Caffeine is the most widely used drug in the world and the most common caffeine delivery vehicle is coffee. Additional vehicles for caffeine delivery include carbonated soft drinks and “energy drinks.” Interestingly, high doses of caffeine are banned from competitive sports as a performance enhancing drug, caffeine is included in a number of over-the-counter pain relievers and there are at least four psychiatric diagnoses associated with caffeine use. Thus, it comes as quite a surprise to most people that drinking coffee is associated with diminished mortality and that protection from death due to liver disease is the best documented contributor to reduced mortality. Although caffeine is the most extensively characterized pharmacologic agent in coffee, there are other active agents as well, and it has been difficult to capitalize on the hepatoprotective qualities of coffee without knowing which ingredient mediates the protective effect.
The recent demonstration by Modi and colleagues [1] that caffeine appears to protect from severe liver fibrosis in a case control study of patients seen at the NIH for treatment of chronic liver disease, primarily hepatitis C, offers at least a partial explanation for the protective effects of coffee. In their study Modi and co-workers observed that patients who consumed more than the 75th percentile of caffeine for the cohort (about 300 mg/day which is equivalent to the caffeine found in 2.25 cups of coffee) were significantly less likely to have severe hepatic fibrosis as defined by Ishak stage ≥3 (OR 0.33). Evidence for a protective effect of caffeine persisted even after correction for age, sex, race, type of liver disease, body mass index and alcohol ingestion (OR 0.25) and the protective effect was no less marked in the patients with HCV infection (OR 0.19). Although prior work had suggested that other agents (kahwol and cafestol) found in coffee might afford protection from hepatic fibrosis by altering the expression of hepatic enzymes involved in activation of agents (e.g. CCl4) that cause fibrosis in animals [2], the authors were unaware of prior work indicating the direct effects of caffeine on hepatic fibrosis in vivo. In this series, coffee was the greatest single source of caffeine and other sources contributed far less caffeine. Because coffee was the dominant source of caffeine in this group of patients, there was only a trend towards an impact for the contribution of other sources of caffeine toward the protection from fibrosis.
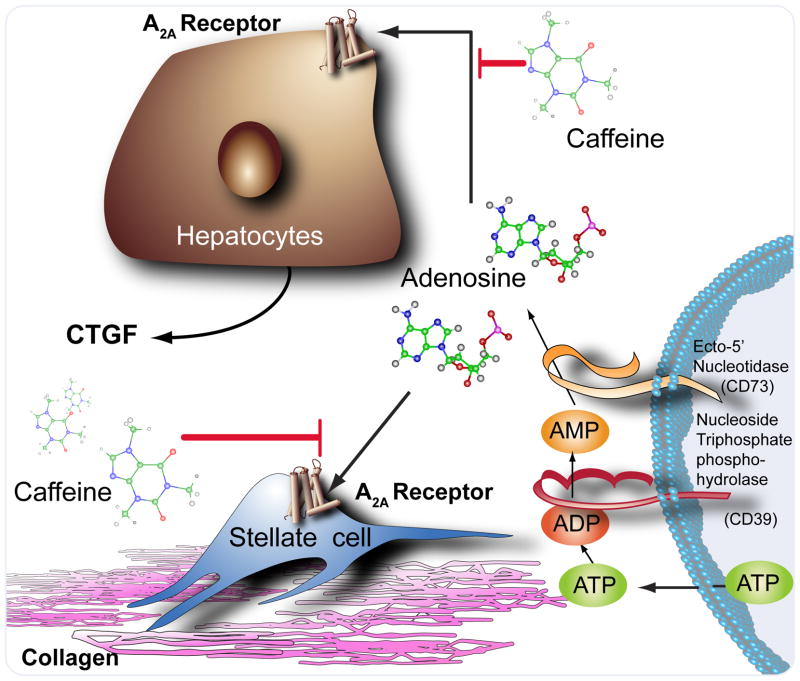
The pharmacologic properties of caffeine have been explored for many years and a variety of different pharmacologic mechanisms have been ascribed to caffeine. The most well known and better understood pharmacologic effect of caffeine is the antagonism of adenosine receptors. Adenosine receptors were first described by Sattin and Rall [3], and it has subsequently become clear that there are four different types of adenosine receptors (A1, A2A, A2B and A3), all of which are members of the G protein coupled family of receptors [4]. Adenosine receptors are ubiquitous in their expression and they regulate a large number of physiologic functions.
