Abstract
Background
Stress can increase impulsivity, and has a negative impact on psychiatric outcome. Norepinephrine is heavily implicated in the stress response, and the alpha-2 antagonist yohimbine is used clinically as a pharmacological stressor. Yohimbine induces mild anxiety and increases impulsivity in healthy volunteers, but has more detrimental effects in some psychiatric populations, triggering mania in bipolar patients and drug-craving in substance-dependent individuals. Understanding the mechanism by which yohimbine affects brain function could provide insight into the heightened reaction to stress seen in these patients.
Methods
The effects of yohimbine were assessed in rats using the five-choice serial reaction time test of attention and impulse control. We then examined whether yohimbine altered CREB activity- a transcription factor implicated in the stress response - in brain areas which regulate impulsivity. The behavioral consequences of any changes in CREB activity were subsequently assessed using viral-mediated gene transfer to regionally over-express CREB or the dominant negative protein mCREB.
Results
Yohimbine increased impulsive responding in rats, and selectively increased CREB phosphorylation within the orbitofrontal cortex (OFC), but not medial prefrontal cortex or nucleus accumbens (NAc). Over-expressing mCREB, a dominant negative CREB antagonist, within the OFC blocked yohimbine's effects on impulse control, whereas over-expressing CREB in this region increased impulsive responding and potentiated the pro-impulsive actions of yohimbine.
Discussion
These data are suggestive of a novel molecular mechanism contributing to impulsivity, which is sensitive to a pharmacological stressor. Such findings may improve our understanding of the neurobiological pathways linking the response to stress and impulsivity in both healthy and psychiatric populations.
Keywords: alpha-2 receptor antagonist, noradrenaline, frontal cortex, five-choice serial reaction time task, phospho-CREB, ERK 42/44
Introduction
High levels of impulsivity are associated with a number of disabling psychiatric disorders including attention-deficit hyperactivity disorder (ADHD), bipolar disorder, substance abuse, and pathological gambling (1–4). Impulsivity can be significantly influenced by the level of stress and arousal an individual experiences (5); stressful life events have a negative impact on the psychiatric outcome of patients with impulse control disorders (e.g. (6), and patients with post-traumatic stress disorder (PTSD) often have impaired impulse control (7). Norepinephrine (NE) plays a key role in regulating the stress-arousal response, and has been implicated in the development and treatment of both impulse control disorders and PTSD (8; 9). NE may therefore be critically involved in mediating the interaction between impulsivity and stress.
Key nodes within the affective corticostriatal loop, such as the nucleus accumbens (NAc) and orbitofrontal cortex (OFC), have been implicated in aspects of impulse control (e.g. (10). A growing body of data suggest that the functions of these areas are altered by stress, and stress-induced increases in catecholamine release within the prefrontal cortex may contribute to the impulse control deficits seen in ADHD (8). Preclinical studies exploring the molecular basis of the stress response also implicate this circuitry. For example, stress increases phosphorylation (activation) of the transcription factor cAMP response element binding protein (CREB) within the rat NAc, and locally increasing CREB levels through viral-mediated gene transfer produces a depression-like phenotype (11). Both of these behavioral and molecular changes can be attenuated by the NE reuptake inhibitor, desipramine, in keeping with the well-known ability of NE to regulate CREB function (12–14).
The alpha-2-adrenoceptor antagonist yohimbine is often used as a pharmacological stressor in neuropsychological experiments. Yohimbine increases NE release via blockade of inhibitory autoreceptors on noradrenergic neurons (15). Its administration heightens arousal, increases heart rate and blood cortisol levels, and can lead to a mildly anxious state (e.g. (16). Yohimbine has also been reported to increase impulsive responding in healthy volunteers (17). The response to yohimbine is more pronounced and detrimental in some psychiatric populations: it can induce the transition to mania in bipolar disorder (18), trigger withdrawal symptoms and craving in opioid-dependent patients (16), and exacerbate panic and other symptoms in PTSD patients (19). Such data suggest that a hypersensitivity to NE may contribute to these psychopathologies. Furthermore, elevated impulsivity may mediate some of the negative consequences of stress on psychiatric outcome. Understanding the mechanism by which yohimbine modulates brain function could provide insight into the nature of this interaction.
In rats, yohimbine increases anxiety and other stress indices, and can also trigger relapse to drug-seeking in the extinction-reinstatement model of addiction (20; 21). However, its effects in preclinical models of impulse control are unknown. The current set of experiments aimed to determine: (a) whether yohimbine increases motor impulsivity in rats parallel to its effects clinically, and (b) whether any alterations in impulsivity could be linked to drug-induced changes in CREB, and upstream regulators such as ERK (extracellular signal regulated kinase), implicated in the emotional response to stress.
