Abstract
Background
The cellular mechanisms of neuropathic pain are inadequately understood. Previous investigations have revealed disrupted Ca2+ signaling in primary sensory neurons after injury. We therefore examined the effect of injury on intracellular Ca2+ stores of the endoplasmic reticulum, which critically regulate the Ca2+ signal and neuronal function.
Methods
Intracellular Ca2+ levels were measured with Fura-2 or mag-Fura-2 microfluorometry in axotomized fifth lumbar (L5) dorsal root ganglion neurons and adjacent L4 neurons isolated from hyperalgesic rats following L5 spinal nerve ligation, compared to neurons from control animals.
Results
Endoplasmic reticulum Ca2+ stores released by the ryanodine-receptor agonist caffeine decreased by 46% in axotomized small neurons. This effect persisted in Ca2+-free bath solution that removes the contribution of store-operated membrane Ca2+ channels, and after blockade of both the mitochondrial, sarco-endoplasmic Ca2+-ATPase, and the plasma membrane Ca2+ ATPase pathways. Ca2+ released by the sarco-endoplasmic Ca2+-ATPase blocker thapsigargin and by the Ca2+-ionophore ionomycin was also diminished by 25% and 41%, respectively. In contrast to control neurons, Ca2+ stores in axotomized neurons were not expanded by neuronal activation by K+ depolarization, and the proportionate rate of refilling by sarco-endoplasmic Ca2+-ATPase was normal. Luminal Ca2+ concentration was also reduced by 38% in axotomized neurons in permeabilized neurons. The adjacent neurons of the L4 dorsal root ganglia showed modest and inconsistent changes after L5 spinal nerve ligation.
Conclusions
Painful nerve injury leads to diminished releasable endoplasmic reticulum Ca2+ stores and a reduced luminal Ca2+ concentration. Depletion of Ca2+ stores may contribute to the pathogenesis of neuropathic pain.
Introduction
The primary afferent neuron is the source of all somatic sensory experience, a target of regional anesthetics, the afferent pathway for pain during surgery, and an origin of abnormal activity in chronic pain. Cytoplasmic Ca2+ is the dominant second messenger in sensory neurons, and regulates diverse processes including neuronal differentiation, neurotransmitter release, excitability, gene expression, enzymatic activation, and programmed cell death (apoptosis). We have previously shown that painful nerve injury leads to prolonged disruption of the Ca2+ signal in primary afferent neurons. Specifically, after transection of their peripheral axons, neurons with their cell bodies in the dorsal root ganglia (DRGs) demonstrate a depressed resting cytoplasmic Ca2+ concentration ([Ca2+]c)1 as well as a diminished temporary increase of [Ca2+]c that follows neuronal activation (the [Ca2+]c transient).2 These findings may in part be attributable to the decrease in activity-related Ca2+ entry through the plasmalemma caused by axotomy of DRG neurons. 3-5 However, the cellular machinery that modulates the sensory neuron Ca2+ signal is complex,6 and its function in the context of injury has not been examined.
Sensory neurons possess channels and pumps that extrude Ca2+ from the cell, sequester Ca2+ into organelles within the cell, and release Ca2+ from these stores. Specifically, the plasma membrane Ca2+-ATPase expels cytoplasmic Ca2+ from the neuron, as does the Na+-Ca2+ exchange pathway. Cytoplasmic Ca2+ is pumped through the action of the sarcoplasmic-endoplasmic reticulum Ca2+-ATPase (SERCA) into high-concentration subcellular reservoirs, predominantly the endoplasmic reticulum (ER),6,7 from which it may be released back to the cytoplasm through channels sensitive to ryanodine (the ryanodine receptor, [RyR]) or inositol triphosphate (IP3). The amount of Ca2+ accumulated in this store is functionally linked to plasma membrane channels (store-operated Ca2+ channels, [SOCCs]) that admit Ca2+ into the cytoplasm at a rate inversely related to the store level.8 Mitochondrial Ca2+ uptake driven by the potential across the mitochondrial inner membrane buffers large Ca2+ loads by uptake when the [Ca2+]c rises above a critical threshold, and subsequently releases this Ca2+ as the [Ca2+]c recovers towards baseline levels. All of these processes are tuned by regulatory systems, and may be sites at which neuronal injury perturbs the final Ca2+ signal.
The quantity of stored Ca2+ critically regulates Ca2+ signaling in neurons. This parameter dictates the ability of the sensory neuron to receive Ca2+ from the cytoplasm,9,10 by which the cell limits excessive [Ca2+]c levels during neuronal activity. Stored Ca2+ may also be released into the cytoplasm through the process of Ca2+-induced Ca2+ release, by which an initial increase in [Ca2+]c activates the RyRs to discharge stored Ca2+ into the cytoplasm, resulting in amplification of [Ca2+]c transients. Thus, the ER is at once both a Ca2+ sink and source,11 critically shaping the Ca2+ signal.
Disordered homeostasis of the Ca2+ store participates in the pathogenesis of various neurological conditions, such as stroke and Alzheimer's disease, as well as painful conditions such as diabetes, human immunodeficiency virus infection, and tissue inflammation.12-14 The ER stores may likewise play a central role in the pathogenesis of neuropathic pain. Accordingly, the experiments reported here explored the influence of injury upon the size of the intracellular Ca2+ store. We employed the spinal nerve ligation (SNL) injury model, since this allows separate evaluation of the axotomized neurons of the fifth lumbar (L5) DRG, for comparison with the L4 neurons that are not directly injured but have axons that transit the sciatic nerve in the company of degenerating distal segments of the L5 neurons, and are thus exposed to mediators of inflammation.15,16 The relative importance of the contribution to pain genesis of these two populations remains unresolved.17
Materials and Methods
All methods and use of animals were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee (Milwaukee, Wisconsin).
Injury model
Male Sprague-Dawley (Taconic Farms Inc., Hudson, NY) rats weighing 160 to 180g were subjected to SNL in a manner derived from the original technique.18 Rats were anesthetized with 2% isoflurane in oxygen and the right paravertebral region was exposed. After removal of the L6 transverse process, the L5 and L6 spinal nerves were ligated with 6-0 silk suture and transected distal to the ligature. The fascia was closed with 4-0 resorbable polyglactin suture and the skin closed with staples. Control animals received anesthesia, skin incision and stapling only. After surgery, the rats were returned to their cages and kept under normal housing conditions with access to pellet food and water ad lib.
Sensory testing
Rats underwent sensory testing for hyperalgesic behavior on three different days between 10d and 17d after surgery, as previously described.1,19 Briefly, right plantar skin was mechanically stimulated with a 22G spinal needle with adequate pressure to indent but not penetrate the skin. Whereas control animals respond with only a brief reflexive withdrawal, rats following SNL may display a complex hyperalgesia response that incorporates sustained licking, chewing, grooming and elevation of the paw. The frequency of hyperalgesia responses was tabulated for each rat. After SNL, only rats that displayed a hyperalgesia-type response after at least 20% of stimuli were used further in this study.
Neuron isolation and plating
Neurons were dissociated from L4 and L5 DRGs after isoflurane anesthesia and decapitation 21 to 27 days after SNL or skin sham surgery. This interval was chosen since hyperalgesia is fully developed by this time.19 DRGs were incubated in 0.0625% trypsin (Sigma Aldrich, St. Louis, MO), 0.0125% DNAse (Invitrogen, Carlsbad, CA) and 0.01% blendzyme 2 (Roche Diagnostics, Indianapolis, IN) in Dulbecco's modified Eagle's medium (DMEM)/F12 with glutaMAX (Invitrogen) for 1.5 hours, centrifuged and triturated with fire-polished pipettes in culture medium containing Neural Basal Media A with B27 supplement (Invitrogen), 0.5mM glutamine, 100ng/ml nerve growth factor 7S (Alomone Labs, Jerusalem, Israel) and 0.02mg/ml gentamicin (Invitrogen). Dissociated neurons were plated onto poly-L-lysine coated glass cover slips (Deutsches Spiegelglas, Carolina Biological Supply, Burlington, NC) and maintained at 37°C in humidified 95% air and 5% CO2 for 2 hours, and were studied no later than 6 hours after harvest.
