Abstract
Imaging of the kidneys can provide valuable information in the work up and management of acute kidney injury. Several different imaging modalities are used to gather information on anatomy of the kidney, to rule out obstruction, differentiate acute kidney injury (AKI) and chronic kidney disease and to obtain information on renal blood flow and GFR. Ultrasound is the most widely used imaging modality used in the initial work up of AKI. The utility of contrast enhanced computerized tomography and magnetic resonance imaging is limited because of toxicities associated with contrast agents used. In this review the basics of ultrasonography are reviewed with an emphasis on findings in AKI. The new developments in different imaging modality and their potential uses in AKI are reviewed as well.
INTRODUCTION
Acute kidney injury (AKI) is characterized by a rapid decline in kidney function within a few hours to a few days. AKI is relatively common among hospitalized patients, especially those in the intensive care units and is associated with higher morbidity and mortality [1, 2]. Causes of AKI vary, and may range from reduced kidney perfusion and prerenal azotemia to direct renal toxicity and urinary tract obstruction. While the end result is similar, i.e., retention of waste products and fluid overload, the anatomy and histology of the kidney and the outcomes differ significantly depending on the cause. Different imaging techniques can potentially provide valuable information with respect to the anatomy of the kidney, the possibility of obstruction, inflammation and edema and the quantity and pattern of renal perfusion. However, depending on the condition of the patient and the severity of kidney injury, not all imaging modalities are suitable for all individuals. In the majority of cases, renal ultrasonography is the imaging study of choice; mainly because of its ease of use, noninvasive nature, safety profile and the fact that practitioners can readily access this technique.
ULTRASOUND
Two-dimensional grey scale ultrasound is the imaging technique most commonly used in initial evaluation of patients with acute or chronic kidney disease. It is widely available, easy to use and free of complications. Portability of ultrasound is also an important advantage, especially for critically ill patients in the ICU setting. Recent advances in ultrasound technology and improved image quality have greatly improved our ability to visualize the kidneys with ultrasound. Although the rate of abnormal ultrasound findings in the setting of AKI is not high (about 10%), these findings can have a significant impact on patient management [3].
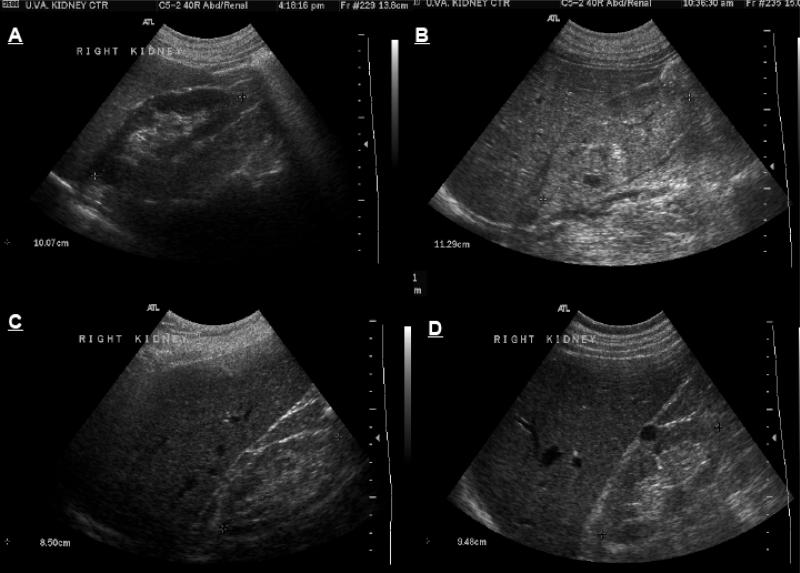
The basic information provided by the B (for brightness) mode ultrasonography of the kidney include renal size and cortical echogenecity (Fig. 1). Kidney size is a useful parameter in differentiating acute from chronic kidney disease, where small size kidneys are expected. The longitudinal length of the kidney is the most helpful measure, while other diameters and the volume of the kidney provide information of lesser value [4]. Enlarged kidneys in the setting of AKI suggest infiltrative diseases such as lymphoma, monoclonal gammapathies and could also be seen in cases of acute proliferative glomerulonephritis (GN), acute tubular necrosis (ATN) and acute interstitial nephritis (AIN) [5]. Kidneys are also expected to be enlarged in renal vein thrombosis. In individuals with a transplanted kidney, an enlarged kidney on ultrasound has sensitivity and specificity greater than 80% for diagnosis of acute rejection [6].
