosphatases. The increased phosphorylation of MLC2 and ensuing contraction of endothelial cells promote the formation of gaps between endothelial cells allowing fluid to go through the endothelial barrier into the interstitial and then alveolar spaces. MLC2 has at least 2 different isoforms which become phosphorylated (Akiyama et al., 1997; Goeckeler and Wysolmerski, 1995). Phosphorylated MLC2 promotes interaction of myosin with filamentous actin and is associated with the formation of stress fibers which alter the morphology of endothelial cells via altering the actin cytoskeleton and focal adhesion distribution (Chihara et al., 1997). Elucidating the phosphorylation of different isoforms of MLC2 in hypoxia should shed some light on their specific involvement in hypoxia-induced permeability of the endothelial barrier.
Rho kinase (ROCK) has been proposed to mediate hypoxia-induced smooth muscle contraction via phosphorylating MYPT1 (Wang et al., 2001b; Wang et al., 2003). The latter is the binding subunit of the three major components of myosin light chain phosphatase (MLCP), which regulates the phosphorylation level of MLC2. Phosphorylation of MYPT1 inhibits the activity of MLCP leading to a higher level of MLC2 phosphorylation. HSP27 has been reported to bind to MYPT1 and increase its phosphorylation by the Rho/ROCK pathway (Patil and Bitar, 2006). The latter interaction could eventually increase the phosphorylation level of MLC2.
In summary, hypoxia activates the p38-MK2 signaling pathway which phosphorylates HSP27, and increases the polymerization of actin and phosphorylation level of MLC2 through phosphorylation of MYPT1. In this manuscript we also demonstrate that hypoxia increases the gaps between endothelial cells resulting in higher permeability of pulmonary endothelial barrier. Our results show that HSP27 plays a critical role in the regulation of the permeability of pulmonary endothelial monolayer. The increased permeability observed in hypoxia appears to correlate with Rho-activated kinase (ROCK) and MLC phosphorylation, while HSP27 phosphorylation might play a role in decreasing permeability.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Media and supplements (RPMI), fetal bovine serum (FBS), penicillin G potassium, streptomycin, fungizone, and glutamine were purchased from Invitrogen (Carlsbad, CA). TGFβ was purchased from R&D Systems (Minneapolis, MN) and activated before use according to manufacturer’s instructions. Thrombin, SB202190, Y27632 and all other reagents and drugs were obtained from Sigma (St. Louis, MO). All drugs were diluted in serum-free medium on the day of the experiments.
Cell Culture
Rat pulmonary microvascular endothelial cells (RPMEC) were cultured in RPMI containing 10% FBS, penicillin, streptomycin, fungizone and glutamine at 37 °C in humidified air containing 5 % CO2. These cells were a gift from Dr. Una Ryan (Avant Immunotherapeutics, Needham, MA) and have been well characterized by us and others (Cote et al., 1996). RPMEC were passaged in 0.25 % trypsin-0.02 % ethylenediaminetetraacetic acid (EDTA) solution, and one day prior to the experiments, cells were maintained in serum-free media. For studying the effects of HSP27 phosphorylation, RPMEC were transfected with the pcDNA3-HSP27PM (Rogalla et al., 1999) to express HSP27 in which the residues, S15D, S78D, S82D, were mutated to mimic phosphorylated HSP27 (pmHSP27). Stable transfectants were made as we described earlier (Kayyali et al., 2002). For hypoxic exposure, cells were placed in humidified airtight incubation chambers (Billups-Rothenberg, Del Mar, CA) and gassed with 3 % O2, 5 % CO2, balanced N2. The hypoxic chambers were kept at 37 °C in a tissue culture incubator. For normoxic exposure (0.5, 1, 2 or 4 h), cells were maintained at 37 °C in humidified air containing 5 % CO2.
Assessment Of Endothelial Permeability
Cells were seeded on Falcon® HTS FluoroBlok™ 1.0 μm Inserts (Becton Dickinson, Bedford, MA), and cultured to get a confluent monolayer. After starvation with serum-free medium for 24 h, apical medium was replaced with 0.35 ml Phenol Red-free medium containing the fluorescent Alexafluor-488-dextran (3 kDa, Molecular Probes, Eugene OR), and basolateral chamber was filled with 0.9 ml Phenol Red-free medium without Alexafluor-488-dextran. The intensity of fluorescence in the basolateral chamber was read from the bottom every 4 min in hypoxic or normoxic conditions using a Tecan plate reader maintained at 37 °C. For hypoxic conditions, the plate reader was enclosed in an air-tight plastic box which was equilibrated with 3 % O2, 5 % CO2, balanced N2. As positive control, the monolayer was pretreated with TGFβ (1ng/ml) for 15 min prior to reading.
Labeling Of Plasma Membrane and Nucleus
In order to determine any alteration in the gaps between cells under different treatments, RPMECs were grown on collagen-coated glass coverslips. Upon confluence cells were treated with hypoxia or TGFβ after being starved overnight on the coverslips. Next, coverslips were rinsed twice with phosphate-buffered saline (PBS), and fixed for 10 minutes with 4% formaldehyde. Next the coverslips were stained using the Image-iT LIVE Plasma Membrane and Nuclear Labeling Kit (Molecular Probes, Eugene OR) according to the manufacturer instruction. Cells were examined using a Zeiss Fluorescence Microscope and imaging system with 40× objective lens.
siRNA Transfection
80%–90% confluent cells were transfected with HSP27 siRNA (Cat # J-005269-06 Dharmacon, IL), and mock control siRNA (D-001810-0X Dharmacon, IL) with Lipofectamine 2000 (Invitrogen, CA) at 100 nM for 36 h according to the manufacturer’s instructions. Western blotting was then performed to test the silencing effect of siRNA on the expression levels of target proteins.
Immunofluorescence Microscopy
After treatment, endothelial cells grown on collagen-coated coverslips were rinsed with PBS, fixed in 4% formaldehyde for 10 min, and permeabilized twice with 0.4% Triton X-100 for 5 min. After being washed twice with PBS, cells were incubated with 5U/ml of Rhodamine-phalloidin (Molecular Probes, Eugene, OR) for 20 min. Coverslips were mounted on slides with Citifluor mounting medium (TED PELLA, Redding, CA) and analyzed with Zeiss Fluorescence Microscope and imaging system at 400× magnification.
SDS-PAGE and Immunoblotting
Cell lysates were assayed for protein using the Bradford protein assay and then diluted with 2x Laemmli loading buffer for SDS-PAGE. For MLC2 detection proteins were first precipitated with 5% TCA. Equal amounts of protein were then loaded in 4–20% Tris/glycine gels, and electrophoresed for 90 min at 300 mA constant current. Next, the gel was blotted onto a PVDF membrane by electrophoretic transfer at 25 V constant voltage overnight. The membrane was then washed, blocked with 5% milk, and probed with primary antibodies. Appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL), and a chemiluminescent substrate (SuperSignal, Pierce, Rockford, IL) were used to visualize immunoreactive bands. The primary antibodies against monophospho-MLC2, MYPT1, phospho-MYPT1 were purchased from Cell Signaling (Danvers, MA), antibodies against MLC2, FAK, HSP27, and Actin were purchased from Santa Cruz (Santa Cruz, CA) and antibody against phospho-FAK was purchased from Upstate (Lake Placid, NY). The donkey anti-mouse and donkey anti-rabbit secondary antibodies were purchased from Pierce (Rockford, IL).
Two-dimensional Electrophoresis
Hypoxia-treated or control cells were homogenized in rehydration buffer (10 M Urea, 2% (w/v) CHAPS, 0.3% (w/v) DTT, 0.002% (w/v) Bromophenol Blue, 0.5% (v/v) pH 4–7 IPG buffer). Next, samples were cleared of interfering material with a sample cleaning kit (Amersham, Piscataway, NJ) prior to running on a 2-D gel. For analytical gels, 11 cm linear pH 4–7 IPG strips were rehydrated for 18 h at room temperature in rehydration buffer containing 100 μg protein. Strips were focused at 17 °C, 500 V step for 1 h, 1000 V gradient for 1 h, 6000 V gradient for 2.5 h, 8000V gradient for 2 h using the Ettan system (GE). For the second dimension, IPG strips were equilibrated for 15 min in equilibration buffer with 65 mM DTT, and alkylated in equilibration buffer with 135 mM iodoacetamide. Molecular weight markers were loaded on the left of each gel. The separation was performed in a 12.5 % constant concentration polyacrylamide slab gels at 100 V for 1 h in the cold room. Transfer to PVDF membrane was performed at 300 mA for 90 min. The membrane was immunostained as above.
Statistical Analysis
Data are presented as means ± standard deviation (SD). Statistically significant differences were determined by ANOVA and Holm-Sidak post hoc analysis. Statistical analysis was carried out using SigmaStat (Systat, San Jose, CA) P<0.05 was considered statistically significant.
