Abstract
Background
Poor fear conditioning characterizes adult psychopathy and criminality, but it is not known whether it is related to aggressive/antisocial behavior in early childhood.
Methods
Using a differential, partial reinforcement conditioning paradigm, electrodermal activity was recorded from 200 male and female children at ages 3, 4, 5, 6, and 8 years. Antisocial/aggressive and hyperactive-inattentive measures were collected at age 8, while social adversity was assessed at age 3.
Results
Poor electrodermal fear conditioning from ages 3 to 8 years was associated with aggressive behavior at age 8 in both males and females.
Conclusions
Results indicate that the relationship between poor fear conditioning and aggression occurs early in childhood. Enhanced electrodermal fear conditioning may protect children against future aggressive/violent behavior. Abnormal amygdala functioning, as indirectly assessed by fear conditioning, may be one of the factors influencing the development of childhood aggression.
Keywords: Fear conditioning, Child, Development, Aggression, Electrodermal
Although deficits in fear conditioning have been found in adult psychopaths and criminals (Hare, 1978), little is known about how the development of conditioning is related to aggressive and disruptive behavior in childhood. To date only two prospective studies have been conducted, showing that poor classical conditioning at age 15 years is associated with both later delinquency at age 25 (Loeb & Mednick, 1977) and also crime by age 29 (Raine, Venables, & Williams, 1996). The poor fear conditioning – aggressive/antisocial relationship may reflect a stable, trait-like risk factor for later aggressive and antisocial behavior. Alternatively, it may reflect a neurodevelopmental abnormality whereby children who later become aggressive/antisocial show poor early fear conditioning, and also fail to manifest the age-related increase in conditioning found in normal children (Gao, Raine, Venables, Dawson, & Mednick, in press a). To date, there have been no developmental tests of these competing predictions. In the current study, these two hypotheses were tested using a longitudinal design.
It is unclear whether poor fear conditioning is specific to aggression or can also be observed for non-aggressive antisocial behavior. Studies have reported large body size, stimulation-seeking, fearlessness (Raine, Reynolds, Venables, Mednick, & Farrington, 1998), autonomic hyporeactivity (Babcock, Green, Webb, & Yerington, 2005; Raine, Venables, & Mednick, 1997), maternal smoking during pregnancy (Räsännen et al., 1999), and birth complications combined with early maternal rejection (Raine, Brennan, & Mednick, 1994) as risk factors for aggressive and violent, but not non-violent, antisocial behavior. Given that reduced fear conditioning is associated with antisocial behavior in adults and that childhood aggression has been found to be one of the best predictors for later aggressive, antisocial, and criminal behavior (Farrington, 1991; Huesmann, Eron, Lefkowitz, & Walder, 1984; Moffitt, 1990), it was hypothesized that reduced conditioning would be a specific correlate of childhood aggression. In contrast, relatively few studies have differentiated types of aggression/violence in their designs (Scarpa & Raine, 1997) or examined the physiological underpinnings of non-aggressive antisocial behavior. In the current study the association between fear conditioning and both non-aggressive and aggressive forms of antisocial behavior are explored.
Another unanswered question concerns whether the same psychophysiology – aggressive/antisocial behavior association is present in both males and females. Moffitt and colleagues (Moffitt & Caspi, 2001; Moffitt, Caspi, Rutter, & Silva, 2001) argue that the causes of life-course persistent antisocial behavior in females and males are essentially the same. However, other studies have argued for a significant sex difference (Silverthorn, Frick, & Reynolds, 2001) and several genetic studies have suggested a sex difference in gene -environment interactions predicting antisocial behavior (Langbehn, Cadoret, Yates, Troughton, & Stewarts, 1998). In the current study sex differences in the association between deficits in fear conditioning and aggressive/antisocial behavior were examined to test between these different perspectives.
Although the poor fear conditioning hypothesis of aggressive and antisocial behavior makes the prediction that those with poor fear conditioning should be aggressive/antisocial, very few if any studies have identified groups high or low in fear conditioning. Instead, almost all studies take a clinical grouping approach in which, for example, psychopaths are compared to non-psychopaths (Flor, Birbaumer, Hermann, Ziegler, & Patrick, 2002). Although this is understandably necessitated by the clinical design of most studies, an optimal design would be a community sample tested on conditioning, and grouped according to both their conditioning status and also their aggression status. Both analytic strategies were adopted here to assess the robustness of the autonomic fear conditioning – aggression association.
Given the significant comorbidity between aggressive behavior and attention-deficit/hyperactivity disorder (ADHD, American Psychiatric Association, 2002), it is of value to know whether hyperactivity-inattention is also associated with poor fear conditioning. Few studies have examined this association in children (Pliszka, Hatch, Borcherding, & Rogeness, 1993). Pliszka et al. compared 6–12-year-old children with ADHD and normal controls and reported no group differences in their electrodermal conditioned responses. It was therefore hypothesized that fear conditioning from ages 3 to 8 years would be unrelated to hyperactivity-inattention.