Fig. 1.
B-mode ultrasound images of (A) normal kidney, (B) enlarged and echogenic kidney with loss of differentiation between cortical, medullary and sinus fat compartments in a case with acute kidney injury, (C) small, slightly echogenic kidney with thin cortex in a patient with chronic kidney disease and (D) normal size but echogenic kidney with a single simple cyst in a patient with chronic kidney disease secondary to diabetic nephropathy.
The echogenecity of tissue, or the brightness of the ultrasound image in B mode, depends on the echoes of the ultrasound from the structures within the tissue. A marked mismatch in impedance between adjacent tissues is a major determinant of the strength of these echoes. In the case of the kidneys, a significant mismatch is seen between normal kidney tissue and the sinus fat surrounding the caliceal system. As a result, more sound reflection results in a bright image in the central areas of the renal ultrasound image. Another determinant of the brightness of the ultrasound image is the number of scatterers per unit of tissue volume or tissue density. For example, edema of the tissue would result in poor echoes and tissue infiltration increases echogenecity. Examples of hyperechoic kidneys in setting of AKI are monoclonal gammapathy, nephrotoxic ATN [4] and acute proliferative and crescentic GN [7].
Cortical thickness not only differentiates between AKI and CKD, the latter being associated with thin cortices, increased cortical thickness may suggest edema or infiltration within the renal cortex. ATN, acute GN and acute interstitial nephritis (AIN) are examples of situations in which enlarged kidneys with thick and echogenic cortices could be seen on renal ultrasonography [4]. However, lack of precision in measuring cortical thickness and the lack of standards for definition of a thick cortex, have limited the use of this parameter except in cases where a baseline ultrasound examination exists.
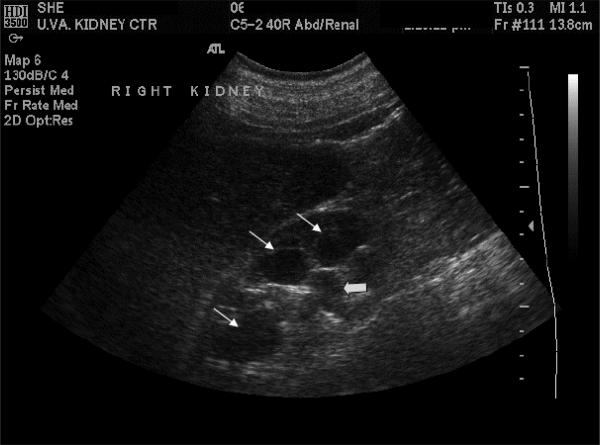
B-mode ultrasonography is a very useful imaging modality to rule out the possibility of urinary tract obstruction as the cause of AKI, if clinically indicated (Fig. 2). While caliceal dilatation suggests urinary tract obstruction, conditions such as pregnancy [8] and diabetes insipidus [9] can also result in caliceal dilation. The sensitivity of ultrasound in diagnosing urinary tract obstruction in a renal allograft is very high (>90%) [10]. The specificity of ultrasound in these cases reaches acceptably high values only in severe cases of hydronephrosis [4].
Fig. 2.
B-mode ultrasound image of the kidney demonstrating hydronephrosis. Dilated calices (thin arrows) and renal hilum and the origin of ureter (thick arrow) are seen in this image.
Doppler ultrasound provides information on renal blood flow. Resistive indices, i.e., peak systolic velocity minus peak dialstolic velocity divided by peak systolic velocity, are calculated by analysis of Doppler waveform in the main renal artery or smaller branches within the kidney. Recent advances in ultrasound technology allow for the study of arteries up to the level of interlobular arteries. However, preparation of patients, body habitus and experience of the operator are major impediments in obtaining reliable results with this technique. In one study of 41 individuals with kidney diseases, comparing Doppler US findings with biopsy results, kidneys with active tubulointerstitial disease had higher RI compared to the kidneys with glomerular disease (0.75 versus 0.58, P<0.01) [3]. Mean RI for patients with ATN and vasculitis/vasculopathy were 0.78 and 0.82, respectively, in another study [3]. Although it has been suggested that RI greater than 0.75 is more likely associated with intrarenal rather than prerenal causes of AKI, the issues associated with the measurement of RI and the fact that it increases with age, make this method less reliable [11].
