Abstract
Importance of the field
Edaravone (Radicut®) is a free radical scavenger marketed in Japan by Mitsubishi Tanabe Pharma Corporation to treat acute ischemic stroke (AIS) patients presenting within 24 hours of the attack. Injectable edaravone ampoules (30mg b.i.d, i.v., 14 days) were first approved on May 23, 2001. On January 19th 2010, as a new innovation, the Radicut BAG was approved by the Japanese Ministry of Health and Welfare. Efficacy of edaravone range from large significant clinical improvements to only modest improvements in clinical function measured using standard stroke scales when administered 6–72 hours following an ischemic stroke. With almost 17 years of edaravone clinical experience, a few adverse events including acute renal failure have been noted.
What the reader will gain
This is the only article to date to critically review available clinical efficacy and toxicology data published in the literature to ascertain whether edaravone should be further pursued as a candidate for development worldwide.
Areas covered in this review
This review covers clinical studies done during the periods 1993–2008.
Take home message
Edaravone may a useful neuroprotective agent to treat more than 15 million worldwide victims devastated by stroke annually. Additional clinical studies are necessary to verify the efficacy of edaravone.
Keywords: neuroprotection, embolism, acute ischemic stroke, clinical, behavior, toxicity, translational science, antioxidant, Radicut, thrombolytic
1. Background
1.1 AIS Incidence
AIS is the third leading cause of death and the leading cause of adult disability in the USA[1] with an estimated cost of $68.9 billion. Annually, approximately 795,000 people suffer a stroke (one every 40 seconds with 1 mortality every 3 minutes), 75% of which are first strokes and the remainder recurrent attacks [2]. The World Health Organization (WHO) estimates that 15 million people suffer strokes worldwide annually and more than 5 million stroke victims die from the brain insult and 5 million are permanently disabled[3] and require medical care.
1.2 AIS subtypes defined
According to the National Institutes of Neurological Disorders and Stroke (NINDS) classification of stroke types and population-based research studies[4, 5], AIS can be classified as the following: (1) cardioembolic stroke (29% of cases), in which the embolism arises from a cardiac source; (2) atheroembolic stroke (16% of cases), which can be associated with narrowing of a cervicocephalic artery; and (3) small vessel lacunar stroke (16% of cases), which are defined as pure motor, sensorimotor or sensory strokes, in addition to ataxic hemiparesis and result from thrombosis in one of the deep penetrating branches from larger cerebral arteries. The majority of strokes (39%) do not have a known cause.
2. Current Treatment: Tissue Plasminogen Activator (tPA, Alteplase®, Activase®, Actilyse®)
The only FDA approved treatment for AIS is the thrombolytic, tissue plasminogen activator (tPA, Alteplase), a plasminogen activator that promotes thrombolysis by activating the endogenous fibrinolytic system[6–9]. In brief, tPA catalyzes the conversion of plasminogen to plasmin, which in turn degrades fibrin and leads to clot lysis and cerebral reperfusion. Alteplase has been shown to be effective up to 4.5 hours after a stroke[10, 11], but is currently only Food & Drug Administration (FDA) approved for use within the first 3 hours of a stroke in patients documenting the absence of an intracerebral hemorrhage(ICH). In alteplase-treated patients, the short-term incidence of symptomatic ICH is significantly higher in tPA-treated patients than in placebo-treated controls [12]. Following the initial approval of alteplase treatment, the treatment was further developed and refined to a point where in the most recent ECASS III trial [10] the investigators showed that tPA could be administered up to 4.5 hours following a stroke without an increased incidence of mortality. The trial showed that the incidence of ICH and sICH was higher with alteplase than with placebo (27.0% vs. 17.6%; P=0.001 and 2.4% vs. 0.2%; P=0.008), respectively. However, the mortality rate did not differ significantly between the alteplase and placebo groups (7.7% and 8.4%, respectively; P=0.68) and there was no significant difference in the rate of other serious adverse events (SAEs)[12]. Clearly, this is a major advancement in the stroke field; however, with only one effective FDA-approved stroke treatment there remains a need to develop treatments for stroke.
3. Free Radicals as a Target
Free radicals are a valid target for therapeutic intervention for the treatment of AIS [13–15], because oxidative stress is a major component of the ischemic stroke cascade [15–17], which is activated following vascular occlusion. However, the ischemic stroke cascade is a multi-component cascade with sequential activation of a complex series of pathophysiological events that evolve temporally [14, 15]. The key to developing clinically effective molecules may lie in the ability of compounds to inhibit multiple ischemic cascade pathways or physiological simultaneously or sequentially! Does edaravone fit the profile of a multi-target drug? Yes, based upon the following evidence.
3.1 Mechanism-Based Translational Research
Edaravone is a low molecular weight lipophilic (i.e. hydrophobic) free radical scavenger that readily crosses the blood brain barrier (BBB) [18, 19]. The anti-oxidant activity of edaravone is its main proposed mechanism of action and an important one, since free radicals may cause injuries following a stroke[16, 20–25]. The oxidative stress that occurs after an ischemic stroke produces reactive oxygen species (ROS) like hydrogen peroxide (H2O2), hydroxyl radical (HO•) and superoxide anion radical (O2•−) that bring about membrane lipid peroxidation and vascular compartment endothelial cell injury[18, 19, 24–26]. The primary focus of edaravone research has revolved around its potential to scavenge hyrdoxyl, peroxyl and superoxide radicals that mediate neuronal and vascular damage [27–30].
Under physiological conditions with neutral or physiological pH, edaravone is present in an anionic form. Due to an electron transfer process, electrons released from the edaravone anion can scavenge radical species containing a free electron, such as a lipid peroxyl radical (OOL•), which is formed after the free radical extraction of a proton from an unsaturated fatty acid. The transfer of electrons from edaravone (E) to a reactive oxygen species produces an edaravone radical (E•). Subsequently, the edaravone radical (E•) forms a peroxyl radical of edaravone (E• •OO), which incorporates the O species to form a 4,5-dione (i.e. 3-methyl-1-phenyl-2-pyrazolin-4,5-dione) and eventually 2-oxo-3-(phenylhydrazone)-butanoic acid (OPB) Details emphasizing the chemical process involved in the antioxidant mechanism of edaravone have been published[19, 31, 32].