We previously found that electrodermal fear conditioning increases from ages 3 to 8 years (Gao et al., in press a). In the current study, latent class analyses were conducted on this sample to identify homogeneous groups based on conditioned responses across ages in relation to aggression. We hypothesized that: (1) compared to good conditioners, poor conditioners across ages 3 to 8 years would score higher on aggressive behavior at age 8; (2) the high aggression group would show poor electrodermal fear conditioning from ages 3 to 8 years; (3) no group differences would be observed for hyperactivity-inattention; and (4) no gender difference would be found in any observed fear conditioning - aggression associations. Finally, the association between fear conditioning and non-aggressive antisocial behavior is explored in the current study.
Method
Participants
Participants were drawn from a cohort of 1,795 children from the African country of Mauritius, a tropical island in the Indian Ocean (Gao et al., in press a; Venables, 1978). All children born in 1969–1970 in two towns were recruited at age 3 years. The ethnic makeup was as follows: 68.7% Indian, 25.6% African, and 5.6% other (Chinese, English, or French descent). Females made up 45.9% of the sample. Two hundred children (100 boys and 100 girls) were selected from the original sample and psychophysiological measures taken repeatedly at ages 3, 4, 5, 6, and 8 years. Informed consent was obtained from the parents of the children, and the research was approved by the institutional review board. Participants and non-participants did not differ on sex, χ2 (1) = 0.490, p = .488, and ethnicity, χ2 (4) = 0.465, p = .501.
Experimental Design
The conditioning paradigm consisted of a set of 12 tones (9 CS+ and 3 CS−). The CS+ tone was reinforced by a loud noise UCS, and a different tone served as the un-reinforced CS−. A 66% partial-reinforcement schedule was used, with 6 of the 9 presentations of the CS+ randomly reinforced. The CS+ was a 1000 Hz, 60 dB, 12.5 sec tone with rise and fall time of 25 msec whereas CS− was a 500 Hz, 60 dB, 12.5 sec tone with rise and fall time of 25 msec. The UCS was an auditory stimulus of 90 dB intensityand 4.5 sec duration recorded from white noise played in a tin can with metal jangling objects (e.g., keys), thus adding low and high frequency components to the stimulus. The ISI was 10 s (onset to onset), with a randomized ITI (inter-trial interval) of 38 s (range 34–42 s). Three un-reinforced CS+/CS− pairs (trial 3/4, 7/8, and 10/12) with complete electrodermal activity components (see below) were used in the current study.
Electrodermal Activity Recording and Data Reduction
Electrodermal data were collected using a Grass Type 79 polygraph with a constant voltage system, Beckman miniature silver/silver chloride electrodes, and an electrolyte consisting of 0.5% KCl in 2% agar–agar. Electrodermal activity was recorded from the medial phalanges of the index and middle fingers of the left hand.
Three types of classically conditioned responses (Dawson & Schell, 1987; Prokasy & Ebel, 1967) were scored: (a) the first interval response (FIR), which was elicited by the CS, with a latency of 1 to 3 s after CS onset, (b) the second interval response (SIR) which was elicited prior to the UCS, with a latency between 6 and 10.5 s after CS onset, and (c) the third interval response (TIR) starting at 11 and ending at 13 s after CS onset on non-reinforced trials. Responses greater than 0.05 μS falling within the above latency windows were scored. To create a measure of conditioning, difference scores were calculated in which responses to the CS− were subtracted from those to the CS+ for the FIR, SIR, and TIR, respectively. Difference scores of zero or less reflect no differential learning, and difference scores above zero reflect greater responses to the CS+ relative to the CS−.1 A global measure of conditioning was formed by averaging the above three components (FIR, SIR, and TIR).
Mean magnitudes of the conditioned responses were recorded at ages 3, 4, 5, 6, and 8 years (psychophysiological data at age 7 were lost). A square root transformation was used before conducting inferential statistical analyses to help attain normality (Dawson, Schell, & Filion, 2007; Venables & Christie, 1980).
Antisocial/Aggressive and Hyperactive-Inattentive Assessment
Antisocial behavior and hyperactivity-inattention were assessed at age 8 years by teachers using the Children’s Behavior Questionnaire (CBQ) (Rutter, 1967; Venables et al., 1983). On average, the teacher had known the children for six months by the time of assessment. The CBQ was originally developed by Rutter to differentiate groups of antisocial, neurotic, and control children (Rutter, 1967). Since then, attempts have been made to examine the factor structure of the CBQ in clinical and normal school age children (Behar & Stringfield, 1974; Fowler & Park, 1979; Venables et al., 1983). Following previous studies (Raine, Yaralian, Reynolds, Venables, & Mednick, 2002), aggressive and non-aggressive antisocial behavior subscale data were used in the current study. In addition, to test the hypothesis on hyperactivity-inattention, three hyperactive-inattentive items from the CBQ were also included. A confirmatory factor analysis conducted using EQS (Multivariate Software, Encino, California) was used to test a three-factor model (aggressive antisocial, non-aggressive antisocial, and hyperactive-inattentive factors) for the overall sample, given that prior studies have shown similar factor structure between male and female groups (Venables et al., 1983). A good fit was found for this model (root mean square error approximation (RMSEA) = .055, non-normed fit index (NNFI) = .946, and comparative fit index (CFI) = .960). The aggressive antisocial subscale had a coefficient alpha of 0.79 and was comprised of the following four items: “often destroys own or others belongs,” “frequently fights with other children,” “irritable and quick to ‘fly off the handle’,” and “bullies other children.” The non-aggressive antisocial subscale had an alpha of 0.67 and was comprised of the following four items: “truants from school,” “is often disobedient,” “often tells lies,” and “has stolen things on one or more occasions.” The hyperactive-inattentive subscale had an alpha of 0.50 and consisted of three items: “very restless; often running about or jumping up and down; hardly ever still,” “squirmy, fidgety child,” and “has poor concentration or short attention span.” Among the sample of 200, complete behavioral measures were available on 143 children (73 males and 70 females); therefore the psychophysiology – aggressive/antisocial/hyperactive-inattentive association and potential moderating effects of sex were tested on this subgroup. Analyses indicated no sex differences between this subgroup and the 57 subjects with incomplete behavioral data, χ2 (1) = 0.129, p = .719. However, compared to the 57 non-participants, participants consisted of more Creole and fewer Moslem and Tamil children, χ2 (4) = 16.833, p < .001. Finally, anxiety/fearfulness was assessed using the CBQ (Venables et al., 1983) to address whether any conditioning–aggressive/antisocial/hyperactive-inattentive associations are moderated by anxiety.