Solutions and agents
Unless otherwise specified, the bath contained Tyrode's solution (in mM): NaCl 140, KCl 4, CaCl2 2, Glucose 10, MgCl2 2, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10, with an osmolarity of 297-300mOsm and pH 7.40. In some experiments, a Ca2+-free Tyrode's was used that contained (in mM): NaCl 140, KCl 4, Glucose 10, MgCl2 2 and, HEPES 10, and ethylene glycol tetraacetic acid (EGTA) 0.2. In experiments with permeabilized neurons, a bath solution modeled upon intracellular solution was used that contained (in mM): KCl 120, EGTA 5, CaCl2 2.25, MgCl2 2, and HEPES 20. Osmolarity was adjusted to 295 with sucrose, and pH was adjusted to 7.40. This clamped Mg2+ and Ca2+ at physiologic concentrations20 of 1.6mM and 39nM respectively, calculated using a web-based EGTA calculator*. Adenosine-triphosphate was omitted in order not to trigger release of stored Ca2+. Agents were obtained as follows: caffeine, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), dimethylsulfoxide, lanthanum chloride, thapsigargin from Sigma Aldrich, saponin from Calbiochem EMD (Gibbstown, NJ), Fura-2-AM, mag-Fura-2-AM, ionomycin, and Pluronic F-127 from Invitrogen. Stock solutions of ionomycin, thapsigargin, FCCP, Fura-2-AM, and mag-Fura-2-AM were dissolved in dimethylsulfoxide, and subsequently diluted in the relevant bath solution such that final bath concentration of dimethylsulfoxide was 0.1% or less, which has no effect on [Ca2+]c (n=20, data not shown). The 500 μl recording chamber was constantly superfused by a gravity-driven bath flow at a rate of 3 ml/min. Agents were delivered by directed microperfusion through a 500 μm diameter hollow quartz fiber 300 μm upstream from the neurons. This flow completely displaced the bath solution, and constant flow was maintained by delivery of bath solution, when specific agents were not being administered, through a computerized valve system. Solution changes were achieved within 200 ms.
Measurement of cytoplasmic Ca2+ concentration
Cover slips holding plated neurons were transferred to a room temperature 5 μM solution of Fura-2-AM that contained 0.04% Pluronic F-127 to aid dispersion of the fluorophore. After 30 min, they were washed three times with regular Tyrode's solution and left in a dark environment for deesterification for 30 min and then mounted onto the recording chamber. The fluorophore was excited alternately with 340 nm and 380 nm wavelength illumination (150W Xenon, Lambda DG-4, Sutter, Novato, CA), and images were acquired at 510 nm using a cooled 12-bit digital camera (Coolsnap fx, Photometrics, Tucson, AZ) and inverted microscope (Diaphot 200, Nikon Instruments, Melville, NY) through a 20× or 40× fluor oil-immersion objective. Neurons were visually examined in the brightfield mode and those showing signs of lysis, crenulation or superimposed glial cells were excluded. Recordings from each neuron were obtained as separate regions of interest by appropriate software (MetaFluor, Molecular Devices, Downingtown, PA). After background subtraction, the fluorescence ratio R for individual neurons was determined as the intensity of emission during 340 nm excitation (I340) divided by I380, on a pixel-by-pixel basis. The calcium concentration was then estimated by the formula [Ca2+]c = Kd•β• (R−Rmin)/(Rmax−R) where β = (I380max)/(I380min). Values of Rmin, Rmax and β were determined by in-situ calibrations of 33 neurons from 3 different preparations as described before1 and were 0.38, 8.49 and 9.54, respectively, and 224 nm was used as Kd.21
Determination of Ca2+ release
Release of Ca2+ from stores was quantified by measuring the size of the resulting [Ca2+]c transient. Using amplitude to quantify large transients, such as those induced by the action of caffeine, poorly reflects the magnitude of Ca2+ release from stores due to the influence of mitochondrial buffering during intervals of high [Ca2+]c. Initial mitochondrial Ca2+ uptake depresses the peak [Ca2+]c during a transient and produces a plateau phase thereafter, during which Ca2+ is released from mitochondria into the cytoplasm.22 Thus, mitochondrial buffering has reciprocal effects on transient amplitude and duration.23 Further, we have previously shown that transient area is proportionate to cytoplasmic Ca2+ load.2 We therefore chose to quantify caffeine-induced transients by calculating the area of the transient.
In other experiments, application of thapsigargin was used to produce transients, for which the amplitude of the rise from baseline to peak [Ca2+]c was measured rather than area, since these transients had a more gradual onset without a clearly discernible beginning and endpoint, unlike those triggered by caffeine. Also, such transients do not reach [Ca2+]c levels that engage mitochondrial buffering, which we estimated from the level of the plateau in large K+- (316 ± 124 nM; n=115) and caffeine-induced transients (310 ± 128 nM, n=541).22,24 Amplitude measurement was also employed for transients induced by ionomycin, since this ionophore acts to equilibrate [Ca2+] gradients between subcellular compartments including cytosol, ER, and mitochondria.25 Calcium was absent from the bath solution during thapsigargin application to remove the contribution of Ca2+ entry through SOCCs,26 and similarly during ionomycin application to avoid entry of Ca2+ through the ionophore.
Measurement of ER luminal Ca2+ concentration
The low-affinity indicator mag-Fura-2-AM was used to measure the Ca2+ concentration contained within the lumina of intracellular compartments ([Ca2+]L).27,28 Dye loading and recording procedures were similar to those used with Fura-2-AM, although loading took place at 37°C to maximize compartmentalization of the fluorophore in intracellular organelles.29 Calibration of the mag-Fura-2 signal was performed in situ on 26 neurons from 3 different dissociated DRGs, and showed values of Rmin, Rmax and β of 0.32, 3.63 and 13.09. A Kd of 53 μM was used for calculation of [Ca2+]L.30 In protocols requiring removal of cytoplasmic mag-Fura-2, whereas attempts using patch-pipette dialysis proved ineffective, successful permeabilization of neurons was achieved by 75-105 s bath application of 0.01% saponin.31 Cytoplasmic washout of the fluorophore was confirmed by following the decline in both the fluorescence intensities at 340 nm and 380 nm and a concurrent increase in the ratio R. Only traces in which the decline in intensity during 380 nm illumination exceeded 40% were included for analysis.
Statistical analysis
Preliminary studies showed no differences between neurons dissociated from L4 and L5 ganglia, removed from the same control animals, in Ca2+ release by caffeine with normal Tyrode's bath solution, (transient area 14.7 ± 11.3 nM•s × 103 in L4, n=19, vs. 15.7 ± 11.4 nM•s × 103, in L5, n=37, P=0.38). We therefore pooled findings from L4 and L5 neurons as the control group. Neurons characterized by size (large > 34 μm, small < 34 μm) were considered separately. Statistical analyses were performed with Statistica (StatSoft Inc, Tulsa, OK). Two-tailed Student's t-test was used for comparing two means. One-way ANOVA was used to detect the influence of injury group on measured parameters. Consideration of additional factors such as time or pharmacologic interventions was achieved using a two-way ANOVA design. An ANCOVA model was used to account for variance due to a covariate after assuring linearity of the influence of the covariate upon the outcome measure. Bonferroni's post-hoc test was used to compare relevant means, and a P value less than 0.05 was considered significant. Averages are reported ± SD.
Results
Upon needle stimulation, SNL animals (n=69) displayed a hyperalgesia response rate of 35 ± 21%, whereas control animals (n=56) showed a hyperalgesic response 0 ± 1% (P<0.001). Consistent with our previous findings,1 sensory neuron axotomy depressed resting [Ca2+]c. Specifically, whereas [Ca2+]c was 66 ± 27 nM in control neurons (n=274), SNL of L5 neurons resulted in a [Ca2+]c of 46 ± 25 nM (n=157; P<0.001 vs. control). SNL had no effect on resting [Ca2+]c of adjacent L4 neurons, in which [Ca2+]c was 63 ± 26 nM (n=110).
Ryanodine receptor-mediated Ca2+ release by caffeine
An estimate of stored Ca2+ may be made by measuring the rise of [Ca2+]c during Ca2+ release from stores triggered by application of a high concentration of caffeine (20 mM), which sensitizes the RyR to Ca2+ to such an extent that resting levels of Ca2+ produce sustained channel opening.32 For caffeine-induced Ca2+ release, we chose to measure transient area rather than amplitude in order to limit the influence of mitochondrial buffering (see methods), and tested assumption in control neurons by comparing transient parameters with and without blockade of mitochondrial Ca2+ buffering with FCCP (1μM).22 Whereas amplitude of the caffeine-induced transient increased by 108% in the presence of FCCP (from 779 ± 807 nM, n=273, to 1615 ± 2075 nM, n=198), area of the transient increased by only 20% (from 18.2 ± 11.1 nM•s × 103, n=268, to 21.9 ± 16.6 M•s × 103, n=198).