Materials and Methods
All experimental protocols were approved by the Institutional Animal Care and Use Committee at UT Southwestern and the Animal Care Committee at UBC.
Subjects
Male Long-Evans rats (initial weight: 300–320g; Charles River, Kingston, NY) were pair-housed with free access to water under a reverse light cycle in a climate-controlled colony (lights on from 21.00-09.00). Animals performing behavioral tests (n = 56) were food restricted (14g standard rat chow daily) and maintained at 85% of their free-feeding weight. Animals used for ex vivo protein analysis (n = 12) had free access to food.
Experiment 1: Determining the effects of yohimbine on 5CSRT performance
Testing took place in 8 standard five-hole operant chambers (Med Associates, Roanoke, VA) between 09.00 and 19.00. 16 rats were trained to perform the 5CSRT as described previously (22; 23). In brief, animals were trained to respond in one of the five holes when the stimulus light located therein was briefly illuminated (0.5s, see supplemental methods). A correct response- a nosepoke into the illuminated hole- was rewarded by delivery of a sugar pellet, whereas a response in any other hole or a failure to respond was scored as incorrect or as an omission respectively, and these were punished by a 5 s time out during which reward could not be earned. Premature responses made before the stimulus light was illuminated provide an index of impulsivity, and were likewise punished by a 5 s time-out. Once stable baseline behaviour was established, yohimbine (0, 1, 2, 5 mg/kg) was administered intraperitoneally according to a Latin square drug design 10 mins prior to the start of the task. Each drug injection day was preceded by a drug-free baseline day and followed by a day during which animals were not tested.
Experiment 2: Determining the effects of yohimbine on CREB signaling within brain areas implicated in impulse control
12 rats were injected with either a dose of yohimbine which increased premature responding (2 mg/kg, n = 6) or vehicle (n = 6). 30 mins later, animals were sacrificed by live decapitation. Tissue samples from the OFC, mPFC and NAc were rapidly dissected, frozen on dry ice and subsequently processed for western blotting (see Supplemental Methods for details). Levels of CREB, pCREB (phospho-CREB), ERK 42/44 and pERK42/44 (phospho-ERK42/44) were determined per sample and normalized relative to GAPDH.
Experiment 3: Determining the effects of modulating CREB activity on impulsivity
37 rats were trained to perform the 1CSRT: a simplified version of the 5CSRT in which the stimulus light always appeared in the central hole. Animals were divided into 5 performance-matched groups (n = 7–8). 2 groups received intra-OFC infusions of adeno-associated viruses (AAVs) designed to over-express either CREB or a CREB mutant (mCREB) (see supplementary methods). mCREB acts as a dominant negative protein to CREB: it binds to CREB but the resulting dimer cannot activate gene transcription. Separate groups received infusions of these AAVs into the NAc, an area implicated in regulating impulsivity and in which CREB modulates the response to affective stimuli, but in which yohimbine did not induce changes in pCREB. The final group received infusions of AAV-GFP (green fluorescent protein ) to control for any non-specific effects of viral-mediated gene transfer (OFC: n = 4; NAc: n = 4). Animals were re-trained on the 1CSRT 8 days later, once AAV expression had peaked. After 15 sessions (3 weeks), a stable post-operative baseline had been established. Animals were then challenged with yohimbine as in experiment 1.
Drugs
Yohimbine hydrocholoride (Sigma, St. Louis, USA) was dissolved in distilled water. Injections were given in a volume of 1ml/kg.
Data analyses
All analyses were conducted using SPSS (version 9.0; SPSS, Chicago, IL).
5CSRT/1CSRT analysis
The following variables were analysed: the percent of trials on which correct, incorrect and premature responses were made, the percentof trials omitted, the total trials completed, and the latency to respond correctly and to collect reward. Any effects of intra-OFC/ intra-NAc AAV CREB or mCREB were determined by ANOVA with 1 repeated measures factor, DAY, and one between subjects factor GROUP (2 levels, GFP vs CREB/mCREB). Data were analysed in 5-day bins. The effects of yohimbine were analysed by ANOVA with 1 repeated measures factor, DRUG (4 levels: three doses plus vehicle) and one between subjects factor, GROUP. If any analyses produced a significant effect of DRUG, mean values for individual doses were compared post hoc to vehicle control values via paired sample Student’s t-tests.
Protein quantification
Density of protein bands were determined using NIH image. Significant differences between vehicle and yohimbine-treated animals were determined through independent-samples Student’s t-tests.