Other ultrasonographic findings that may be useful in differential diagnosis of AKI are a finding of a hypodense band surrounding the kidney in acute cortical necrosis.
COMPUTERIZED TOMOGRAPHY
Significant advances in CT technology have occurred since its introduction almost 40 years ago. Scans are acquired in a single spiral fashion with very thin slices. The new volumetric scanners with several rows of detectors have revolutionized CT imaging. In particular, this technology has provided the opportunity for reconstructing high resolution 3D images. This is particularly significant in the field of CT angiography, which is quickly replacing catheter angiography [12].
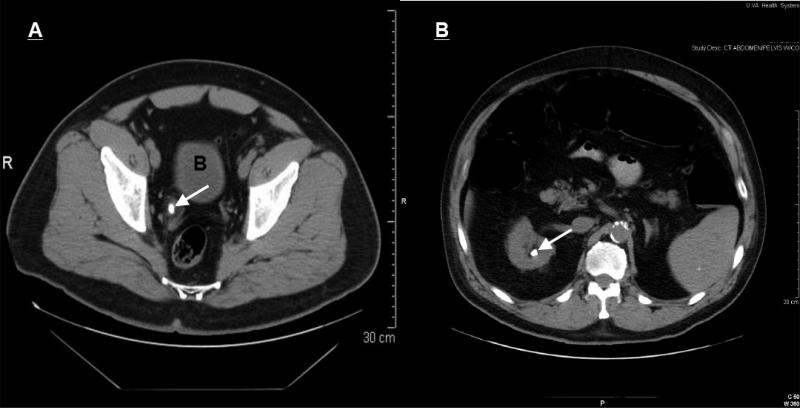
Non-contrast helical CT is currently considered the gold-standard for the diagnosis of Nephrolithiasis (Fig. 3). All types of stones, except for indinavir stones, are detected by non-contrast CT. This imaging technique provides information on the location of the stone, the degree of stenosis and the general anatomy of the kidneys without the use of contrast agents. This provides for an ideal imaging modality for those suspected of having AKI induced by obstruction as a result of a stone. Other findings, such as the presence of hydronephrosis or hydroureter with or without perinephric or periureteric stranding of the fat on the same side as the stone, increase the specificity of CT. Stranding of a periureteric fat may persist even after a stone is passed and symptoms are resolved.
Fig. 3.
Unehanced computerized tomography (CT) images of (A) distal right ureteral stone (arrow) with periureteric stranding and (B) large kidney stone (arrow). B = bladder.
Other potential causes of acute kidney injury, such as a leaking abdominal aortic aneurysm or an intra-abdominal inflammatory or infectious process (localized stranding of intra-abdominal fat), could be detected using non-enhanced CT. However, intravenous contrast agents are required to enhance the CT image in all other situations. Contrast-enhanced CT is useful in the diagnosis of renal tumors, renal artery stenosis and parenchymal renal disease. After injection of the contrast agent using a triphasic helical CT, also known as functional CT, glomerular filtration rate and renal blood flow can be measured as well [13]. However, due to the high risk nephrotoxicity associated with the use of contrast agents, CT scans are only used for unenhanced studies in cases suspicious of kidney stones not detected by ultrasonography.
MAGNETIC RESONANCE (MR) IMAGING
More recent advances in MR imaging technology have provided potentials for future utility of this imagin g technique in the setting of AKI. All of the currently FDA-approved contrast agents for MR imaging are gadolinium based and thus unsuitable for use in patients with low GFR because of the risk of nephrogenic systemic sclerosis (NSF). However, newly developed contrast agents have been shown to be useful and safe in the setting of reduced kidney function in animal and human studies. MR angiography (MRA) using ultra-small particles of iron oxide (USPIO) could be used for the study of RBF and renal blood volume [14]. These agents are macromolecular structures based on iron molecules that are not filtered through glomeruli and serve as true blood pool agents. They provide enhancement characteristics similar to gadolinium-based contrast agents.