To measure stores, caffeine was administered continuously until the [Ca2+]c returned to baseline to assure that the stores were fully emptied, which was confirmed by the observation that reapplication of caffeine 30s after washout produced no further Ca2+ release (n=10; data not shown). Sustained application also precluded SERCA function so that this process did not affect the measure of Ca2+ release. Application of caffeine (20 mM) in this fashion produced a rapidly rising transient that included a prolonged plateau phase followed by resolution to a level close to resting [Ca2+]c (fig. 1A). All but one of 542 neurons responded with an increase of at least 50% above resting [Ca2+]c.
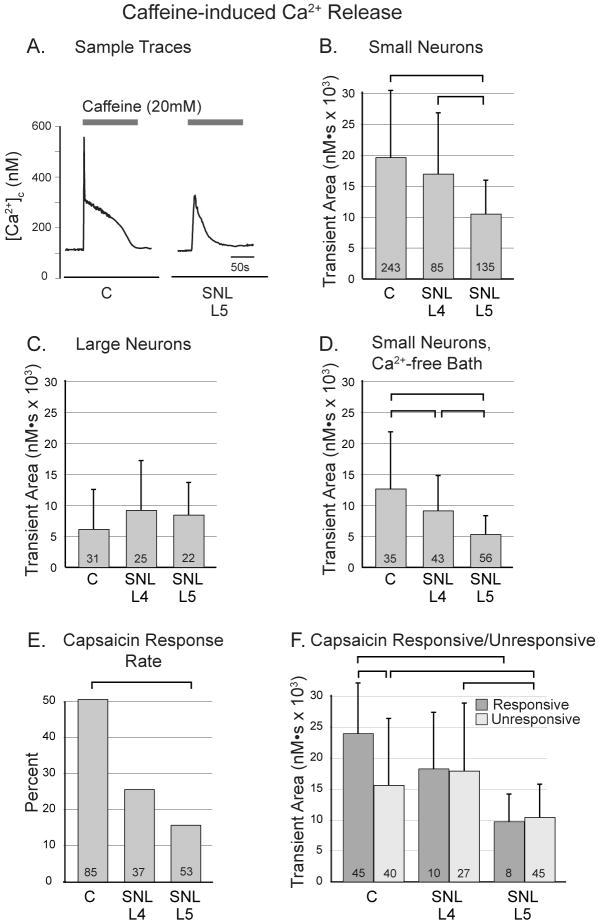
Figure 1.
Response of cytoplasmic Ca2+ concentration ([Ca2+]c) in sensory neurons to application of caffeine (20mM). A. Characteristic transients show a rapid peak and sustained plateau, with diminished size after spinal nerve ligation (SNL) in axotomized small neurons of the fifth lumbar (L5) dorsal root ganglion. B. In small neurons (diameter less than 34μm), transient area is decreased in L5 neurons compared to control (C). C. In large neurons, there are no differences between injury groups. D. When Ca2+ is removed from the bath solution, transients in small neurons are reduced in size compared to Ca2+-containing solution (panel B.), but injury still reduces transient in L5 neurons, as well as in L4 neurons. E. The frequency of neuronal sensitivity to capsaicin (10nM) is decreased in L5 neurons after SNL. F. The area of caffeine-induced transients is less in neurons insensitive to capsaicin than in responsive ones in control neurons but not in L4 or L5 neurons after SNL. Transient area is reduced in L5 neurons compared to control in both capsaicin sensitive and insensitive populations. For panels B-E, one-way ANOVA with Bonferroni post-hoc testing was used. For panel F, two-way ANOVA with Bonferroni post-hoc testing was used. For all panels, numbers in the bars indicate n for number of neurons, brackets above bars connect groups that are significantly different (P<0.05), and error bars show standard deviation.
DRG neurons are a heterogeneous population representing different sensory modalities. While small neurons are broadly associated with nociceptive sensory modality, neurons with large somata conduct low-threshold sensory information.33,34 In small neurons, the transient areas evoked by caffeine were significantly decreased by axotomy in L5 neurons after SNL, as well as in adjacent L4 neurons (fig. 1B). Caffeine-induced transients had areas in control large neurons that were smaller than in control small neurons (P<0.001). However, there was no effect of injury on large neuron Ca2+ release measured this way (fig. 1C).
Caffeine release of stored Ca2+ triggers influx of Ca2+ across the plasmalemma through store-operated Ca2+ channels (SOCCs),35 which could be independently influenced by injury. We therefore performed additional experiments in small neurons eliminating this factor by using Ca2+-free bath solution.36 Under these conditions, transients triggered by caffeine application showed influences of injury that were congruent with the findings above (fig. 1D). The transient area in all groups was less in the absence of bath Ca2+ compared to experiments conducted with bath Ca2+, which likely indicates a contribution by SOCC to the transient generated by caffeine release of Ca2+ stores.35
To further compare Ca2+ stores and injury effect in putative nociceptors versus low-threshold afferents, we examined additional small neurons that were further categorized by sensitivity to capsaicin. Sensitivity to capsaicin is typical of peptidergic polymodal nociceptors, while insensitivity is a characteristic of IB4-binding mechanosensitive nociceptors.37 In a separate preliminary study (data not shown), the EC50 for capsaicin necessary to achieve a 50% increase in [Ca2+]c was 7.9 nM (total n=175 neurons, used only for this determination). Using10 nM, 45/85 (53%) of control neurons responded to capsaicin by this criterion (fig. 1E), comparable to the typical proportion of nociceptive neurons.38 In control neurons, [Ca2+]c transient areas were significantly larger in capsaicin-responsive cells than in nonresponsive ones (P<0.001; fig. 1F), as reported previously. SNL decreased the proportion of neurons sensitive to capsaicin in the L4 group (10/37, 27%) and especially the L5 group (8/53, 15%; P<0.001), as expected from previous findings that axonal injury causes loss of the capsaicin receptor, transient receptor potential vanilloid-1.1,39 In both capsaicin-responsive and non-responsive populations, axotomy reduced the area of the caffeine-induced transient (fig. 1F), indicating loss of Ca2+ stores. Specifically, in capsaicin-responsive neurons, the average area under the transient curve was decreased by 59% in axotomized compared to control neurons, and axotomy of capsaicin-nonresponsive neurons decreased the area by 33%. This indicates that both subgroups of small nociceptive sensory neurons have decreased releasable Ca2+ stores after injury.
Ca2+ release during inhibition of sequestration and extrusion
In order to examine Ca2+ release in the absence of the buffering by uptake into mitochondria, we applied caffeine (20 mM) during the continued presence of FCCP (1μM; fig. 2A). Peak of the transient in L4 neurons was not different from controls, while the axotomized L5 neurons decreased by 51% compared to controls (fig. 2B). This indicates that smaller caffeine-induced [Ca2+]c transients in axotomized neurons are not due to greater mitochondrial buffering in this neuronal group.
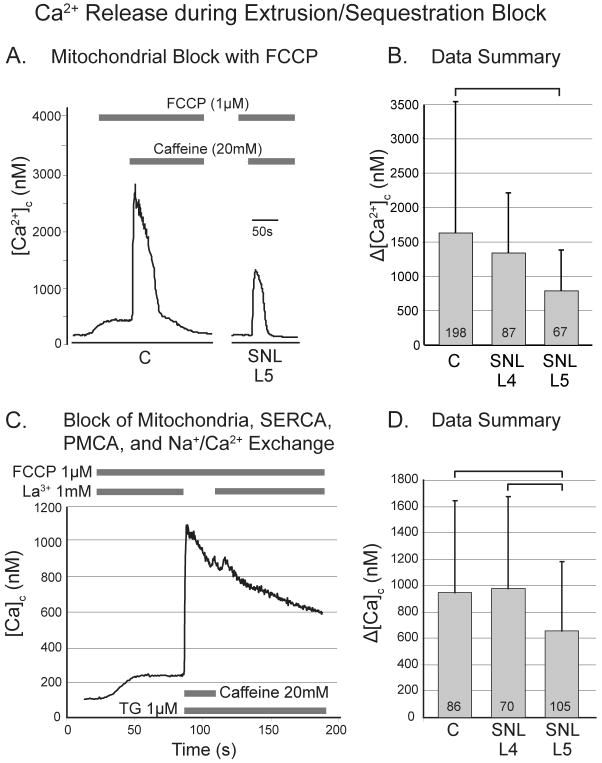
Figure 2.