Results
Experiment 1: Yohimbine increased impulsivity and speed of responding on the 5CSRT
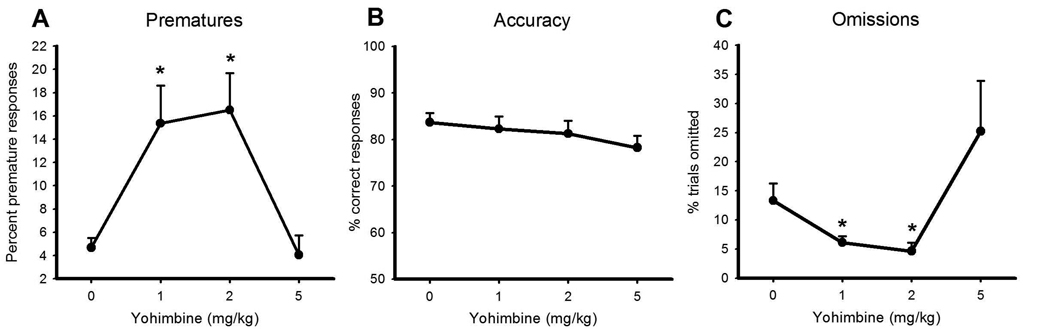
Yohimbine significantly increased premature responding at both 1 and 2 mg/kg, yet had no effect at the highest dose tested, such that the dose-response function resembled a typical U-shaped curve (Figure 1A, dose: F3, 39 = 15.630, p< 0.0001; vehicle vs 1 mg/kg: F1, 13 25.431, p< 0.0001; vehicle vs 2 mg/kg: F1, 13 = 17.996, p< 0.001; vehicle vs 5 mg/kg: F1, 13 = 1.644, NS). There was no effect of the drug on accuracy of target detection (Figure 1B, dose: F3,39 = 2.062, NS), indicating that yohimbine had not impaired attentional processes. The lower doses of yohimbine also decreased omissions (dose: F3, 39 = 5.561, p< 0.003; vehicle vs 1 mg/kg: F1, 13 = 7.380, p< 0.018; vehicle vs 2 mg/kg: F1, 13 = 20.101, p< 0.001), and reduced the latency to respond correctly and to collect food reward (correct latency- vehicle: 0.62s ± 0.043, 1 mg/kg yohimbine 0.55 ± 0.02. 2 mg/kg yohimbine 0.55 ± 0.03 - dose: F3, 39 = 5.684, p< 0.002; vehicle vs 1 mg/kg: F1, 13 5.182, p< 0.04; vehicle vs 2 mg/kg: F1, 13 = 5.528, p< 0.035; reward collection latency - vehicle: 2.06s ± 0.12, 2 mg/kg yohimbine 1.83 ± 0.09- dose: F3, 39 = 7.389, p< 0.0001; vehicle vs 2 mg/kg: F1, 13 = 6.780, p< 0.022). It should be noted that the response to the highest dose of yohimbine was highly variable, with 3 animals (~20%) showing a dramatic increase in omissions and a reduction in trials completed. However, these effects were not significant across the group (Figure 1C, omissions: vehicle vs 5 mg/kg: F1, 13 = 1.257, NS; trials completed- dose: F3, 39 = 2.648, NS, data not shown). Yohimbine did not affect the number of perseverative responses animals made (dose: F3, 39 = 1.799, NS, data not shown).
Figure 1. The effects of yohimbine on 5CSRT performance.
Yohimbine significantly increased premature responding on the 5CSRT (panel A). Accuracy of target detection was unaffected by the drug, whereas lower doses decreased omissions. Data shown are mean ± SEM. * indicates a significant difference (p< 0.05) as determined by one-way ANOVA comparing vehicle to drug dose.