RESULTS
Hypoxia-induced permeability of pulmonary microvascular endothelial monolayer is p38-dependent
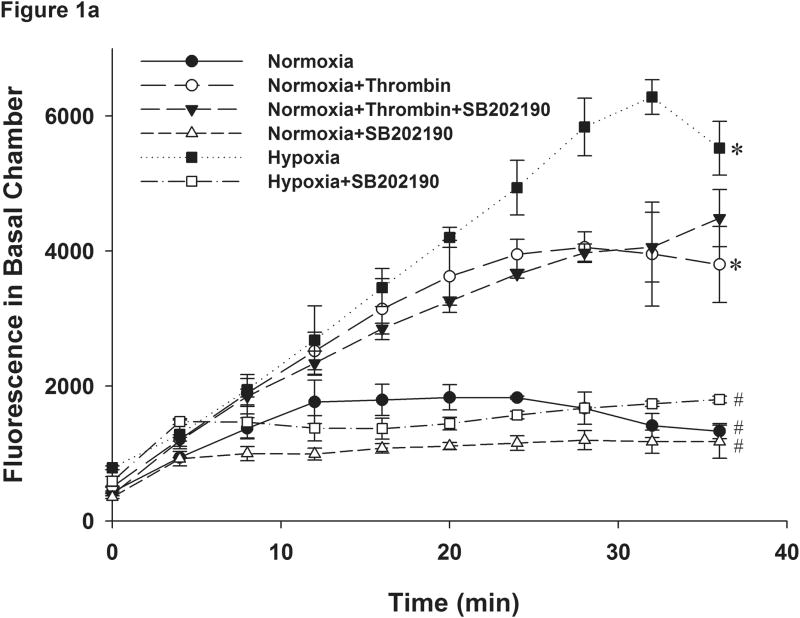
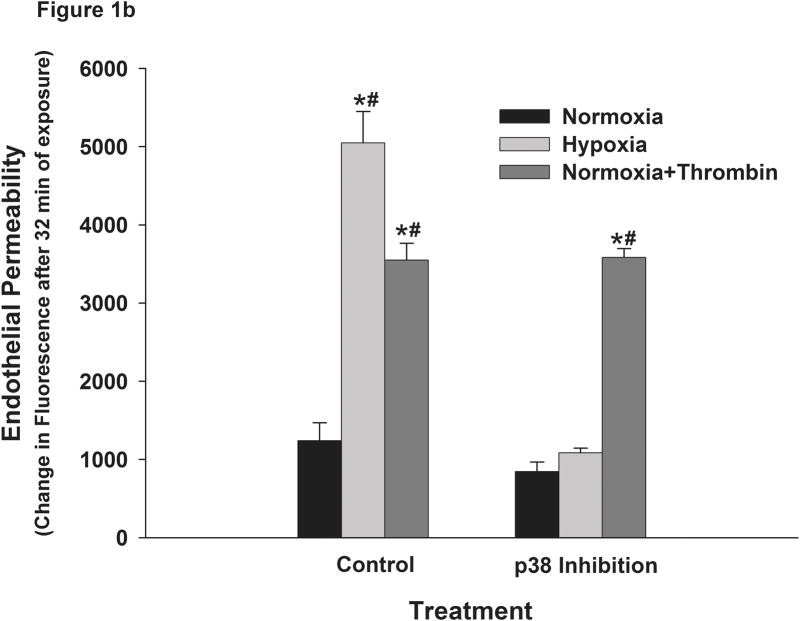
In order to determine the effects of hypoxia on pulmonary endothelial permeability we plated RPMEC on Fluoroblock cell inserts which were impervious to light. The use of these inserts allowed continuous recording of the time-course of altered fluorescence from the lower chambers without interference from the upper chamber to which the fluorescently-tagged dextran is added. Hence, the need to draw samples from upper and lower chambers at different time points was obviated. The latter approach increased the risk of physically disrupting the endothelial barrier, and in the case of hypoxia resulted in interruption of hypoxic exposure. The plate reader was also placed in a hypoxic box maintained at 3% oxygen to isolate the effects of hypoxia from those due to hypoxia-reoxygenation. Continuous measurement of fluorescence beyond 45 minutes was limited by decay of the signal. The reason for this decay could be that fluorescence of the dye in the bottom chamber was quenched by repeated flashes of light during each measurement as we have also observed that decay when fluorescent-dextran was added to filter inserts on which no cells were grown. Hence, we did not include time points beyond 45 minutes for comparison. These data suggest that the fluorescence measured reflects both the rate of transfer minus the rate of decay. Our results showed that the permeability of the RPMEC monolayer as reflected by fluorescence reading in the lower chamber increased significantly with exposure to hypoxia (Figure 1a). Thrombin, an agent known to increase endothelial barrier permeability was used as a positive control in these experiments and as shown in Figure 1, it also increased RPMEC monolayer permeability. Pretreatment with SB202190 (2μM), an inhibitor of p38, blocked the increased permeability due to exposure to hypoxia. However, SB202190 did not block the thrombin-induced endothelial permeability (Figure 1a and Figure 1b). These results indicate that p38 plays a specific and critical role in elevating the permeability of RPMEC monolayers in response to hypoxia.
Figure 1.
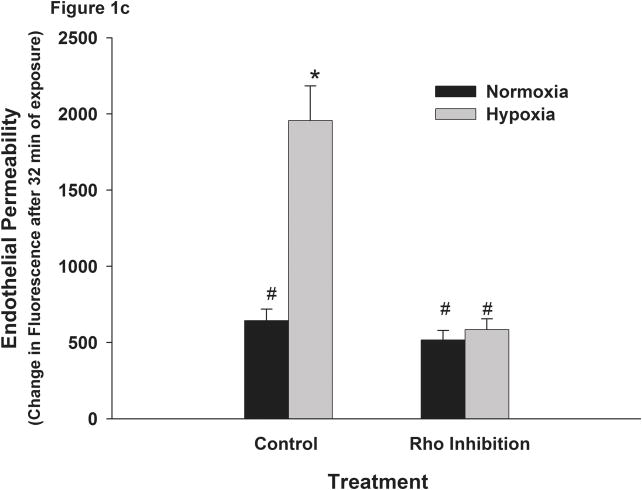
Monolayer permeability of RPMEC was increased by exposure to hypoxia. RPMEC monolayers were grown on filter inserts and exposed to normoxia, hypoxia (3% O2) or Thrombin (1U/ml). The transfer of Alexa Fluor-dextran (3 kDa) through the monolayer was measured over time. (a) Hypoxia and thrombin increased the amount of Alexa Fluor-dextran in the basolateral chamber at a higher rate than normoxia. An inhibitor of p38 (SB202190, 1 μM) reduced the fluorescence transfer in response to hypoxia. Data are presented as means ± standard deviation (n =4 samples). (b) Represents the change in fluorescence after 32 minutes of exposure. (c) Inhibition of ROCK abolished hypoxia-induced permeability in RPMEC. * Statistically significant difference from normoxia control mean, # statistically significant difference from normoxia with p38 inhibition mean. P <0.05 in ANOVA and Holm-Sidak post hoc analysis.
ROCK mediates hypoxia-induced permeability in RPMEC
Activation of the Rho/ROCK pathway has been known to lead to cell contractility in smooth muscle cells through its effect on MLC phosphorylation. The same pathway has been shown to be important in endothelial cell contractility and barrier regulation (Patil and Bitar, 2006; Wang et al., 2001b; Wang et al., 2003). Our laboratory has recently shown that hypoxia-induced endothelial cell contractility could be blocked by ROCK inhibition. To evaluate the role of ROCK in hypoxia-induced permeability, we assayed the permeability of RPMEC monolayer which was exposed to hypoxia in the presence of the ROCK inhibitor Y27632. As shown in Figure 1c, pretreatment with Y27632 (2 μM) significantly attenuated the increase of permeability induced by hypoxia (Figure 1c). These data suggest that Rho/ROCK pathway plays an important role in mediating hypoxia-induced permeability in RPMEC monolayer.