However, the therapeutic benefit of edaravone may be due to more than its antioxidant activity and may be directly related to its multi-target pharmacology and the ability of edaravone to regulate numerous signaling pathways. For example, recent mechanistic research has suggested that edaravone can suppress delayed neuronal death [26, 31, 33–35], counteract microglia-induced neurotoxicity [29] and reduce the long-term inflammation [36]. It has also been shown that edaravone can inhibit lipoxygenase, an enzyme responsible for lipid oxidation damage[31] and could also directly suppress the oxidation of low-density lipoprotein[32]. In addition to the attenuating the deleterious effects mediated by the ischemic cascade, it has been suggested that may prevent the development of edema following a stroke by inhibiting astrocyte production of expression of vascular endothelial growth factor [37]. Moreover, Kituchi et al. [38] hypothesized that edaravone may reduce aquaporin-4 levels following an ischemic event, thereby reducing edema. Last, Yagi et al. showed that edaravone may reduce the activity of matrix metalloproteinase-9, and further protect against vascular damage and intracerebral hemorrhage (ICH) [39–41]. Taken together, edaravone may prevent neuronal degeneration, vascular compartment damage and behavioral deficits resulting from both ischemia progression and edema. Thus, edaravone may fit into a class of multi-target compounds that may be useful to treat stroke. Is this borne out by preclinical and clinical stroke studies?
3.2 Preclinical Translational Research
3.2.1 Rodent Studies
The first peer-reviewed rodent stroke study with edaravone was published in 1989 by Oishi and colleagues [42]. While the authors did not show any behavioral improvement or cell survival, they showed that edaravone attenuated polyvinyl acetate (PVA)-induced changes in brain monoamine metabolism [42] and suggested that edaravone was acting as a free radical scavenger to counteract the effects of PVA-induced ischemia. Nishi et al also used the PVA model and showed that edaravone prevented ischemia-induced edema[43]. These 2 seminal studies sparked the development and subsequent approval of edaravone for the treatment of AIS in Japan.
Following the early PVA studies, additional stroke studies were done using accepted rodent stroke models. For example, Jin et al. [44] used a standard ischemia model in gerbils to show that edaravone could increase cerebral blood flow following ischemia and reduce edema, but there was no significant effect of neuronal survival or mortality rate compared to the control group. In an intraluminal stroke model, Nito et al.[45] also showed that edaravone reduced edema. Watanabe from Mitsubishi Kasei Corp., the developers of edaravone published a report[46] showing that pre-treatment with edaravone (3 mg/kg i.v.) prolonged survival time in rats subjected to global ischemia and reduced BBB dysfunction, while post-treatment decreased cortical infarct size, an effect that was postulated to be associated with edaravone-induced inhibition of iron-dependent peroxidation of mitochondrial membranes. In a rodent ischemia/reperfusion model, Wu et al [47] provided additional evidence that edaravone could reduce ischemia-induced infarct size. There have been numerous other rodent studies showing similar effects when edaravone is given either as a pre-treatment or post-treatment. Since they have recently been reviewed in detail, please refer to the original review articles [18, 27, 48–51]. However, important information regarding therapeutic window analyses in preclinical models, which are recommended by STAIR[52] and thereafter by Lapchak[13], have not been reported for rodents.
3.2.2 Rabbit Studies
The only available translational study used the rabbit small clot embolic stroke model (RSCEM), which is a useful model and possibly a predictor of treatments that may show efficacy in human clinical trials[13]. The RSCEM was the preclinical animal model used in the development and FDA approval of tPA, the development profile which has recently been reviewed in detail by Lapchak[13], but see[53–57] for the original publications. The RSCEM uses embolization to cause multifocal cerebral ischemia, which results in graded behavioral deficits that can be quantified quantitatively [13, 58–60]. The primary behavioral endpoint is based upon motor function components of the NIHSS scale [61–64]. In the RSCEM, edaravone administered subcutaneously decreased behavioral deficits when up to 3 hours post-embolization [59]. Moreover, the study showed that edaravone could be administered in combination with standard dose intravenous tPA therapy, but the combination using maximally effective doses of both drugs did not result in either a synergistic or additive effect, most likely due to the achievement of maximal benefit by each drug as monotherapy[59]. Nevertheless, the study indicates that had a therapeutic widow of at least 3 hours in rabbits. In a recent hypothesis driven translational research publication by Lapchak[13], a 3hour therapeutic window in the RSCEM was calculated to represent approximately 7.3–9 hours in AIS patients. Is Edaravone effective in AIS patients within that projected therapeutic window? The following section will address the efficacy and therapeutic window data documented by clinical researchers in Japan and India.
3.3 Clinical Research Efficacy
3.3.1 Thrombotic/Embolic Strokes
The original 2003 Otomo clinical trial report from the Edaravone Acute Ischemic Stroke Group[65] is the only randomized, placebo-controlled, double-blind multicenter study using a 14 day treatment regimen. As shown in Table 1, the study was conducted between December 1993 and March 1996 and Mitsubishi Pharma Corp both supplied the test drugs and supported the studies. The dose used in the Otomo study[66] was cited to be based upon early phase 2 dose-finding and efficacy studies[66, 67], which used doses from 10–60 mg b.i.d. In all patients entered into the trial, with mean time to treatment of 35.2 ± 26.6 hours and 37.3 ± 22.6 hours for placebo and edaravone respectively, a significant difference using the Wilcoxon's 2-tailed rank sum test between edaravone-treated and placebo-treated groups measured using the mRS (p=0.0382) was reported (Table 1). In addition, Figure 1 presents the primary clinical score data from the study when patients received edaravone within 24 hours of stroke onset. The subset analysis data clearly shows a significant (p=0.0001) shift in the proportion of patients with mRS scores of 0 and 1 following edaravone treatment and this was true when all study patients were assessed either 3, 6 or 12 months after stroke onset[65]. Clearly in this trial, edaravone produced significant clinical improvement in all patients receiving the drug, independent of the time to treatment time interval (i.e.: ≤24, 25–48 or 48–72hours), however, the most pronounced effect was in the ≤24 hours group. It should be noted that in this trial, the majority of patients in each group were identified as thrombotic strokes (78–81%) with 17–19% being embolic strokes.
Table 1.
Edaravone- Efficacy in AIS Patients- Randomized, Placebo-Controlled Study
|
Otomo[65] December 1993 - March 1996 |
Control (C) (125) Edaravone (E) (125) |
Neurological Deficit Baseline Median (C) 6 (4,11) (E) 6, (4,10) |
|
Study Type: Randomized Placebo-Controlled Double-Blind, Multicenter |
Result: Significant clinical improvement using mRS Assessment |
|
|
Stroke Type: Thrombotic & Embolic |
mRS (0–2) 3 month score (C) 66% |
|
| (E) 73% (p=0.048) | ||
| Dose- 30mg i.v., b.i.d. | 6 month score (C) 67% | |
| (E) 73% (p=0.0112) | ||
| Treatment duration:14 days | 12 month score (C) 66% | |
| (E) 72% (p= 0.0248) | ||
| Data Analysis: mRS | ||
|
TT: (C) 35.2 ± 26.6 hours (E) 37.3 ± 22.6 hours |
Figure 1.