Statistical Analyses
Latent Class Growth Analyses
Latent class growth analyses (LCGA) was conducted on the conditioned responses from ages 3 to 8 years to identify homogeneous clusters of individual trajectories with different estimated parameters (Nagin, 1999). Analyses were conducted in the following four steps. First, a latent growth curve model was tested in which one initial level (μ0i, indicating the individual’s initial level of conditioning), one slope (μ1i, indicating the individual’s linear change of conditioning over time), a correlation coefficient (ρ0si, a correlation between initial level and slope), a set of basis coefficients that define the timing of shape of the trajectory over time, variance (σ0i2 and σ1i2, for initial level and slope, respectively), covariance between level and slope (σ0si), and unique variance (σei2) terms (i is the number of groups) were estimated for the overall sample (n = 200). Second, a two-class model was estimated in which two homogeneous groups with different parameters between the groups were assumed. Three- and four- class models were then estimated in a similar way. Akaike information criterion (AIC), Bayesian information criterion (BIC), and adjusted BIC were used to evaluate models. The model with the smaller AIC, BIC, or adjusted BIC was accepted within model comparisons, together with the relative size of each resulting profile so that, ideally, no one class held less than approximately 5% of the total sample.
After the optimal number of latent classes was determined, multiple group latent growth analyses were conducted to compare the estimated parameters between classes (McArdle, 2006). Starting with an invariant model in which the same parameter values were restrained to be equal between classes, progressive relaxations were then made to improve the model fit based on the following sequence: 1) latent means (μ0i and μ1i), 2) latent variances and covariances (σ0i2, σ1i2, σ0si), 3) latent basis coefficients, and 4) the unique variances (σei2). Since these models were nested, χ2 was used to compare model fit between steps.
Data were analyzed using Version 2 of Mplus (Muthén & Muthén, 1998). One benefit of its use is a maximum likelihood estimation procedure that uses all observations within the data set, including those with data missing at one or more waves. This reduces the sampling bias that occurs in approaches such as listwise or pairwise deletion that excludes participants with incomplete data.
Biological Approach
Once the optimal number of latent classes (e.g., conditioning groups) was established, aggressive, non-aggressive antisocial, and hyperactive-inattentive measures were compared between the conditioning groups using multivariate analysis of variance (MANOVA). An N (number of conditioning groups) × 2 (sex) MANOVA with aggressive, non-aggressive antisocial, and hyperactive-inattentive scores as dependent measures was conducted.
Clinical Approach
Following procedures of previous studies (Raine et al., 1998; Raine et al., 1997; Raine et al., 2002), high- (n = 25) and low-scoring (n = 93) aggressive antisocial groups were created using a cutoff of as close as possible to 1 SD above and below the mean on the aggressive antisocial subscale. Similarly, high- and low- scoring non-aggressive antisocial (n = 25 and 97) and hyperactive-inattentive groups (n = 25 and 42) were formed using the same criterion. A 2 (antisocial group) × 2 (sex) repeated measures analysis variance (ANOVA) with Age as a within-subject factor was conducted for aggressive antisocial, non-aggressive antisocial, and hyperactive-inattentive measures, respectively. Finally, Cohen’s d (Cohen, 1988) was reported for effect sizes of group differences.
Results
Descriptive Statistics
Males and females did not differ on electrodermal fear conditioning across ages, F (1, 168) = 0.075, p = .784. Compared to females, males scored significantly higher on aggressive, t (141) = 2.323, p = .022, d = 0.391, and non-aggressive antisocial behavior scales, t (141) = 2.222, p = .028, d = 0.797, but not on the hyperactive-inattentive scale, t (141) = 1.546, p =.124, d = 0.261. The means, standard deviations, and correlations between study variables are listed in Table 1. Specifically, anxiety/fearfulness was not significantly correlated with conditioning at each age or with the overall conditioning measure, and therefore was not included in the following analyses.
Table 1.