Response of cytoplasmic Ca2+ concentration ([Ca2+]c) in sensory neurons to application of caffeine (20mM) during pharmacological blockade of processes that extrude and sequester Ca2+. A. Typical traces of caffeine-induced Ca2+ release in the presence of the mitochondrial protonophore p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP), which eliminates buffering of cytoplasmic Ca2+ by mitochondria, and increases amplitude of the transient while eliminating the plateau phase. B. Spinal nerve ligation (SNL) depresses the amplitude of caffeine-induced transients during FCCP in the axotomized neurons of the fifth lumbar (L5) dorsal root ganglion. C. Typical traces of caffeine-induced Ca2+ transients in the presence of FCCP, and thapsigargin (TG), which blocks sequestration of Ca2+ by the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA), as well as La3+, which blocks extrusion of Ca2+ from the neuron by the plasma membrane Ca2+ ATPase (PMCA) and the Na+/Ca2+ exchanger. D. SNL depresses amplitude of the resulting caffeine-induced transient in the L5 population. For panels B and D, one-way ANOVA with Bonferroni post-hoc testing was used, numbers in the bars indicate n for number of neurons, and brackets above bars connect groups that are significantly different (P<0.05), and error bars show standard deviation.
We additionally eliminated the potential influence of variable Ca2+ reuptake by the SERCA, Ca2+ extrusion via the plasma membrane Ca2+-ATPase and Ca2+-Na+ exchange mechanism, and Ca2+ influx by inducing Ca2+ release with caffeine (20 mM) in the context of multiple pharmacological blockers (FCCP, 1μM; thapsigargin, 1μM to block SERCA, administered only with and after caffeine in order to avoid an initial thapsigargin-induced store release; and La3+, 1mM to block plasma membrane Ca2+-ATPase, Ca2+-Na+ exchange, and Ca2+ entry pathways, discontinued during caffeine in order to avoid precipitation). This resulted in transients in which an initial increase of [Ca2+]c was not followed by recovery (fig. 2C). Injury had a significant effect on the caffeine-induced transient amplitude (fig. 2D), in which the L4 group was unchanged but the L5 group showed 30% less Ca2+ release compared to control.
Constitutive Ca2+ release revealed by thapsigargin
It is unclear whether ryanodine receptor-sensitive Ca2+ stores fully overlap with Ca2+ stores sensitive to inositol-3P.6 For this reason, and because receptor-mediated Ca2+ release may be influenced by altered expression of the receptor after injury without actually affecting Ca2+ stores per se, we additionally determined the effect of injury on Ca2+ stores using techniques that do not rely on receptor activation. Thapsigargin provides such an alternate method by blocking SERCA, which exposes a constitutive Ca2+ leak from stores. This effect is dose related,26 and although a prior study using 20 nM thapsigargin failed to produce transients in sensory neurons,40 we observed slow [Ca2+]c transients with application of 1μM thapsigargin in all neurons (fig. 3A). Full emptying of RyR-sensitive stores by thapsigargin was demonstrated by reduction of the subsequent caffeine-induced Ca2+ release transient area in control neurons to 165 ± 353 nM•s (n=20), which is 1% of caffeine-induced Ca2+ release reported above. However, thapsigargin-sensitive Ca2+ stores encompass more than just the RyR-sensitive pool, since a thapsigargin-induced transient (amplitude 23 ± 17 nM, n=19) persists after caffeine-induced Ca2+ release, which constitutes 22% of the thapsigargin-induced transient in the absence of preceding caffeine (107 ± 69, n=85). An effect of injury is evident in the thapsigargin-sensitive pool, since the thapsigargin-induced transient in SNL L5 neurons had a 25% lower transient amplitude than control or SNL L4 neurons (fig. 3B).
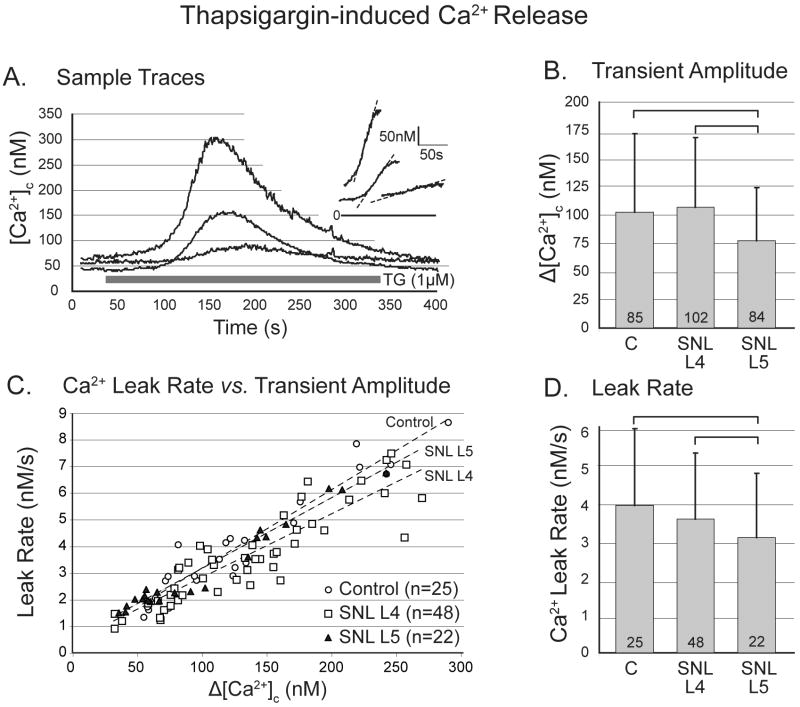
Figure 3.
Response of cytoplasmic Ca2+ concentration ([Ca2+]c) in sensory neurons to application of thapsigargin (TG). A. Sample traces from control (C) neurons demonstrate variable amplitude of response but a linear rising limb of the transient (inset). B. Spinal nerve ligation (SNL) depresses the response in axotomized neurons of the fifth lumbar (L5) dorsal root ganglion. (One-way ANOVA with Bonferroni post-hoc testing.) C. The rate of Ca2+ leak during TG application, measured as the slope of the rising limb, is linearly dependent on the transient amplitude for all injury groups. D. Leak rate is depressed in L5 neurons. For panels C and D, an ANCOVA model with Bonferroni post-hoc testing was employed. For panels B and D, numbers in the bars indicate n for number of neurons, brackets above bars connect groups that are significantly different (P<0.05), and error bars show standard deviation.
The rise of [Ca2+]c during thapsigargin application was reliably a straight line (fig. 3A inset) indicative of a zero order process, consistent with the observation that the leak rate from Ca2+ stores is independent of [Ca2+]L until the stores are nearly exhausted.10 In order to evaluate the rate of constitutive release from thapsigargin-sensitive stores, we measured the slope of this rising phase of the thapsigargin-induced [Ca2+]c transient. Leak rate measured this way showed a tight dependence upon total releasable Ca2+ content as indicated by amplitude of the thapsigargin release transient (R2 = 0.85 for all groups together, P<0.001; n=95 neurons, fig 3C). For this reason, an analysis of covariance (ANCOVA) model was used to examine the average leak rate, which was depressed in SNL L5 neurons compared to control and SNL L4 neurons (fig. 3D). This was attributable to the effect of the depressed releasable Ca2+ in the L5 neurons, as demonstrated by the comparable relationship between leak rate and releasable Ca2+ in control and L5 neurons (fig. 3C). However, L4 neurons had a slightly depressed relationship of leak versus level of releasable Ca2+.