Edaravone Efficacy in AIS Patients treated within 24 hours: Data extracted from the 1993–1996 Randomized, Placebo-Controlled, Double-Blind, Multicenter Trial. There was a significant shift toward lower mRS scores of 0–1 for patients treated within 24 hours. Data from[65].
The Inatomi[68] retrospective historically-controlled study was differentiated from the Otomo trial[65] because the authors limited analysis to patients with embolic strokes. This study showed that edaravone treatment for 7 days could only produce a modest clinical improvement in AIS patients with NIHSS ≤ 7 upon entry into the trial (Table 2); efficacy that was not seen in the moderate to severe AIS patients (NIHSS >7) patients. Specifically, the data shows that edaravone-treated patients in the NIHSS ≤ 7 improved by 2 points whereas the control group became worse by 2 points. Moreover, the authors report that 6 months after AIS onset, patients who became independent (mRS <2) were more likely to have received edaravone than placebo, (41% versus 28%; p=0.066). Thus, the reports suggest that edaravone may only have utility in AIS patients with NIHSS ≤ 7.
Table 2.
Edaravone- Efficacy in AIS Patients- Retrospective Analyses
| Study[REF] (Alphabetical) |
Trial Information | Clinical Endpoints | |||||
|---|---|---|---|---|---|---|---|
|
Inatomi [68] October 2000 - September 2002 |
Control (C) (101) | NIHSS SCORES | |||||
| Edaravone (E) (141) | |||||||
| 0–7 | 8–15 | 16–20 | 21–40 | mean | |||
| Study Type: Retrospective | (C) | 4/6 (27) | 12/8 (27) | 18/14 (24) | 30/31(23) | 15/14 | |
| Historical Controlled | (E) | 4/2 (31)* | 12/9 (34) | 18/16 (39) | 26/26(37) | 16/14 | |
| Stroke Type: Cardioembolic |
Result: Data given as NIHSS on admittance (Day 0 /Day 10). *Significantly different from control for NIHSS 0–7 (p=0.013). A 2 point NIHSS improvement was noted with edaravone treatment. |
||||||
| Dose- 30mg i.v., b.i.d. | |||||||
| Treatment duration: 7 days | |||||||
|
Data Analysis: NIHSS (Day 0)/NIHSS (Day 10) | |||||||
| TT: Undefined, but ≤24hours | |||||||
|
Mishina[69] June 1999 - May 2002 |
Control (C) (49) | NIHSS BASELINE SCORE | |||||
| Edaravone (E) (21) | |||||||
| (C) 5.1 ± 4.5 | |||||||
| Study Type: Retrospective | (E) 3.3 ± 2.8 | ||||||
| Stroke Type: Lacunar |
Result: With edaravone treatment, there was an odds ratio of 6.49 (95% confidence interval, 1.35 to 50.32; p=0.04) for good outcome, i.e. mRS ≤ 2 for patients with NIHSS ≤ 8. |
||||||
| Dose- 30mg i.v., b.i.d. | |||||||
| Treatment duration: 14 days | |||||||
|
Data Analysis: NIHSS and mRS 30 day | |||||||
| TT: ≤24hours | |||||||
|
Ohta[71] January 2004 - June 2007 |
Control (C) (65) | NIHSS BASELINE SCORE | |||||
| Edaravone (E) (59) | |||||||
| (C) 2.0 ± 1.3 Admit to 1.0 ± 1.2 Discharge | |||||||
| Study Type: Retrospective | (E) 2.2 ± 1.0 Admit to 0.7 ± 10.7 Discharge | ||||||
| Stroke Type: Lacunar |
Result: Mean NIHSS reduction in the edaravone- treated group was significantly different (p=0.007) from control (1.5 ± 1.0 vs. 1.0 ± 1.1). There was also a modest, but significant (p=0.006) reduction of NIHSS palsy scores in the edaravone-treated group (1.0 ± 1.0 vs. 0.5 ± 1.1). Moreover, a larger population (91.5%) of edaravone-treated patients at discharge had a favorable outcome compared to control (78.5%) (p=0.044 chi-square). |
||||||
| Dose- 30mg i.v., b.i.d. | |||||||
|
Treatment duration: 2–10 days; mean duration 5.9 ± 1.9 days. | |||||||
|
Data Analysis: NIHSS entry and discharge | |||||||
| TT: (C) 9.8 ± 7.0 hours | |||||||
| (E) 9.2 ± 5.8 hours | |||||||
|
Shinohara[72] August 2004 - October 2006 |
Edaravone (199) | NIHSS BASELINE SCORE | |||||
|
Study Type: Open-label unblinded, uncontrolled |
(E) 3.7 ± 2.3 Admittance | ||||||
| 14 day score 0–1 98/190 patients (51.6%) | |||||||
|
Stroke Type: Thrombotic and Lacunar |
1 month score 0–1 117/191 patients (61.6%) | ||||||
| 3 month score 0–1 135/190 patients (70.7%) | |||||||
| Dose- 30mg i.v., b.i.d. |
Result: Trend for improvement from mean baseline to 0–1 score. |
||||||
| Treatment duration: 14 days | |||||||
|
Data Analysis: NIHSS entry and discharge | |||||||
| TT: (E) 13.2 ± 6.0 hours | |||||||
|
Sinha[73] January 2008 - July 2008 |
Edaravone (22) | (a) NIHSS BASELINE SCORE 10.62 ± 8.86 | |||||
|
Study Type: Unblinded Uncontrolled Trial. |
7 day 6.62 ± 7.48 (p<0.05) | ||||||
| 14 day 5.12 ± 6.03 (p<0.05) | |||||||
| 30 day 4.12 ± 4.64 (p<0.05) | |||||||
| Stroke Type: Not given | 90 day 3.12 ± 4.71 (p<0.005) | ||||||
| Dose- 30mg i.v., b.i.d. | (b) mRS BASELINE 4.01 ± 0.92 | ||||||
| Treatment duration: 14 days | 7 day 3.62 ± 1.18 (p<0.05) | ||||||
| 14 day 2.87 ± 1.55 (p<0.05) | |||||||
| Data Analysis: mRS and BI | 30 day 2.25 ± 1.28 (p<0.005) | ||||||
| 90 day 1.86 ± 1.07 (p<0.005) | |||||||
| TT: 26.5 ± 21.27 hours | |||||||
|
Result: Significant decrease in both NIHSS and mRS compared to baseline up to 90 days following the stroke. |
|||||||
|
Toyoda[74] October 1999 - July 2002 |
Control (C) (31) | NIHSS BASELINE SCORE | |||||
| Edaravone (E) (30) | |||||||
| (C) 21 ± 3 | |||||||
|
Study Type: Retrospective Historical Control |
(E) 21 ± 4 | ||||||
|
Stroke Type: Internal carotid artery occlusion |
Result: Frequency of independence (i.e. mRS grade of 1 or 2) increased in edaravone-treated patients compared to control (p<0.1). Temporary functional outcome improvement (p<0.03) in edaravone-treated patients. Improved acute survival rate. |
||||||
| Dose- 30mg i.v., b.i.d. | |||||||
| Treatment duration: 14 days | |||||||
|
Data Analysis: NIHSS and mRS | |||||||
| TT: Undefined, but ≤6hours | |||||||
|
Unno[76] July 2006 - November 2007 |
Edaravone (E) (72) | Dosing Regimen: Tertile | |||||
|
Study Type: Retrospective, Historical Control |
0–14 ampoules (n=21) | ||||||
| 15–23 ampoules (n=27) | |||||||
| 24–33 ampoules (n=24) | |||||||
|
Stroke Type: cardioembolic thrombotic, lacunar |
Result: Regression analysis performed indicated a significant correlation between number of edaravone ampoules used and FIMM and BI outcome. p=0.003 for FIMM and p=0.0005 for BI in cardioembolic patients. There was no significant correlation for other stroke subtypes. |
||||||
| Dose- 30mg i.v., b.i.d. | |||||||
| Treatment duration: 14 days | |||||||
|
Data Analysis: Functional Independence Measure Motor (FIMM) and Barthel Index (BI) | |||||||
| TT: Undefined, but ≤24hours | |||||||
NIHSS- National Institutes of Health Stroke Scale, mRS- Modified Rankin Scale, BI- Barthel Index, b.i.d- twice a day, TT- time to treatment.