Phosphomimicking HSP27 abolishes hypoxia-induced permeability
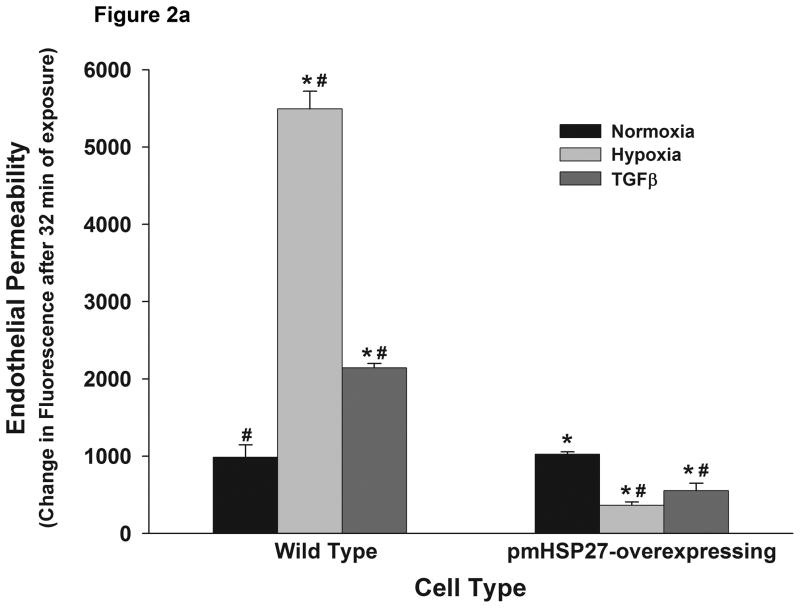
We had previously demonstrated that activation of p38 by hypoxia in RPMEC results in actin polymerization and stress fiber formation via activation of MK2 and the latter’s phosphorylation of HSP27 (Kayyali et al., 2002). Since increased actin stress fiber formation has been reported to reflect increased contractility and to be associated with increased endothelial barrier permeability in response to several agents, we speculated that overexpressing phosphomimicking HSP27 (pmHSP27) in RPMEC would reduce their barrier capacity. Thus we evaluated the effect of hypoxia and TGFβ on RPMEC stably transfected with pmHSP27. When cells that were stably transfected with pmHSP27 were exposed to hypoxia they were surprisingly resistant to its permeability enhancing effect (Figure 2a). TGFβ, another agent reported to induce permeability via activating the p38 pathway (Goldberg et al., 2002; Lee et al., 2007; Lu et al., 2006) also failed to increase endothelial permeability in pmHSP27-overexpressing RPMEC (Figure 2a). Both hypoxia and TGFβ (1 ng/ml) increased permeability of wild type RPMEC (Figure 2a) within 30 min in our experiments. Other researchers reported earlier that endothelial monolayer integrity started to deteriorate between 1 and 2 h after TGFβ treatment (Hurst et al., 1999). The different time-course reported could be due the different types of endothelial cells used in different laboratories. In summary, our results show that overexpression of pmHSP27 abolished the increase and even decreased the permeability induced by hypoxia or TGFβ treatment compared with the normoxic condition (Figure 2a).
Figure 2.
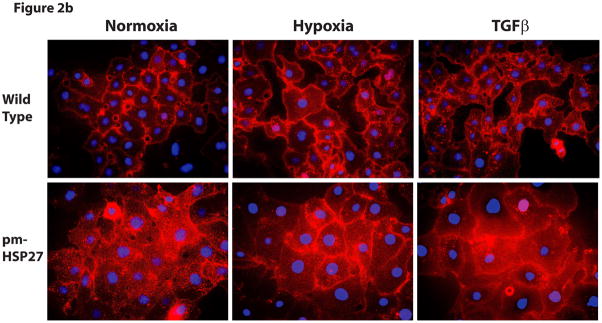
Overexpression of pmHSP27 abolished the increased permeability and gap formation induced by hypoxia or TGFβ in RPMEC monolayer. (a) RPMEC were stably transfected with a plasmid carrying phosphomimicking mutant pmHSP27 sequence. These cells were then exposed to hypoxia or TGFβ (1 ng/mL) for the duration shown. Overexposing pmHSP27 reversed the response of RPMEC to hypoxia or TGFβ. Data are presented as means ± standard deviation (n =4 samples). * Statistically significant difference from normoxia wild type mean, # statistically significant difference from normoxia pmHSP27-overexpressing mean. P <0.05 in ANOVA and Holm-Sidak post hoc analysis. (b) The borders between endothelial cells were visualized by staining plasma membranes and nuclei after treatment with 3% oxygen for 1 h or TGFβ (1 ng/mL) for 15 min. Both hypoxia and TGFβ increased the number and size of gaps forming between RPMEC, and overexpression of pmHSP27 reversed the increased intercellular gaps induced by hypoxia or TGFβ.
Hypoxia increases gap formation between endothelial cells while overexpressing pmHSP27 reduces these gaps
Gaps between different endothelial cells play an important role in increasing the permeability of the endothelial barrier by allowing fluid and macromolecular proteins to permeate to the interstitial space. The increase in gap formation in response to several stimuli has been correlated with the ability of these agents to increase endothelial barrier permeability (Hastie et al., 1998). In order to visualize the gaps between endothelial cells, RPMEC grown on coverslips were allowed to reach confluence and then treated with hypoxia or normoxia. Next, they were fixed and stained with red-fluorescent Alexa Fluor 594-tagged wheat germ agglutinin, which binds to the sialic acid residues on the plasma membrane. The nuclei of endothelial cells were also labeled with blue-fluorescent Hoechst 33342 dye. The results we obtained demonstrated that both hypoxia (3% oxygen for 1hr) and TGFβ (1ng/ml for 15 min) increased the intercellular gaps significantly, and caused these gaps to increase in size (Figure 2b). However, neither hypoxia nor TGFβ induced any alteration of the gaps between the pmHSP27-overxpressing cells. These results suggest that HSP27 protein phosphorylation inhibits hypoxia- or TGFβ-induced gap formation which is consistent with its observed effect on RPMEC monolayer permeability.
Actin stress fiber formation correlates with pmHSP27 expression as well as with decreased rather than increased permeability
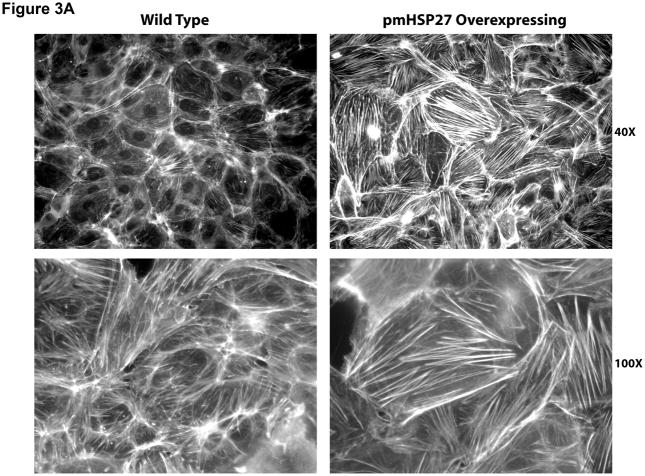
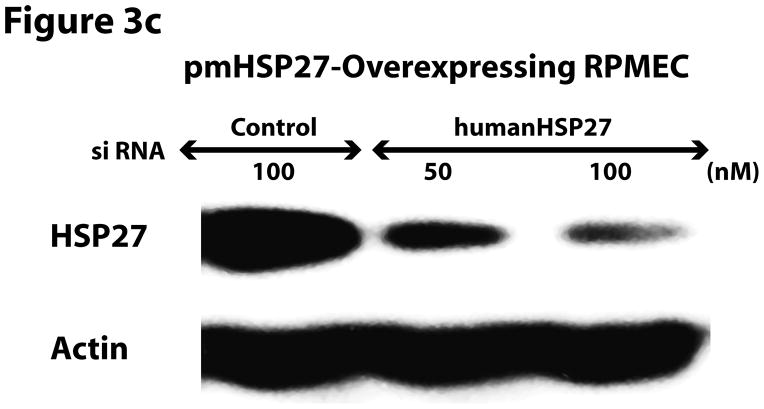
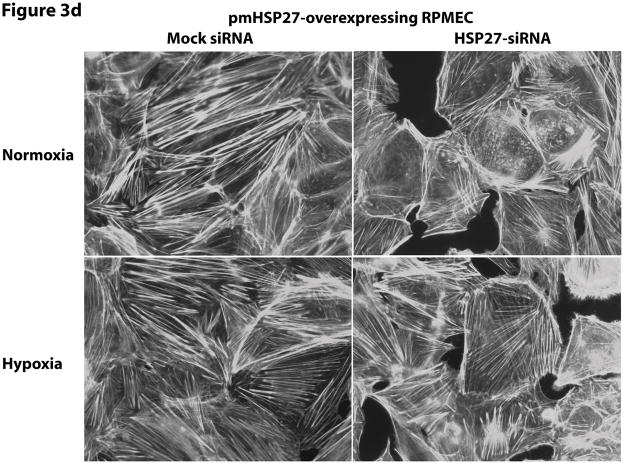
Since the effect of pmHSP27 on permeability and intercellular gap formation was opposite to what would be expected based on reported effects of HSP27 phosphorylation on actin stress fiber formation and the effect of the latter on endothelial permeability we decided to investigate these relations further. To verify the effect of pmHSP27 on actin stress fiber formation these cells were stained with Rhodamine-phalloidin which labels filamentous actin. Overexpressing pmHSP27 in stably transfected RPMEC indeed caused increased stress fiber formation compared to mock-transfected cells as we have previously reported (Figure 3a). The level of stress fibers did not change significantly in response to exposure to TGFβ or hypoxia. In order to further test the link between actin stress fiber formation and stable pmHSP27-overexpression we transfected these cells with siRNA against human HSP27. Based on its sequence the latter siRNA is expected to knockdown the expression of the overexpressed human pmHSP27 form but not the endogenous rat form of the protein. As shown in Figure 3b human HSP27 siRNA did not affect the level of endogenous rat HSP27 in wild type RPMEC. On the other hand human HSP27 siRNA reduced the level of HSP27 in pmHSP27 overexpressing cells bringing it down to a level comparable to wild type cells (Figure 3c). At the same time actin stress fiber formation was dramatically reduced by knocking down pmHSP27 with siRNA (Figure 3d). Thus, these results suggest that pmHSP27-induced actin stress fiber formation in RPMEC can be reversed or rescued by knocking down HSP27.