Ca2+ release by ionomycin
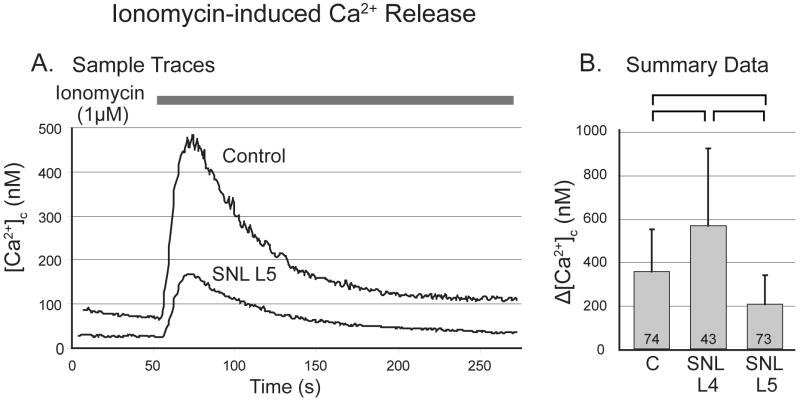
Application of the Ca2+ ionophore ionomycin (1μM) is an additional technique that releases stored Ca2+ without requiring receptor activation12 and eliminates the influence of mitochondria.25 A Ca2+-free bath solution was used for 3min preceding ionomycin application and thereafter, in order to avoid the influence of Ca2+ entry through the plasmalemma. Transients showed an exponential resolution that lacked the plateau attributable to mitochondria (fig. 4A). After SNL, ionomycin released more Ca2+ in the adjacent L4 neurons, but releasable Ca2+ was reduced in axotomized L5 neurons compared to control (fig. 4B).
Figure 4.
Response of cytoplasmic Ca2+ concentration ([Ca2+]c) in sensory neurons to application of ionomycin in bath solution not containing Ca2+. A. Sample traces from a control (C) neuron and another from the fifth lumbar (L5) dorsal root ganglion after spinal nerve ligation (SNL). B. Application of ionomycin produces transients with reduced amplitude in L5 neurons, but increased amplitude in L4 neurons (one-way ANOVA with Bonferroni post-hoc testing). Numbers in the bars indicate n for number of neurons, brackets above bars connect groups that are significantly different (P<0.05), and error bars show standard deviation.
SERCA-dependent recovery of Ca2+ stores at rest
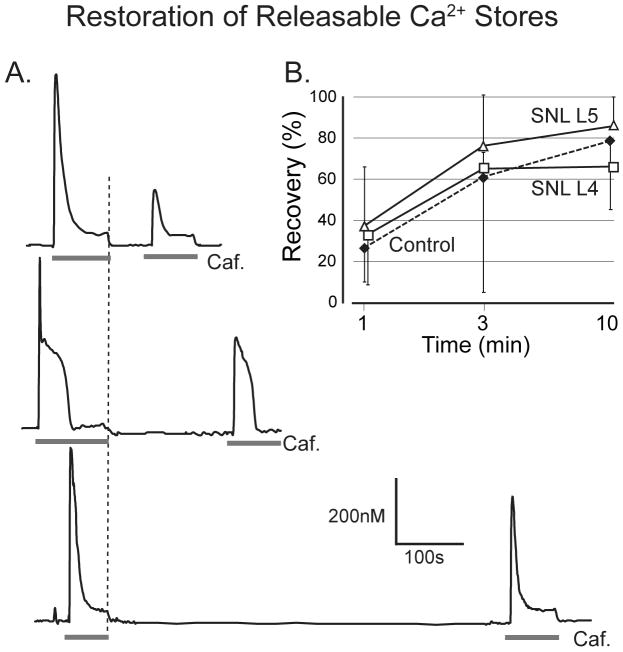
One possible reason that Ca2+ stores cannot be filled in injured neurons may be dysfunction of the SERCA mechanism that pumps Ca2+ into the ER. Repeat application of caffeine (20 mM) produces a diminished Ca2+ release until adequate time has elapsed to allow refilling of the stores (fig. 5A). We therefore tested the pace at which neurons in the different groups recovered from caffeine-induced depletion of stored Ca2+. Complete release of stored Ca2+ was achieved by sustained application of caffeine (20 mM), which was followed by a 1, 3 or 10-minute recovery period, and then caffeine was applied again. Each neuron was used to test only one time interval. After 10min recovery time, the area of the second transient in control neurons had recovered to 79 ± 23% of the first (fig. 5B), and there was no difference in the proportionate rate of recovery after injury, normalized to the initial baseline Ca2+ release. We confirmed that replenishment during rest was SERCA-dependent, as thapsigargin applied during the refilling interval reduced 10-min recovery to 0.3 ± 1.4% (n=20). These findings indicate that SERCA function is adequate following neuronal injury to provide recovery to the original baseline level of stores.
Figure 5.
Recovery of caffeine-induced Ca2+ release during rest. A. A second application of caffeine (Caf., 20mM) at intervals of 1, 3, and 10min following the offset of the previous application (dotted line) shows progressive recovery of transient area in three different neurons. B. Proportionate recovery is comparable in control neurons and in fourth lumbar (L4) and L5 neurons after spinal nerve ligation (SNL). Two-way ANOVA shows a significant main effect for time interval, but not for injury. Groups at each data point include 4 to 14 neurons. Error bars show standard deviation.
Role of activity in regulating Ca2+ stores
Ca2+ enters sensory neurons predominantly through voltage-gated Ca2+ channels during neuronal activity. After SNL, axotomized L5 neurons receive no afferent activity generated from natural stimulation of the receptive field, and this loss may thereby lead to depleted Ca2+ stores. We therefore examined whether membrane depolarization with K+, which in turn leads to Ca2+ influx across the plasmalemma and store repletion, might increase Ca2+ stores in axotomized neurons to the levels of control neurons. Priming transients were generated by 5s application of 50 mM K+ followed 3min later by sustained application of 20 mM caffeine. Compared to caffeine-induced transients without prior in vitro activation, caffeine-induced transients that followed activation by K+ (fig. 6) were larger by 94% in control neurons and by 182% in SNL L4 neurons, whereas activation produced no expansion (0%) of the caffeine-induced transient in axotomized L5 neurons. However, the K+-induced transient in SNL L5 neurons was also smaller (13.0 ± 12.6 × 103 nM•s, n=24) than in control neurons (31.4 ± 27.9 × 103 nM•s, n=40) or SNL L4 neurons (44.1 ± 23.5 × 103 nM•s, n=14), indicating that these groups may have received a greater Ca2+ influx and boost in stores than the SNL L5 population. Application of K+ lasting only 2 s resulted in K+-induced transients in control neurons (10.2 ± 7.2 × 103 nM•s, n=30) that were comparable in area to those produced by 5 s K+ activation in SNL L5 neurons, but these similar Ca2+ loads still showed a greater priming effect in control neurons (28.7 ± 20.1 × 103 nM•s, n=29; 58% greater than baseline caffeine-induced transient) than in axotomized L5 neurons (0%). These observations indicate that the deficit in Ca2+ stores following injury cannot be repaired by activity, and therefore is not due to neuronal disuse.
Figure 6.
Recovery of caffeine-induced Ca2+ release after Ca2+ loading during activation with depolarization by application of K+ (50mM, 5s) compared to transients without prior activation (same data as Fig. 1B). Although a priming Ca2+ load increases releasable Ca2+ in control (C) neurons and fourth lumbar (L4) neurons after spinal nerve ligation (SNL), L5 neurons after SNL show no increased Ca2+ release after priming by activation. Two-way ANOVA with Bonferroni post-hoc testing, numbers in the bars indicate n for number of neurons, brackets above bars connect groups that are significantly different (P<0.05), and error bars show standard deviation. After K+ priming, transient area is reduced in L5 neurons compare to both control and L4 neurons (significance bar not shown).
Direct measurement of [Ca2+]L
Pharmacological control of Ca2+ release, sequestration and extrusion is inevitably imperfect, due to incomplete drug specificity and efficacy. We therefore devised a technique for directly measuring Ca2+ concentration in the subcellular stores using the low-affinity Ca2+ fluorophore mag-Fura-2.31 Although this approach defines the concentration of unbound Ca2+ in the membrane delimited Ca2+ stores rather than their net mass, it can be assumed that the concentration of free luminal Ca2+ is directly related to total stored Ca2+.
We initially determined [Ca2+]L in nonpermeabilized neurons.41 Although such neurons contain mag-Fura-2 in the cytoplasm, the conditions of loading (see methods) were optimized to preferentially accumulate mag-Fura-2 in organelle compartments. Under these conditions, calculated [Ca2+]L was found to be significantly lower in L4 neurons and especially L5 neurons after SNL compared to control (fig. 7A). Caffeine (20mM) was applied to the loaded neurons to validate the location of mag-Fura-2 in the stored Ca2+ compartment. This produced a decrease of calculated [Ca2+]L by a least 10% in 107/131 (82%) of the neurons, although this may be an underestimate due to the influences of caffeine.42 If the principal source of fluorescence emission were in fact cytoplasmic rather than compartmentalized mag-Fura-2, release of Ca2+ stores would have produced an increase in the calculated [Ca2+] value, so these findings confirm that the fluorophore is preferentially located in the compartment from which Ca2+ is released by caffeine.
