Summary
Leishmania synthesize abundant phosphoglycan-containing molecules made up of [Gal-Man-PO4] repeating units, including the surface lipophosphoglycan (LPG), and the surface and secreted proteophosphoglycan (PPG). The vector competence of Phlebotomus duboscqi and Lutzomyia longipalpis sand flies was tested using L. major knockout mutants deficient in either total phosphoglycans (lpg2− or lpg5A−/5B−) or LPG alone (lpg1−) along with their respective gene add-back controls. Our results confirm that LPG, the major cell surface molecule of Leishmania promastigotes known to mediate attachment to the vector midgut, is necessary to prevent the loss of infection during excretion of the blood meal remnants from a natural vector, P. duboscqi, but not an unnatural vector, L. longipalpis. Midgut digestive enzymes induced by blood feeding pose another potential barrier to parasite survival. Our results show that 36–72 h after the infective feed, all parasites developed well except the lpg2− and lpg5A−/5B− mutants, which showed significantly reduced survival and growth. Protease inhibitors promoted the early survival and growth of lpg2− in the blood meal. PPG was shown to be the key molecule conferring resistance to midgut digestive enzymes, as it prevented killing of lpg2− promastigotes exposed to midgut lysates prepared from blood-fed flies. The protection was not associated with inhibition of enzyme activities, but with cell surface acquisition of the PPG, which appears to function similar to mammalian mucins to protect the surface of developing promastigotes against proteolytic damage.
Introduction
Parasitic protozoa of the genus Leishmania are the aetiologic agents of a spectrum of human diseases that remain of substantial public health importance in tropical and subtropical regions. Leishmania have a dimorphic life cycle consisting of extracellular promastigotes that multiply and develop within the digestive tract of a female sand fly, and intracellular amastigotes that reside and multiply within the phagolysosomal vacuoles of host macrophages. The identification of molecules that enable the parasite to survive within these harsh, diverse environments continues to be the focus of much of the research devoted to these organisms. Based largely on the analysis of null mutants deficient in a single or related group of glycoconjugates, a number of different molecules have been implicated in various steps of the infectious cycle (Naderer et al., 2004). With respect to the invertebrate stages of the parasite, a family of surface and secreted phosphoglycan-containing molecules have been shown to be essential for growth and development in the sand fly vector (Sacks and Kamhawi, 2001; Turco et al., 2001). These molecules have in common their expression of linear or branched Gal(β1,4)Man(α1-PO4)]-derived repeating units, secreted or released as free phosphoglycan chains (PG) (Greis et al., 1992), linked directly to the cell surface via a glycosylphosphatidylinositol (GPI) anchor, as found in the abundant surface lipophosphoglycan (LPG) (Turco and Descoteaux, 1992; McConville and Ferguson, 1993), or attached to the Ser/Thr-rich regions on a protein backbone, as found in the surface and secreted proteophosphoglycan (PPG) and secretory acid phosphatase (sAP) (Ilg, 2000). The repeating units are synthesized in the Golgi lumen by the sequential transfer of mannose 1-phosphate and galactose from their respective nucleotide donors. Mutants deficient in the Golgi nucleotide-sugar transporters for GDP-Man and UDP-Gal, encoded by LPG2 and LPG5A + LPG5B, respectively, have been generated by homologous gene replacement that are devoid of all phosphoglycan-containing molecules (Spath et al., 2003; Capul et al., 2007a). By contrast, null mutants of the L. major gene LPG1, which encodes a putative galactofuranosyl (Galf) transferase involved in biosynthesis of the LPG glycan core, are defective only in LPG, but otherwise synthesize normal levels of related glycoconjugates (Spath et al., 2000).
Comparisons of Leishmania–sand fly interactions in permissive and non-permissive vector species have revealed a number of barriers to parasite growth and development that have likely selected for expression of surface and secreted phosphoglycan-containing structures (Sacks and Kamhawi, 2001). Two main barriers have been described: (i) the digestive enzymes produced by midgut epithelial cells that are induced by blood feeding and to which parasites are exposed during their early stages of transformation and growth in the posterior midgut, and (ii) the excretion of the digested blood meal that necessitates anchoring of promastigotes to the gut wall in order to avoid being passed out along with the blood meal remnants. The roles for LPG in midgut attachment seem especially convincing based on its specific binding to midgut epithelial cells (Pimenta et al., 1992) and on the fact that a L. major LPG1 deficient mutant fails to persist in the midgut of a natural vector beyond the time of blood meal excretion (Sacks et al., 2000; Myskova et al., 2007). The role of both LPG and protein-bound phosphoglycans in conferring resistance to digestive enzymes in the blood-fed midgut is suggested by the compromised early growth of LPG2 deficient L. major and L. donovani mutants in their respective sand fly vectors (Sacks et al., 2000), although neither the molecules themselves nor their influence on proteolytic activities in the gut was directly investigated. The purpose of the present studies was to extend the phenotypic analysis of L. major LPG2, LPG5A/B and LPG1 null mutants to other vector species, and to explore directly the role of PPG, LPG, and PG in promoting L. major survival within the blood-fed midgut.