Figure 3.
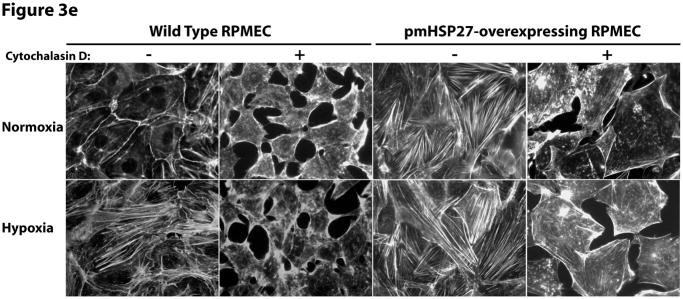
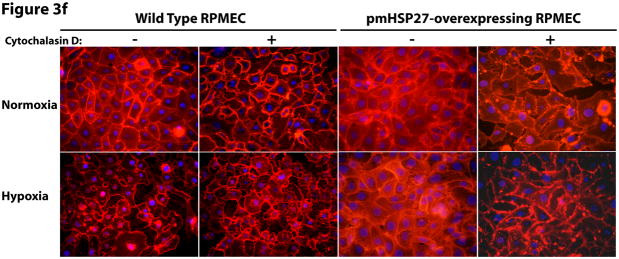
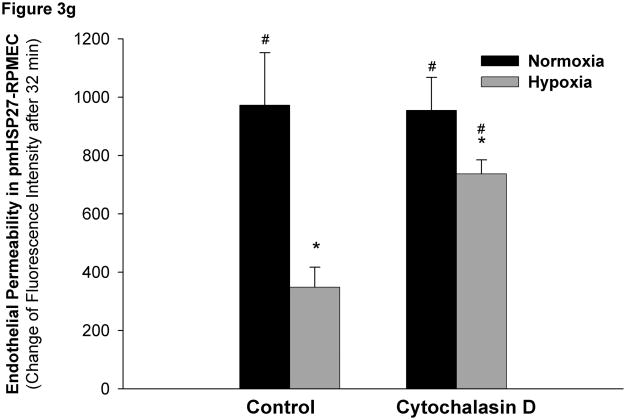
HSP27, actin stress fibers, and endothelial barrier integrity. (a) Over-expression of pmHSP27 increased the formation of actin stress fiber in PRMEC. pmHSP27-overexpressing RPMECs grown on coverslips were stained with Rhodamine-phalloidin for 20 min, actin stress fibers was visualized with fluorescence microscopy. (b) Human HSP27 siRNA did not affect the expression level of endogenous rat HSP27. Wild type RPMEC was transfected with human HSP27 siRNA and mock control siRNA, then protein level of HSP27 was assayed after 36 h by Western blotting. (c) Human HSP27 siRNA reduced the expression of pmHSP27 in pmHSP27-overexpressing RPMEC. Transfection was performed at the indicated concentrations of siRNA, then expression level of pmHSP27 was tested after 36 h by Western blotting. (d) Transfection of pmHSP27 siRNA attenuated the formation of actin stress fiber in pmHSP27-overexpressing RPMEC. After transfection of pmHSP27 siRNA, actin stress fibers were visualized in pmHSP27-overexpressing RPMEC grown on cover slips by Rhodamine-phalloidin staining. Magnification was 400X. (e) Cytochalasin D inhibited the formation of actin stress fiber. Stress fibers were visualized by Rhodamine-phalloidin staining after treatment with an inhibitor of actin filament formation, Cytochalasin D (2 μM) Magnification was 400X. (f) Cytochalasin D inhibited the formation of intercellular gaps. Gaps between cells were visualized with Image-iT LIVE Plasma Membrane and Nuclear Labeling Kit. Magnification was 400X. (g) Cytochalasin D reversed the reduced permeability in pmHSP27-overexpressing RPMEC. Permeability was tested after 32 min treatment with Cytochalasin D. Data are presented as means ± standard deviation (n =4 samples). * Statistically significant difference from normoxic control mean, # statistically significant difference from hypoxic control mean. P <0.05 in ANOVA and Holm-Sidak post hoc analysis.
As pmHSP27 likely affects other cell proteins we further investigated if its effect on actin stress fiber formation is linked to its effects on permeability and intercellular gap formation. Thus, we treated cells with cytochalasin D, an agent that inhibits actin filament formation directly and independently of HSP27. As shown in Figure 3e, Cytochalasin D inhibited actin stress fiber formation in both wild type and pmHSP27 overexpressing cells under normoxic and hypoxic conditions. Moreover, cytochalasin D increased intercellular gap formation and more importantly abolished the resistance of pmHSP27 overexpressing cells to hypoxia (Figure 3f). Finally, treatment with cytochalasin D reversed the reduced permeability observed in hypoxic pmHSP27-overexpressing RPMEC (Figure 3g).
Taken together these results indicate that the phosphorylation of HSP27 strongly correlates with actin stress fiber formation, and that abolishing actin stress fiber formation reverses the barrier augmenting effect of pmHSP27 on permeability and intercellular gap formation. These findings raise caution against the use of stress fiber formation as an endpoint related to increased permeability. Furthermore, these results suggest that increased HSP27 phosphorylation and actin stress fiber formation in hypoxia could not explain the p38 and ROCK-mediated permeability.
Hypoxia increases the phosphorylation level of MLC2
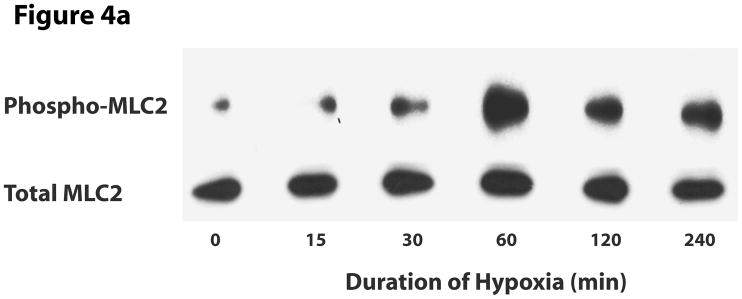
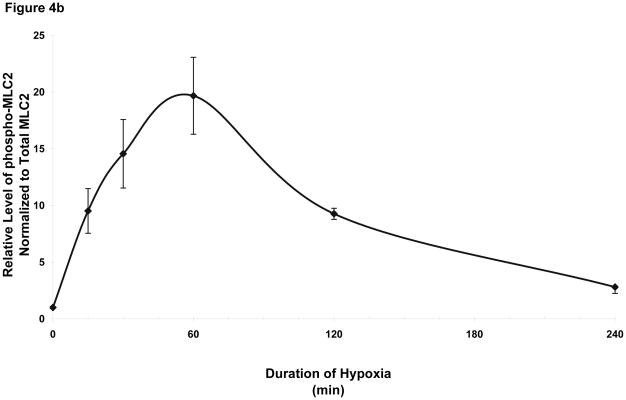
We next investigated signaling events that could explain the hypoxia-induced permeability in wild type cells and its lack in pmHSP27-overexpressing cells. Since several studies have reported that phosphorylation of MLC2 was related to increased permeability of endothelial monolayer (Bogatcheva et al., 2007; Dudek and Garcia, 2001; Mehta and Malik, 2006; Mucha et al., 2003), we tested the phosphorylation of MLC2 in response to hypoxia. Immunoblotting with an antibody against monophospho-MLC2 (ser 19) indicated that the level of phosphorylation of MLC2 increased with time in hypoxia, peaked at 1 h, then recovered after 4 h (Figure 4). Immunoblotting with an antibody against diphospho-MLC2 (ser 19, thr 18) did not detect any bands in normoxic or hypoxic samples.
Figure 4.
Increased phosphorylation of MLC2 in hypoxia. RPMEC were treated with hypoxia for different times, then proteins were precipitated with 5% TCA. (a) The samples were run on SDS-PAGE gel. The target protein was detected with antibodies against phospho-MLC2 and total MLC2. The picture is representative of three different experiments. (b) Time-course of altered MLC2 phosphorylation in hypoxia. The intensity of every band of phospho-MLC2 protein was normalized to that of corresponding total MLC2. The data represent average ± standard deviation of three different experiments.