USPIO, Ferumoxtran-10 and ferumoxytol, were developed for iron replacement in patients with advanced CKD and phase III studies using ferumoxytol are completed. In those studies, USPIO resulted in a good safety profile [15]. While ferumoxtran requires slow infusion to avoid adverse reaction, ferumoxytol is free of systemic adverse events and can be administered as a bolus for MRA studies [15]. USPIO are negatively charged and are picked up by activated monocytes and macrophages. Therefore, increased localized tissue uptake in a late film (24 hours after injection), may indicate the presence of inflammation [14, 16]. This has been demonstrated in acute cellular rejection in renal transplantation, acute glomerulonephritis and in animal models of ischemia reperfusion [14, 15].
Using deoxyhemoglobin as an endogenous contrast agent, blood oxygen level dependent (BOLD) MR imaging is a novel and noninvasive imaging method to assess tissue oxygen delivery. Reduced partial pressure of oxygen in tissues, and as a result of increased deoxyhemoglobin concentration, results in a decreased intensity on T2-weighted MR images. Small human studies have shown that this technique is useful in assessing changes in renal medullary oxygenation in response to ingestion of different drugs and water loading [17-19]. This technique is also useful to detect altered medullary oxygenation in disease states such as acute ureteral obstruction[20] and vascular occlusive disease [19].
NUCLEAR MEDICINE IMAGING
99m Technetium (Tc) is the preferred isotope for nuclear medicine imaging of the kidney, due to its good imaging capability with low radiation doses and its relatively short half-life of 6 hours. Dimethylenetriaminepentaacetic acid (DTPA) and mercaptoacetyltriglycine (MAG3) bound 99mTc are commonly used to measure GFR and RBF, respectively. Study of RBF using 99mTc-MAG3 scan may be helpful in the setting of AKI, but is not widely used. While the renal uptake in the first 1 – 2 minutes is normal in prerenal azotemia, it is expected to be reduced in vascular and parenchymal diseases and in ATN [21]. After 20 minutes, the uptake is increased in cases with prerenal azotemia, vascular disease and ATN and is expected to be reduced in obstructive uropathy and parenchymal renal disease. Excretion of the radioisotope is reduced in AKI irrespective of the cause [21].
Positron emission tomography (PET) provides significantly better spatial resolution than conventional scintigraphy and in addition has the capacity to provide data on the function and molecular composition of an organ. Combination of PET and CT scanning provides an opportunity to add functional and quantitative data with anatomical and spatial information to be able to localize lesions. Tracers used for PET scans are either limited to the tissue such as Rb-82, N-13 and Cu-62 PTSM (category II) or can freely diffuse between the blood pool and the tissue, like O-15 (category I) [22]. Potential clinical uses for PET scans in the future based on the results of few studies of kidney imaging include, determination of RBF and GFR, diagnosis of renal artery stenosis, determination of the function of tubular peptide, cation and anion transporters, tissue activity of enzymes such as angiotensin converting enzyme and regulation of receptors such as angiotensin type 1 receptors within the kidney [22]. In particular, the changes in expression of molecules such as reactive oxygen species that control cell response and repair after injury in the setting of AKI could potentially be determined by PET scanning [23].
Traditionally, PET scanning has required an arterial line and not been widely available. Most of the PET imaging studies of the kidneys are performed for research purposes at this time.
CONTRAST-ENHANCED ULTRASOUND
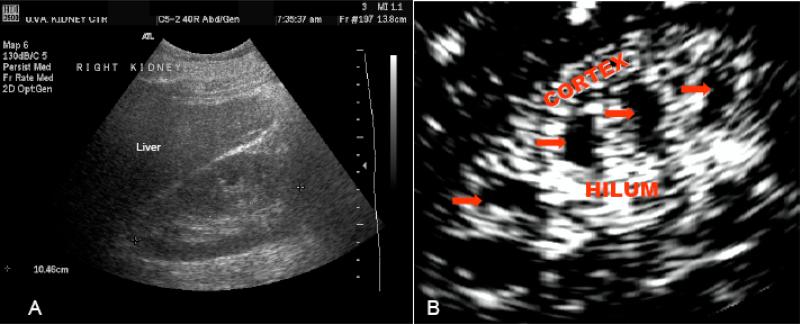
Contrast enhanced ultrasonography (CEU) has been used extensively in cardiology to assess myocardial perfusion during echocardiography [24-29]. In this technique, stabilized gas bubbles are injected intravenously to enhance the ultrasound image. Contrast agents used for ultrasonography are microbubbles made of a protein, saccharide or lipid shell and a high molecular weight gas such as perfluorocarbons [30]. Reflection of ultrasound waves off of these gas filled microbubbles results in a bright image of the vasculature within the organ (Fig. 4).
