Abstract
Cholesterol status and dietary fat alter several metabolic pathways reflected in lipoprotein profiles. To assess plasma lipoprotein response and mechanisms by which cholesterol and dietary fat type regulate expression of genes involved in lipoprotein metabolism we developed an experimental model system using F1B hamsters fed diets (12 weeks) enriched in 10% (w/w) coconut, olive or safflower oil with either high cholesterol (0.1%; cholesterol-supplemented) or low cholesterol coupled with cholesterol lowering drugs 10-days prior to killing (0.01% cholesterol, 0.15% lovastatin, 2% cholestyramine; cholesterol-depleted). Irrespective of dietary fat, cholesterol-depletion, relative to supplementation, resulted in lower plasma non-high density lipoprotein (HDL) and HDL cholesterol, and triglyceride concentrations (all P<0.05). In the liver, these differences were associated with higher sterol regulatory element binding protein (SREBP)-2, low density lipoprotein (LDL) receptor, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and 7-α hydroxylase mRNA levels; higher scavenger receptor B1 and apolipoprotein (apo) A-I mRNA and protein levels; and lower apo E protein levels and in intestine modestly lower sterol transporters ATP binding cassette (ABC) A1, ABCG5 and ABCG8 mRNA levels. Irrespective of cholesterol status, coconut oil, relative to olive and safflower oils, resulted in higher non-HDL cholesterol and triglyceride concentrations (both P<0.05) and modestly higher SREBP-2 mRNA levels. These data suggest that in F1B hamsters, differences in plasma lipoprotein profiles in response to cholesterol depletion are associated with changes in the expression of genes involved in cholesterol metabolism, whereas the effect of dietary fat type on gene expression was modest which limits the usefulness of the experimental animal model.
Keywords: fatty acids, plasma cholesterol, statin, triglyceride, gene expression
1. Introduction
Cholesterol homeostasis plays an important role in the regulation of whole body cholesterol content by balancing absorption (intestinal and biliary) and synthesis (hepatic and extra-hepatic), thereby preventing the net accumulation of cholesterol in circulation and tissues [1, 2]. Dietary fatty acid chain length and degree of saturation have been shown to alter several metabolic pathways involving cholesterol throughout the body, the combined effect of which is reflected in plasma lipid and lipoprotein profiles [3–5]. The availability of an intact and unmodified animal model would allow for the simultaneous assessment of dietary fat type induced changes in multiple tissue systems within the context of altered cholesterol homeostasis and facilitate the development of a more complete understanding of the complex set of factors known to regulate lipid and lipoprotein metabolism and ultimately atherosclerotic lesion development.
To achieve this, we chose the hamster to develop an experimental animal model because it has a low rate of endogenous cholesterol synthesis, and is one of the few rodents to have cholesteryl ester transfer protein activity and possess tissue specific editing of apo B mRNA [6–9]. Hamsters, like humans, take up approximately 80% of LDL cholesterol via the LDL receptor pathway [7]. The F1B strain was chosen because it had been reported to respond to saturated fat and cholesterol by increasing the non-HDL fraction to a greater extent than the HDL fraction [6, 7, 10–13]. Using the FIB hamster, whole body cholesterol status was altered by feeding either a low cholesterol diet followed by acute cholesterol depletion induced by plasma cholesterol lowering drugs (3-hydroxy-3-methyl-glutaryl [HMG]-CoA reductase inhibitor and bile acid sequestrant), or a high cholesterol diet. This experimental approach has been used successfully in multiple animal species to address other experimental questions [12, 14, 15].