Results
Growth of PG null mutants in P. duboscqi and L. longipalpis sand flies
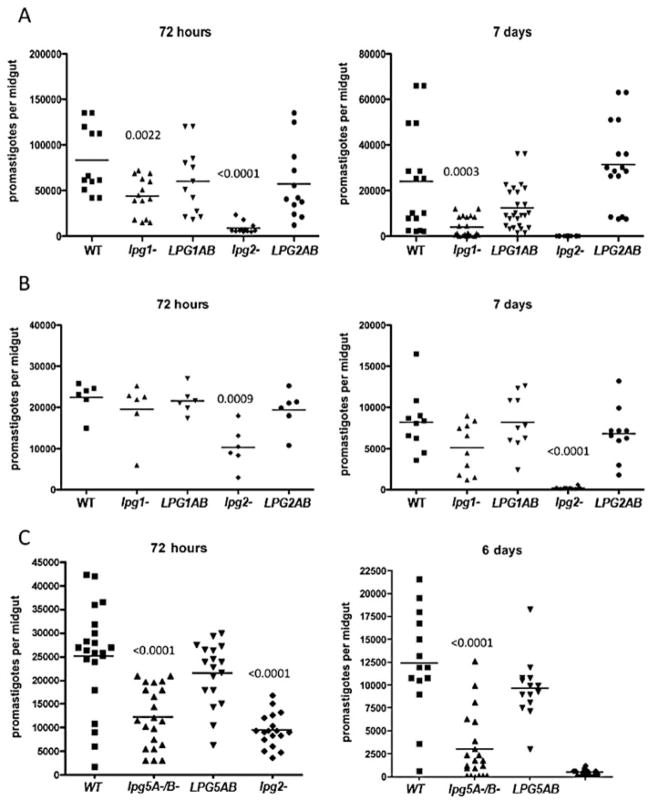
The sand flies were artificially infected using the following cloned lines of L. major: wild-type (WT), the null mutants lpg1− and lpg2−, and their ‘add-back controls’ lpg1−/+LPG1, and lpg−/+LPG2. For P. duboscqi, 100% of the flies in each group were positive for viable promastigotes 72 h after the infective blood meal (Fig. 1A). The WT, lpg1−/+LPG1 and lpg2−/+LPG2 control lines showed the highest parasite loads per gut, the lpg1− mutant showed a slight but significant decrease (P = 0.002), while lpg2− showed a pronounced decrease (> 80%) in survival or growth of promastigotes when compared with the WT (P = 0.0005). Seven days after infection, following completion of the digestive process and excretion of the blood-meal remnants in most flies, the P. duboscqi flies infected with the WT and add-back lines showed persistence of > 10 000 parasites per gut. By contrast, flies infected with the lpg1− promastigotes retained fewer than 1000 parasites (P = 0.0003), and the lpg2− promastigotes were completely lost. At two later time points examined, 15 and 21 days, the WT and add-back control lines had successfully colonized the anterior midgut with approximately 10 000 promastigotes, while virtually all of the flies infected with the lpg1− or lpg2− promastigotes had completely lost their infections (data not shown).
Fig. 1.
Growth and survival of L. major PG-mutants in sand fly vectors. P. duboscqi (A and C) and L. longipalpis (B) were membrane fed on mouse blood containing 4 × 106 ml−1 logarithmic phase promastigotes of the following lines: lpg1−, lpg2−, lpg5A−/5B−, lpg1−/+LPG1 (LPG1AB), lpg2−/+LPG2 (LPG2AB), and lpg5A−/5B−/+LPG5A/B (LPG5AB). At the indicated times following the infective feed, their midguts were dissected, homogenized, and the number of viable promastigotes determined by counting under a haemacytometer. Data shown in (A) and (B) are representative of two independent experiments. Data in (C) are pooled from two independent experiments. P-values shown were calculated in comparison to flies infected with WT promastigotes.
Lutzomyia longipalpis is a natural vector of L. chagasi infantum, but permits the growth and development of most Leishmania species, including L. major (Walters, 1993). Seventy-two hours after the infective feed, 100% of the flies were positive for viable promastigotes, with each group containing an average of 20 000 promastigotes per midgut (Fig. 1B). The exception was the flies infected with the lpg2−, which showed a > 50% reduction in parasite growth or survival (P = 0.0009). At 7 days post infection, following blood meal digestion and excretion, the WT, lpg1−/+LPG1, and lpg2−/+LPG2 control lines all maintained strong and comparable infections, approximately 9000 promastigotes per gut, while the lpg1−, in contrast to its substantial loss in P. duboscqi, was retained in the L. longipalpis midgut at levels only slightly reduced compared with the WT and add-back controls. Consistent with the results in P. duboscqi, the lpg2− was completely lost, save for one fly that retained a low number of parasites. These results indicate that LPG is not required for either the early growth or the persistence of L. major in the midgut of an unnatural vector species, confirming recent findings by others (Myskova et al., 2007). By contrast, the absence of all phosphoglycan containing molecules was lethal to the parasite in both vector species, an effect that in each case became apparent prior to blood meal excretion, during early exposure to the blood induced, digestive enzymes in the gut.
Mutants deficient in PG biosynthesis have also been generated by knocking out the genes encoding the nucleotide sugar transporters for UDP-Gal (Capul et al., 2007a). The lpg5A−/5B− double gene mutant is completely abrogated for UDP-Gal uptake into the Golgi apparatus, and thus can be used as an independently derived PG-deficient line for comparison to the lpg2− mutant. Following infection of P. duboscqi, the lpg5A−/5B− and lpg2− mutants showed a 54% and 66% reduction, respectively, in the number of viable midgut promastigotes at 72 h post infection in comparison to WT (Fig. 1C, left panel). The early growth of the lpg5A−/5B−/+LPG5A/B control was restored to a level comparable to the WT. At day 6, following blood meal excretion, lpg5A−/5B− suffered a further loss of infection from the midgut, though most flies remained positive for low numbers of promastigotes, while the lpg2− promastigotes were again completely lost from the majority of flies (Fig. 1C, right panel). The lpg5A−/5B−/+LPG5A/B control line persisted normally.
Protease inhibitors enhance the early survival of lpg2− promastigotes in P. duboscqi
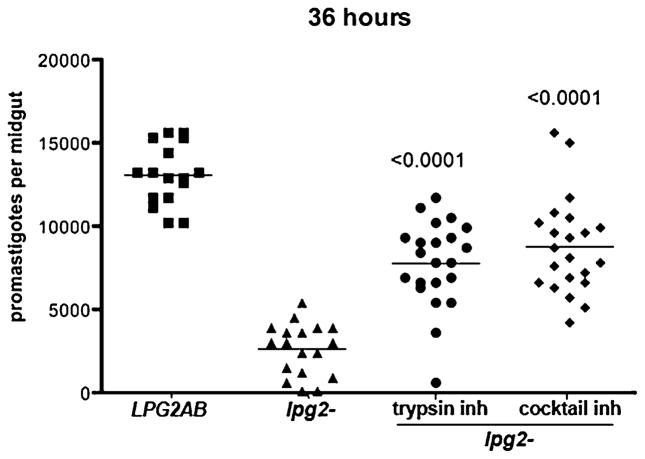
To determine if susceptibility to midgut proteases was, at least in part, responsible for the deficient growth of the lpg2− promastigotes in the vector, P. duboscqi sand flies were fed on blood meals containing lpg2− or lpg2−/+LPG2 promastigotes, with or without soybean trypsin inhibitor, or a cocktail of protease inhibitors. At 36 h, flies infected with the lpg2− showed a significant reduction in parasite survival or growth (80%) compared with the lpg2−/+LPG2 (Fig. 2), revealing how quickly conditions in the midgut pose a threat to the parasite in the absence of PG-containing molecules. The early growth of the lpg2− was substantially rescued by the addition of either trypsin inhibitor or the cocktail of protease inhibitors to the blood meal (P < 0.0001).