Fig. 4.
B-mode (A), and contrast-enhanced (B) ultrasound images of the kidney. Note the marked improvement in image quality especially in clear distinction between the cortex and medulla in the contrast enhanced image. This technique can provide useful information on the quantity and pattern of blood flow, volume and velocity to different regions within the kidney tissue.
Ultrasound contrast agents are superior to contrast agents used in magnetic resonance or computed tomography for imaging of the vasculture because they behave the same as red blood cells and do not diffuse out of the vascular space [25]. The small diameter of microbubbles (1 and 6 μm), makes it possible for them to pass through all capillary beds including the pulmonary capillaries [31]. As a result, ultrasonographic imaging of the vasculature is possible after intravenous infusion of these contrast agents.
Ultrasound contrast agents have been proven to be safe and free of hemodynamic effects. CEU is an ideal imaging modality for the study of microcirculation as well as the study of blood flow patterns and blood velocity in larger vessels and tissue blood flow [25, 32].
CEU involves continuous Power Modulation ultrasound imaging of the tissue (kidney) with low mechanical index (MI 0.1) during constant intravenous infusion of the ultrasound contrast agent. After about 2 minutes, when the microbubbles reach the steady state, all the microbubbles within the kidney are disrupted using high energy ultrasound (MI 1.0). Elimination of the contrast agent from the tissue results in a sharp decrease in the video intensity immediately after the high energy pulse. Microbubbles will then start to reappear in the tissue at a constant rate until they reach a plateau which is considered the tissue volume or reserve. Continuous ultrasound imaging using Power Modulation with low mechanical index of 0.1 (to avoid further disruption of microbubbles) after application of the high energy pulse provides information on the rate of reappearance of microbubbles in the kidney tissue. This information is used to estimate RBC velocity (Fig. 5) [33].
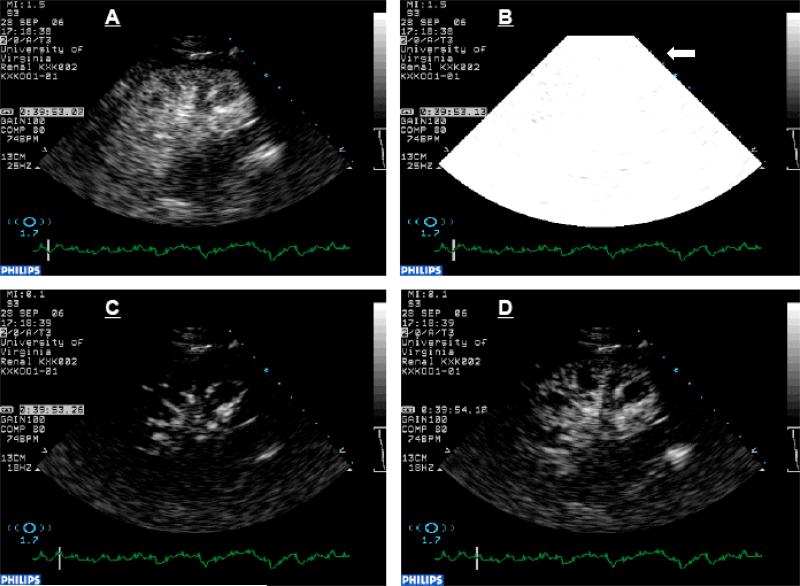
Fig. 5.
Sequential contrast-enhanced images of kidney with frames selected from (A) steady state during infusion of contrast agent, (B) high energy pulse and destruction of tissue microbubbles within the kidney, (C) replenishment of the kidney tissue with contrast agent observed first in the main, interlobar and arcuate arteries in a fraction of a second and later in the renal cortex (after about 1 second) (D).