The liver plays a major role in lipoprotein metabolism. Hepatic cholesterol levels are controlled by a balance between cholesterol synthesis, uptake and secretion, primarily through the activities of HMG-CoA reductase, low-density lipoprotein (LDL) receptor and 7α- hydroxylase, respectively, and are important determinants of plasma lipoprotein profiles [6, 16, 17]. Sterol regulatory element binding protein (SREBP)- 2, a member of the SREBP family of transcription factors, regulates cholesterol synthesis and uptake through alterations in the expression of HMG-CoA reductase and LDL receptor [1, 18]. A sterol sensor in the endoplasmic reticulum modulates SREBP-2 transcriptional activity in response to changes in intracellular free cholesterol levels [1]. Fatty acid chain length and degree of saturation indirectly regulate SREBP-2 transcriptional activity by altering the free cholesterol regulatory pool [19]. The rate of acyl-CoA cholesterol acyl transferase (ACAT) activity alters intracellular free cholesterol levels and formation of cholesteryl ester for hepatic intracellular de novo lipoprotein synthesis [20]. The cell surface receptors ATP-binding cassette transporter (ABC) A1 and scavenger receptor class B type 1 (SR-B1) activities are determinants of hepatic substrate availability and circulating lipoproteins [21–24]. Additional factors regulating hepatic lipoprotein synthesis include the availability of triglyceride [6, 25, 26], apolipoprotein (apo) B-100 and apo E [27, 28], and microsomal triglyceride transfer protein (MTP) activity [29–31].
Intestinal cholesterol absorption also modulates lipoprotein metabolism, primarily through a family of ABC transporters, ABCA1, ABGG5 and ABCG8 [32]. These transporters control the trafficking of intestinal sterol balance by facilitating the efflux of sterols from the apical (ABCG5/8) [33] or basolateral (ABCA1) [34] membrane of the enterocyte. Niemann-Pick C1 Like1 (NPC1L1) facilitates intestinal sterol uptake on the apical side of the enterocyte [35].
Dietary fatty acid type and cholesterol have been shown to modulate the level and activity of transcription factors, which regulates the expression of genes involved in cholesterol and lipoprotein metabolism [3]. PUFA and cholesterol metabolites are the ligands for transcription factors PPAR, LXR and FXR, which play a role in regulating plasma lipoprotein profiles [3, 36–38]. Dietary fatty acids and cholesterol regulate SREBP activity through altering levels and cellular location of the transcription factor [1, 19, 39, 40].
To assess the effect of fatty acid chain length and degree of saturation, both cholesterol-supplemented and cholesterol-depleted F1B hamsters were fed diets enriched in fats high in saturated, monounsaturated or polyunsaturated fatty acids. Using this approach, our aim was to simultaneously assess the mechanisms by which whole body cholesterol status and dietary fat type regulate the expression of genes involved in hepatic and intestinal cholesterol and lipoprotein metabolism.
2. Materials and methods
2.1. Animals and diets
Ninety-six 8 week-old male F1B hamsters (BioBreeders, Watertown, MA) were divided into six diet groups on the basis of body weight and housed in stainless steel suspended cages (4 hamsters /cage) with a reverse 12:12 light: dark cycle. Hamsters were given free access to LM-485 mouse/rat diet (Harlan-Teklad, Madison, WI) and water during a two-week acclimation period. Thereafter, the hamsters were switched to ad libitum semi-purified diets containing 10% (w/w) coconut oil (saturated fatty acids), olive oil (monounsaturated fatty acids) or safflower oil (n-6 polyunsaturated fatty acids), in combination with 0.1% (w/w) cholesterol or 0.01% (w/w) cholesterol for 12 weeks (Tables 1 and Table 2, diet composition and diet fatty acid profile, respectively). The analytical data were consistent with the intended diet composition. To determine the effect of acute whole body cholesterol depletion on gene expression and protein synthesis, during the last ten days of the dietary period, 0.15% lovastatin (Merck & Co., Inc. Rahway, NJ) and 2% cholestyramine (Bristol-Myers Squibb Co., Princeton, NJ) were added to the low cholesterol diets. Hence, the 0.01% cholesterol plus lipid lowering drug diets and 0.1% cholesterol were designed to deplete (−C) and supplement (+C) cholesterol, respectively, in the animals with the intent to alter cholesterol homeostasis, (coconut −C, olive −C, safflower −C, coconut +C, olive +C and safflower +C).
Table 1.