Experiment 2: Yohimbine selectively increased pCREB and pERK 42 within the OFC
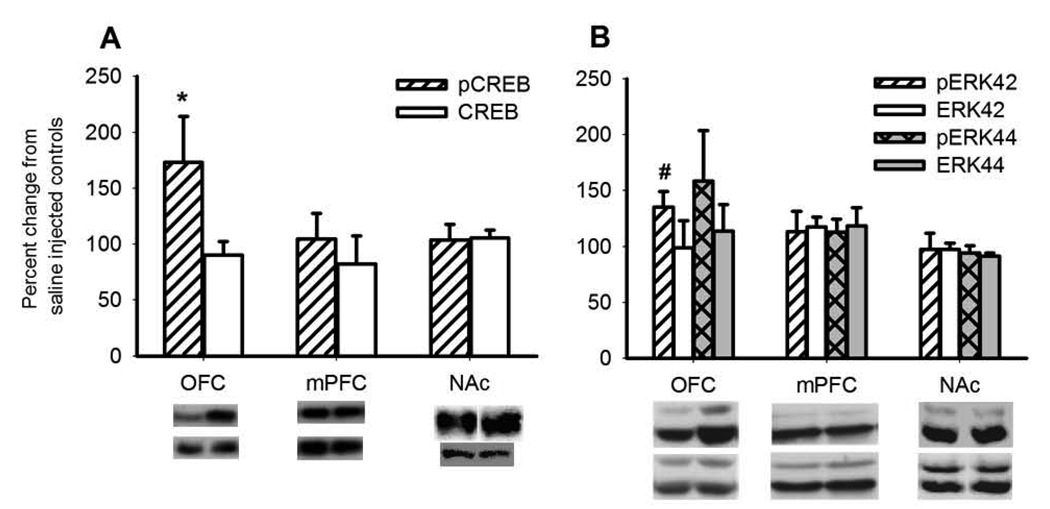
As shown in Figure 2A, yohimbine increased levels of pCREB within the OFC (t(8) = −2.260, p< 0.054), but not within the mPFC (t(8) = -0.699, NS) or NAc (t(8) = 1.177, NS). Levels of total CREB remained unaltered in all regions. There was also a trend for an increase in pERK42, but not total ERK42, selectively within the OFC (Figure 2B, pERK42, OFC: t(8) = −2.148, p< 0.06; mPFC t(8) = <1.175, NS; NAc: t(8) = 0.138, NS; ERK 42, OFC: t(8) = 0.042, NS; mPFC: t(8) = 0.0001, NS; NAc: t(8) = 0.250, NS). There were no changes in levels of phospho- or total ERK44 within any region tested.
Figure 2. Western blot analysis of protein expression within the OFC, mPFC and NAc 30 mins after yohimbine administration.
Yohimbine selectively increased phosphorylation of CREB and ERK42 in the OFC, but not in the mPFC or NAc. No change in the levels of unphosphorylated proteins were observed. Data are shown as the mean fold change from control ± SEM. * indicates a significant difference (p< 0.05), # indicates a trend level of significance (p< 0.1) as determined by independent samples Student's t-tests.
Experiment 3a: Over-expressing CREB within the OFC, but not NAc, transiently increases impulsive responding on the 1CSRT
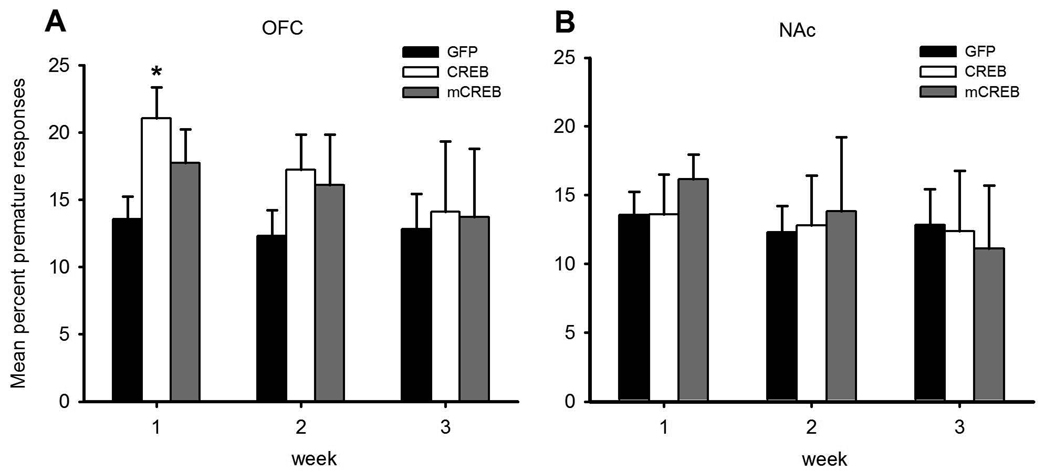
During the first week of post-operative testing, increasing CREB expression in the OFC, by use of viral-mediated gene transfer, significantly increased premature responding (Figure 3A, pre-op vs post-op performance- CREBOFC vs GFP, surgery × group: F1, 12 = 6.383, p< 0.027; surgery × day × group: F1, 48 = 2.589, p< 0.023; post-operative data only- CREBOFC vs GFP, group: F1, 12 = 7.896, p< 0.016; CREBOFC group-surgery: F1,5 = 8.326, p< 0.034; GFP group- surgery: F1,7 = 0.395, NS). This increase in impulsivity was transient, and was no longer observed in week 2 (CREBOFC vs GFP, post-operative data only- group: F1, 12 = 2.501, NS). In contrast, increasing CREB levels in the NAc had no effect on impulsivity (Figure 3B, pre-op vs post-op performance-CREBNAc vs GFP surgery × group: F1, 14 = 0.012, NS). Infusions of AAV mCREB did not alter behavior regardless of the region targeted (week 1 pre-op vs post-op performance- mCREBOFC vs GFP surgery × group: F1, 13 = 1.553, NS; mCREBNAc vs GFP surgery × group: F1, 14 = 0.585, NS). No other variable was significantly affected by viral injections.