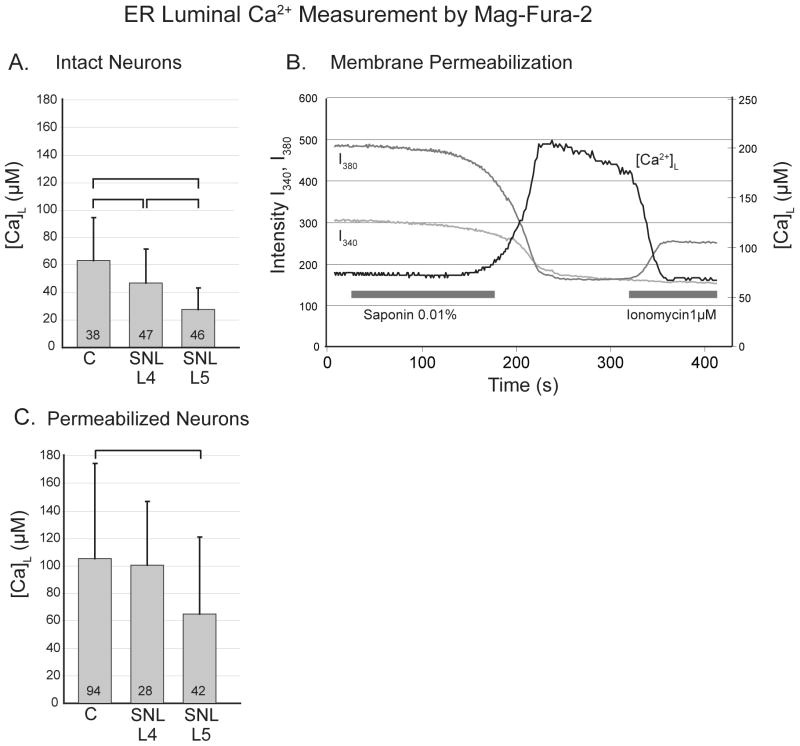
Figure 7.
Determination of the Ca2+ concentration in the endoplasmic reticulum (ER) lumen ([Ca2+]L) using the low affinity fluorophore mag-Fura-2. A. In non-permeabilized neurons, the estimated [Ca2+]L is lower than control in neurons from the fourth lumbar (L4) dorsal root ganglion after spinal nerve ligation (SNL), and lower in L5 neurons than both other groups. B. Application of saponin in a bath solution resembling intracellular solution (free [Ca2+] ∼ 39 nM) allows washout of cytoplasmic mag-Fura-2, indicated by a decrease in intensity of fluorescence emission during both 380nm (I380) and 340nm (I340) excitation. The resulting [Ca2+]L calculated from the I340/I380 ratio increased to a peak indicative of [Ca2+]L. Subsequent application of ionomycin releases Ca2+ from the stores, represented by decreased [Ca2+]L. C. The [Ca2+]L in permeabilized neurons is depressed in axotomized L5 neurons after SNL compared to control. For panels A and C, one-way ANOVA with Bonferroni post-hoc testing was used, numbers in the bars indicate n for number of neurons, brackets above bars connect groups that are significantly different (P<0.05), and error bars show standard deviation.
It has been argued the determination of [Ca2+]L may be vitiated by even small amounts of residual mag-Fura-2 in the relatively low Ca2+ concentration environment of the cytoplasm.28 This view is supported by our observation of [Ca2+]L in these intact neurons that are lower than the range reported by others.43,44 We therefore permeabilized the neurons to provide a more accurate estimation of [Ca2+]L. Using this measurement technique (fig. 7B), [Ca2+]L measurements were higher than in non-permeabilized neurons. A luminal origin of the signal was confirmed by demonstration of decreased [Ca2+]L upon ionomycin application (1μM) in a subset of neurons (fig. 7B), during which [Ca2+]L decreased by 42 ± 16% (n=6, P<0.01). This incomplete equilibration of Ca2+ between stores and cytoplasm may be due to either an equilibration rate slower than our technique could observe, or the existence of sub-compartments that ionomycin failed to affect. Other studies using the same ionomycin concentration have shown comparable levels of store depletion.35,45 Small neurons again showed a marked decrease after SNL in L5 neurons compared to control (fig. 7C). These [Ca2+]L findings, combined with observations on Ca2+ release, reveal a deficit of stored Ca2+ following injury of sensory neurons.
As has been done in other studies,43,44 we included Mg2+ in the bath to maintain a natural intracellular concentration20 following permeabilization. However, entry of bath Mg2+ acting on residual cytoplasmic mag-Fura-2 could contribute to our observed increase of [Ca2+]L during permeabilization, and bias our determination of [Ca2+]L. This is an unlikely explanation, since this would also cause fluorescence intensities to increase during permeabilization, whereas we observed them to decrease. However, to additionally test this factor, permeabilization was performed in a set of control neurons using Mg2+-free bath solution, which produced a [Ca2+]L (132 ± 90 μM, n=28) that was minimally different from experiments in which the bath solution contained Mg2+ (105 ± 69 μM, n=94; P=0.16).
Discussion
The somata of sensory neurons are known to be substantially affected by injury of their peripheral axons.46 Our present findings extend this recognition by showing that a fundamental feature, the intracellular Ca2+ store, is depleted after injury. Specifically, the amount of Ca2+ that can be released from stores through a variety of pathways is diminished after axotomy, and the concentration of Ca2+ in the luminal compartment is also reduced as estimated by direct imaging.
Intracellular Ca2+ stores in neurons reside in several organelles. The best characterized is the ER store, where Ca2+ also critically regulates the luminal processes of protein assembly and folding.47,48 Additional sites of Ca2+ storage include the nuclear envelope49,50 and the Golgi apparatus.51 The contribution of storage in secretory vesicles52 and so-called calciosomes53 remains poorly defined. Recent evidence indicates the dominant site for the Ca2+ pool is in the ER lumen.7 Although subcellular localization may play a role in directing released Ca2+ to particular targets,54 current techniques do not allow these stores to be functionally distinguished.
We employed standard techniques to identify the magnitude of the Ca2+ store.11 Caffeine freely diffuses through the cell membrane and, by activating the RyR, completely discharges Ca2+ from the stores available to this release pathway. Although caffeine interacts with Ca2+ sensitive fluorophores, these effects are minor when using ratiometric indicators such as in the present studies.42,55 Stored Ca2+ measured this way is affected selectively for specific subgroups of neurons. Axotomy by SNL has no effect on caffeine-releasable Ca2+ in large neurons that mostly transduce and transmit low threshold sensory information. However, axotomy substantially depresses caffeine-releasable stores in the small neuron population, especially in capsaicin-sensitive putative nociceptors. Capsaicin non-responsive small neurons, which probably represent a population of nonpeptidergic, isolectin B4-binding mechanosensitive nociceptors,37 also have depleted stores after injury, but are less affected than capsaicin sensitive neurons. Loss of Ca2+ store in only certain neuron subtypes after axotomy may be due to ER damage selectively in these neurons, as is explored in the companion paper.
Sensory neurons possess additional intracellular Ca2+ stores that may be released through activation of an IP3-sensitive channel. There is not full agreement whether the Ca2+ pools sensitive to IP3 and caffeine overlap in peripheral sensory neurons,6,56 although experiments using sequential activation of these pathways mostly support the view that the pools are one and the same.57,58 We did not measure Ca2+ release by IP3 because injury of peripheral sensory neurons directly affects IP3 signaling,59,60 and membrane permeant or caged IP3 compounds are not readily available. We therefore turned to an alternate strategy of discharging stores indiscriminately either by the Ca2+ ionophore ionomycin or by constitutive leak from stores during SERCA inhibition by thapsigargin. Observations using both of these techniques support the findings from caffeine experiments and show an axotomy-induced deficit of Ca2+ in stores that is independent of the release mechanism.