Fig. 2.
Effect of protease inhibitors on L. major infections in P. duboscqi. Flies were infected with 4 × 106 ml−1 logarithmic phase LPG2AB promastigotes, or lpg2− promastigotes with or without soybean trypsin inhibtor (1 mg ml−1), or a cocktail of protease inhibitors (1 mg ml−1). P-values shown were calculated in comparison to flies infected with lpg2− without inhibitors.
lpg2− sensitivity to killing by blood-fed midguts in vitro
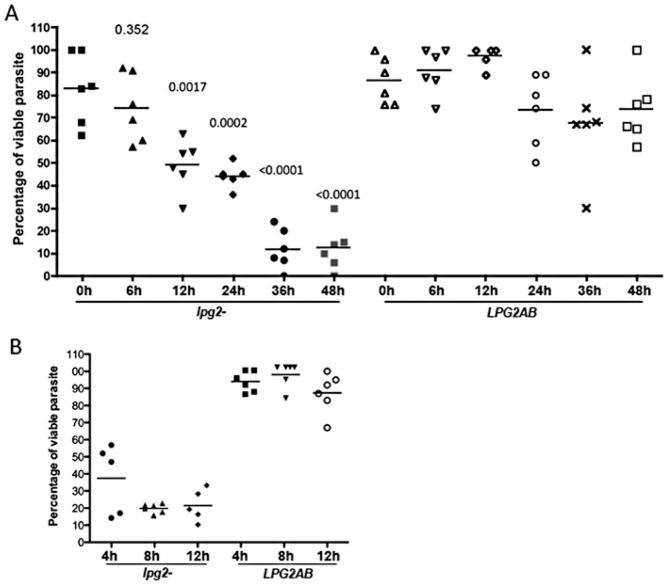
The susceptibility of the lpg2− promastigotes to damage by the contents of the blood-fed midgut was further investigated by in vitro exposure of parasites to the lysate of a single midgut obtained from P. duboscqi at different intervals following membrane feeding on normal, uninfected mouse blood. An 8 h incubation of the lpg2− promastigotes with unfed midguts, or with midguts obtained 6 h after blood feeding, did not result in significant loss of viability compared with the parasites incubated alone. Following exposure to midguts obtained 12 h after engorgement, approximately 50% of the lpg2− parasites were killed, while exposure to the 36 h and 48 h midgut lysates killed over 80% of the cells (Fig. 3A). Importantly, the killing of the lpg2−/+LPG2 never exceeded 20% of the average number of cells remaining viable after incubation alone, even following exposure to lysates prepared from 36 and 48 h blood-fed midguts. Employing 36 h midgut lysates only, it was revealed that the lpg2− promastigotes were killed as early as 4 h after lysate exposure, while the LPG2AB promastigotes remained resistant for up to 12 h (Fig. 3B).
Fig. 3. Susceptibility of lpg2− mutants to killing by lysates from blood-fed midguts in vitro.
A. lpg2− and LPG2AB logarithmic phase promastigotes (5 × 104 in 1 μl aliquots) were exposed for 8 h at 26°C to freezed-thawed lysates prepared from individual P. duboscqi midguts obtained from non-blood fed flies (0 h), or from flies at various times (6–48 h) after feeding on uninfected mouse blood.
B. Percentage of viable promastigotes following exposure of lpg2− mutants to individual 36 h blood-fed, P. duboscqi midguts for 4, 8 and 12 h at 26°C. Bars represent the mean number of viable parasites present after incubation with midgut lysates calculated as the percentage of the mean number of viable promastigotes present in six 1 μl aliquots of the organisms incubated alone. P-values shown were calculated in comparison to lpg2− incubated with midgut lysate from non-blood fed flies (0 h).
Phosphoglycan-containing molecules confer resistance to killing by blood-fed midguts in vitro
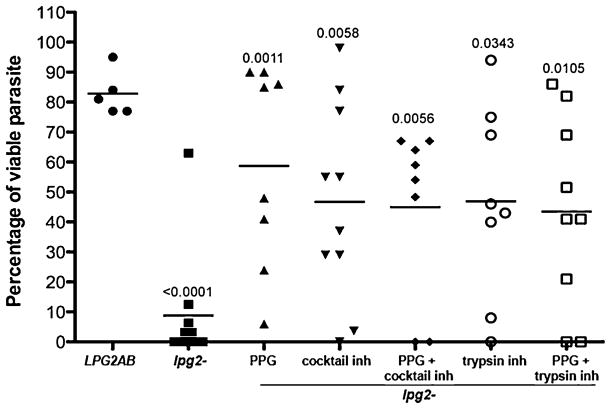
The ability of specific phosphoglycan-containing molecules to inhibit the in vitro killing of the lpg2− promastigotes was determined by adding purified PPG, LPG or PG to the mixture of promastigotes and lysate prepared from a single 36 h blood-fed midgut. The per cent viability was calculated based on the number of viable promastigotes following incubation of parasites with each of the molecules alone. The lpg2− mutant again showed high susceptibility to killing following an 8 h exposure to the midgut lysate (> 70%) (Fig. 4A). The PPG showed significant protection at all concentrations tested (5–40 μg ml−1), with the highest concentration providing close to full recovery of parasite survival in 6 of the 8 individual midgut lysates tested. The LPG, on the other hand, provided only slight and non-significant protection at the highest concentration (40 μg ml−1). Since the LPG glycolipid is likely to form micelles in aqueous solutions, its behaviour in these assays may be difficult to compare with the PPG, whose purification involved removal of glycolipid contaminants by ocytlsepharose chromatography. The protection assays were repeated using PG prepared from LPG delipidated by PI-PLC hydrolysis. The PG showed significant protection at the higher concentrations tested (40 and 20 μg ml−1, P = 0.0012 and P < 0.0001 respectively) (Fig. 4B), demonstrating that the water-soluble PG chains, free of any lipid- or protein-linked moieties, are able to promote the viability of pan PG-deficient L. major in the vector midgut. The ability of the PPG and water-soluble PG chains to reconstitute lpg2− resistance to killing by midgut lysates relative to the natural resistance displayed by WT and LPG2AB promastigotes is shown in Fig. 4C. While both molecules again provided significant protection against killing (P = 0.0018 and P = 0.0225 respectively), pre-incubation with PPG was necessary to confer optimal protection. The LPG was again ineffective in these protection assays.