Composition of experimental diets
| 0.01% (w/w) | 0.1% (w/w) | |
|---|---|---|
| −C diet† | +C diet | |
| Ingredient | ||
| g/kg diet | ||
| Casein | 204 | 203 |
| L-Methionine | 4 | 4 |
| Maltodextrin | 102 | 102 |
| Cornstarch | 281 | 281 |
| Sucrose | 129 | 129 |
| Cellulose | 122 | 122 |
| Coconut, olive, or safflower oil | 100 | 100 |
| Soybean oil | 20 | 20 |
| AIN93G mineral mix | 28 | 28 |
| AIN93 vitamin mix | 8.0 | 8.0 |
| Choline bitartrate | 2.0 | 2.0 |
| Cholesterol | 0.1 | 1 |
| Tert-butylhydroquinone | 0.02 | 0.02 |
Semi-purified diets were prepared by Research Diets, New Brunswick, NJ.
Lovastatin (0.15% w/w) and cholestyramine (2.0% w/w) were added to the 0.01% (w/w) cholesterol diet the last ten days of treatment.
Table 2.
Fatty acid and cholesterol composition of the diets
| Coconut oil | Olive oil | Safflower oil | ||
|---|---|---|---|---|
| Percent of total fatty acids | ||||
| Total SFA | ||||
| −C | 78.3 | 19.4 | 14.4 | |
| +C | 78.5 | 19.4 | 14.4 | |
| 12:0 | ||||
| −C | 36.8 | 0.04 | 0.04 | |
| +C | 37.2 | 0.04 | 0.04 | |
| 14:0 | ||||
| −C | 15.9 | 0.1 | 0.2 | |
| +C | 16.5 | 0.1 | 0.2 | |
| 16:0 | ||||
| −C | 11.2 | 13.1 | 8.5 | |
| +C | 11.6 | 13.1 | 8.6 | |
| 18:0 | ||||
| −C | 6.7 | 6.1 | 5.5 | |
| +C | 7.1 | 6.1 | 5.4 | |
| Total MUFA | ||||
| −C | 10.1 | 62.4 | 16.8 | |
| +C | 10.1 | 62.4 | 16.8 | |
| 18:1 | ||||
| −C | 10.1 | 61.8 | 16.7 | |
| +C | 10.1 | 61.8 | 16.8 | |
| Total PUFA | ||||
| −C | 11.6 | 18.3 | 68.8 | |
| +C | 11.4 | 18.3 | 68.7 | |
| 18:2n-6 | ||||
| −C | 9.9 | 16.1 | 66.9 | |
| +C | 9.7 | 16.1 | 66.8 | |
| 18:3n-3 | ||||
| −C | 1.1 | 1.5 | 1.1 | |
| +C | 0.9 | 1.5 | 1.1 | |
| 20:5n-3 | ||||
| −C | 0.6 | 0.7 | 0.7 | |
| +C | 0.8 | 0.7 | 0.8 | |
| Cholesterol | % w/w | |||
| −C | 0.01 | 0.01 | 0.01 | |
| +C | 0.08 | 0.08 | 0.08 | |
Values are averages of duplicate measurements of diet samples.
SFA=saturated fatty acids, MUFA=monounsaturated fatty acids, PUFA=polyunsaturated fatty acids.
After 12 weeks hamsters were fasted for 16 hours and killed by CO2 inhalation. Livers were removed, rinsed with PBS and divided into segments. A portion was immediately used for nuclear and membrane protein extraction and the remaining segments were frozen in liquid nitrogen and stored at −80°C until analysis. The small intestine was removed, flushed with PBS and the jejunum placed in ‘RNA later’ (Qiagen, Valencia, CA) and stored at −80°C. The animal protocol was approved by the Institutional Animal Care and Use Committee of the Jean Mayer Human Nutrition Research on Aging, Tufts University.
2.2. Plasma lipid and lipoprotein analysis
Retro-orbital blood was collected into EDTA-coated tubes from fasted hamsters (16 hours) under isoflurane anesthesia at 0, 6 and 12-week time points. Plasma was separated from red blood cells by centrifugation at 1500 × g for 20 minutes at 4°C. Plasma total cholesterol, high density lipoprotein (HDL) cholesterol and triglyceride concentrations were determined on a Cobas Mira automated analyzer using enzymatic reagents (Roche Diagnostics, Indianapolis, IN). Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL cholesterol. Additionally, 4 plasma pools per diet group were created by combing plasma from 4 animals to be used for fast protein liquid chromatography (FPLC) analysis using two Superose 6 columns (Amersham Biosciences, Piscataway, NJ) as previously described [41, 42]. The total cholesterol concentration of the FPLC fractions was measured using enzymatic reagents (Wako, Richmond, VA).