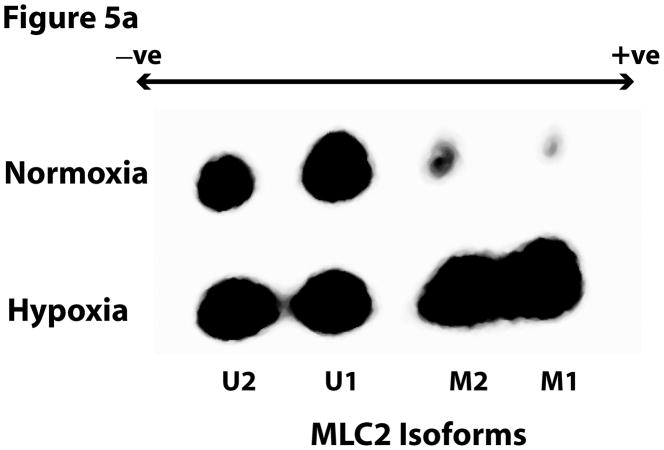
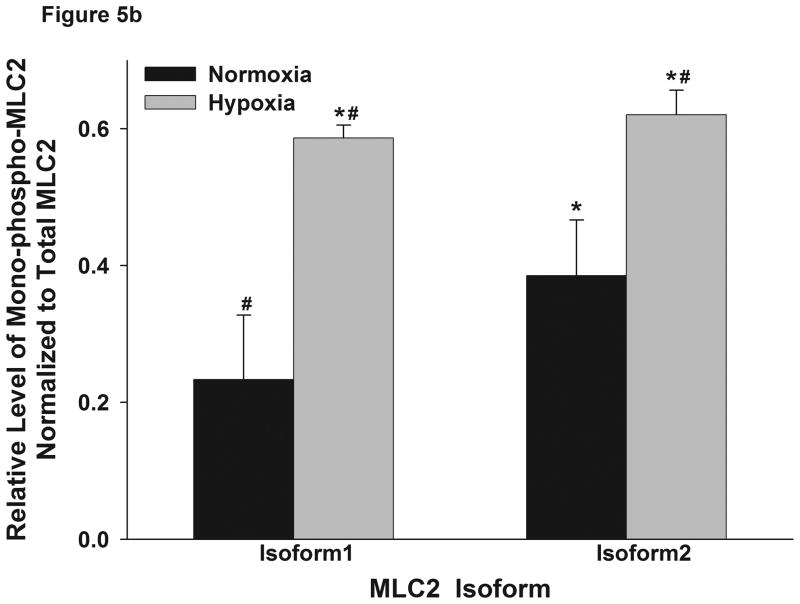
MLC2 has been reported to have at least 2 isoforms which can be distinguished by 2-D electrophoresis, a technique that can also be used to distinguish between mono- and di-phosphorylated forms of the two isoforms (Kumar et al., 1989). Thus, we performed 2-D electrophoresis to elucidate how hypoxia affected the phosphorylation of different MLC2 isoforms. The 2-D Western blots were probed with an anti-total MLC2 antibody. The results showed that the two isoforms were increasingly phosphorylated in hypoxia (Figure 5); U1 = unphosphorylated isoform 1, M1 = monophosphorylated isoform 1, U2 = unphosphorylated isoform 2, M2 = monophosphorylated isoform 2). Hypoxia seemed to increase mostly the monophosphorylation of the isoforms of MLC2 leading to stronger M1 and M2 spots. These findings are consistent with the immuoblotting with phosphospecific antibodies described above. Moreover, hypoxia increased the monophosphorylation of isoform U1 to M1 by 157% while it increased the phosphorylation of isoform U2 to M2 by 59%. These results raise the possibility that isoform 1 contributes more than isoform 2 to the MLC2-mediated contractility in hypoxia.
Figure 5.
Hypoxia increased the phosphorylation level of different isoforms of MLC2. (a) RPMEC were exposed to hypoxia for 1 hour, cell lysates were subjected to 2-D electrophoresis, and immunoblotted with anti-total MLC2 antibody. Phosphorylation causes MLC2 to become more acidic. Two isoforms of MLC2 were observed (1 and 2), and only monophosphorylated forms were found to be increased with hypoxia (M1 and M2 = monophosphorylated isoforms 1 and 2; U1 and U2 = unphosphorylated isoforms 1 and 2). No changes in diphosphorylated MLC2 were observed by 2-D electrophoresis. (b) For each isofrom the intensity of the monophospho-protein spot was normalized to that of total protein of corresponding isoform. The data represent average ± standard deviation of three different experiments. * Statistically significant difference from normoxia isoform 1 mean, # statistically significant difference from normoxia isoform 2 mean. P <0.05 in ANOVA and Holm-Sidak post hoc analysis.
pmHSP27 overexpression inhibits hypoxia-induced phosphorylation of MLC2 and MYPT1
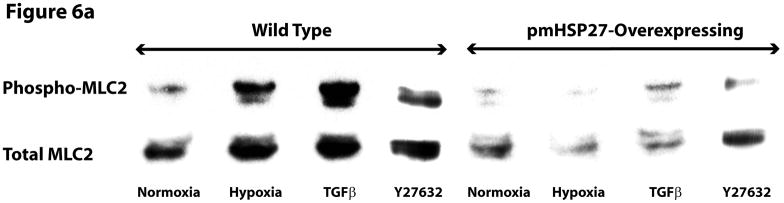
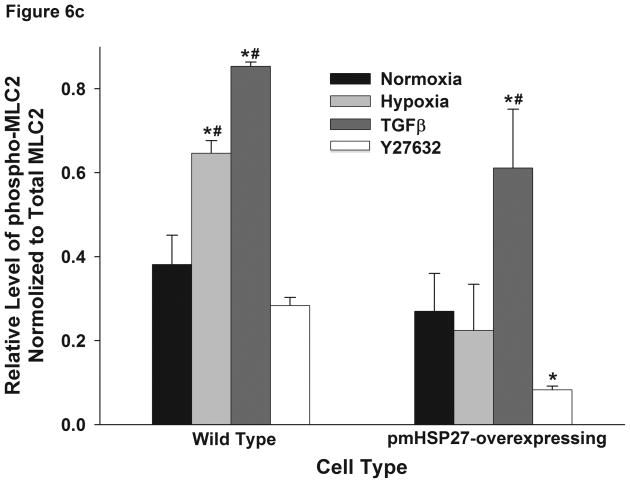
Since pmHSP27 overexpression in RPMEC can abolish the hypoxia-induced permeability which is associated with the increased phosphorylation of MLC2, we tested the effect of pmHSP27 overexpression on MLC2 phosphorylation. The results shown in Figure 6(A and C) indicated that hypoxia-induced phosphorylation of MLC2 was attenuated in pmHSP27-overexpresssing RPMEC, and suggested that HSP27 plays a critical role in regulating phosphorylation of MLC2 induced by hypoxia.
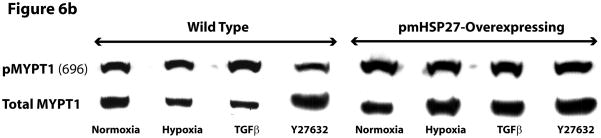
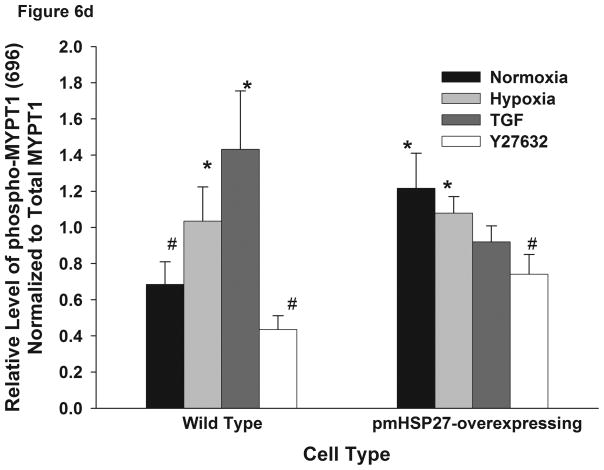
Figure 6.
Over-expression of pmHSP27 attenuates MLC2 and MYPT1 phosphorylation. RPMEC and pmHSP27-overexpressing RPMEC were exposed to hypoxia (3%) for 1 h or TGFβ (1 ng/ml) for 10 min. Cell lysates were then immunoblotted to test the phosphorylation of MLC2 (a) and MYPT1 (b). The bar graph shows quantitation of the intensity of bands of phospho-MLC2 (c) and phospho-MYPT1-696 (d) normalized to corresponding total protein. While hypoxia and TGFβ increased the phosphorylation of MLC2 and MYPT1 in wild type RPMEC, they did not induce these effects in pmHSP27 overexpressing RPMEC. Inhibiting Rho kinase with Y27632 (2μM) also inhibited MLC2 and MYPT1 phosphorylation in response to hypoxia. The data represent average ± standard deviation of three different experiments. * Statistically significant difference from normoxia wild type mean, # statistically significant difference from normoxia pmHSP27-overexpressing mean. P <0.05 in ANOVA and Holm-Sidak post hoc analysis.