3.3.2 Lacunar Strokes
The Mishina[69] report is a small retrospective analysis study that included both control and edaravone-treated patients. This study, unlike the 2 previous studies described above, this study only included patients with identified lacunar infarctions using previously published criteria[70]. There was stratification of time to treatment for patients: ≤ 6 and 12–24 hours. Three month mRS rating scores were used as primary clinical criteria and an mRS score for improvement of ≤ 2 was defined as a good outcome. Final analysis only included 21 patients in the edaravone group compared to 49 patients in the control group (Table 2). Using a backward stepwise logistic regression analysis the authors concluded that here was a significant odds ratio effect in patients with edaravone treatment (p=0.035) that was also correlated with NIHSS ≤ 8, but not time to treatment. The effect appears to confirm that reported by Otomo and suggests that only patient with mild strokes may benefit from edaravone treatment.
Using a separate lacunar stroke patient population, Ohta[71] also retrospectively studied the effects of edaravone on functional outcome. A significant difference to note in this study is an acute time to treatment of 9.8 ± 7.0 and 9.2 ± 5.8 hours in the control and edaravone-treated groups respectively, with a mean treatment duration of 5.9 ± 1.9 days. The authors provide detailed statistical analysis using both the MANN-Whitney test and chi-square test and present highly significant improvements in both the edaravone and control groups. Further statistical analysis shows that there is no significant difference between mean NIHSS scores at either admission or discharge. However, statistical analysis shows that there was a larger reduction in palsy score in the edaravone-treated group compared to the control group (p=0.006).
Shinohara[72] also studied the effects of edaravone in a mixed population of stroke patients including thrombotic and lacunar stroke. The study was part of a multicenter randomized parallel-group open-label design comparing edaravone to ozagrel (Table 1). The main conclusion is that edaravone was at least as effective as ozagrel. The data shows that there is a trend for decreased NIHSS scores in edaravone-treated patients.
In the 2009 Sinha trial, the first published experience with edaravone outside of Japan[73], twenty two AIS patients were given 30 mg of edaravone twice daily for 14 days by infusion (Table 1). The mean time to treatment following onset was 26.5 ± 21.27 hours. The study used mRS and Barthel Index (BI) assessed up to 90 days following treatment. In the study, 68% of patients had a favorable outcome where the mean mRS score decreased from 4.01 ± 0.92 at baseline to 1.86 ± 1.07 at day 90 (p<0.005) and the mean BI increased from 40.00 ± 30.11 at baseline to 75.62 ± 22.86 at day 90 (p<0.005). This was the first confirmation that edaravone had efficacy in patients and that the effect could be observed with a long therapeutic window.
The Toyoda study[74] enrolling internal carotid artery (ICA) occlusion patients is an extreme test for the efficacy of edaravone because of the fatal nature of ICA occlusions in the majority of patients[75]. In the study, stroke patients with baseline NIHSS scores ≥ 15 (Mean ± SEM was 21 ± 4) were treated for 14 days with edaravone and they were compared to a historical control (Table 2). The most obvious effect of edaravone was decreased mortality during the acute stage following ICA occlusion, where only 20% of edaravone-treated patients compared to 45% of control patients died from the stroke (P<0.03). Moreover, there was temporary functional outcome improvement (p<0.03) in edaravone-treated patients, which was not significant in all ICA occlusion survivors, but 8% of edaravone-treated patients were considered independent within 8 weeks of onset. These results are significant considering the devastating nature of this particular type of stroke.
Last, the Unno trial[76], which is a dose-finding trial, attempted to correlate number of ampoules of edaravone administered to patients with thrombotic, embolic and lacunar strokes with improved functional outcome. The study used a sophisticated treatment regimen design where the uncontrolled study stratified the population of enrolled patients into Tertiles with the mean number of ampoules administered as follows: Tertile 1: 4±5 (Mean dose, 120mg, n=29), Tertile 2: 19±3 (Mean dose, 570mg, n=23), Tertile 3: 26±2 (Mean dose, 780mg, n=20). This should be compared to the previous design used by Otomo[65], where a cumulative dose of 840mg was used (i.e.: 30 mg b.i.d., 14 days = 840 mg). Using regression correlative analysis, the author’s document a significant correlation between number of edaravone ampoules or cumulative dose used, and Functional Independence Measure Motor (FIMM) and Barthel Index (BI) outcome. Significance levels, p=0.003 for FIMM and p=0.0005 for BI were noted for cardioembolic patients, but this was not carried over to other stroke subtypes (p>0.05). It is interesting to note that even in the 3rd Tertile, the mean cumulative is less than that used in previous studies. This should be considered a study design shortcoming because it does not allow for direct comparison to previous studies using a standard dosing regimen.