Adenosine is a short-lived but abundant purine nucleoside that is released as a result of adenine nucleotide catabolism. Although hypoxia is the most well known stimulus for increasing extracellular adenosine levels, it has long been acknowledged that other types of cellular injury also induce adenosine release as adenine nucleotides are turned over and released. In the liver, adenosine is released as a result of the exposure to toxins, including ethanol and the hepatic fibrosing agents CCl4 and thioacetamide [5]. Recent work from our laboratory has demonstrated that adenosine and its receptors (A1 and A2B receptors) play a central role in the development of hepatic steatosis in mice that chronically ingest high doses of ethanol [6]. In prior work, we and others have used a combination of specific adenosine receptor antagonists and adenosine receptor knockout mice to demonstrate a critical role for adenosine A2A receptors in murine models of hepatic fibrogenesis [5, 7]. Indeed, caffeine itself prevented hepatic fibrosis in response to CCl4 and thioacetamide by, presumably, blocking adenosine receptor-mediated fibrosis [7]. Adenosine A2A receptors are expressed in hepatic stellate cells and hepatocytes where they directly stimulate collagen production and induce [8–10]. Interestingly, all four adenosine receptors are expressed in human liver and the expression of adenosine A2A and A2B receptors is markedly increased in cirrhotic and steatotic livers [6].
Thus, the demonstration by Modi and colleagues [1] that caffeine ingestion prevents advanced liver fibrosis provides further supportive evidence for a role for adenosine receptors in hepatic fibrosis. Moreover, these findings suggest that more selective adenosine receptor agonists with more favorable pharmacokinetics might offer even greater protection from the development of hepatic fibrosis and cirrhosis.
Figure 1.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR54897, AR56672), OSI Pharmaceuticals and the Vilcek Foundation.
Footnotes
Disclosures: Dr. Cronstein holds or has filed applications for patents on the use of adenosine A2A receptor agonists to promote wound healing and use of A2A receptor antagonists to inhibit fibrosis; use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone; the use of adenosine A1 and A2B Receptor antagonists to treat fatty liver, and; the use of adenosine A2A receptor agonists to prevent prosthesis loosening. Consultant (within the past two years) King Pharmaceutical (licensee of patents on wound healing and fibrosis above). CanFite Biopharmaceuticals, Savient Pharmaceuticals, Bristol-Myers Squibb, Roche Pharmaceuticals, Cellzome, Tap (Takeda) Pharmaceuticals, Prometheus Laboratories, Regeneron (Westat, DSMB), Sepracor, Amgen, Endocyte, Protalex, Allos, Inc., Combinatorx, Kyowa Hakka.,. Honoraria/Speakers’ Bureaus: Tap (Takeda) Pharmaceuticals. Stock: CanFite Biopharmaceuticals received for membership in Scientific Advisory Board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 51:201–209. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KJ, Choi JH, Jeong HG. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2007;45:2118–2125. doi: 10.1016/j.fct.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Sattin A, Rall TW. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3′, 5′-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970;6:13–23. [PubMed] [Google Scholar]
- 4.Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, Cronstein BN. Ecto-5′-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 6.Peng Z, Borea PA, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol. 2008;49:758–767. doi: 10.1016/j.jhep.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Che J, Chan ES, Cronstein BN. Adenosine A2A Receptor Occupancy Stimulates Collagen Expression by Hepatic Stellate Cells via Pathways Involving Protein Kinase A, Src, and Extracellular Signal-Regulated Kinases 1/2 Signaling Cascade or p38 Mitogen-Activated Protein Kinase Signaling Pathway. Mol Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.038760. [DOI] [PubMed] [Google Scholar]
- 10.Hashmi AZ, Hakim W, Kruglov EA, Watanabe A, Watkins W, Dranoff JA, Mehal WZ. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G395–401. doi: 10.1152/ajpgi.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]