Fig. 4.
Inhibition of in vitro killing of lpg2− promastigotes by PG-containing molecules. lpg2− logarithmic phase promastigotes (5 × 104 in 1 μl aliquots) were exposed for 8 h at 26°C to freezed-thawed lysates prepared from individual P. duboscqi midguts obtained from flies at 36 h after feeding on uninfected mouse blood, in the absence or presence of various concentrations (5–40 μg ml−1) of PPG or LPG (A), or LPG or PG prepared by PI-PLC hydrolysis of LPG (B). WT, LPG2AB or lpg2− logarithmic phase promastigotes were exposed to individual midgut lysates in the absence or presence of 40 μg ml−1 PPG, PG or LPG (C). Bars represent the mean number of viable parasites present after incubation with midgut lysates, calculated as the percentage of the mean number of viable promastigotes present in six 1 μl aliquots of the organisms incubated with the respective concentrations of PG-containing molecules alone. P-values shown were calculated in comparison to lpg2− incubated with midgut lysate in the absence of added molecules.
The killing of the lpg2− promastigotes by the 36 h midgut lysate was also inhibited by addition of protease inhibitors (Fig. 5). As observed in the in vivo infections (Fig. 2), the presence of either the trypsin inhibitor or the cocktail of protease inhibitors partially rescued the survival of the lpg2− mutants during incubation with the majority of the midgut lysates tested. The protection conferred by the protease inhibitors was highly variable depending on the individual midgut used, and the presence of both the inhibitors and PPG did not provide an additive survival benefit to the cells.
Fig. 5.
Inhibition of in vitro killing of lpg2− promastigotes by PPG or protease inhibitors. LPG2AB or lpg2− logarithmic phase promastigotes (5 × 104 in 1 μl aliquots) were exposed for 8 h at 26°C to freezed-thawed lysates prepared from individual P. duboscqi midguts obtained from flies at 36 h after feeding on uninfected mouse blood, in the absence or presence of PPG (20 μg ml−1), and/or soybean trypsin inhibitor (1 mg ml−1), and/or the cocktail of protease inhibitors (1 mg ml−1). Bars represent the mean number of viable parasites present after incubation with midgut lysates, calculated as the percentage of the mean number of viable promastigotes present in six 1 μl aliquots of the organisms incubated with the respective concentrations of PPG and/or inhibitors alone. P-values shown were calculated in comparison to lpg2− incubated with midgut lysate in the absence of added PPG and/or inhibitors.
Enzymatic activities in the blood-fed midguts
Trypsin serine proteases are the main digestive proteases in Dipteran midguts, including sand flies, and their activities have been previously shown to increase following a blood meal. The trypsin activities in infected sand flies were assayed using a chromogenic substrate. Trypsin activity induced by the blood meal was detected as early as 6 h post feeding, and peaked at approximately 24–36 h, consistent with prior studies (Fig. 6A). No significant difference in total midgut trypsin activity was observed between infected and uninfected flies, or between flies infected with lpg2− and the WT or LPG2AB promastigotes. Thus, despite the ability of soybean trypsin inhibitor to rescue lpg2− survival in vivo (Fig. 2) and ex vivo (Fig. 5), there was no association between the levels of trypsin activities and the growth of the respective promastigotes lines in the infected midguts. Chymotrypsin activity was also monitored, and while its activity rose more gradually and peaked at roughly 48 h post blood meal, again there was no apparent difference between infected and uninfected flies or between flies infected with the different lines (Fig. 6B). The spike in activity in the lpg2− infected flies at 36 h was not a consistent finding in three independent experiments.
Fig. 6.
Effect of infection with lpg2− mutants on the early kinetics of serine protease activities in blood-fed midguts. P. duboscqi were membrane fed on mouse blood containing 4 × 106 ml−1 logarithmic phase promastigotes, and at various times post feeding dissected midguts were assayed using chromogenic substrates for trypsin (A) or chymotrypsin (B). Values represent mean (±1 SD) enzyme concentration per midgut, 6–8 midguts per group. Data are from one of three independent experiments. Trypsin (C) and chymotrypsin (D) activities in individual midgut lysates (MG) prepared from flies at 36 h post feeding on uninfected mouse blood, in the absence or presence of LPG2AB or lpg2− logarithmic phase promastigotes (50 000 in 1 μl aliquots), lpg2− +PPG (20 μg ml−1), lpg2− +soybean trypsin inhibitor (1 mg ml−1), lpg2− +chymotrypsin inhibitor (1 mg ml−1). Enyzme activities present in parasites alone are also shown. Data are from one of two independent experiments.
Enzymatic activities were also assayed in the 36 h, uninfected, blood-fed midguts used in the in vitro killing assays. Neither the trypsin (Fig. 6C) nor chymotrypsin (Fig. 6D) activities present in the midgut lysates were affected by the presence of the lpg2− or lpg2−/+LPG2 promastigotes. Importantly, the addition of PPG did not inhibit the enzymatic activities, either when added to the lysate alone, or when added along with the lpg2− promastigotes. The addition of either the trypsin or chymotryspin inhibitors completely abrogated the activity of the respective enzymes in the midgut lysate, as expected. Thus the PPG, despite protecting lpg2− promastigotes from killing by digestive enzymes the gut, did not appear to confer protection by inhibiting the enzymatic activities directly.