4.1 Clinical Research Pharmacokinetics
Shibata and colleagues originally published the Phase I safety and pharmacokinetic profile of edaravone[77]. In normal volunteers, edaravone is well tolerated following single or multiple dose I.V. administration, which may be due to a short half life in the range of 0.15–5.16 hours depending on the administration regimen. The Phase I study did not evaluate edaravone using the most common treatment regimen for stroke patients (i.e.: 30mg b.i.d, 14 days). Edaravone is excreted as unmetabolized drug (~1%) or metabolized by sulfation (5–13%) or glucuronidation (68–83%) and excreted in urine within 24hours of administration. In normal volunteers, there were no adverse events (AEs).
4.2 Preclinical & Clinical Toxicology
Preclinical CeeTox™ analysis of Edaravone using an extensive panel of toxicity markers shows that the drug has no acute toxicities when a rat H4IIE hepatoma cell system was used for analysis[78]. In the assay, the estimated CTox value or sustained concentration expected to produce toxicity in a rat 14 day repeat dose study is >300 µM. However, the study noted that Edaravone was metabolically unstable and quickly metabolized by pooled microsomes from non-induced male Sprague-Dawley rats with only 28% of parent remaining after a 30 minute incubation period, which is indicative of rapid phase I metabolism via cytochrome P450 enzymes[78].
However, clinically a number of incidences of AEs have been observed in stroke trials [79]. Of the 8 stroke clinical trials with edaravone, only the Otomo study[65] and Shinohara study[72] reported AEs and SAEs in their published reports. In the Otomo study[65], the AEs were abnormal liver function, itching and nausea and SAEs were death due to exacerbation of brain infarction, sudden cardiac arrest, pneumonia and depression-induced suicide. Interestingly, in the Shinohara study[72], edaravone-induced renal AEs occurred in 5.7% of patients, hepatic AEs in 21.6% of patients and cerebrovascular disorders in 13.4% of patients. Noteworthy are death in 5 patients due to pneumonia, lymphoma and depression-related suicide. The major edaravone AE is reported to be an increased risk of renal toxicity associated [80, 81], which is reversible in 45% of patients after edaravone treatment is stopped [80]. However, 11 adverse side effects are now listed on the drug information sheet for the drug, including fulminant hepatitis[82], thrombocytopenia, rhabdomyolysis and acute lung injury. There is also a report of an increased frequency of hemorrhagic transformation when edaravone was administered to patients with cardiogenic embolism [83].
5. Edaravone in combination with thrombolytics
A few of the published clinical studies document the use of edaravone in combination with thrombolytics such as tPA and urokinase. The following studies note tPA (IV) and urokinase (UK, intra-arterial) use in patients receiving edaravone: Inatomi [68], Toyoda [74] and Unno [76]. Inatomi indicated that one patient was partially recanalized with UK. The remaining studies did not specifically comment on the adverse effects or efficacy of combination therapy. The following studies noted tPA and UK as an exclusion criteria: Ohta [71], Otomo [65] and Mishina [69].
The most intriguing finding regarding combination therapy of Edaravone and tPA was documented by Yoshifumi[84]. The authors reported preliminary findings of a clinical trial showing that patients treated with edaravone prior to administration of intravenous tPA had a reduced ICH compared to tPA-treated patients. If this edaravone effect can be reproduced, then combination therapy using a multi-arm clinical design should be considered for in the next clinical trial.
6. Prospects for the Use of Edaravone to Treat Stroke & Conclusion
Based upon 1 randomized, placebo-controlled, double-blind multicenter trial and numerous retrospective trials with a total of 669 patients with embolic, thrombotic and lacunar strokes treated with edaravone, the cumulative data suggests that some patients may benefit from the administration of edaravone. Who should receive edaravone and when?
As can be gleaned from Tables 1 and 2, edaravone has not been systematically tested for efficacy using a standard treatment regimen, excepto for a dose of 30mg i.v. b.i.d, but even that dose, which has not been used for a consistent duration of treatment, may not be optimal. Figure 2 presents the time to treatment for all currently available edaravone trials. In every trial to date, independent of the time to treatment, the data has been presented to show a significant and positive effect of edaravone on a clinical, functional or survival parameter measured in the study. Based upon the clinical trial data, Edaravone is most effective when administered within 24 hours following a stroke.
Figure 2.
Edaravone Time to Treatment indicated by Study Author. In all studies, there was a significant positive effect of Edaravone on a clinical outcome independent of the Time to Treatment. When only a single bar is provided, the study did not have a placebo control. References for author are as follow: Otomo[65], Inatomi[68], Mishina[69], Ohta[71], Shinohara[72], Sinha[73], Toyoda[74], Unno[76].
MCI-186 is currently being studied for safety and pharmacokinetic profile in patients with AIS in a Phase IIa multi-centre, randomized, double-blind, placebo-controlled, clinical study [85]. It is interesting to note that the trial is a 2-cohort study with 1000mg and 2000mg doses for the study and that patients will be enrolled within 24 hours of a stroke. This suggests that the standard 30mg b.i.d dosing used in all previous studies, including the Unno study[76] may not have been optimal. The original dose used in the Otomo study[65] was cited to be based upon early phase 2 dose-finding and efficacy studies[66, 67], which used doses from 10–60 mg b.i.d. The higher end of the studies[66, 67] is consistent with the proposed 2nd tier cohort to use 2000mg dose in patients.
There appears to be some consensus in the edaravone clinical trial literature[76] that an extended edaravone treatment period is required for maximum efficacy and this may be based upon the original Otomo study[65]. Certainly with treatment durations of 6–14 days for the clinical efficacy studies assessed in this article, it is difficult to conclude that a particular treatment duration is most beneficial. As reported by Unno[76], the specific treatment regimen prescribed by Mitsubishi Pharmaceutical in unclear and may depend upon the severity of stroke upon admission. That should not be a factor in the further development of this therapeutic agent if a blinded randomized clinical trial is to be carried out. According to the Kageyama [86] post-marketing survey, clinically there is widespread variation in the duration of treatment and this can be seen in the studies cited in Table 2.
It is surprising that the phase IIa edaravone trial design[85] has specifically excluded thrombolytic such as tPA, UK, streptokinase, reteplase and tenecteplase. The current state-of the-art clinical trial design, which was first attempted for NXY-059[87], but failed due to inferior pharmacological properties of the drug[13, 88], used a 4-arm design to include placebo, tPA, study drug and the combination of study drug plus tPA [87]. It is conceivable that a tPA-treated population of patients that would have partial or full recanalization [10, 11] plus the benefit of a multi-target drug to attenuate the deleterious effects of ischemic cascade activation[26, 59] and possibly also reduce tPA-induced edema[40, 84], if it were to occur, would have a better outcome(see [59]).