Means, Standard Deviations, Sample Sizes, and Correlations between Study Variables
| Mean | SD | N | CC 3 | CC 4 | CC 5 | CC 6 | CC 8 | CC | Agg. | Non-agg. | Hyper. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC 3 (in MicroSiemens) | 0.065 | 0.071 | 200 | 1 | ||||||||
| CC 4 | 0.058 | 0.077 | 198 | .096 | 1 | |||||||
| CC 5 | 0.068 | 0.067 | 194 | .211** | .142* | 1 | ||||||
| CC 6 | 0.094 | 0.083 | 185 | .079 | .122 | .035 | 1 | |||||
| CC 8 | 0.100 | 0.077 | 182 | .134 | .076 | .091 | .361** | 1 | ||||
| CC | 0.080 | 0.043 | 182 | 0.534*** | 0.553*** | 0.510*** | 0.651*** | 0.660*** | 1 | |||
| Agg. | 0.700 | 1.207 | 143 | −.098 | −.081 | −.105 | −.155 | −.130 | −0.194* | 1 | ||
| Non-agg. | 0.631 | 1.174 | 143 | .010 | −.038 | −.079 | −.115 | −.055 | −0.119 | .494*** | 1 | |
| Hyper. | 1.403 | 1.228 | 143 | −.103 | .028 | −.189* | −.069 | −.042 | −0.130 | .409*** | .361*** | 1 |
| Anx./Fear. | 2.769 | 2.556 | 143 | −.027 | −.030 | −.051 | −.115 | .017 | −.074 | .207* | .191* | .436*** |
Note. CC 3 – 8 = Classical conditioning at ages 3 to 8, CC = Average classical conditioning across ages, Agg. = Aggressive antisocial behavior, Non-agg. = Non-aggressive antisocial behavior, Hyper. = Hyperactivity- inattention, Anx./Fear. = Anxiety/Fearfulness.
p < .05,
p < .01,
p < .001.
Good and Poor Conditioning Groups
Latent Class Growth Analyses
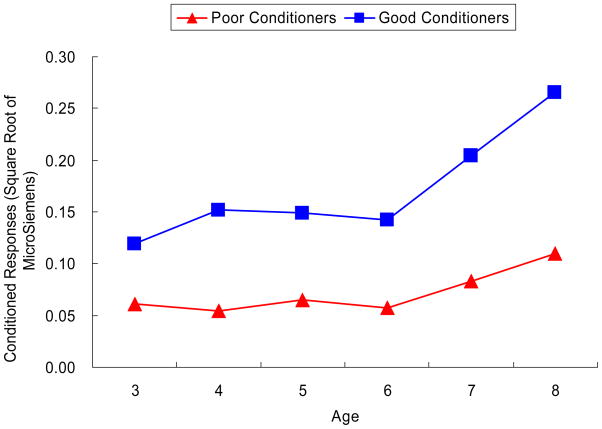
Model evaluations are displayed in Table 2. In comparing model fit among the one-, two-, and three-class models2, a two-class model had the smallest AIC, BIC, and adjusted BIC and was thus selected. For descriptive purposes the latent classes were labeled as “good conditioners” (n = 39, 19.5% of the sample) and “poor conditioners” (n = 161).
Table 2.
Fit of the Latent Class Growth Analysis Models with Different Classes
| Test | C1 | C2 | C3 |
|---|---|---|---|
| AIC | −2323.430 | −2431.309 | −2429.513 |
| BIC | −2293.479 | −2381.391 | −2377.145 |
| Adjusted BIC | −2321.995 | −2428.917 | −2420.875 |
Note. C1 = One-class model; C2 = Two-class model; C3 = Three-class model. AIC = Akaike information criterion; BIC = Bayesian information criterion.
Results of the multiple group latent growth analyses are listed in Table 3. First, an invariant model with equality constraints imposed on all parameters for good and poor conditioners was fitted (M0). Second, when the equality of initial level (μ0i) between groups was relaxed (M1), a significant χ2 change compared to M0 was observed, Δχ2= 128.495, Δdf = 1, p < .01. Third, the relaxation on slope (μ1i) led to a better fit compared to M1, Δχ2= 4.926, Δdf = 1, p < .05. When the variance/covariance (M3), basis coefficients (M4), and error variances (M5) were relaxed sequentially, model fit substantially improved in comparison to the preceding model (for each step, Δχ2> 17, p < .01; see Table 3). Therefore, based on the best-fitting model (M5), good and poor conditioners showed different trajectories of conditioning across ages (i.e., the good conditioners showed higher initial levels of conditioning and a stronger increase in conditioning across ages). The parameter estimations for each group are as follows: for good conditioners, μ01 = 0.119, SE = 0.010, p < .01, μ11 = 0.147, SE = 0.050, p < .05, ρ0s = −.001, SE = .005, p > .05, σ02 < .001, σs2 < .001, σe2 = .014, SE = .002, p < .01; for poor conditioners μ02 = 0.061, SE = 0.004, p < .01, μ12 = 0.049, SE = 0.013, p < .05, ρ0s = −.003, SE = .001, p < .05, σ02 < .001, σs2 = .021, SE = .004, p < .05, σe2 = .003, SE < .001, p < .01. Predicted developmental trajectories of fear conditioning for each class based on the final model are shown in Figure 1.
Table 3.