MLC2 phosphatase (MLCP) regulates the level of phospho-MLC2 through dephosphorylation of MLC2. Recent studies have highlighted the importance of regulation of cell contractility via regulating the activity of MLCP. To investigate the role MLCP plays in the regulation of MLC2 phosphorylation in response to hypoxia or TGFβ, we tested the phosphorylation of MYPT1. The latter protein constitutes the myosin-binding unit of the MLCP protein complex, and once phosphorylated it inhibits the phosphatase activity of the enzyme. Immunoblotting for phospho-MYPT1 demonstrated that hypoxia or TGFβ increased the phosphorylation level of MYPT1 in wild type RPMEC (Figure 6B, D). Inhibition of ROCK, which is believed to phosphorylate MYPT1 in hypoxic cells (Wang et al., 2001a; Wang et al., 2003), by the inhibitor Y27632 abolished the increased phosphorylation of MLC2 and MYPT1 due to hypoxia or TGFβ (Figure 6). Furthermore, overexpressing pmHSP27 attenuated or blocked the increase in MYPT1 phosphorylation in response to hypoxia or TGFβ (Figure 6B, D). These results suggest that HSP27 also plays an important role in regulating the phosphorylation of MYPT1 which is regulated by Rho/ROCK kinase pathway.
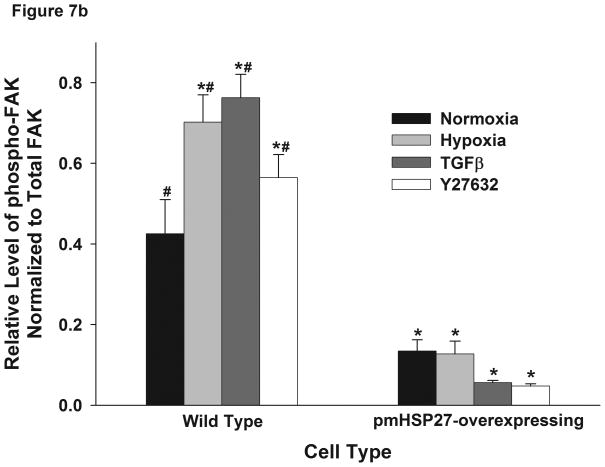
HSP27 alters the phosphorylation of FAK induced by hypoxia
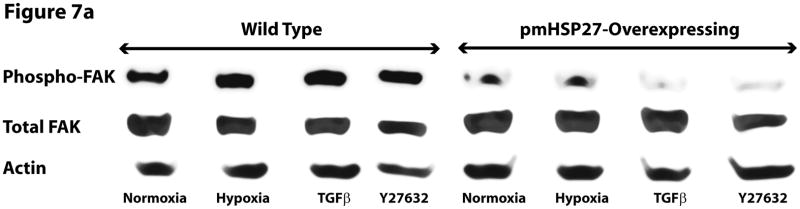
FAK is an important regulator of focal adhesion function which is believed to contribute to endothelial barrier permeability through mediating cell-extracellular matrix interactions. Increased FAK phosphorylation has been reported in response to permeability-inducing agents (Mehta et al., 2002). To test if FAK contributed to the increased endothelial permeability in hypoxia, we examined the phosphorylation level of FAK by immunoblotting RPMEC lysates after exposure to hypoxia or TGFβ. The results showed that hypoxia and TGFβ increased the phosphorylation level of FAK in wild type cells but not in pmHSP27-overexpressing cells (Figure 7). These results suggested that HSP27 also plays a critical role in FAK phosphorylation under conditions of hypoxia or TGFβ treatment.
Figure 7.
Hypoxia increased the phosphorylation level of FAK in wild type but not pmHSP27-overexpressing RPMEC. Wild type RPMEC and pmHSP27-RPMEC were exposed to hypoxia (3%) for 1 h or TGFβ (1ng/ml) for 10 min, then (a) Western blotting was performed to test the phosphorylation of FAK. (b) The bar graph shows quantitation of the intensity of phospho-MLC2 bands normalized to that of total FAK. The data represent average ± standard deviation of three different experiments. * Statistically significant difference from normoxia wild type mean, # statistically significant difference from normoxia pmHSP27-overexpressing mean. P <0.05 in ANOVA and Holm-Sidak post hoc analysis.
DISCUSSION
Hypoxia is known to affect the permeability of the pulmonary endothelial barrier leading to pulmonary edema. It is also believed to contribute to the increased endothelial barrier permeability in other tissues such as, tumor vasculature, brain vasculature and gastrointestinal tract epithelium (Brown et al., 2003; Koto et al., 2007; Tappenden, 2002). Understanding the mechanism through which hypoxia alters endothelial permeability could be beneficial in protecting patients from hypoxia-induced injury. While several signaling pathways might be activated by hypoxia in different tissues, we have previously demonstrated that the pathway involving p38 and downstream components plays an important role in modulating the cytoskeleton and biomechanical properties of hypoxic pulmonary microvascular endothelial cells (An et al., 2005; Kayyali et al., 2002). Thus we have used an in vitro endothelial cell monolayer model to monitor the alteration of barrier permeability over time and found that hypoxia increased the permeability starting 4 minutes after exposure to hypoxia. To the best of our knowledge, this is the first time the time-course of the altered permeability due to hypoxia has been monitored continuously. Further investigation of the role of p38 pathway in regulating endothelial permeability revealed that its downstream target, HSP27, plays an important role through affecting many proteins that are known to be important for barrier function. Our data suggest that actin stress fiber formation in response to HSP27 phosphorylation correlate with barrier augmentation rather than barrier disruption as previously reported.
Several studies have reported increased permeability in the lung or in cultured monolayers in response to hypoxia (Ali et al., 1999; Baudry et al., 1998; Carpenter et al., 1998; Carpenter and Stenmark, 2001; Hansen et al., 1996; Hassoun et al., 1998; Lewis et al., 1997; Ogawa et al., 1990a; Ogawa et al., 1992; Ogawa et al., 1990b; Partridge, 1995; Pinsky et al., 1995; Stelzner et al., 1988; Wojciak-Stothard et al., 2006). Increased permeability in monolayers in response to hypoxia has also been described in other cell types, such as oral, lung, and kidney epithelial cell monolayers as well as skin microvascular endothelial cells(Furuta et al., 2001). Fluid and macromolecules cross endothelial barriers either through the cells (transcellular route), or through pores or gaps between these cells (paracellular route). The latter route is believed to be the major contributor to tissue edema (Dudek and Garcia, 2001). Thus the size and number of gaps forming between cells determine the permeability of pulmonary microvascular endothelial barrier. Our data show that staining of plasma membranes reveals significantly increased gaps between cells in response to hypoxia or TGFβ. The latter observations are consistent with the increased endothelial barrier permeability in response to these two agents.
While the effect of hypoxia on barrier permeability appears to suggest consistent increased permeability in different cell types, the mechanism of that increased permeability appears to be complex. One of the most commonly reported effects related to increased permeability is increased actin stress fiber formation. Several agents that induce endothelial permeability, e.g., thrombin, H2O2, have been associated with increased actin stress fiber formation. We have indeed demonstrated that hypoxia can induce actin stress fiber formation in RPMEC via activation of p38 and MK2 and phosphorylation of HSP27 (Kayyali et al., 2002). However, our current findings challenge the correlation of stress fiber formation with increased endothelial permeability. Although inhibiting p38 blocked the hypoxia-induced permeability, our data suggest that actin stress fiber formation alone could not explain the increased endothelial permeability. Stably transfected pmHSP27-overexpressing endothelial cells contained significantly more actin stress fibers than mock-transfected endothelial cells as expected (Kayyali et al., 2002), a phenotype that could be rescued by siRNA against the pmHSP27 (Figure 3). Yet as we show in this report these cells appear more resistant to permeability inducing stimuli. Consistent with the permeability findings pmHSP27 overexpressing monolayers also contained significantly less and smaller intercellular gaps than wild type. Inhibiting the actin stress fibers in pmHSP27 overexpressing cells with Cytochalasin D abolished the reduced monolayer intercellular gaps and permeability. These results are also consistent with our earlier study where we measured endothelial cell contractility (stiffness) and adhesiveness (tethering) in hypoxic and pmHSP27 overexpressing cells (An et al., 2005). In that study we found that hypoxia-induced p38-mediated actin stress fiber formation correlated with increased adhesiveness but not contractility. Moreover, pmHSP27 overexpressing RPMEC were more adhesive but not more contractile than wild type cells (An et al., 2005). We also observed in the study that hypoxia indeed induces increased contractility in a ROCK dependent manner which precedes the increased adhesiveness correlated with actin stress fiber formation. Thus it would appear that in hypoxia an initial response might involve MLC2 phosphorylation which would result in actomyosin formation and contraction independently of actin stress fiber formation. Indeed, MLC2 phosphorylation and actomyosin bridge cycling in smooth muscle cells appear to be independent of actin polymerization (Gunst and Zhang, 2008). As to why p38 inhibition blocked hypoxia-induced permeability, other p38 substrates might be involved which are beyond the scope of this report, but HSP27 and actin stress fibers could be ruled out as mediators. In summary, our data suggest that actin stress fiber formation correlates with barrier augmentation and might occur to counterbalance transient increased contractility induced by permeability-inducing stimuli. This conclusion runs counter to reports that associate actin stress fibers with increased contractility and increased permeability. However, in blood vessels actin stress fibers are known to arise in endothelial cells in response to sheer stress from blood flow which when disrupted results in disruption of these stress fibers and increased atherogenesis that has been associated with increased permeability (White et al., 1983)(for review see (Li et al., 2005)). In endothelial cell culture models of laminar shear stress, which is associated with increased actin stress fibers, endothelial barrier augmentation has been reported in brain endothelial cell (Colgan et al., 2007) and HUVEC monolayers (Garcia-Cardena et al., 2001).