7. Expert Opinion
Based upon the efficacy profile of Edaravone in patients with multiple stroke subtypes and stroke severity, the drug should be further pursued to determine its utility as a neuroprotective treatment. To do that, Edaravone should be further studied in a well controlled clinical trial with standardization of dose, treatment duration and time to treatment in order to unequivocally ascertain the efficacy of Edaravone. In fact, if Mitsubishi Pharmaceuticals aims to develop Edaravone worldwide, then a recommended time to treatment window in a double blind randomized clinical trial would be in the range of 7–10 hours based upon trends of efficacy in patients with various types of stroke in the Otomo study[65] and a preclinical embolic stroke translational study[13, 59]. The goal of the initial trial should be to provide evidence of significant efficacy using an optimal drug dose within a reasonable therapeutic window and not obscure efficacy by using an overly broad therapeutic window. Based upon the current literature, it does not appear that the optimal drug dose for durable neuroprotection has been established. However, with respect to patient safety, the 30 mg i.v., b.i.d. 14 days regimen used by Otomo[65] may be near the upper end of the safety limit, because renal dysfunction, which is estimated to occur in 0.04% of the treated population[80], is associated with Edaravone use at this dose.
Drug Summary Box
| Drug name | Edaravone |
|---|---|
| Phase | Launched |
| Launched indication | Cerebral infarction |
| Pharmacology description | Free radical scavenger |
| Route of administration | Parenteral, intravenous |
| Chemical structure | 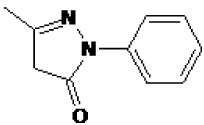 |
| Pivotal trial(s) | Edaravone Acute Ischemic Stroke Group |
Pharmaprojects - copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Informa-Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
Footnotes
Declaration of interest:
This paper was funded by a NINDS Translational Research Grant (U01 NS60685-01). The author declares no conflicts of interest and was not compensated by any pharmaceutical or biotechnology company to contribute this article to the peer-reviewed scientific literature.
References
- 1.Ingall T. Stroke--incidence, mortality, morbidity and risk. J Insur Med. 2004;36(2):143–152. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.The internet stroke center- stroke statistics. [cited; Available from: http://www.strokecenter.org/patients/stats.htm]
- 4.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000 May;31(5):1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 5.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999 Dec;30(12):2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 6.Lapchak PA. Development of thrombolytic therapy for stroke: a perspective. Expert Opin Investig Drugs 2002. 2002;11(11):1623–1632. doi: 10.1517/13543784.11.11.1623. [DOI] [PubMed] [Google Scholar]
- 7.Schellinger PD, Fiebach JB, Mohr A, Ringleb PA, Jansen O, Hacke W. Thrombolytic therapy for ischemic stroke--a review. Part II--Intra- arterial thrombolysis, vertebrobasilar stroke, phase IV trials, and stroke imaging. Crit Care Med. 2001;29(9):1819–1825. doi: 10.1097/00003246-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Schellinger PD, Fiebach JB, Mohr A, Ringleb PA, Jansen O, Hacke W. Thrombolytic therapy for ischemic stroke--a review. Part I--Intravenous thrombolysis. Crit Care Med. 2001;29(9):1812–1818. doi: 10.1097/00003246-200109000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Verstraete M. Newer thrombolytic agents. Ann Acad Med Singapore. 1999;28(3):424–433. [PubMed] [Google Scholar]
- 10. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317–1329. doi: 10.1056/NEJMoa0804656..**Extremely important study showing that alteplase could be used within 4.5 hours of a stroke.
- 11.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009 Dec;8(12):1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 12.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995 Dec 14;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 13.Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Translational Stroke Research. 2010 doi: 10.1007/s12975-010-0018-4. http://dx.doi.org/10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 15.Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin Emerg Drugs. 2007 Mar;12(1):97–112. doi: 10.1517/14728214.12.1.97. [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18(6):667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999 Jan;9(1):119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008 Summer;26(2):101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006 Spring;12(1):9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222(3):236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima M, Niwa M, Iwai T, Uematsu T. Involvement of free radicals in cerebral vascular reperfusion injury evaluated in a transient focal cerebral ischemia model of rat. Free Radic Biol Med. 1999;26(5–6):722–729. doi: 10.1016/s0891-5849(98)00257-3. [DOI] [PubMed] [Google Scholar]
- 22.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005 Oct 1;39(7):841–852. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Lapchak PA, Araujo DM. Development of the Nitrone-Based Spin Trap Agent NXY-059 to Treat Acute Ischemic Stroke. CNS Drug Rev. 2003 Fall;9(3):253–262. doi: 10.1111/j.1527-3458.2003.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siesjo BK, Katsura K, Zhao Q, Folbergrova J, Pahlmark K, Siesjo P, et al. Mechanisms of secondary brain damage in global and focal ischemia: a speculative synthesis. J Neurotrauma. 1995;12(5):943–956. doi: 10.1089/neu.1995.12.943. [DOI] [PubMed] [Google Scholar]
- 25.Siesjo BK, Siesjo P. Mechanisms of secondary brain injury. Eur J Anaesthesiol. 1996;13(3):247–268. [PubMed] [Google Scholar]
- 26.Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain Res. 2010 Jan 11;1307:22–27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higashi Y. Edaravone for the treatment of acute cerebral infarction: role of endothelium-derived nitric oxide and oxidative stress. Expert Opin Pharmacother. 2009 Feb;10(2):323–331. doi: 10.1517/14656560802636888. [DOI] [PubMed] [Google Scholar]
- 28.Kono H, Woods CG, Maki A, Connor HD, Mason RP, Rusyn I, et al. Electron spin resonance and spin trapping technique provide direct evidence that edaravone prevents acute ischemia-reperfusion injury of the liver by limiting free radical-mediated tissue damage. Free Radic Res. 2006 Jun;40(6):579–588. doi: 10.1080/10715760600606374. [DOI] [PubMed] [Google Scholar]