Currently CEU is only approved by the FDA for myocardial blood flow studies. However, a few human studies have demonstrated its utility as a quick, practical and safe method of monitoring changes in renal blood flow. The pattern and quantity of blood flow and tissue blood volume in different regions within the organ, i.e., cortex separately from medulla, can be determined within a few minutes with this relatively noninvasive technique, which is particularly desirable in clinical situations in which patient transfer out of the intensive care unit is an issue. By providing information on renal hemodynamis, as well as regional changes in renal blood flow, CEU has the potential to serve as a helpful tool in the diagnostic work up of patients with AKI.
SUMMARY
Renal ultrasonography remains the safest, most useful and most widely used imaging technique in the initial assessment of patients with AKI. Information relating to kidney size and echogenicity are valuable in directing physicians towards appropriate decision making pathways in the management of AKI. One of the main utilities of ultrasound in this setting is eliminating the possibility of significant obstruction.
Contrast enhanced ultrasonography has the potential of serving as a safe and practical tool in the study of renal hemodynamics in the future. Development of new contrast agents for MR imaging, although not yet in clinical use, can potentially make this modality desirable in this setting. PET scans provide valuable information on tissue composition and function, but require further research and development and more widespread availability before they become a significant contributor to the routine diagnostic work up of patients.
REFERENCES
- 1.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 3.Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154:1223–7. doi: 10.2214/ajr.154.6.2110732. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill WC. B-mode sonography in acute renal failure. Nephron Clin Pract. 2006;103:c19–23. doi: 10.1159/000090604. [DOI] [PubMed] [Google Scholar]
- 5.Pozzi Mucelli R, Bertolotto M, Quaia E. Imaging techniques in acute renal failure. Contrib Nephrol. 2001:76–91. doi: 10.1159/000060076. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson ML, Bell A, Burton PR, Donnelly PK, Veitch PS, Bell PR. Probability of rejection predicted from ultrasonographic measurement of renal transplant swelling. Br J Surg. 1993;80:1059–62. doi: 10.1002/bjs.1800800846. [DOI] [PubMed] [Google Scholar]
- 7.Longmaid HE, 3rd, Rider E, Tymkiw J. Lupus nephritis: New sonographic findings. J Ultrasound Med. 1987;6:75–9. doi: 10.7863/jum.1987.6.2.75. [DOI] [PubMed] [Google Scholar]
- 8.Peake SL, Roxburgh HB, Langlois SL. Ultrasonic assessment of hydronephrosis of pregnancy. Radiology. 1983;146:167–70. doi: 10.1148/radiology.146.1.6849041. [DOI] [PubMed] [Google Scholar]
- 9.Stevens S, Brown BD, McGahan JP. Nephrogenic diabetes insipidus: a cause of severe nonobstructive urinary tract dilatation. J Ultrasound Med. 1995;14:543–5. doi: 10.7863/jum.1995.14.7.543. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb RH, Voci SL, Cholewinski SP, Hartley DF, Rubens DJ, Orloff MS, et al. Sonography: a useful tool to detect the mechanical causes of renal transplant dysfunction. J Clin Ultrasound. 1999;27:325–33. doi: 10.1002/(sici)1097-0096(199907/08)27:6<325::aid-jcu3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Quaia E, Bertolotto M. Renal parenchymal diseases: is characterization feasible with ultrasound? Eur Radiol. 2002;12:2006–20. doi: 10.1007/s00330-002-1360-z. [DOI] [PubMed] [Google Scholar]
- 12.Read S, Allen C, Hare C. Applications of computed tomography in renal imaging. Nephron Clin Pract. 2006;103:c29–36. doi: 10.1159/000090606. [DOI] [PubMed] [Google Scholar]
- 13.Hackstein N, Wiegand C, Rau WS, Langheinrich AC. Glomerular filtration rate measured by using triphasic helical CT with a two-point Patlak plot technique. Radiology. 2004;230:221–6. doi: 10.1148/radiol.2301021266. [DOI] [PubMed] [Google Scholar]