Since hypoxia induced endothelial permeability which is not explainable by HSP27 phosphorylation and actin stress fiber formation, we turned our attention to signaling that could explain that increased permeability. Based on our previous study demonstrating a role for ROCK in hypoxia-induced contractility we assessed MLC2 phosphorylation in hypoxic endothelial cells. Another report on porcine pulmonary artery endothelial cells suggested that hypoxia-induced Rho activation mediates increased barrier permeability (Wojciak-Stothard et al., 2005). Rho is a small G protein, which is known to regulate smooth muscle cell contractility. Compromised endothelial permeability in response to different stimuli has also been suggested to be mediated by Rho activation, e.g., sodium fluoride acting through ROCK (Wang et al., 2001a), and IL-8 through Rho and Rac (Schraufstatter et al., 2001). Once activated, Rho can activate ROCK, which may phosphorylate MLC2 directly (Amano et al., 1996; Feng et al., 1999), but more likely increases MLC2 phosphorylation through phosphorylating and inactivating myosin light chain phosphatase MLCP (Essler et al., 1998; Robertson et al., 2000). While MLC2 phosphorylation has been correlated with increased endothelial permeability in response to a variety of agents, the role of MLC2 phosphorylation in hypoxia-induced permeability change has not been thoroughly investigated. The results described in this manuscript demonstrate that hypoxia indeed induces MLC2 phosphorylation, and that phosphorylation of MLC2 correlates with permeability of endothelial cells. We further characterize the MLC2 isoforms that become phosphorylated in response to hypoxia. Consistent with our findings in endothelial cells, hypoxia has been shown to activate Rho and ROCK in pulmonary artery smooth muscle cells leading to MLC2 phosphorylation (Wang et al., 2001b; Wang et al., 2003). Our results also suggest that hypoxia promotes the phosphorylation of one MLC2 isoform more than the other. The significance of these differences is not clear but is reminiscent of the variable phosphorylation of different isoforms of MLC2 in skeletal muscle. It is possible that the greater phosphorylation of the slower migrating isoform 1 compared to isoform 2 relates to the contractile properties of the RPMEC we are working with as has been described in different types of skeletal muscles (Bozzo et al., 2005; Toursel et al., 2000). Both phosphospecific antibodies and 2-D analysis indicated that hypoxia causes monophosphorylation rather than diphosphorylation of MLC2 in response to hypoxia. Previous studies have suggested that monophosphorylation of MLC2 is associated with the initiation of contraction, while diphosphorylation of MLC2 is associated with sustained contraction (Goeckeler and Wysolmerski, 1995). Thus it is possible that monophosphorylation represents a transient increase in contractility which precedes the increased tethering strength we saw in our previous study (An et al., 2005).
Phosphorylation of MLC2 is believed to contribute to the contraction of cells through promoting myosin interaction with polymerized actin (Garcia et al., 1995). Our results reveal significantly increased phosphorylation of MLC2 peaking by 1 hour of exposure to hypoxia. This result is consistent with the rapid increase in permeability and intercellular gap formation that we observed above. As to the mechanism by which hypoxia alters MLC2 phosphorylation, previous work by our group and others suggests that MLCP might play an important role (An et al., 2005; Dakshinamurti et al., 2005; Wardle et al., 2006). MLCP plays a critical role in maintaining the proper phosphorylation level of MLC. The activity of MLCP itself is regulated by the phosphorylation of its targeting subunit MYPT1. Hypoxia and TGFβ increased the phosphorylation of MYPT1 at site 696 in wild type RPMEC. These results combined with the reduction of hypoxia-induced permeability by ROCK inhibition suggest that hypoxia increases endothelial barrier permeability through activating ROCK, which inactivates MLCP through phosphorylating its MYPT1 subunit, resulting in increased MLC2 phosphorylation and endothelial contractility.
Since pmHSP27 overexpressing cells were resistant to hypoxia-induced permeability we investigated the relation of pmHSP27 overexpression to MLC2 phosphorylation. When MLC2 and MYPT1 phosphorylation were assessed in pmHSP27-overexpressing RPMEC, we found that they were decreased in these cells compared to wild type, and that the levels did not increase in response to hypoxia. The fact that pmHSP27-overexpressing RPMEC were resistant to hypoxia-induced permeability and intercellular gap formation further supports the idea that MYPT1 and MLC2 phosphorylation mediate hypoxia-induced permeability. Future studies will explore whether HSP27 phosphorylation directly alters MYPT1 phosphorylation — a scaffolding role for HSP27 which facilitates the translocation of ROCK and RhoA to the contractile apparatus at the membrane and the association with MYPT1 has been suggested by other researchers (Patil and Bitar, 2006). It is also possible that actin stress fiber formation contribute to altered signaling via focal adhesions.
Recent publications have indicated that FAK plays an essential role in mediating endothelial adhesion, contraction and migration under physical stress and chemical stimulation (Gerthoffer and Gunst, 2001). Phosphorylation of FAK might contribute to pulmonary endothelial cell response to hypoxia since it has been described to occur in cardiac myocytes in response to hypoxia (Seko et al., 1999). Activated FAK and c-Src complex can activate cytoskeletal remodeling pathways through phosphatidylinositol-3 kinase and paxillin p130 cascade and initiate the biophysical and morphological changes (Gerthoffer and Gunst, 2001); (Imai and Clemmons, 1999). In our experiments hypoxia and TGFβ increased the phosphorylation level of FAK only in wild type RPMEC but not in pmHSP27-overexpressing RPMEC. Future experiments will examine whether HSP27 interacts directly with FAK or whether it alters its phosphorylation through other cytoskeletal components including actin stress fiber formation.
In conclusion, hypoxia activates pathways in RPMEC leading to the phosphorylation of HSP27 and MYPT1 which increases the phosphorylation level of MLC2 and possibly the phosphorylation of FAK, both of which likely contribute to endothelial cells contraction and intercellular gap formation. The contraction and gap formation would explain the increased endothelial barrier permeability in response to hypoxia. However, the increased actin stress fiber formation in response to activation of the p38-MK2-HSP27 pathway appears to contribute to barrier augmentation rather than disruption. It is possible that actin stress fiber formation in endothelial cells is an adaptive response to barrier disruption caused by MLC2 and FAK phosphorylation and that in the long run it is reflective of barrier repair rather than disruption. As our previous study indicated that actin stress fiber formation and pmHSP27 correlate with increased endothelial tethering but not stiffness or contractility (An et al., 2005; Kayyali et al., 2002), we postulate that actin stress fiber formation is a response to counterbalance increased contractility. Since actin stress fiber formation and increased tethering peak after increased contractility in response to hypoxia, HSP27 phosphorylation could lead to augmenting the barrier after it becomes compromised by MLC2 phosphorylation-mediated contractility. It is worth investigating whether other reports of increased actin stress fiber formation in response to permeability-inducing agents represent the increased adhesivenss and barrier recovery phase in response to contractility induced by these agents. Such a possibility would shift the focus on actin stress fiber formation as a desirable effect rather than an effect that should be blocked. Our results suggest that targeting HSP27 and related signaling molecules might be a useful approach in managing endothelial permeability and pulmonary edema.
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: HL-79320.