- 29.Banno M, Mizuno T, Kato H, Zhang G, Kawanokuchi J, Wang J, et al. The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology. 2005 Feb;48(2):283–290. doi: 10.1016/j.neuropharm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Shichinohe H, Kuroda S, Yasuda H, Ishikawa T, Iwai M, Horiuchi M, et al. Neuroprotective effects of the free radical scavenger Edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004 Dec 17;1029(2):200–206. doi: 10.1016/j.brainres.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 31.Higashi Y, Jitsuiki D, Chayama K, Yoshizumi M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Patents Cardiovasc Drug Discov. 2006 Jan;1(1):85–93. doi: 10.2174/157489006775244191. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida H, Sasaki K, Namiki Y, Sato N, Tada N. Edaravone, a novel radical scavenger, inhibits oxidative modification of low-density lipoprotein (LDL) and reverses oxidized LDL-mediated reduction in the expression of endothelial nitric oxide synthase. Atherosclerosis. 2005 Mar;179(1):97–102. doi: 10.1016/j.atherosclerosis.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Amemiya S, Kamiya T, Nito C, Inaba T, Kato K, Ueda M, et al. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005 Jun 1;516(2):125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 34.Xiao B, Bi FF, Hu YQ, Tian FF, Wu ZG, Mujlli HM, et al. Edaravone neuroprotection effected by suppressing the gene expression of the Fas signal pathway following transient focal ischemia in rats. Neurotox Res. 2007 Oct;12(3):155–162. doi: 10.1007/BF03033912. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi K, Kawahara K, Tancharoen S, Matsuda F, Morimoto Y, Ito T, et al. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J Pharmacol Exp Ther. 2009 Jun;329(3):865–874. doi: 10.1124/jpet.108.149484. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005 Oct;36(10):2220–2225. doi: 10.1161/01.STR.0000182241.07096.06. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa A, Yoshida H, Metoki N, Toki T, Imaizumi T, Matsumiya T, et al. Edaravone inhibits the expression of vascular endothelial growth factor in human astrocytes exposed to hypoxia. Neurosci Res. 2007 Dec;59(4):406–412. doi: 10.1016/j.neures.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Kikuchi K, Tancharoen S, Matsuda F, Biswas KK, Ito T, Morimoto Y, et al. Edaravone attenuates cerebral ischemic injury by suppressing aquaporin-4. Biochem Biophys Res Commun. 2009 Dec 25;390(4):1121–1125. doi: 10.1016/j.bbrc.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, et al. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008 Feb;39(2):463–469. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- 40.Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, et al. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009 Feb;40(2):626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 41.Kano T, Harada T, Katayama Y. Attenuation of extravasation of tissue plasminogen activator by the free radical scavenger, edaravone: evaluation in a rat thromboembolic stroke model. Neurol Res. 2005 Jul;27(5):499–502. doi: 10.1179/016164105X17387. [DOI] [PubMed] [Google Scholar]
- 42.Oishi R, Itoh Y, Nishibori M, Watanabe T, Nishi H, Saeki K. Effect of MCI-186 on ischemia-induced changes in monoamine metabolism in rat brain. Stroke. 1989 Nov;20(11):1557–1564. doi: 10.1161/01.str.20.11.1557. [DOI] [PubMed] [Google Scholar]
- 43.Nishi H, Watanabe T, Sakurai H, Yuki S, Ishibashi A. Effect of MCI-186 on brain edema in rats. Stroke. 1989 Sep;20(9):1236–1240. doi: 10.1161/01.str.20.9.1236. [DOI] [PubMed] [Google Scholar]
- 44.Jin YJ, Mima T, Raicu V, Park KC, Shimizu K. Combined argatroban and edaravone caused additive neuroprotection against 15 min of forebrain ischemia in gerbils. Neurosci Res. 2002 May;43(1):75–79. doi: 10.1016/s0168-0102(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 45.Nito C, Kamiya T, Amemiya S, Katoh K, Katayama Y. The neuroprotective effect of a free radical scavenger and mild hypothermia following transient focal ischemia in rats. Acta Neurochir Suppl. 2003;86:199–203. doi: 10.1007/978-3-7091-0651-8_43. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994 Mar;268(3):1597–1604. [PubMed] [Google Scholar]
- 47.Wu TW, Zeng LH, Wu J, Fung KP. MCI-186: further histochemical and biochemical evidence of neuroprotection. Life Sci. 2000 Sep 29;67(19):2387–2392. doi: 10.1016/s0024-3205(00)00824-9. [DOI] [PubMed] [Google Scholar]
- 48.Tanahashi N, Fukuuchi Y. Treatment of acute ischemic stroke: recent progress. Intern Med. 2002;41(5):337–344. doi: 10.2169/internalmedicine.41.337. [DOI] [PubMed] [Google Scholar]
- 49.Wang CX, Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs Aging. 2007;24(7):537–546. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- 50.Kitagawa Y. Edaravone in acute ischemic stroke. Intern Med. 2006;45(5):225–226. doi: 10.2169/internalmedicine.45.0143. [DOI] [PubMed] [Google Scholar]
- 51.Abe K. [Edaravone] Nippon Rinsho. 2006 Oct 28;64 Suppl 7:548–553. [PubMed] [Google Scholar]
- 52. STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–2758. doi: 10.1161/01.str.30.12.2752.. *Original recommendations for the use of multiple species to develop new stroke treatments.
- 53.Chehrazi BB, Seibert JA, Kissel P, Hein L, Brock JM. Evaluation of recombinant tissue plasminogen activator in embolic stroke. Neurosurgery. 1989 Mar;24(3):355–360. doi: 10.1227/00006123-198903000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Gross CE, Raymond SJ, Howard DB, Bednar MM. Delayed tissue-plasminogen activator therapy in a rabbit model of thromboembolic stroke. Neurosurgery. 1995 Jun;36(6):1172–1177. doi: 10.1227/00006123-199506000-00017. [DOI] [PubMed] [Google Scholar]
- 55.Phillips DA, Davis MA, Fisher M. Selective embolization and clot dissolution with tPA in the internal carotid artery circulation of the rabbit. AJNR Am J Neuroradiol. 1988 Sep;9(5):899–902. [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips DA, Fisher M, Smith TW, Davis MA. The safety and angiographic efficacy of tissue plasminogen activator in a cerebral embolization model. Ann Neurol. 1988 Apr;23(4):391–394. doi: 10.1002/ana.410230414. [DOI] [PubMed] [Google Scholar]
- 57.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985 Dec 13;230(4731):1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 58.Lapchak PA, Araujo DM, Zivin JA. Comparison of Tenecteplase with Alteplase on clinical rating scores following small clot embolic strokes in rabbits. Exp Neurol. 2004;185(1):154–159. doi: 10.1016/j.expneurol.2003.09.009. 2004/01// [DOI] [PubMed] [Google Scholar]
- 59. Lapchak PA, Zivin JA. The lipophilic multifunctional antioxidant edaravone (radicut) improves behavior following embolic strokes in rabbits: a combination therapy study with tissue plasminogen activator. Exp Neurol. 2009 Jan;215(1):95–100. doi: 10.1016/j.expneurol.2008.09.004.. *The only translational study to document a therapeutic window.