Summary of Sequential Goodness-of-Fit for Multiple Group Latent Growth Models
| Model | Step | df | χ2 | Δdf | Δχ2 |
|---|---|---|---|---|---|
| M0 | Invariance | 31 | 406.871 | - | - |
| M1 | +Free μ0 | 30 | 278.376 | 1 | 128.495** |
| M2 | +Free μ1 | 29 | 273.450 | 1 | 4.926* |
| M3 | +Free σ2 | 26 | 250.136 | 3 | 23.314** |
| M4 | +Free coefficients | 23 | 232.970 | 3 | 17.166** |
| M5 | + Free σe2 | 22 | 107.209 | 1 | 125.761** |
Note.
p < .05,
p < .01.
Figure 1.
Predicted developmental profiles of electrodermal conditioned responses for good and poor conditioners.
Among the 143 subjects with complete behavioral data, 32 children were “good conditioners” and 111 were “poor conditioners”. Good and poor conditioners did not differ on sex, χ2 (1) = 0.287, p = .592.
Poor and Good Conditioning Groups – Biological Approach
The 2 (conditioning group) × 2 (sex) MANOVA on the three behavioral variables revealed a significant main effect of conditioning group, F (1, 133) = 3.472, p < .05. No other significant main effect or interaction was found, F < 1.747, p > .250.
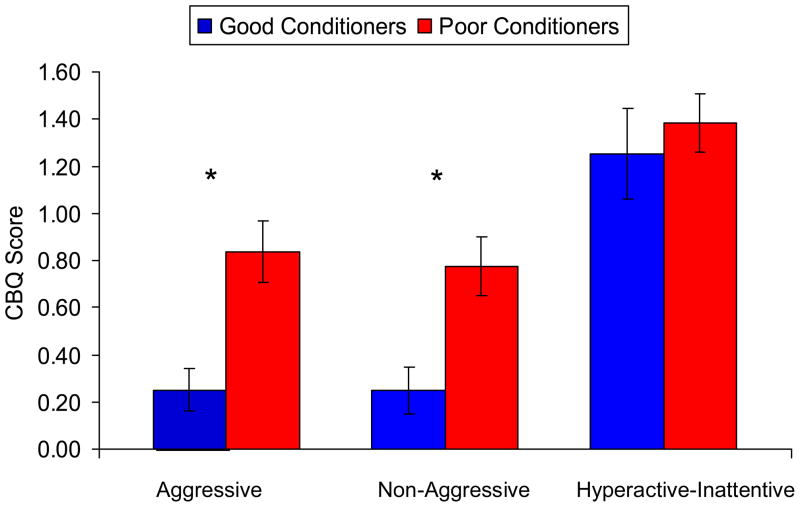
Univariate analyses revealed that poor conditioners scored significantly higher on aggressive antisocial behavior (see Figure 2), F (1, 135) = 5.648, p < .05, d = 0.572, and non-aggressive antisocial behavior, F (1, 135) = 4.570, p < .05, d = 0.518. However, no group difference was found on the hyperactive-inattentive score, F (1, 135) = 0.454, p = .501, d = 0.078.
Figure 2.
Good conditioners showed significantly lower aggressive and non-aggressive antisocial scores compared to poor conditioners (indicated by *), but not on hyperactive-inattentive score.
Low and High Antisocial/Hyperactive-Inattentive Groups – Clinical Approach
All repeated measures ANOVAs on the dependent conditioning measures showed significant main effects of age, all F > 3.206, p < .05, indicating a change in fear conditioning across ages. No group × age interaction was significant for any of the three behavioral measures, all F < 0.579, p > .679.
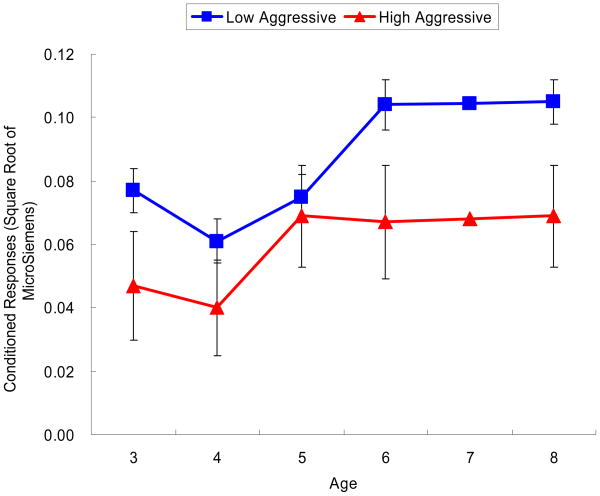
Compared to low aggressive children, high aggressive children showed significantly lower combined conditioned responses across all ages, F (1, 114) = 7.319, p < .01. The effect sizes from ages 3 to 8 years ranged from 0.194 (age 3) to 0.563 (age 6). There were no main effects or interactions involving sex, all F < 2.110, p > .085. The electrodermal conditioned responses from ages 3 to 8 years for aggressive groups are displayed in Figure 3.
Figure 3.
High aggressive children showed lower electrodermal responses across ages than the low aggressive children.
High and low non-aggressive antisocial children did not differ in the measure of electrodermal conditioned responses, F (1, 118) = 2.105, p = .149. The effect sizes from ages 3 to 8 years ranged from 0 (age 4) to 0.314 (age 6). No interaction effects involving sex were significant, all F < 2.210, p > .072. Fear conditioned responses were not different between high and low hyperactive-inattentive children, F (1, 63) = 1.368, p = .247. The effect sizes ranged from 0 (age 4) to 0.321 (age 5) across ages. No interaction effects involving sex were significant, all F < 2.029, p > .103.