Cell surface acquisition of PPG by lpg2− promastigotes
The ability of exogenous PPG to rescue lpg2− survival in the absence of any measurable inhibitory effect on serine protease activities suggested that the molecules might reconstitute the surface of the lpg2− cells to protect them from proteolytic damage. Pre-incubation of the PPG with lpg2− promastigotes, followed by thorough washing of the cells, resulted in significant protection of the promastigotes when subsequently exposed to individual midgut lystes (Fig. 7A). By contrast, the pre-absorption of the PPG with the lpg2− promastigotes resulted in the loss of any protection afforded by the supernatant material when added to a fresh population of lpg2− promastigotes (Fig. 7A). By comparison with PPG, pre-incubation with LPG followed by washing was unable to protect lpg2− promastigotes against killing by midgut lysates (Fig. 7B). The findings suggest that the PPG but not LPG became passively adsorbed onto the surface of the lpg2− cells. This was directly demonstrated by surface staining and flow cytometric analysis of the promastigotes following pre-incubation with PPG (Fig. 7C). The top panel shows strong, uniform staining of the lpg2−/+LPG2 parasite relative to the weak staining of the lpg2− promastigotes with WIC79.3, specific for the Gal-containing side-chains that branch off the Gal-Man-P backbone units expressed in most L. major strains (de Ibarra et al., 1982). Staining of the PPG pre-incubated and washed lpg2− promastigotes resulted in a strong and uniform shift in surface staining roughly equivalent to the lpg2−/+LPG2 control, while fresh lpg2− cells incubated with the pre-adsorbed material stained as poorly as the un-incubated, lpg2− control (bottom left panel). Interestingly, when fresh lpg2− cells were incubated with PPG that had been pre-adsorbed with WT promastigotes, only a slight shift in staining relative to the un-incubated lpg2− control was observed, suggesting that PPG can be passively adsorbed onto the surface of WT cells (data not shown). Finally, pre-incubation of the lpg2− promastigotes with LPG-derived PG also resulted in strong surface staining of the cells, indicating efficient cell surface acquisition of PG, while pre-incubation with intact LPG resulted in a slight, but uniform increase in surface staining (bottom right panel).
Fig. 7. Cell surface acquisition of PPG protects lpg2− promastigotes from killing by midgut lysates in vitro.

A. Lysates from individual 36 h normal blood-fed midguts were incubated for 8 h at 26°C with 1 μl aliquots of LPG2AB, lpg2−, lpg2− plus 40 μg ml−1 PPG, lpg2− pre-incubated with 40 μg ml−1 PPG and washed, and fresh lpg2− plus supernatant from the high-speed centrifugation of the lpg2− cells pre-incubated with PPG.
B. Lysates from individual 36 h normal blood-fed midguts were incubated for 8 h at 26°C with 1 μl aliquots of lpg2−, lpg2− pre-incubated with 40 μg ml−1 PPG and washed, lpg2− pre-incubated with 40 μg ml−1 LPG and washed. Bars represent the mean number of viable parasites present after incubation with midgut lysates calculated as the percentage of the mean number of viable promastigotes present in six 1 μl aliquots of the respective treatment groups incubated alone. P-values shown were calculated in comparison to lpg2− incubated with midgut lysate alone.
C. LPG2AB and lpg2− L. major parasites were stained for surface expression of the Gal-substituted Gal-Man-P repeat units recognized by mAb WIC79.3 either before (top panels), or following incubation alone or pre-incubation with either PPG (+pre inc PPG) (left lower panel), PG (+pre inc PG) or LPG (+pre inc LPG) (right lower panel), each at 40 μg ml−1. Fresh lpg2− parasites were also stained following incubation with the high-speed supernatant derived from lpg2− pre-incubated with PPG (middle panel) or PG (bottom panel).
Discussion
Leishmania mutants deficient in genes controlling the biosynthesis of LPG and related cell surface and released glycoconjugates have provided some of the more striking phenotypes in Leishmania biology. As reported previously for P. papatasi (Sacks et al., 2000; Myskova et al., 2007), and confirmed in these studies for P. duboscqi, LPG1 mutants, which are deficient solely in LPG biosynthesis, are unable to persist in the midguts of their natural vectors beyond the time of blood meal excretion. Based on a series of in vitro binding studies, the defect is strongly associated to the role that LPG plays in mediating attachment of the parasite to lectin-like molecules lining the midgut epithlelium (Pimenta et al., 1992; 1994; Kamhawi et al., 2004). By contrast, the LPG1 mutant did persist in the midgut following blood meal excretion in an unnatural vector, L. longipalpis, confirming studies by Myskova et al. (2007) suggesting an LPG independent mechanism of midgut attachment in permissive flies.
Our findings also confirm the equally prominent phenotype previously observed for LPG2 mutants (Sacks et al., 2000; Spath et al., 2003; Boulanger et al., 2004), which are deficient in expression of all phosphoglycan-containing molecules and display an early growth defect in the blood-fed midgut. We extend these findings in an important way by defining the killing mechanisms involved and the specific parasite glycoconjugates that confer survival. Employing two additional vector species, as well as an independently derived mutant deficient in expression of LPG and protein-linked PGs, our current studies confirm the extreme susceptibility of PG-deficient mutants to the damaging conditions in the posterior midgut shortly after blood feeding. The studies attribute these lethal effects to the activity of gut digestive enzymes, and directly demonstrate a role for PPG- and LPG-derived molecules in protecting the parasite surface from proteolytic damage.
Because the lpg2− null mutants are deficient in expression of all PG repeat-modified molecules, including free PG chains, lipid-containing LPG and protein-containing PPGs, the nature of the specific molecules able to promote early parasite survival in the blood-fed midgut has remained open to question. The lpg1− mutant specifically lacking LPG shows only a modest defect in early survival (Fig. 1A), implicating other LPG2-dependent molecules more strongly in this process. Since the PPG expressed by the lpg1− mutant is detectable in both normal size and amount (Spath et al., 2000; 2003), the relative dispensability of LPG for early midgut survival cannot be attributed to a compensatory increase in PPG. However, LPG2 is a multispecific GDP-sugar transporter, with specificities for GDP-D-Arap and GDP-L-Fuc in addition to GDP-Man (Hong et al., 2000), and previous studies have suggested that its loss may affect glycoconjugates unrelated to PGs (Capul et al., 2007b). For this reason we also tested the lpg5A−/5B− mutant, which affects PG synthesis independently by ablating UDP-Gal transport into the Golgi apparatus (Capul et al., 2007a). The similarity in the in vivo fly survival phenotypes of the lpg2− and lpg5A−/5B−(Fig. 1C), as well as the biochemical studies implicating PPG and PG in this process (Figs 4, 5 and 7), provides convincing evidence that it is the PG-containing molecules that are required for normal survival and growth in the early blood-fed midgut. Of note, the sAP is either undetectable or produced in only low abundance by L. major sp. (Shakarian and Dwyer, 2000), thereby leaving the LPG and PPG as the major PG-containing molecules that are lacking in the lpg2− and lpg5A−/5B− mutants.