2.3. Liver lipid composition
Liver lipids were extracted [43] and total cholesterol, free cholesterol and triglyceride were determined using enzymatic reagents (Wako and Roche Diagnostics) [44]. Esterified cholesterol was calculated as the difference between total and free cholesterol. Delipidated liver tissue was digested in 1N NaOH for the determination of protein using the bicinchoninic acid (BCA) assay (Pierce Inc., Rockford, IL).
2.4. Fatty acid profiles
Red blood cell membranes were prepared by washing the cells three times with 0.9% NaCl (pH 7.4). Fatty acid profiles of red blood cell membranes and the experimental diets were determined by gas chromatography as previously described [45]. Peaks of interest were identified by comparison with authentic standards (Nu-Chek Prep, Inc. Elysian, MN) and expressed as mol% of total fatty acids.
2.5. Cholesterol content of experimental diets
Lipid was extracted from freeze-dried aliquots of diet overnight and total cholesterol was determined by gas chromatography (GC) as previously described [46].
2.6. Quantitative real time PCR
Total RNA was extracted from a portion of liver and small intestine (jejunum) using the Qiagen RNeasy Mini kit. Added to the protocol was an on column DNase digestion step to eliminate contamination with genomic DNA. RNA was reverse transcribed using SuperScript II reverse transcriptase with random hexamers according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Primers for ACAT-2, apo A-I, apo B-100, beta-actin, 7α-hydroxylase, HMG-CoA reductase, LDL receptor, MTP, PPAR alpha and SREBP-2 were designed using Primer Express software (Applied BioSystems, Foster City, CA), and primer specificity and amplification efficiency were verified before use. Real time PCR was conducted in an Applied Biosystems 7300 Sequence detection system using SYBR green reagents (Applied BioSystems) with the appropriate primers (Table 3). Reaction conditions were 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Values were normalized using beta-actin as an endogenous control.
Table 3.
Primers for quantitative real time PCR
| Gene | Forward Primer | Reverse Primer | Accession No./ Reference |
|---|---|---|---|
| ABCA1† | ATAGCAGGCTCCAACCCTGAC | GGTACTGAAGCATGTTTCGATGTT | [47] |
| ABCG5† | TGATTGGCAGCTATAATTTTGGG | GTTGGGCTGCGATGGAAA | [47] |
| ABCG8† | TGCTGGCCATCATAGGGAG | TCCTGATTTCATCTTGCCACC | [47] |
| NPC1L1† | CCTGACCTTTATAGAACTCACCACAGA | GGGCCAAAATGCTCGTCAT | [47] |
| SREBP-2‡ | GCAAGGTGTTCCTGCATGAA | TGGTGTTCTGACTGGTACGCC | GU12330 |
| LDL receptor‡ | GCAGTGTTTCTGTGGCTGACAC | GCCATGCACAGGGTCCA | M94387 |