Finally, it is possible that group differences could be a function of general overall responsivity to stimuli. We tested this by comparing clinical groups on the overall size of electrodermal responses (i.e., the average of CS+ and CS−). No group differences were found at any age (F < 1.7, p > .18), or across ages (F < 1.4, p > .15).
Discussion
The key findings from this longitudinal study are that poor electrodermal fear conditioning from ages 3 to 8 years is associated with aggression at age 8 years. Two different designs examining the fear conditioning - aggression relationships indicate a robust and bidirectional effect: individuals with good conditioning from ages 3 to 8 years are less aggressive at age 8, and also less-aggressive children are better conditioners. To our knowledge, only one prior study has demonstrated such bidirectionality in the relationship between a biological risk factor (low resting heart rate) and aggression in children (Raine et al., 1997). Moreover, the observed conditioning – aggression relationship applies to both males and females and could not be accounted for by the personality traits of anxiety and fearfulness. Findings constitute the first longitudinal study linking early fear conditioning deficits with aggressive behavior in children.
Neurological and brain-imaging research has shown that the amygdala and the prefrontal cortex are crucial for both the acquisition and expression of electrodermal conditioned fear in humans (Büchel, Morris, Dolan, & Friston, 1998; Knight, Nguyen, & Bandettini, 2005). Furthermore, neuroimaging studies have reported amygdala and prefrontal cortical deficits in aggressive and violent adults (Critchley et al., 2000; Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000). Future child developmental studies which assess both amygdala and prefrontal functioning in conjunction with electrodermal fear conditioning could help substantiate the hypothesis that poor fear conditioning in aggressive children is predicated on dysfunction of these brain structures.
Until functional imaging studies are more easily able to scan young children in the 3–8 year age range, a relative strength of the electrodermal fear conditioning paradigm for child clinical researchers is that it allows for an indirect assessment of the brain circuit implicated in fear conditioning, including the amygdala and the orbitofrontal cortex. Given the technical ease with which it can be measured, the short test time duration (c. 10 minutes), its low cost (c. $5,000), and moderate aversive quality required for the UCS (90 dB), it is recommended that electrodermal fear conditioning should be increasingly assessed in both laboratory and field studies of disruptive behavior problems in children where it can be integrated with other variables to better understand the etiology of disruptive behavior disorders. It is noteworthy that intercorrelations between conditioning and antisocial/aggressive behavior at each ages were non-significant, whereas the average of conditioning across ages was significantly related to aggressive antisocial behavior at age 8 (see Table 1). These finding are in accordance with the findings of LCGA, and suggest that conditioning measurement at multiple time points provides a more reliable assessment of conditioning.
While poor conditioners had significantly higher non-aggressive antisocial scores than good conditioners, children with high non-aggressive antisocial scores did not significantly differ on conditioning compared to those with low scores. This failure to find significant bidirectionality could be due to the moderate internal reliability of the non-aggressive antisocial scale (alpha = .67) and reduced statistic power. Nevertheless, children with poor conditioning showed significantly increased antisocial behavior using the biological design. On balance, the reduced conditioning - non-aggressive antisociality relationship should be interpreted with caution.
Using different sub-samples of the same larger cohort, prior studies have reported early psychophysiology in relation to later aggression and psychopathic personality (Gao, Raine, Venables, Dawson, & Mednick, in press b; Glenn, Raine, Venables, & Mednick, 2007; Raine, Reynolds, Venables, & Mednick, 1997; Raine, Venables et al., 1997). Specifically, among the original cohort of 1,795 subjects, low resting heart rate at age 3 was related to high aggression at age 11 (Raine, Venables et al., 1997), while deficient electrodermal conditioning at age 3 predisposed to criminal offending at age 23 years (Gao et al., in press b). In addition, reduced electrodermal orienting responses at age 3 have been found to be associated with high aggression at age 11 only among children from high socioeconomic homes (Raine, Reynolds et al., 1997). Glenn et al. (2007) found that long electrodermal half-recovery times to aversive stimuli and increased arousal and orienting at age 3 years were associated with high psychopathy scores in adulthood. However, only 29 out of the 200 subjects in the current sample were assessed on psychopathy at ages 25–28, and consequently we could not test for conditioning – psychopathy relations. The age 8 behavioral data were the focus of the current study because only the “200” (out of 1,795) were followed up on conditioning every year from ages 3 to 8, and at age 8, behavioral data were available for most of the subjects (143 out of 200) at the same time-point as the end of conditioning data collection, thus providing adequate statistical power. Importantly, to our knowledge this is the only study to date examining the longitudinal changes of autonomic conditioning at any age and its association with any child or adult behavior problem.