Using an in vitro assay involving lysates from a single blood-fed midgut, PPG showed a remarkable capacity to protect lpg2− promastigotes from killing. The protection was associated with efficient adsorption of the PPG onto the surface of the cells, presumably as a consequence of ionic interactions between the high negatively charged PPG with positively charged molecules exposed on the surface of the lpg2− promastigotes. Alternatively, the negatively charged polysaccharides might bind to negatively charged surface molecules in the presence of divalent cations. Importantly, the protection was not associated with inhibition of midgut digestive enzymes, either during infection in vivo or during the co-culture with midgut lysate in the in vitro killing assays. Adler (1938) was the first to suggest that serum-induced proteolytic factors might prevent the development of an unnatural strain of Leishmania in P. papatasi, based on his observation that lowering the rabbit serum concentration enhanced parasite survival (Adler, 1938). In the studies by Borovsky and Schlein (1987), the addition of soybean trypsin inhibitor to the blood meal enhanced the survival of L. donovani in the midgut of P. papatasi, and recent studies using soybean trypsin inhibitor or RNAi to silence trypsin 1 gene expression in blood-fed L. longipalpis enhanced the midgut survival of L. mexicana promastigtoes (Rogers et al., 2008; Sant’anna et al., 2009). In the present studies, the ability of trypsin inhibitor or a cocktail of serine and cysteine protease inhibitors to prevent the killing of the lpg2− promastigotes in the midgut is clear. What is at issue is how the PPG functions in this capacity. We have been unable to reproduce early findings in P. papatasi (Schlein and Romano, 1986; Dillon and Lane, 1993) or the more recent findings in L. longipalpis suggesting that L. major or L. mexicana infections, respectively, suppress blood meal-induced protease activities. The levels of trypsin and chymotrypsin activities induced by blood feeding were not reduced at any time point in infected versus uninfected P. duboscqi sand flies, and no significant difference between the lpg2− parasites and the other infection groups was observed. Furthermore, the levels of serine protease activities present in the 36 h blood-fed midgut lysates were not reduced by the addition of the PPG. It is possible that the PPG may have inhibited the activity of other digestive enzymes that were not directly assayed using the chromogenic substrates, and whose upregulation has been inferred by transcriptome analysis of blood-fed, P. papatasi midguts (Ramalho-Ortigao et al., 2007). Nevertheless, we favour the hypothesis that the protection afforded the parasites by secreted phosphoglycans results from an abundance of negatively charged molecules in the vicinity of, or reconstituted on, the surface of the parasite, which protects the cell from enzymatic attack, similar to the protective function of gastric mucins in human intestinal cells. The filamentous PPG released by L. major promastigotes in vitro was originally described as having an overall structure with many similarities to mammalian mucins (Ilg et al., 1996). Finally, while our findings pertain to the released form of PPG, they have not specifically addressed the possible protection afforded by the GPI membrane anchored PPG, previously described for another strain of L. major (Ilg et al., 1999). In this regard, we have been unable to detect PPG in the Triton membrane fraction of the LV39 WT strain, or to surface label PPG on the lpg1− mutant cells (Spath et al., 2000). Thus, the water-soluble, secreted form is likely the principle PPG conferring protection, at least for this strain of L. major.
The fact that the lpg1− null mutants of both L. major and L. donovani, deficient only in LPG biosynthesis, survive well in their natural vectors so long as the blood meal is still present (Sacks et al., 2000; Myskova et al., 2007), has tended to minimize the contribution of surface LPG to the resistance of developing promastigotes to early killing in the midgut. Yet the demonstration that LPG is not required for early survival in the midgut cannot be taken as evidence that when present, it does not also function in this capacity. In the current studies, the L. major lpg1− phenotype in P. duboscqi did in fact reveal a modest defect in early growth that was rescued in the add-back line, consistent with the behaviour of the same mutant in P. papatasi, and an L. donovani LPG1 deficient mutant in P. argentipes (Sacks et al., 2000). The protection of the lpg2− with the PG derived by LPG lipid hydrolysis provides additional evidence that the phosphoglycan domain can confer some resistance against killing by midgut proteases. Whether free PG chains released by promastigotes in culture are present during infection in the fly has not been possible to determine. Released molecules bearing the phosphoglycan epitope have been detected in abundance in L. major-infected P. papatasi midguts as early as day 2 (Davies et al., 1990), although it is not known what fraction, if any, of these molecules were free PG chains as opposed to secreted PPG. Using an antibody specific for the phosphoglycan terminal mannose oligosaccharide caps of L. mexicana PPG, Rogers et al. (2004) demonstrated the presence of secreted PPG in infected L. longipalpis sand flies as early as day 3. Nonetheless, since the protection afforded by either the PPG or PG against killing by the midgut lysate appeared to be explained by their cell surface association, it seems likely that the densely organized glycocalyx formed by the high copy number and three-dimensional solution structure of the surface LPG (Homans et al., 1992) would itself provide a barrier against proteolytic attack. Together, the expression of surface and released phosphoglycosylated protein and lipid molecules ensures that the developing promastigotes are well protected against the hydrolytic environment of the blood-fed midgut.
Experimental procedures
Parasites
The WT L. major strain used in all experiments was LV39 clone 5 (MHOM/SU/59/P) (Marchand et al., 1987). The LPG1, LPG2 and LPG5A/B null mutants (lpg1−, lpg2−, lpg5A−/5B−) and their respective LPG1, LPG2, LPG5A/B add-back control lines (lpg1−/+LPG1, lpg2−/+LPG2, lpg5A−/5B−/+LPG5A/B) were generated in this strain background as previously described (Spath et al., 2000; 2003; Capul et al., 2007a). All parasites were grown at 26°C in medium 199 supplemented with 20% heat-inactivated FCS (Gemini Bio-Products), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 2 mM L-glutamine, 40 mM Hepes, 0.1 mM adenine (in 50 mM Hepes), 5 mg ml−1 hemin (in 50 % triethanolamine), and 1 mg ml−1 6-biotin. The lpg1−/+LPG1 and lpg2−/+LPG2 lines were grown in the presence of 50 μg ml−1 Geneticin (G418) (Sigma).
Sand fly infections
Three- to five-day-old female P. duboscqi or L. longipalpis sand flies, obtained from colonies initiated from field specimens collected in Senegal and Brazil, respectively, were fed through a chick skin membrane on heparinized mouse blood containing 4 × 106 ml−1 logarithmic phase promastigotes (WT, lpg1−, lpg2−, lpg5A/B−, lpg1−/+LPG1, lpg2−/+LPG2 and lpg5A−/5B−/+LPG5A/B). The red blood cells were washed twice in 0.86% saline and added back to the plasma, which was heat inactivated at 56°C for 45 min. In some experiments, trypsin inhibitor from soybean (Sigma-Aldrich) and protease inhibitor cocktail (Roche Applied Science), inhibiting a broad spectrum of serine and cysteine proteases, were added to the blood meal at the concentration of 1 mg ml−1. Blood engorged flies were separated and maintained at 26°C with 30% sucrose. The sand flies were anaesthetized with CO2, their midguts dissected, frozen until use, or homogenized, and the number of viable promastigotes determined by counting under a Neubauer improved haemacytometer.