Figure 3. Over-expressing CREB within the OFC increases premature responding in the 1CSRT.
As shown in panel A, a transient increase in premature responding was observed in animals over-expressing CREB within the OFC during the first week of post-operative testing, whereas over-expressing mCREB did not affect performance. In contrast, behaviour was not altered by increasing levels of CREB or mCREB within the NAc (panel B). Data are presented as the average from each week of post-operative testing. * indicates a significant difference (p< 0.05) from control.
Experiment 3b: Modulating CREB within the OFC affects yohimbine's ability to increase impulsive responding
Two animals, one in the GFP control group and one in the OFC mCREB group, were excluded from the experiment at this point due to ill health (final n per group: GFP: n = 7, CREBOFC: n = 6, mCREBOFC: n = 6; CREBNAc n = 8; mCREBOFC: n = 8). As in experiment 1, the highest dose of yohimbine tested lead to high numbers of omissions (30–100%) in around 20% of rats, regardless of surgery condition. Given the smaller n per group in this phase of the experiment, this dose was not included in the analyses due to the low number of trials completed by some individuals.
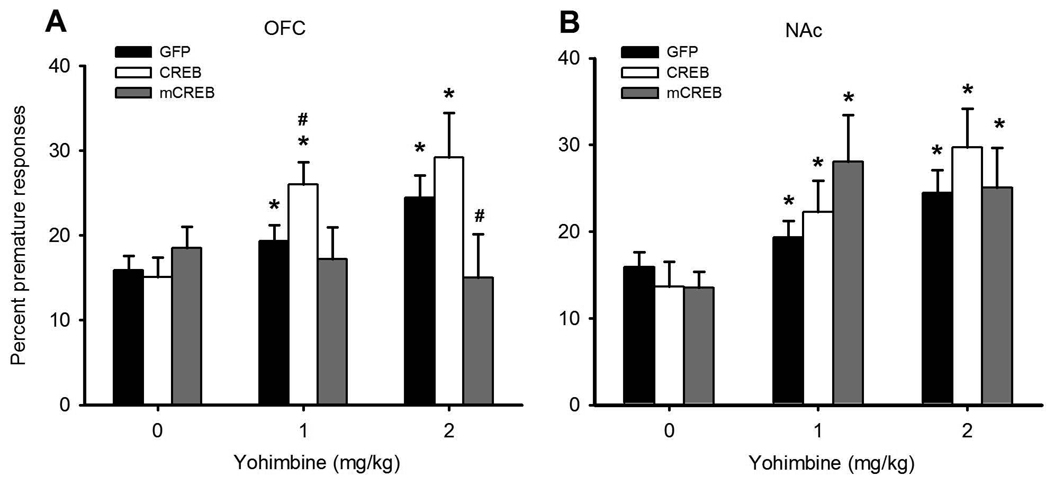
In parallel to data obtained using the 5CSRT, yohimbine significantly increased premature responding (Figure 4, dose: F2, 60 = 21.677, p< 0.0001, vehicle vs 1 mg/kg: F1, 30 = 43.459, p< 0.0001; vehicle vs 2 mg/kg: F1,30 = 26.669, p< 0.0001). This effect of yohimbine was dose-dependently affected by regional over-expression of CREB and mCREB (dose × group: F8, 60 = 2.323, p< 0.03; vehicle vs 1 mg/kg: dose×group: F1,30 = 3.097, p< 0.03; vehicle vs 2 mg/kg: dose×group F1,30 = 2.378, p< 0.074). When the effect of yohimbine was analysed separately in each group, a significant dose effect was observed in all cohorts except those which had received intra-OFC AAV mCREB, as these rats appeared unaffected by the drug (Figure 4A, Dose- GFP:F2, 12 = 7.516, p< 0.008; CREBNAC: F2, 14 = 6.503, p< 0.01; mCREBNAC F2, 14 = 10.062, p< 0.002; CREBOFC = F2, 10 = 14.087, p< 0.001; mCREBOFC: F2,10 = 0.259, NS). Comparing mCREBOFC rats to control subjects revealed that this null effect was most pronounced at the 2 mg/kg dose (all doses, mCREBOFC vs GFP, dose × group: F2,22 = 4.369, p< 0.025; water vs. 2 mg/kg: dose < group F1, 11 = 5.804, p< 0.035).