This study has complementary strengths and limitations. A potential limitation is that this sample is composed of community- recruited children from the African sub-continent. Nevertheless, findings on these non-Western children broadly confirm findings from adult studies in Western cultures. This is the only study to the authors’ knowledge investigating the early development of autonomic fear conditioning in relation to aggressive/antisocial behavior. This longitudinal assessment of conditioning fills a critical gap in the literature and provides an initial step towards clarifying the early links between fear conditioning and aggression in children. Furthermore, findings provide further support for the view that at least for early childhood behavior problems, there are common biological correlates to male and female aggressive behavior (Moffitt et al., 2001). Behavioral measures were limited to teacher ratings and future multi-informant studies would be desirable. Importantly, it can neither be claimed nor concluded that conditioning deficits are specific to antisocial/aggressive behavior because the internal reliability of the hyperactive-inattentive scale was relatively low, and Type II error is possible; nevertheless, findings are consistent with prior studies showing normal conditioning in ADHD children (Pliszka et al., 1993). Finally, preliminary evidence has shown that reactively aggressive children show heightened physiological responses to anger provocation (Hubbard et al., 2002; Pitts, 1997), suggesting that poor fear conditioning may not be found in all aggressive children. We were not able to measure reactive vs. proactive aggression to assess whether the poor fear conditioning – aggression relationship may be specific to proactive aggression, a form of aggression linked to psychopathy (Raine et al., 2006). Although the current findings are suggestive, the question of whether or not poor fear conditioning in early childhood could be considered a developmental precursor to adult antisocial and aggressive behavior requires further substantiation from longitudinal research.
The future promise of these findings for child psychopathology research is that the utilization of the electrodermal fear conditioning paradigm in a longitudinal context offers a relatively unique window into the interface between developmental science, neuroscience, and clinical science. This interdisciplinary convergence may potentially allow clearer elucidation of the etiology of child, adolescent, and adult behavior problems in future research.
Key points.
Previous studies have associated poor fear conditioning with adult psychopathy and criminality, but little is known on whether poor fear conditioning early in life is related to childhood aggressive/antisocial behavior.
Findings from this longitudinal study indicated that poorer electrodermal fear conditioning from ages 3 to 8 years is associated with the development of aggressive behavior at age 8 years.
Results suggest that abnormal amygdala functioning, as indirectly assessed by fear conditioning, may influence the development of childhood aggression.
The utilization of the electrodermal fear conditioning paradigm in future studies of young children may help elucidate the etiology of both child and adult clinical disorders.
Acknowledgments
The initial data collection was made possible by grants to Peter H. Venables from the Medical Research Council (UK) and the Welcome Trust (UK). This study was also supported by grants to Adrian Raine from NIH (Independent Scientist Award K02 MH01114). We thank all the local members of the Mauritius Joint Child Health Project, especially Cyril Dalais, for help with data collection.
Footnotes
Conditioning is successfully elicited given that SCRs to the CS+ were significantly larger than to the CS− (Gao et al., in press a).
Four and five classes LCGA were also conducted but none of these models converged. Therefore the results were not reported.
References
- Babcock JC, Green CE, Webb SA, Yerington TP. Psychophysiological profiles of batterers: Autonomic emotional reactivity as it predicts the antisocial spectrum of behavior among intimate partner abusers. Journal of Abnormal Psychology. 2005;114:444–455. doi: 10.1037/0021-843X.114.3.444. [DOI] [PubMed] [Google Scholar]
- Behar L, Stringfield S. A behavior rating scale for the pre-school child. Developmental Psychology. 1974;10:601–610. [Google Scholar]
- Büchel C, Morris RJ, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Critchley HD, Simmons A, Daly EM, Russell A, van Amelsvoort T, Robertson DM, et al. Prefrontal and medial temporal correlates of repetitive violence to self and others. Biological Psychiatry. 2000;47:928–934. doi: 10.1016/s0006-3223(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM. Human autonomic and skeletal classical conditioning: The role of conscious cognitive factors. In: Davey G, editor. Cognitive processes and Pavlovian conditioning in humans. New York: Oxford University Press; 1987. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- Farrington DP. Childhood aggression and adult violence: Early precursors and later-life outcomes. In: Pepler DJ, Rubin KH, editors. The development and treatment of childhood aggression. Hillsdale, NJ: Lawrence Erlbaum; 1991. pp. 5–29. [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Fowler PC, Park RM. Factor structure of the pre-school behavior questionnaire in a normal population. Psychological Reports. 1979;45:599–606. [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. The development of electrodermal fear conditioning in children from ages 3 to 8 years. Developmental Science. doi: 10.1111/j.1467-7687.2009.00874.x. in press a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Poor childhood fear conditioning predisposes to adult crime. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2009.09040499. in press b. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine R, Venables PH, Mednick SA. Early temperamental and psychophysiological precursors of adult psychopathic personality. Journal of Abnormal Psychology. 2007;116:508–518. doi: 10.1037/0021-843X.116.3.508. [DOI] [PubMed] [Google Scholar]