Preparation of phosphoglycan-containing molecules
Proteophosphoglycan was purified from 2–4 × 1010 late log phase, WT promastigotes. Purification was achieved by a modification of Ilg et al. (1996). The conditioned medium was separated from cells by centrifugation at 5000 g for 30 min and passed through a column of octyl-Sepharose (40 ml packed) equilibrated in 1 M ammonium sulfate, 20 mM Tris-HCl, pH 7.5. Any LPG or lipid-anchored PPG binds to the octyl-Sepharose and is removed from the soluble PPG. Fractions (2.5 ml) were collected and unbound PPG material was monitored spotting aliquots of the eluant on silica and then carbohydrate staining with orcinol/H2SO4 (Skipski and Barclay, 1969). Any LPG or lipid- anchored PPG binds to the octyl-Sepharose and is removed from the soluble PPG. To further ensure complete removal of lipid-anchored glycoconjugates, the soluble PPG-containing fractions were pooled and reapplied to another column of octyl-Sepharose equilibrated in the same buffer. The unbound PPG material was pooled, desalted by passage over a column of Sephadex G25 equilibrated in water, dried and resuspended in water. PPG was quantitated by phenol/sulfuric acid assays (Dubois et al., 1956) and the presence of PPG in the samples was confirmed by Western blotting using WIC79.3. LPG was purified from WT promastigotes in late logarithmic phase and delipidation of LPG by PI-PLC hydrolysis was performed as described elsewhere (Orlandi and Turco, 1987).
Assay for parasite susceptibility to lysates from blood-fed midguts
Midguts were obtained aseptically from P. duboscqi sand flies membrane-fed 0–48 h previously on uninfected, heat-inactivated mouse blood, and stored individually in a microfuge tube without medium, then freezed-thawed 15 times and stored at −20°C until use. A 1 μl aliquot of a parasite suspension containing 50 000 logarithmic-phase promastigotes in Dulbecco’s modified Eagle’s medium (DMEM) was added directly to the microfuge tube containing the single freeze-thawed midgut, and parasites were exposed to the lysate for 4, 8 or 12 h at 26°C. The suspension was then diluted in 20 μl DMEM containing 10% FBS, and the percentage of viable parasites was determined by counting under a haemacytometer and comparing with the number of viable promastigotes following incubation of the parasites alone. In some experiments, the parasites were exposed to a single freeze-thawed, 36 h blood-fed midgut for 8 h in the presence of purified PPG, LPG or PG prepared from WT promastigotes, or in the presence of soybean trypsin inhibitor (1 mg ml−1) or protease inhibitor cocktail (1 mg ml−1). To avoid dilution of the midgut enzymes, the 1 μl aliquots of added parasites were obtained from parasite suspensions in DMEM containing the relevant PG-containing molecules and/or protease inhibitors. For analysis of cell surface acquisition and adsorption of PPG, a 10 μl aliquot of a suspension of LPG2KO containing 5 × 105 promastigotes, with or without purified PPG at 40 μg ml−1, were pre incubated for 5 h at 26°C. The suspension was spun in a microcentrifuge at 3000 g for 10 min, the supernatant removed and 1 μl aliquots used for addition to a fresh preparation of lpg2− promastigotes exposed to lysates from a single 36 h blood-fed midgut. The pelleted cells were washed twice in DMEM, resuspended in 10 μl DMEM, and 1 μl aliquots were incubated with a single 36 h blood-fed midgut lysate, or surface stained for analysis of PPG acquisition.
Staining for surface expression of LPG/PPG
Logarithmic phase lpg2− or lpg2−/+LPG2 promastigotes, prepared as described above, were incubated without fixation with an anti-Fc-γ III/II (CD16/32) receptor Ab (2.4G2, BD Biosciences) in RPMI without phenol red (Gibco) containing 0.5% FCS for 10 min followed by incubation for 20 s with Alexafluor 488 anti-LPG/PPG (mAb WIC79.3) (de Ibarra et al., 1982). The isotype control employed was rat IgG1 (R3-34). For each sample, at least 2 × 104 parasites were collected using a FACS Canto flow cytometer (BD Biosciences) and analysed using FlowJo Software (Stanford University).
Enzymatic assays
Dissected midguts were stored frozen individually at −20°C. Thawed guts were homogenized in 50 μl DMEM. For trypsin activity, 100 μl of 0.5–1 mM Nα-benzoyl-DL-arginine p-nitroanilide (BApNA; Sigma) was used per assay. For chymotrypsin activity, 0.5–1 mM of N-succinyl-ala-ala-pro-phe p-nitroanilide substrate (Sigma) was used. Immediately before use, substrates were diluted in 0.1 M Tris-HCl, pH 8.0 from a 100 mM stock in dimethylsulfoxide and mixed with the equivalent of 1/10 of a midgut. For chymotrypsin assays, 0.1 or 0.5 mM of the specific inhibitor 2-nitro-4-carboxyphenyl-N, N-diphenylcarbamate (Sigma) was also used. Assays were done at 26°C; chromogen release was followed for 45 min using a kinetics module in a spectrophotometric microplate reader (Molecular Devices) set at 405 nm.
Statistical analyses
Comparisons in which the data represented replicate samples were carried out using t-tests. All P-values are two-sided, and were calculated using Prism 4 (Graphpad Software, San Diego, CA, USA).
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIAID, NIH, and in part by NIAID Grant Support (SMB and SJT AI031078).
Footnotes
Publisher's Disclaimer: This article is a US Government work and is in the public domain in the USA
Since submission of this article, a report by Svárovská has been published confirming the susceptibility of lpg2− promastigotes to early killing following infection of three different sand fly species; Svárovská A, Ant TH, Seblová V, Jecná L, Beverley SM, and Volf P. (2010) Leishmania major glycosylation mutants require phosphoglycans (lpg2−) but not lipophosphoglycan (lpg1−) for survival in permissive sand fly vectors. PLoS Negl Trop Dis. 4: e580.