Figure 4. Modulating CREB activity within the OFC, but not the NAc, affects the increase in premature responding caused by yohimbine.
Yohimbine increased premature responding in control animals, and this effect was potentiated in rats over-expressing CREB within the OFC and attenuated in those over-expressing mCREB within this region (panel A). Over-expression of CREB or mCREB within the NAc did not affect the response to yohimbine (panel B), with both groups showing a drug-induced increase in premature responding comparable to the control group. * indicates a significant within-group difference (p< 0.05) as determined by one-way ANOVA comparing vehicle to drug dose. # indicates a significant between-group difference (p< 0.05) as compared to GFP controls via independent samples Student's t-tests.
In contrast, over-expression of CREB in the OFC tended to potentiate the effects of yohimbine at the lowest dose tested (all doses, CREBOFC vs GFP, group: F1,11 = 3.426, p< 0.091; water vs 1 mg/kg- group: F1,11 = 5.088, p< 0.045; water- group: F1,11 = 1.640, NS; 1mg/kg- group: F1,11 = 5.575, p< 0.038). Over-expression of CREB or mCREB in the NAc had no effect on the response to yohimbine (Figure 4B, all doses, CREBNAc vs GFP, dose × group: F2, 26 = 0.088, NS; group: F1,13 = 2.582, NS; mCREBNAc vs GFP, dose × group: F2, 26 = 2.354, NS; group: F1, 13 = 1.811, NS). Although visual inspection of the data indicated that increasing mCREB in the NAc potentiated the response to 1 mg/kg yohimbine, this was not statistically significant due to high variability between individuals.
In contrast to data from the 5CSRT, yohimbine no longer decreased the level of omissions, though this likely reflects the fact that animals omitted far fewer trials on this simpler task (mean omissions across all groups: 3.02% ± 0.49, Dose: F2, 60 = 0.124, NS; Dose × group: F8, 60 = 0.642, NS; group: F1, 30 = 1.609, NS). No other behavioural variable was significantly affected by yohimbine.
Discussion
Here we have shown that yohimbine increases motor impulsivity in rats, paralleling its effects in humans (17), and that this is mediated at least partly by an increase in CREB activity within the OFC: locally over-expressing the dominant negative protein, mCREB, blocked yohimbine's effects on impulse control, whereas raising CREB levels in the OFC increased impulsive responding and potentiated the pro-impulsive actions of yohimbine. These findings support the hypothesis that acute activation of the noradrenergic system can increase impulsivity, potentially as part of the stress-arousal response, and indicate a novel molecular mechanism by which impulse control is regulated.
The extent to which the pro-impulsivity effects of yohimbine relate to feelings of anxiety or hyper-arousal is unclear. Healthy volunteers did not report high levels of anxiety following doses of yohimbine which increased impulsive responding (17) . In rats, the most robust anxiogenic effects are obtained using higher doses of yohimbine (24). Hence, in the current study, the most anxiogenic dose (5 mg/kg) did not affect impulsive responding, while doses lower than those typically used to trigger anxiety (1–2 mg/kg) significantly increased impulsivity. These data suggest that yohimbine's effects on impulsivity and anxiety are dissociable, and may be experienced at different points along the arousal continuum. The ability of yohimbine to increase impulsivity could also contribute to its ability to trigger relapse to drug-seeking in rats (20; 25). Although the latter effect has been attributed to a yohimbine-induced activation of the stress response, impulsivity can engender relapse in human cocaine addicts (4), and drugs which inhibit premature responding on the 5CSRT in rats can likewise reduce reinstatement of drug-seeking (e.g. M100907, (26). The relationship between stress-arousal and impulsivity in models of addiction may warrant further investigation.
It is unlikely that yohimbine is increasing CREB phosphorylation in the OFC via activation of post-synaptic α2A receptors; not only are the majority of α2A receptors presynaptic, but activation of α2A receptors decreases adenylyl cyclase activity through activation of G(i), thereby reducing cAMP production and decreasing CREB activation (27). In contrast, β adrenoreceptors activate adenylyl cyclase (28). Given that yohimbine increases NE release, the increase in OFC pCREB may arise through activation of postsynaptic β receptors in this region. Indirect support for this suggestion comes from the finding that the β receptor antagonist propranalol blocks the increase in premature responding caused by methylphenidate via a centrally-acting mechanism (29). Theoretically, the effects of yohimbine reported here may stem from the drug's actions at non-adrenergic receptors, as yohimbine can act as an antagonist at dopamine D2 and an agonist at 5-HT1A receptors (30; 31). However, neither eticlopride (a selective D2 antagonist) nor 8-OH-DPAT (a selective 5-HT1A receptor agonist) increase premature responding on the 5CSRT, making such interpretations unlikely (22; 32).