The Ca2+ transient during release of stores is supplemented by Ca2+ influx through SOCCs.26 Therefore, a loss of this capacitative Ca2+ entry process due to injury could create the appearance of a deficit in stores measured by release. However, we observed that the depressant effect of axotomy on store release persists in Ca2+-free bath solution, so this explanation is unlikely. Also, we considered whether neuronal inactivity might have led to store depletion after axotomy. However, cytoplasmic Ca2+ loads provided by activation of voltage-gated Ca2+ channels minimally expanded stores in axotomized neurons compared to control neurons. Together, our findings support the conclusion that the capacity of the intracellular Ca2+ storage reservoir is compromised after axotomy in sensory neurons.
The amount of stored Ca2+ is dictated by the concentration of Ca2+ in the store, [Ca2+]L, the bound Ca2+ in equilibrium with this free fraction, and the extent of the compartment. Therefore, although mag-Fura-2 microfluorimetry reflects [Ca2+]L, it cannot indicate the total mass of stored Ca2+ per se. Although the concentration of the free fraction should be proportionate with the total Ca2+ store, this method cannot account for changes in the amount or performance of various Ca2+ binding proteins in the compartment, which could alter also the mass of stored Ca2+ without changing [Ca2+]L. Our findings with mag-Fura-2 reveal a decrease in resting [Ca2+]L associated with axotomy. Since caffeine produces a fall in [Ca2+]L measured by mag-Fura-2, it is likely that the mag-Fura-2 technique quantifies the state of the same Ca2+ pool as does caffeine-induced release. The combined results of these two approaches point to a model in which axotomy decreases the ability of the storage compartment, predominantly the ER, to concentrate Ca2+ within its lumen. This level of [Ca2+]L is set by the dynamic balance between SERCA and constitutive Ca2+ leakage from the ER.10 Although resting axotomized neurons show no delay in fractional recovery of Ca2+ stores back to their baseline following release, this is nonetheless compatible with compromised SERCA function since baseline store levels are comparatively depleted. We showed that the relationship between constitutive Ca2+ leak rate and Ca2+ store level is normal in axotomized neurons, which points to a normal leak pathway. This leaves compromised SERCA function as the cause of decreased [Ca2+]L, which could be due to altered regulation by kinases.41 Our data do not reveal whether injury also diminishes the extent of the stores, which is addressed in the companion paper.
The effect of painful conditions on neuronal Ca2+ stores has previously been examined only in models of diabetes mellitus. Those studies found that Ca2+ transients induced by ionomycin12 or caffeine61,62 are decreased in diabetic animals, similar to our observations after peripheral nerve trauma. However, a substantially different pathogenic mechanism in diabetes is suggested by differences in other aspects of the Ca2+ signaling pathway in sensory neurons, including an increased resting [Ca2+]c12,61,63,64 and prolonged activity-induced transients,61,62,64 which contrast with decreased resting [Ca2+]c and decreased activity-induced transients after axotomy.1,2,65 Like diabetes, painful peripheral inflammation is associated with elevated resting [Ca2+]c and transients in DRG neurons,14 but the state of the Ca2+ stores in this condition has not been determined.
The SNL model provides the opportunity to separately evaluate neurons in the L5 DRG that are axotomized, versus the adjacent L4 neurons. Following SNL, features of Ca2+ storage and release in these two populations are divergent. In contrast to axotomized L5 neurons, ionomycin release of stored Ca2+ is elevated in L4 neurons, although this may represent increased release from the mitochondrial component of stored Ca2+ released. The L4 neurons also show an expanded Ca2+ storage capacity, as shown by a supranormal level of releasable Ca2+ after cytoplasmic Ca2+ loading. Finally, whereas axotomy diminishes Ca2+ release by caffeine or thapsigargin and decreases [Ca2+]L measured by mag-Fura-2 in permeabilized L5 neurons, these are unaffected in the L4 population. These divergent findings may result from the difference in predominant influences of SNL on the L5 versus L4 neurons. Whereas the SNL results in axotomy of the L5 population, the peripheral axons of the adjacent L4 neurons remain intact but are exposed to inflammatory mediators triggered by Wallerian degeneration of detached L5 axonal segments.15,16,66 Other studies have shown divergent influences on Ca2+ handling in inflammation compared to axotomy. Specifically, models of inflammatory pain reveal increased activity-induced Ca2+ transients in DRG neurons,14 whereas the opposite occurs after axotomy.2 Our present findings also suggest that Ca2+ stores are also differentially regulated by axotomy and inflammation. A degree of inconsistency in our L4 findings may be attributable to a variable admixture of axotomy among the L4 neuronal population following L5 SNL.67
Disruption of the level of Ca2+ stores in neuronal subcellular compartments is a fundamental pathogenic factor in a variety of diseases including ischemia, acquired immunodeficiency syndrome, and Alzheimer's disease.6 In addition to storing Ca2+, the ER is the site assembly and post-translational modification of proteins by glycosylation and folding. Depletion of luminal Ca2+ triggers highly conserved stress responses involving accumulation of unfolded protein, global suppression of protein synthesis, and activation of a variety of transcription factors, resulting in neuronal dysfunction and death.68 For instance, Ca2+ store depletion by caffeine or thapsigargin exposure suppresses protein synthesis and induces neuronal apoptosis independent of increased [Ca2+]c.69,70 The present findings suggest that similar processes may take place in sensory neurons after peripheral nerve injury, and that they may contribute to long-term changes associated with chronic pain.
Acknowledgments
a) grant NS-42150 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland to QH; b) Erwin Schroedinger Fellowship from the Austrian Science Fund, Vienna, Austria (project J2695) to MR.
Footnotes
(http://brneurosci.org/egta.html), last accessed April 18th, 2009
References
- 1.Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102:1217–25. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs A, Rigaud M, Hogan QH. Painful nerve injury shortens the intracellular Ca2+ signal in axotomized sensory neurons of rats. Anesthesiology. 2007;107:106–16. doi: 10.1097/01.anes.0000267538.72900.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86:43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- 4.McCallum JB, Kwok WM, Mynlieff M, Bosnjak ZJ, Hogan QH. Loss of T-type calcium current in sensory neurons of rats with neuropathic pain. Anesthesiology. 2003;98:209–16. doi: 10.1097/00000542-200301000-00032. [DOI] [PubMed] [Google Scholar]
- 5.McCallum JB, Kwok WM, Sapunar D, Fuchs A, Hogan QH. Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons. Anesthesiology. 2006;105:160–8. doi: 10.1097/00000542-200607000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–79. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht MA, Colegrove SL, Hongpaisan J, Pivovarova NB, Andrews SB, Friel DD. Multiple modes of calcium-induced calcium release in sympathetic neurons I: Attenuation of endoplasmic reticulum Ca2+ accumulation at low [Ca2+](i) during weak depolarization. J Gen Physiol. 2001;118:83–100. doi: 10.1085/jgp.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 9.Usachev YM, Thayer SA. Ca2+ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive Ca2+ stores. J Physiol. 1999;519(Pt 1):115–30. doi: 10.1111/j.1469-7793.1999.0115o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mogami H, Tepikin AV, Petersen OH. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17:435–42. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992;450:217–46. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N. Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch. 2004;448:395–401. doi: 10.1007/s00424-004-1263-8. [DOI] [PubMed] [Google Scholar]
- 13.Medina I, Ghose S, Ben-Ari Y. Mobilization of intracellular calcium stores participates in the rise of [Ca2+]i and the toxic actions of the HIV coat protein GP120. Eur J Neurosci. 1999;11:1167–78. doi: 10.1046/j.1460-9568.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu SG, Gold MS. Inflammation-induced increase in evoked calcium transients in subpopulations of rat dorsal root ganglion neurons. Neuroscience. 2008;153:279–88. doi: 10.1016/j.neuroscience.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: Differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–62. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- 16.Ramer MS, French GD, Bisby MA. Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain. 1997;72:71–8. doi: 10.1016/s0304-3959(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 17.Gold MS. Spinal nerve ligation: What to blame for the pain and why. Pain. 2000;84:117–20. doi: 10.1016/s0304-3959(99)00309-7. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 19.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–87. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Henrich M, Buckler KJ. Effects of anoxia, aglycemia, and acidosis on cytosolic Mg2+, ATP, and pH in rat sensory neurons. Am J Physiol Cell Physiol. 2008;294:C280–94. doi: 10.1152/ajpcell.00345.2007. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 22.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–24. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friel DD. Mitochondria as regulators of stimulus-evoked calcium signals in neurons. Cell Calcium. 2000;28:307–16. doi: 10.1054/ceca.2000.0172. [DOI] [PubMed] [Google Scholar]