References
- Adler S. Factors determining the behaviour of Leishmania sp. in sandflies. Harefuah. 1938;14:1–6. [Google Scholar]
- Borovsky D, Schlein Y. Trypsin and chymotrypsin-like enzymes of the sandfly Phlebotomus papatasi infected with Leishmania and their possible role in vector competence. Med Vet Entomol. 1987;1:235–242. doi: 10.1111/j.1365-2915.1987.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Boulanger N, Lowenberger C, Volf P, Ursic R, Sigutova L, Sabatier L, et al. Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect Immun. 2004;72:7140–7146. doi: 10.1128/IAI.72.12.7140-7146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capul AA, Barron T, Dobson DE, Turco SJ, Beverley SM. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J Biol Chem. 2007a;282:14006–14017. doi: 10.1074/jbc.M610869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capul AA, Hickerson S, Barron T, Turco SJ, Beverley SM. Comparisons of mutants lacking the Golgi UDP-galactose or GDP-mannose transporters establish that phosphoglycans are important for promastigote but not amastigote virulence in Leishmania major. Infect Immun. 2007b;75:4629–4637. doi: 10.1128/IAI.00735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CR, Cooper AM, Peacock C, Lane RP, Blackwell JM. Expression of LPG and GP63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi. Parasitology. 1990;101 (Part 3):337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Lane RP. Bloodmeal digestion in the midgut of Phlebotomus papatasi and Phlebotomus langeroni. Med Vet Entomol. 1993;7:225–232. doi: 10.1111/j.1365-2915.1993.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gillis KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Biochem. 1956;28:350–356. [Google Scholar]
- Greis KD, Turco SJ, Thomas JR, McConville MJ, Homans SW, Ferguson MA. Purification and characterization of an extracellular phosphoglycan from Leishmania donovani. J Biol Chem. 1992;267:5876–5881. [PubMed] [Google Scholar]
- Homans SW, Mehlert A, Turco SJ. Solution structure of the lipophosphoglycan of Leishmania donovani. Biochemistry. 1992;31:654–661. doi: 10.1021/bi00118a004. [DOI] [PubMed] [Google Scholar]
- Hong K, Ma D, Beverley SM, Turco SJ. The Leishmania GDP-mannose transporter is an autonomous, multi-specific, hexameric complex of LPG2 subunits. Biochemistry. 2000;39:2013–2022. doi: 10.1021/bi992363l. [DOI] [PubMed] [Google Scholar]
- de Ibarra AA, Howard JG, Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982;85 (Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- Ilg T. Proteophosphoglycans of Leishmania. Parasitol Today. 2000;16:489–497. doi: 10.1016/s0169-4758(00)01791-9. [DOI] [PubMed] [Google Scholar]
- Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous, mucin- like proteophosphoglycan secreted by Leishmania parasites. J Biol Chem. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- Ilg T, Montgomery J, Stierhof YD, Handman E. Molecular cloning and characterization of a novel repeat-containing Leishmania major gene, ppg1, that encodes a membrane-associated form of proteophosphoglycan with a putative glycosylphosphatidylinositol anchor. J Biol Chem. 1999;274:31410–31420. doi: 10.1074/jbc.274.44.31410. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, et al. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Ferguson MA. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand M, Daoud S, Titus RG, Louis J, Boon T. Variants with reduced virulence derived from Leishmania major after mutagen treatment. Parasite Immunol. 1987;9:81–92. doi: 10.1111/j.1365-3024.1987.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Myskova J, Svobodova M, Beverley SM, Volf P. A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microbes Infect. 2007;9:317–324. doi: 10.1016/j.micinf.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderer T, Vince JE, McConville MJ. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr Mol Med. 2004;4:649–665. doi: 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Jr, Turco SJ. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987;262:10384–10391. [PubMed] [Google Scholar]
- Pimenta PF, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, Sacks DL. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Pimenta PF, Saraiva EM, Rowton E, Modi GB, Garraway LA, Beverley SM, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc Natl Acad Sci USA. 1994;91:9155–9156. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Ortigao M, Jochim RC, Anderson JM, Lawyer PG, Pham VM, Kamhawi S, Valenzuela JG. Exploring the midgut transcriptome of Phlebotomus papatasi: comparative analysis of expression profiles of sugar-fed, blood-fed and Leishmania-major-infected sandflies. BMC Genomics. 2007;8:300. doi: 10.1186/1471-2164-8-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Ilg T, Nikolaev AV, Ferguson MA, Bates PA. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430:463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Hajmova M, Joshi MB, Sadlova J, Dwyer DM, Volf P, Bates PA. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008;10:1363–1372. doi: 10.1111/j.1462-5822.2008.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Modi G, Rowton E, Spath G, Epstein L, Turco SJ, Beverley SM. The role of phosphoglycans in Leishmania-sand fly interactions. Proc Natl Acad Sci USA. 2000;97:406–411. doi: 10.1073/pnas.97.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’anna MR, Diaz-Albiter H, Mubaraki M, Dillon RJ, Bates PA. Inhibition of trypsin expression in Lutzomyia longipalpis using RNAi enhances the survival of Leishmania. Parasit Vectors. 2009;2:62. doi: 10.1186/1756-3305-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein Y, Romano H. Leishmania major and L. donovani: effects on proteolytic enzymes of Phlebotomus papatasi (Diptera, Psychodidae) Exp Parasitol. 1986;62:376–380. doi: 10.1016/0014-4894(86)90045-7. [DOI] [PubMed] [Google Scholar]
- Shakarian AM, Dwyer DM. Structurally conserved soluble acid phosphatases are synthesized and released by Leishmania major promastigotes. Exp Parasitol. 2000;95:79–84. doi: 10.1006/expr.2000.4511. [DOI] [PubMed] [Google Scholar]
- Skipski VP, Barclay M. Thin layer chromatography of lipids. Methods Enzymol. 1969;14:530–598. [Google Scholar]
- Spath GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci USA. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003;301:1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Turco SJ, Spath GF, Beverley SM. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 2001;17:223–226. doi: 10.1016/s1471-4922(01)01895-5. [DOI] [PubMed] [Google Scholar]
- Walters LL. Leishmania differentiation in natural and unnatural sand fly hosts. J Eukaryot Microbiol. 1993;40:196–206. doi: 10.1111/j.1550-7408.1993.tb04904.x. [DOI] [PubMed] [Google Scholar]