References
- Akiyama K, Akopian G, Jinadasa P, Gluckman TL, Terhakopian A, Massey B, Bing RJ. Myocardial infarction and regulatory myosin light chain. J Mol Cell Cardiol. 1997;29(10):2641–2652. doi: 10.1006/jmcc.1997.0493. [DOI] [PubMed] [Google Scholar]
- Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol. 1999;277(5 Pt 1):L1057–1065. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am J Physiol Cell Physiol. 2005;289(3):C521–530. doi: 10.1152/ajpcell.00429.2004. [DOI] [PubMed] [Google Scholar]
- Baudry N, Danialou G, Boczkowski J, Vicaut E. In vivo study of the effect of systemic hypoxia on leukocyte-endothelium interactions. Am J Respir Crit Care Med. 1998;158(2):477–483. doi: 10.1164/ajrccm.158.2.9701074. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Adyshev D, Mambetsariev B, Moldobaeva N, Verin AD. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L487–499. doi: 10.1152/ajplung.00217.2006. [DOI] [PubMed] [Google Scholar]
- Bozzo C, Spolaore B, Toniolo L, Stevens L, Bastide B, Cieniewski-Bernard C, Fontana A, Mounier Y, Reggiani C. Nerve influence on myosin light chain phosphorylation in slow and fast skeletal muscles. Febs J. 2005;272(22):5771–5785. doi: 10.1111/j.1742-4658.2005.04965.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs AR, Davis TP. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NFkappaB. J Cell Sci. 2003;116(Pt 4):693–700. doi: 10.1242/jcs.00264. [DOI] [PubMed] [Google Scholar]
- Carpenter TC, Reeves JT, Durmowicz AG. Viral respiratory infection increases susceptibility of young rats to hypoxia-induced pulmonary edema. J Appl Physiol. 1998;84(3):1048–1054. doi: 10.1152/jappl.1998.84.3.1048. [DOI] [PubMed] [Google Scholar]
- Carpenter TC, Stenmark KR. Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. Am J Physiol Lung Cell Mol Physiol. 2001;281(4):L941–948. doi: 10.1152/ajplung.2001.281.4.L941. [DOI] [PubMed] [Google Scholar]
- Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal Rearrangements and Transcriptional Activation of c-fos Serum Response Element by Rho-kinase. J Biol Chem. 1997;272(40):25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292(6):H3190–3197. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulmonol. 2005;40(5):398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279(23):24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273(34):21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne DJ, Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274(6):3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193(9):1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98(8):4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163(3):510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91(2):963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130(3):613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg PL, MacNaughton DE, Clements RT, Minnear FL, Vincent PA. p38 MAPK activation by TGF-beta1 increases MLC phosphorylation and endothelial monolayer permeability. Am J Physiol Lung Cell Mol Physiol. 2002;282(1):L146–154. doi: 10.1152/ajplung.2002.282.1.L146. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295(3):C576–587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Kanstrup IL, Richalet JP, Olsen NV. High altitude-induced albuminuria in normal man is enhanced by infusion of low-dose dopamine. Scand J Clin Lab Invest. 1996;56(4):367–372. doi: 10.3109/00365519609090589. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, Yu FS, Cote CG, Zulueta JJ, Sawhney R, Skinner KA, Skinner HB, Parks DA, Lanzillo JJ. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1, and hypoxia. Role in acute lung injury. Am J Respir Crit Care Med. 1998;158(1):299–305. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- Hastie LE, Patton WF, Hechtman HB, Shepro D. Metabolites of the phospholipase D pathway regulate H2O2-induced filamin redistribution in endothelial cells. J Cell Biochem. 1998;68(4):511–524. [PubMed] [Google Scholar]
- Hurst VI, Goldberg PL, Minnear FL, Heimark RL, Vincent PA. Rearrangement of adherens junctions by transforming growth factor-beta1: role of contraction. Am J Physiol. 1999;276(4 Pt 1):L582–595. doi: 10.1152/ajplung.1999.276.4.L582. [DOI] [PubMed] [Google Scholar]
- Imai Y, Clemmons DR. Roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in stimulation of vascular smooth muscle cell migration and deoxyriboncleic acid synthesis by insulin-like growth factor-I. Endocrinology. 1999;140(9):4228–4235. doi: 10.1210/endo.140.9.6980. [DOI] [PubMed] [Google Scholar]
- Kayyali US, Pennella CM, Trujillo C, Villa O, Gaestel M, Hassoun PM. Cytoskeletal changes In hypoxic pulmonary endothelial cells are dependent on the MAP kinase-associated protein kinase MK2. J Biol Chem. 2002;28:28. doi: 10.1074/jbc.M205863200. [DOI] [PubMed] [Google Scholar]
- Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170(4):1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC, Mohan SR, Zavodny PJ, Narula SK, Leibowitz PJ. Characterization and differential expression of human vascular smooth muscle myosin light chain 2 isoform in nonmuscle cells. Biochemistry. 1989;28(9):4027–4035. doi: 10.1021/bi00435a059. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kayyali US, Sousa AM, Rajan T, Lechleider RJ, Day RM. Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinase/Src. Am J Respir Cell Mol Biol. 2007;37(4):485–493. doi: 10.1165/rcmb.2006-0439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DM, Bradwell AR, Shore AC, Beaman M, Tooke JE. Capillary filtration coefficient and urinary albumin leak at altitude. Eur J Clin Invest. 1997;27(1):64–68. doi: 10.1046/j.1365-2362.1997.740627.x. [DOI] [PubMed] [Google Scholar]
- Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38(10):1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol. 2006;101(2):375–384. doi: 10.1152/japplphysiol.01515.2005. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol. 2002;539(Pt 3):779–789. doi: 10.1113/jphysiol.2001.013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha DR, Myers CL, Schaeffer RC., Jr Endothelial contraction and monolayer hyperpermeability are regulated by Src kinase. Am J Physiol Heart Circ Physiol. 2003;284(3):H994–H1002. doi: 10.1152/ajpheart.00862.2002. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990a;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Koga S, Kuwabara K, Brett J, Morrow B, Morris SA, Bilezikian JP, Silverstein SC, Stern D. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am J Physiol. 1992;262(3 Pt 1):C546–554. doi: 10.1152/ajpcell.1992.262.3.C546. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Shreeniwas R, Butura C, Brett J, Stern DM. Modulation of endothelial function by hypoxia: perturbation of barrier and anticoagulant function, and induction of a novel factor X activator. Adv Exp Med Biol. 1990b;281:303–312. doi: 10.1007/978-1-4615-3806-6_32. [DOI] [PubMed] [Google Scholar]
- Partridge CA. Hypoxia and reoxygenation stimulate biphasic changes in endothelial monolayer permeability. Am J Physiol. 1995;269(1 Pt 1):L52–58. doi: 10.1152/ajplung.1995.269.1.L52. [DOI] [PubMed] [Google Scholar]
- Patil SB, Bitar KN. RhoA- and PKC-alpha-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006;290(1):G83–95. doi: 10.1152/ajpgi.00178.2005. [DOI] [PubMed] [Google Scholar]
- Pinsky DJ, Yan SF, Lawson C, Naka Y, Chen JX, Connolly ES, Jr, Stern DM. Hypoxia and modification of the endothelium: implications for regulation of vascular homeostatic properties. Semin Cell Biol. 1995;6(5):283–294. doi: 10.1006/scel.1995.0038. [DOI] [PubMed] [Google Scholar]
- Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131(1):5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Schneider GB, Hamano H, Cooper LF. In vivo evaluation of hsp27 as an inhibitor of actin polymerization: hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J Cell Physiol. 1998;177(4):575–584. doi: 10.1002/(SICI)1097-4652(199812)177:4<575::AID-JCP8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1094–1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- Seko Y, Takahashi N, Sabe H, Tobe K, Kadowaki T, Nagai R. Hypoxia induces activation and subcellular translocation of focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;262(1):290–296. doi: 10.1006/bbrc.1999.1185. [DOI] [PubMed] [Google Scholar]
- Stelzner TJ, O’Brien RF, Sato K, Weil JV. Hypoxia-induced increases in pulmonary transvascular protein escape in rats. Modulation by glucocorticoids. J Clin Invest. 1988;82(6):1840–1847. doi: 10.1172/JCI113800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden KA. Provision of phosphorylatable substrate during hypoxia decreases jejunal barrier function. Nutrition. 2002;18(2):168–172. doi: 10.1016/s0899-9007(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Toursel T, Bastide B, Stevens L, Rieger F, Mounier Y. Alterations in Contractile Properties and Expression of Myofibrillar Proteins in Wobbler Mouse Muscles. Experimental Neurology. 2000;162(2):311–320. doi: 10.1006/exnr.1999.7349. [DOI] [PubMed] [Google Scholar]
- Wang P, Verin AD, Birukova A, Gilbert-McClain LI, Jacobs K, Garcia JG. Mechanisms of sodium fluoride-induced endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001a;281(6):L1472–1483. doi: 10.1152/ajplung.2001.281.6.L1472. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001b;25(5):628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lanner MC, Jin N, Swartz D, Li L, Rhoades RA. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells: Role of Rho-kinase. Am J Respir Cell Mol Biol. 2003;24:24. doi: 10.1165/rcmb.2002-0157OC. [DOI] [PubMed] [Google Scholar]
- Wardle RL, Gu M, Ishida Y, Paul RJ. Ca2+-desensitizing hypoxic vasorelaxation: pivotal role for the myosin binding subunit of myosin phosphatase (MYPT1) in porcine coronary artery. J Physiol. 2006;572(Pt 1):259–267. doi: 10.1113/jphysiol.2005.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White GE, Gimbrone MA, Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L749–760. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Tsang LY, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1173–1182. doi: 10.1152/ajplung.00309.2005. [DOI] [PubMed] [Google Scholar]