- 60.Lapchak PD, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15 lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007 Dec 12;150(3):585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 61.Broderick JP, Lu M, Kothari R, Levine SR, Lyden PD, Haley EC, et al. Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS tPA stroke trial. Stroke. 2000 Oct;31(10):2335–2341. doi: 10.1161/01.str.31.10.2335. [DOI] [PubMed] [Google Scholar]
- 62.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31(4):811–816. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 63. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994 Nov;25(11):2220–2226. doi: 10.1161/01.str.25.11.2220.. **The first study to validate a stroke scale in patients treated with tPA
- 64.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, et al. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999 Nov;30(11):2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 65.Otomo E. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15(3):222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 66.Otomo E. Late phase 2 clinical trial of MCI-186 on acute cerebral infarction: A dose-finding double-blind study. J Clin Exp Med. 1998;185:841–863. [Google Scholar]
- 67.Otomo E, Tohgi H, Kogure K, Hirai S, Terashi A, Gotoh F, et al. Clinical efficacy of a free radical scavenger, MCI-186, on acute cerebral infarction: Early phase II clinical trial. Ther Res. 1998;19:1311–1332. [Google Scholar]
- 68.Inatomi Y, Takita T, Yonehara T, Fujioka S, Hashimoto Y, Hirano T, et al. Efficacy of edaravone in cardioembolic stroke. Intern Med. 2006;45(5):253–257. doi: 10.2169/internalmedicine.45.1423. [DOI] [PubMed] [Google Scholar]
- 69.Mishina M, Komaba Y, Kobayashi S, Tanaka N, Kominami S, Fukuchi T, et al. Efficacy of edaravone, a free radical scavenger, for the treatment of acute lacunar infarction. Neurol Med Chir (Tokyo) 2005 Jul;45(7):344–348. doi: 10.2176/nmc.45.344. discussion 8. [DOI] [PubMed] [Google Scholar]
- 70.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST.Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 71.Ohta Y, Takamatsu K, Fukushima T, Ikegami S, Takeda I, Ota T, et al. Efficacy of the free radical scavenger, edaravone, for motor palsy of acute lacunar infarction. Intern Med. 2009;48(8):593–596. doi: 10.2169/internalmedicine.48.1871. [DOI] [PubMed] [Google Scholar]
- 72.Shinohara Y, Saito I, Kobayashi S, Uchiyama S. Edaravone (radical scavenger) versus sodium ozagrel (antiplatelet agent) in acute noncardioembolic ischemic stroke (EDO trial) Cerebrovasc Dis. 2009;27(5):485–492. doi: 10.1159/000210190. [DOI] [PubMed] [Google Scholar]
- 73.Sinha MK, Anuradha HK, Juyal R, Shukla R, Garg RK, Kar AM. Edaravone in acute ischemic stroke, an Indian experience. Neurology Asia 2009. 2009;14:7–10. [Google Scholar]
- 74.Toyoda K, Fujii K, Kamouchi M, Nakane H, Arihiro S, Okada Y, et al. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J Neurol Sci. 2004 Jun 15;221(1–2):11–17. doi: 10.1016/j.jns.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996 Apr;53(4):309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 76.Unno Y, Katayama M, Shimizu H. Does functional outcome in acute ischaemic stroke patients correlate with the amount of free-radical scavenger treatment?: a retrospective study of edaravone therapy. Clin Drug Investig. 2010;30(3):143–155. doi: 10.2165/11535500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 77.Shibata H, Shigenori A, Izawa M, Murksaki M, Takamatsu Y, Izawa O, et al. Phase I clinical study of MCI-186 (Edaravone, 3-methyl-1-phenyl-2-pyrazolin-5-one) in healthy volunteers: safety and pharmacokinetics of single and multiple administrations. Jpn J Clin Pharmacol Ther. 1998;29(6):863–876. [Google Scholar]
- 78.Lapchak PA, KcKim JM. CeeTox™ analysis of CNB-001 a novel curcumin-based neurotrophic/neuroprotective lead compound to treat stroke: comparison with NXY-059 and Radicut. Translational Stroke Research. 2010 doi: 10.1007/s12975-010-0034-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Risk/benefit assessment of drugs Analysis and Response. [Cited; available from http://www.rad-ar.or.jp]
- 80.Hishida A. Clinical analysis of 207 patients who developed renal disorders during or after treatment with edaravone reported during post-marketing surveillance. Clin Exp Nephrol. 2007 Dec;11(4):292–296. doi: 10.1007/s10157-007-0495-2. [DOI] [PubMed] [Google Scholar]
- 81.Hishida A. Determinants for the prognosis of acute renal disorders that developed during or after treatment with edaravone. Clin Exp Nephrol. 2009 Apr;13(2):118–122. doi: 10.1007/s10157-008-0108-8. [DOI] [PubMed] [Google Scholar]
- 82.Abe M, Kaizu K, Matsumoto K. A case report of acute renal failure and fulminant hepatitis associated with edaravone administration in a cerebral infarction patient. Ther Apher Dial. 2007 Jun;11(3):235–240. doi: 10.1111/j.1744-9987.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- 83.Mishina M, Komaba Y, Kobayashi S, Kominami S, Fukuchi T, Mizunari T, et al. Administration of free radical scavenger edaravone associated with higher frequency of hemorrhagic transformation in patients with cardiogenic embolism. Neurol Med Chir (Tokyo) 2008 Jul;48(7):292–297. doi: 10.2176/nmc.48.292. [DOI] [PubMed] [Google Scholar]
- 84.Yoshifumi T. Benefits of Pre-treatment with edaravone in tPA intravenous therapy for acute cerebral infarction; XXIIIrd International Symposium on Cerebral Blood Flow (abstract); 2007. 2007. [Google Scholar]
- 85.Safety and Pharmacokinetics of MCI-186 in Subjects With Acute Ischemic Stroke. [Cited; Available from http://www.clinicaltrials.gov/ct2/show/NCT00821821]
- 86.Kageyama M, Toriyama S, Tsuboshita A. A post-marketing drug use survey of a neuroprotective drug Radicut injection 30mg (nonproprietary name: edaravone) for acute ischmic stroke. J New Rem Clin. 2009;58:1212–1226. [Google Scholar]
- 87. Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007 Aug 9;357(6):562–571. doi: 10.1056/NEJMoa070240.. *Important study as far as an optimal clinical design, which tested the effects of a small molecule in combination with tPA.
- 88.Bath PM, Gray LJ, Bath AJ, Buchan A, Miyata T, Green AR. Effects of NXY-059 in experimental stroke: an individual animal meta-analysis. Br J Pharmacol. 2009 Aug;157(7):1157–1171. doi: 10.1111/j.1476-5381.2009.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