Although this is the first demonstration that modulating CREB activity can directly affect impulsivity, the hypothesis that dysfunction within the NE-CREB pathway contributes to impulse control disorders is not new. In bipolar disorder, peripheral NE levels positively correlate with the severity of mania, and decrease when mania is successfully treated with lithium (33). In rats, chronic lithium treatment decreases CREB phosphorylation in the frontal cortex, striatum, and hippocampus (34), whereas repeated administration of valproic acid attenuates stress-induced changes in tyrosine hydroxylase expression in noradrenergic neurons (e.g. (35). Furthermore, mice which lack an endogenous inhibitor of the CREB pathway have been suggested as a putative model of ADHD due to their hyperactivity and elevated exploratory behavior (36). This phenotype has been attributed to alterations in stress-responsivity arising through deficits in regulation of the pineal hormone melatonin, release of which depends on NE-induced CREB activation (37).
The mechanism by which increased orbitofrontal CREB activity potentiates impulsive responding remains to be identified. Over-expressing CREB within the NAc and several other neuronal cell types increased membrane excitability, potentially by modifying the behavior of sodium and potassium ion channels (38; MARIE; HAN), but its effects on cortical pyramidal cells have yet to be determined. Many genes contain CRE-binding sites, therefore the potential transcriptional targets through which pCREB can act are numerous (~820 in the rat) (ADD REF FOR HIS). Increasing CREB in the NAc did not enhance impulsive responding, highlighting the regional specificity of this effect. Significant differences have been observed in the function of CREB targets unique to the frontal cortex vs the striatum (42): those unique to the frontal cortex were relatively enriched in the presenilin pathway implicated in cognitive function and neurodegeneration. Whether such a pathway could play a role in impaired impulse control as a result of increases in stress/arousal is highly speculative, although increases in a truncated presenilin protein were observed in post-mortem frontal cortex samples from some BD patients (43).
Despite the extensive pharmacological characterisation of the 5CSRT, only a limited number of systemically-administered compounds have been found to increase premature responding: the psychostimulant drugs cocaine and d-amphetamine, the selective 5-HT2C receptor agonist SB242,084, the glutamate NMDA receptor antagonists dizocilpine, and Ro 63–1908 (32; 44; 45); yet these drugs operate through very different pharmacological mechanisms. Identifying the intracellular pathways through which these drugs affect impulsivity may indicate critical points of functional convergence, information which may inform the design of treatments for impulse control disorders. Whether activation of CREB in the OFC is one such critical point remains to be determined, but increases in striatal pCREB has been suggested as a common mechanism underlying the antidepressant action of pharmacologically distinct drugs (13).
In summary, we have translated a clinical finding - that yohimbine increases impulsive responding- into a preclinical model, and determined the underlying mechanism of action in terms of the brain region and molecular signaling pathway involved. Such research may contribute to an improved understanding of the super-sensitivity to yohimbine and other stressors observed in psychiatric populations.
Supplementary Material
Acknowledgements
This work was supported by grants from NIMH and NIDA (EJN), a National Sciences and Engineering Research Council Discovery Grant (CAW), and two Undergraduate Student Research Awards (HS).
Footnotes
Financial Disclosures
EJN reports financial interests in PsychoGenics, Inc., Merck Research Laboratories, and AstraZeneca.