- 24.Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramov AY, Duchen MR. Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium. 2003;33:101–12. doi: 10.1016/s0143-4160(02)00203-8. [DOI] [PubMed] [Google Scholar]
- 26.Kwan CY, Takemura H, Obie JF, Thastrup O, Putney JW., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990;258:C1006–15. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- 27.Hofer AM, Schlue WR, Curci S, Machen TE. Spatial distribution and quantitation of free luminal [Ca] within the InsP3-sensitive internal store of individual BHK-21 cells: Ion dependence of InsP3-induced Ca release and reloading. Faseb J. 1995;9:788–98. doi: 10.1096/fasebj.9.9.7601343. [DOI] [PubMed] [Google Scholar]
- 28.Hofer AM, Schulz I. Quantification of intraluminal free [Ca] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: Some considerations. Cell Calcium. 1996;20:235–42. doi: 10.1016/s0143-4160(96)90029-9. [DOI] [PubMed] [Google Scholar]
- 29.Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–23. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- 30.Raju B, Murphy E, Levy LA, Hall RD, London RE. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989;256:C540–8. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- 31.Solovyova N, Verkhratsky A. Monitoring of free calcium in the neuronal endoplasmic reticulum: An overview of modern approaches. J Neurosci Methods. 2002;122:1–12. doi: 10.1016/s0165-0270(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 32.Neering IR, McBurney RN. Role for microsomal Ca storage in mammalian neurones? Nature. 1984;309:158–60. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- 33.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–44. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- 34.Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–75. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 35.Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: A study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J Cell Biol. 1998;140:325–34. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience. 1993;57:845–59. doi: 10.1016/0306-4522(93)90029-f. [DOI] [PubMed] [Google Scholar]
- 37.Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–24. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- 38.Holzer P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 39.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–54. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shmigol A, Kostyuk P, Verkhratsky A. Dual action of thapsigargin on calcium mobilization in sensory neurons: Inhibition of Ca2+ uptake by caffeine-sensitive pools and blockade of plasmalemmal Ca2+ channels. Neuroscience. 1995;65:1109–18. doi: 10.1016/0306-4522(94)00553-h. [DOI] [PubMed] [Google Scholar]
- 41.Usachev YM, Marsh AJ, Johanns TM, Lemke MM, Thayer SA. Activation of protein kinase C in sensory neurons accelerates Ca2+ uptake into the endoplasmic reticulum. J Neurosci. 2006;26:311–8. doi: 10.1523/JNEUROSCI.2920-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muschol M, Dasgupta BR, Salzberg BM. Caffeine interaction with fluorescent calcium indicator dyes. Biophys J. 1999;77:577–86. doi: 10.1016/S0006-3495(99)76914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solovyova N, Fernyhough P, Glazner G, Verkhratsky A. Xestospongin C empties the ER calcium store but does not inhibit InsP3-induced Ca2+ release in cultured dorsal root ganglia neurones. Cell Calcium. 2002;32:49–52. doi: 10.1016/s0143-4160(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 44.Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. Ca(2+) dynamics in the lumen of the endoplasmic reticulum in sensory neurons: Direct visualization of Ca(2+)-induced Ca(2+) release triggered by physiological Ca(2+) entry. Embo J. 2002;21:622–30. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan AJ, Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994;300(Pt 3):665–72. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devor M. In: Response of nerves to injury in relation to neuropathic pain, Wall and Melzack's Textbook of Pain. 5th. McMahon S, Koltzenburg M, editors. London: Churchill Livingston; 2006. pp. 905–27. [Google Scholar]
- 47.Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: Molecular, structural and functional properties. Prog Neurobiol. 2001;65:309–38. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 48.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–78. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 49.Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signalling. J Cell Sci. 2004;117:3087–94. doi: 10.1242/jcs.01295. [DOI] [PubMed] [Google Scholar]
- 50.Petersen OH, Gerasimenko OV, Gerasimenko JV, Mogami H, Tepikin AV. The calcium store in the nuclear envelope. Cell Calcium. 1998;23:87–90. doi: 10.1016/s0143-4160(98)90106-3. [DOI] [PubMed] [Google Scholar]
- 51.Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. Embo J. 1998;17:5298–308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen OH. Can Ca2+ be released from secretory granules or synaptic vesicles? Trends Neurosci. 1996;19:411–3. [PubMed] [Google Scholar]
- 53.Rossier MF, Putney JW., Jr The identity of the calcium-storing, inositol 1,4,5-trisphosphate-sensitive organelle in non-muscle cells: Calciosome, endoplasmic reticulum … or both? Trends Neurosci. 1991;14:310–4. doi: 10.1016/0166-2236(91)90143-i. [DOI] [PubMed] [Google Scholar]
- 54.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 55.Nohmi M, Hua SY, Kuba K. Basal Ca2+ and the oscillation of Ca2+ in caffeine-treated bullfrog sympathetic neurones. J Physiol. 1992;450:513–28. doi: 10.1113/jphysiol.1992.sp019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thayer SA, Perney TM, Miller RJ. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988;8:4089–97. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crawford JH, Wootton JF, Seabrook GR, Scott RH. Activation of Ca2+-dependent currents in dorsal root ganglion neurons by metabotropic glutamate receptors and cyclic ADP-ribose precursors. J Neurophysiol. 1997;77:2573–84. doi: 10.1152/jn.1997.77.5.2573. [DOI] [PubMed] [Google Scholar]
- 58.Solovyova N, Verkhratsky A. Neuronal endoplasmic reticulum acts as a single functional Ca2+ store shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurones. Pflugers Arch. 2003;446:447–54. doi: 10.1007/s00424-003-1094-z. [DOI] [PubMed] [Google Scholar]
- 59.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: Effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–68. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 60.Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–60. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 61.Huang TJ, Sayers NM, Fernyhough P, Verkhratsky A. Diabetes-induced alterations in calcium homeostasis in sensory neurones of streptozotocin-diabetic rats are restricted to lumbar ganglia and are prevented by neurotrophin-3. Diabetologia. 2002;45:560–70. doi: 10.1007/s00125-002-0785-x. [DOI] [PubMed] [Google Scholar]
- 62.Li F, Obrosova IG, Abatan O, Tian D, Larkin D, Stuenkel EL, Stevens MJ. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 2005;288:E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 63.Kostyuk E, Svichar N, Shishkin V, Kostyuk P. Role of mitochondrial dysfunction in calcium signalling alterations in dorsal root ganglion neurons of mice with experimentally-induced diabetes. Neuroscience. 1999;90:535–41. doi: 10.1016/s0306-4522(98)00471-0. [DOI] [PubMed] [Google Scholar]
- 64.Kostyuk E, Voitenko N, Kruglikov I, Shmigol A, Shishkin V, Efimov A, Kostyuk P. Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons. Diabetologia. 2001;44:1302–9. doi: 10.1007/s001250100642. [DOI] [PubMed] [Google Scholar]
- 65.Fuchs A, Rigaud M, Sarantopoulos CD, Filip P, Hogan QH. Contribution of calcium channel subtypes to the intracellular calcium signal in sensory neurons: the effect of injury. Anesthesiology. 2007;107:117–27. doi: 10.1097/01.anes.0000267511.21864.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheth RN, Dorsi MJ, Li Y, Murinson BB, Belzberg AJ, Griffin JW, Meyer RA. Mechanical hyperalgesia after an L5 ventral rhizotomy or an L5 ganglionectomy in the rat. Pain. 2002;96:63–72. doi: 10.1016/s0304-3959(01)00429-8. [DOI] [PubMed] [Google Scholar]
- 67.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–92. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 2001;29:1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- 69.Wei H, Wei W, Bredesen DE, Perry DC. Bcl-2 protects against apoptosis in neuronal cell line caused by thapsigargin-induced depletion of intracellular calcium stores. J Neurochem. 1998;70:2305–14. doi: 10.1046/j.1471-4159.1998.70062305.x. [DOI] [PubMed] [Google Scholar]
- 70.Doutheil J, Gissel C, Oschlies U, Hossmann KA, Paschen W. Relation of neuronal endoplasmic reticulum calcium homeostasis to ribosomal aggregation and protein synthesis: implications for stress-induced suppression of protein synthesis. Brain Res. 1997;775:43–51. doi: 10.1016/s0006-8993(97)00899-8. [DOI] [PubMed] [Google Scholar]