- Hare RD. Electrodermal and cardiovascular correlates of psychopathy. In: Hare RD, Schalling D, editors. Psychopathic behavior: Approaches to research. New York: Wiley; 1978. pp. 107–143. [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsde SR, Parker EH, Flanaga KD, Dearing KF, et al. Observational, physiological, and self-report measures of children’s anger: Relations to reactive versus proactive aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Huesmann LR, Eron LR, Lefkowitz MM, Walder LO. Stability of aggression over time and generations. Developmental Psychology. 1984;20:1120–1134. [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Cadoret RJ, Yates WR, Troughton EP, Stewarts MA. Distinct distributions of conduct and oppositional defiant symptoms to adult antisocial behavior: Evidence from an adoption study. Archives of General Psychiatry. 1998;55:821–829. doi: 10.1001/archpsyc.55.9.821. [DOI] [PubMed] [Google Scholar]
- Loeb J, Mednick SA. A prospective study of predictors of criminality: Electrodermal response patterns. In: Mednick SA, Christiansen KO, editors. Biosocial bases of criminal behavior. New York: Gardener Press; 1977. pp. 245–254. [Google Scholar]
- McArdle JJ. Dynamic structural equation modeling in longitudinal experimental studies. In: van Montfort K, Oud H, Satorra A, editors. Longitudinal models in the behavioural and related sciences. Mahwah, NJ: Erlbaum; 2006. pp. 159–188. [Google Scholar]
- Moffitt TE. Juvenile delinquency and attention deficit disorder: Boys’ developmental trajectories from age 3 to age 15. Child Development. 1990;61:893–910. doi: 10.1111/j.1467-8624.1990.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Development and Psychopathology. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva P. Sex differences in antisocial behaviour: Conduct disorder, delinquency and violence in the Dunedin Longitudinal Study. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles, CA: Muthén & Muthén; 1998. [Google Scholar]
- Nagin DS. Analyzing development trajectories: A semi-parametric, group-based approach. Psychological Methods. 1999;4:139–157. [Google Scholar]
- Pitts TB. Reduced heart rate levels in aggressive children. In: Raine A, Brennan PA, Farrington DP, Mednick SA, editors. Biosocial bases of violence. New York: Plenum; 1997. pp. 317–320. [Google Scholar]
- Pliszka SR, Hatch JP, Borcherding SH, Rogeness GA. Classical conditioning in children with attention deficit hyperactivity disorder (ADHD) and anxiety disorders: A test of Quay’s model. Journal of Abnormal Child Psychology. 1993;21:411–423. doi: 10.1007/BF01261601. [DOI] [PubMed] [Google Scholar]
- Prokasy WF, Ebel HC. Three components of the classically conditioned GSR in human subjects. Journal of Experimental Psychology. 1967;73:247–256. [Google Scholar]
- Raine A, Brennan PA, Mednick SA. Birth complications combined with early maternal rejection at age 1 year predispose to violent crime at age 18 years. Archives of General Psychiatry. 1994;51:984–988. doi: 10.1001/archpsyc.1994.03950120056009. [DOI] [PubMed] [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke-Kopp L, Lynam DR, Reynolds C, et al. The reactive-proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior. 2006;32:159–171. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Raynolds C, Venables PH, Mednick SA. Biosocial bases of aggressive behavior in childhood: Resting heart rate, skin conductance orienting, and physique. In: Raine A, Brennan PA, Farrington DP, Mednick SA, editors. Biosocial bases of violence. New York: Plenum; 1997. pp. 107–126. [Google Scholar]
- Raine A, Reynolds C, Venables PH, Mednick SA, Farrington DP. Fearlessness, stimulation-seeking, and large body size at age 3 years as early predispositions to childhood aggression at age 11 years. Archives of General Psychiatry. 1998;55:745–751. doi: 10.1001/archpsyc.55.8.745. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Better autonomic conditioning and faster electrodermal half-recovery time at age 15 years as possible protective factors against crime at age 29 years. Developmental Psychology. 1996;32:624–630. [Google Scholar]
- Raine A, Yaralian PS, Reynolds C, Venables PH, Mednick SA. Spatial but not verbal cognitive deficits at age 3 years in persistently antisocial individuals. Development and Psychopathology. 2002;14:25–44. doi: 10.1017/s0954579402001025. [DOI] [PubMed] [Google Scholar]
- Räsännen P, Hakko H, Isohanni M, Hodgins S, Järvelin M, Tiihonen J. Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in the northern Finland 1966 birth cohort. American Journal of Psychiatry. 1999;156:857–862. doi: 10.1176/ajp.156.6.857. [DOI] [PubMed] [Google Scholar]
- Rutter M. A children’s behavior questionnaire for completion by teachers: Preliminary findings. Journal of Child Psychology and Psychiatry. 1967;8:1–11. doi: 10.1111/j.1469-7610.1967.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Scarpa A, Raine A. Psychophysiology of anger and violent behavior. Psychiatric Clinics of North America. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Silverthorn P, Frick PJ, Reynolds R. Timing of onset and correlates of severe conduct problems in adjudicated girls and boys. Journal of Psychopathology and Behavioral Assessment. 2001;23:171–181. [Google Scholar]
- Venables PH. Psychophysiology and psychometrics. Psychophysiology. 1978;15:302–315. doi: 10.1111/j.1469-8986.1978.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. New York: Wiley & Sons; 1980. pp. 3–67. [Google Scholar]
- Venables PH, Fletcher RP, Dalais JC, Mitchell DA, Schulsinger F, Mednick SA. Factor structure of the Rutter ‘Children’s behavior questionnaire’ in a primary school population in a developing country. Journal of Child Psychology and Psychiatry. 1983;24:213–222. doi: 10.1111/j.1469-7610.1983.tb00570.x. [DOI] [PubMed] [Google Scholar]