References
TWO NEW REFS:
- Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolaños CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein (CREB) in the rat locus coeruleus: Regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Association AP. Diagnostic and Statistical Manual IV. 4th ed. Washington D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 2.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 3.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 4.Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KJ, Revelle W. Impulsivity and time of day: is rate of change in arousal a function of impulsivity? J Pers Soc Psychol. 1994;67:334–344. doi: 10.1037//0022-3514.67.2.334. [DOI] [PubMed] [Google Scholar]
- 6.Roberts RE, Roberts CR, Chan W. One-year incidence of psychiatric disorders and associated risk factors among adolescents in the community. J Child Psychol Psychiatry. 2008 doi: 10.1111/j.1469-7610.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 7.Casada JH, Roache JD. Behavioral inhibition and activation in posttraumatic stress disorder. J Nerv Ment Dis. 2005;193:102–109. doi: 10.1097/01.nmd.0000152809.20938.37. [DOI] [PubMed] [Google Scholar]
- 8.Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depression and anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 10.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- 13.Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, et al. Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 15.Szemeredi K, Komoly S, Kopin IJ, Bagdy G, Keiser HR, Goldstein DS. Simultaneous measurement of plasma and brain extracellular fluid concentrations of catechols after yohimbine administration in rats. Brain Res. 1991;542:8–14. doi: 10.1016/0006-8993(91)90990-d. [DOI] [PubMed] [Google Scholar]
- 16.Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- 17.Swann AC, Birnbaum D, Jagar AA, Dougherty DD, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry. 2005;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Price LH, Charney DS, Heninger GR. Three cases of manic symptoms following yohimbine administration. Am J Psychiatry. 1984;14:1267–1268. doi: 10.1176/ajp.141.10.1267. [DOI] [PubMed] [Google Scholar]
- 19.Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 20.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 22.Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- 23.Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, et al. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole BJ, Hillmann M, Seidelmann D, Klewer M, Jones GH. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology (Berl) 1995;121:118–126. doi: 10.1007/BF02245598. [DOI] [PubMed] [Google Scholar]
- 25.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 27.Repaske MG, Nunnari JM, Limbird LE. Purification of the alpha 2-adrenergic receptor from porcine brain using a yohimbine-agarose affinity matrix. J Biol Chem. 1987;262:12381–12386. [PubMed] [Google Scholar]
- 28.Brandt DR, Asano T, Pedersen SE, Ross EM. Reconstitution of catecholamine-stimulated guanosinetriphosphatase activity. Biochemistry. 1983;22:4357–4362. doi: 10.1021/bi00288a002. [DOI] [PubMed] [Google Scholar]
- 29.Milstein J, Dalley J, Robbins T. Methylphenidate-induced impulsivity: pharmacological antagonism by {beta}-adrenoreceptor blockade. J Psychopharmacol. 2008 doi: 10.1177/0269881108098146. [DOI] [PubMed] [Google Scholar]
- 30.Scatton B, Zivkovic B, Dedek J. Antidopaminergic properties of yohimbine. J Pharmacol Exp Ther. 1980;215:494–499. [PubMed] [Google Scholar]
- 31.Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- 32.van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 33.Greenspan K, Schildkraut JJ, Gordon EK, Baer L, Aronoff MS, Durell J. Catecholamine metabolism in affective disorders: III. MHPG and other catecholamine metabolites in patients treated with lithium carbonate. J Psychiatric Res. 1970;7:171–183. doi: 10.1016/0022-3956(70)90004-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen B, Wang JF, Hill BC, Young LT. Lithium and valproate differentially regulate brain regional expression of phosphorylated CREB and c-fos. Brain Research Molecular Brain Research. 1999;70:45–53. doi: 10.1016/s0169-328x(99)00125-4. [DOI] [PubMed] [Google Scholar]
- 35.Sands SA, Guerra V, Morilak DA. Changes in tyrosine hydroxylase mRNA expression in the rat locus coeruleus following acute or chronic treatment with valproic acid. Neuropsychopharmacology. 2000;22:27–35. doi: 10.1016/S0893-133X(99)00072-X. [DOI] [PubMed] [Google Scholar]
- 36.Lahti TA, Partonen T. CREM mutations and ADHD symptoms. Med Hypotheses. 2009;72:544–545. doi: 10.1016/j.mehy.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 37.Schomerus C, Korf HW. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann N Y Acad Sci. 2005;1057:372–383. doi: 10.1196/annals.1356.028. [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- 39.Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell JB, Gratton A. Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci. 1992;12:3609–3618. doi: 10.1523/JNEUROSCI.12-09-03609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winstanley CA, Green TA, Theobald DE, Renthal W, Laplant Q, Dileone RJ, et al. DeltaFosB induction in orbitofrontal cortex potentiates locomotor sensitization despite attenuating the cognitive dysfunction caused by cocaine. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanis KQ, Duman RS, Newton SS. CREB binding and activity in brain: regional specificity and induction by electroconvulsive seizure. Biol Psychiatry. 2008;63:710–720. doi: 10.1016/j.biopsych.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MJ, Sharples RA, Evin G, McLean CA, Dean B, Pavey G, et al. Expression of truncated presenilin 2 splice variant in Alzheimer's disease, bipolar disorder, and schizophrenia brain cortex. Brain Res Mol Brain Res. 2004;127:128–135. doi: 10.1016/j.molbrainres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and "impulsive-like" behaviours produced by NMDA receptor antagonism. Psychopharmacology. 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- 45.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.