- 14.Choyke PL, Kobayashi H. Functional magnetic resonance imaging of the kidney using macromolecular contrast agents. Abdom Imaging. 2006;31:224–31. doi: 10.1007/s00261-005-0390-9. [DOI] [PubMed] [Google Scholar]
- 15.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, Jacobs PM, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75:465–74. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharfuddin AA, Sandoval RM, Molitoris BA. Imaging techniques in acute kidney injury. Nephron Clin Pract. 2008;109:c198–204. doi: 10.1159/000142929. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann L, Simon-Zoula S, Nowak A, Giger A, Vock P, Boesch C, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney. Int. 2006;70:144–50. doi: 10.1038/sj.ki.5000418. [DOI] [PubMed] [Google Scholar]
- 18.Tumkur SM, Vu AT, Li LP, Pierchala L, Prasad PV. Evaluation of intra-renal oxygenation during water diuresis: a time-resolved study using BOLD MRI. Kidney Int. 2006;70:139–43. doi: 10.1038/sj.ki.5000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780–8. doi: 10.1681/ASN.2007040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoeny HC, Kessler TM, Simon-Zoula S, De Keyzer F, Mohaupt M, Studer UE, et al. Renal oxygenation changes during acute unilateral ureteral obstruction: assessment with blood oxygen level-dependent mr imaging--initial experience. Radiology. 2008;247:754–61. doi: 10.1148/radiol.2473070877. [DOI] [PubMed] [Google Scholar]
- 21.Haufe SE, Riedmuller K, Haberkorn U. Nuclear medicine procedures for the diagnosis of acute and chronic renal failure. Nephron Clin Pract. 2006;103:c77–84. doi: 10.1159/000091576. [DOI] [PubMed] [Google Scholar]
- 22.Szabo Z, Xia J, Mathews WB, Brown PR. Future direction of renal positron emission tomography. Semin Nucl Med. 2006;36:36–50. doi: 10.1053/j.semnuclmed.2005.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabo Z, Xia J, Mathews WB. Radiopharmaceuticals for renal positron emission tomography imaging. Semin Nucl Med. 2008;38:20–31. doi: 10.1053/j.semnuclmed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Kaul S, Senior R, Dittrich H, Raval U, Khattar R, Lahiri A. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography. Circulation. 1997;96:785–92. [PubMed] [Google Scholar]
- 25.Lindner J, Wei K. Contrast Echocardiography. Curr Probl Cardiol. 2002;27:449–520. doi: 10.1067/mcd.2002.129364. (abstract) [DOI] [PubMed] [Google Scholar]
- 26.Swinburn JM, Lahiri A, Senior R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J Am Coll Cardiol. 2001;38:19–25. doi: 10.1016/s0735-1097(01)01317-1. [DOI] [PubMed] [Google Scholar]
- 27.Ragosta M, Camarano G, Kaul S, Powers ER, Sarembock IJ, Gimple LW. Microvascular integrity indicates myocellular viability in patients with recent myocardial infarction. New insights using myocardial contrast echocardiography. Circulation. 1994;89:2562–9. doi: 10.1161/01.cir.89.6.2562. [DOI] [PubMed] [Google Scholar]
- 28.Lepper W, Hoffmann R, Kamp O, Franke A, de Cock CC, Kuhl HP, et al. Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty. Circulation. 2000;101:2368–74. doi: 10.1161/01.cir.101.20.2368. [DOI] [PubMed] [Google Scholar]
- 29.Balcells E, Powers ER, Lepper W, Belcik T, Wei K, Ragosta M, et al. Detection of myocardial viability by contrast echocardiography in acute infarction predicts recovery of resting function and contractile reserve. J Am Coll Cardiol. 2003;41:827–33. doi: 10.1016/s0735-1097(02)02962-5. [DOI] [PubMed] [Google Scholar]
- 30.Jakobsen JA, Correas JM. Ultrasound contrast agents and their use in urogenital radiology: status and prospects. Eur Radiol. 2001;11:2082–91. doi: 10.1007/s003300000817. [DOI] [PubMed] [Google Scholar]
- 31.Correas JM, Bridal L, Lesavre A, Mejean A, Claudon M, Helenon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001;11:1316–28. doi: 10.1007/s003300100940. [DOI] [PubMed] [Google Scholar]
- 32.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 33.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–40. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]