| HMG-Co A | L00173 | ||
| GAGCTACATTTGTGCTTGGCG | TTCATTAGGCCGAGGCTCAC | ||
| reductase‡ | |||
| SR-B1‡ | AAGCCTGCAGGTCTATGAAGC | AGAAACCTTCATTGGGTGGGTA | [48] |
| ACAT-T§ | GGTGGAATTATGTGGCCAAGA | CATGTTGGCAAAGACAGGGAC | NM_153728 |
| 7α- | L04690 | ||
| CACTCTGCACCTTGAGGATGG | GGGTCTGGGTAGATTGCAGG | ||
| hydroxylase‡ | |||
| MTP‡ | ACATGCTGACCTTTGTGCGA | ACGGTCATAATTGTGGGCAAC | U14995 |
| Apo A-I‡ | GGCGGGAGATGAACAAGGA | GGCGGTAAAGAGCCACTTCC | AF046919 |
| Apo B-100‡ | TGATTATCTGAATGCATCTGACTGG | TCCTTGGCATTGGCTACTTGT | AF176576 |
| Beta actin‡ | TGCTGTCCCTGTATGCCTCTG | AGGGAGAGCGTAGCCCTCAT | AJ312092 |
| PPAR alpha‡ | GGCCAATGGCATCCAAAATA | CCTTGGCGAATTCTGTGAGC | AJ555631 |
Mouse sequence
Hamster sequence
Rat sequence
2.7. Immunoblotting analysis
Freshly excised liver tissue from 2 hamsters was pooled, and nuclear and membrane proteins were extracted as described [15]. All buffers contained 24 µg/mL pefabloc, 5 µg/mL pepstatin A, 10 µg/mL leupeptin, 2 µg/mL aprotinin and 50 µg/mL N-acetylleucylnorleucinal (reagents from Roche Diagnostics). Liver cell lysates were prepared by homogenizing liver tissue in 5 volumes of buffer [25 mM HEPES (pH 7.5), 1.5 mM MgCl2, 300 mM NaCl, 5 mM DTT, 1 mM EDTA, 10% (v/v) glycerol, 0.5% (v/v) Triton X-100, 5 µg/mL Na3VO4, 5 µg/mL NaF (reagents from Sigma, St. Louis, MO) and protease inhibitors (Roche Diagnostics)] followed by 1 hour of agitation at 4°C. Homogenates were then centrifuged and supernatants collected. Protein concentration in the nuclear and membrane fractions, and cell lysate were determined using the BCA assay.
Proteins from nuclear and membrane fractions (50 µg) and cell lysates (40µg) were separated by SDS-PAGE and transferred to PVDF membranes using a wet transfer system. Proteins were detected as previously described [48]. Relative protein levels were normalized to the density of beta actin.
2.8. Statistical analysis
Prior to statistical analysis, data were checked for normality and appropriate transformations performed when necessary. Differences between cholesterol-supplemented (+C) and cholesterol-depleted (−C) hamsters within a dietary fat group were assessed using an unpaired Students t-test and among dietary fat groups using ANOVA. Tukey’s honestly significant difference test was used for post hoc analysis. Differences were considered significant at P ≤ 0.05. All statistical analyses were performed using SAS (Version 9.1, SAS Institute, Cary, NC).
3. Results
3.1. Plasma lipid and lipoprotein profiles
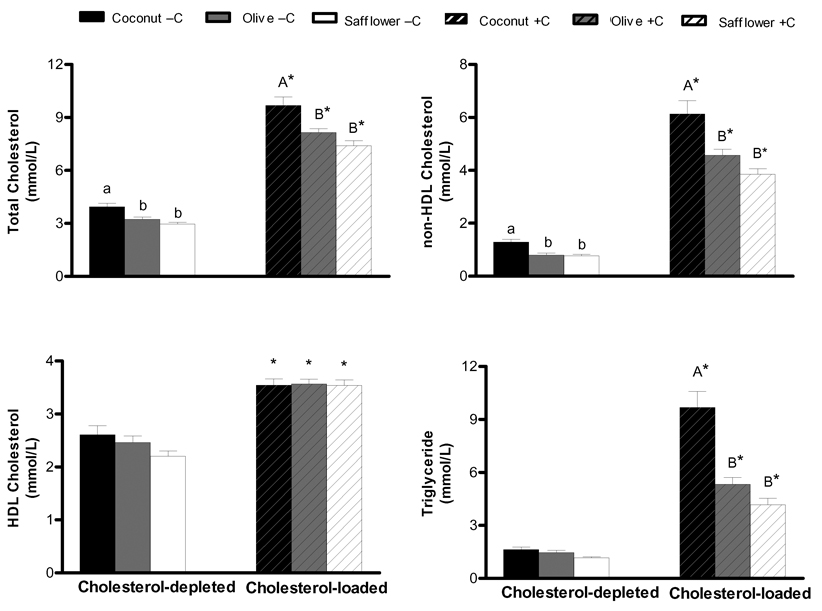
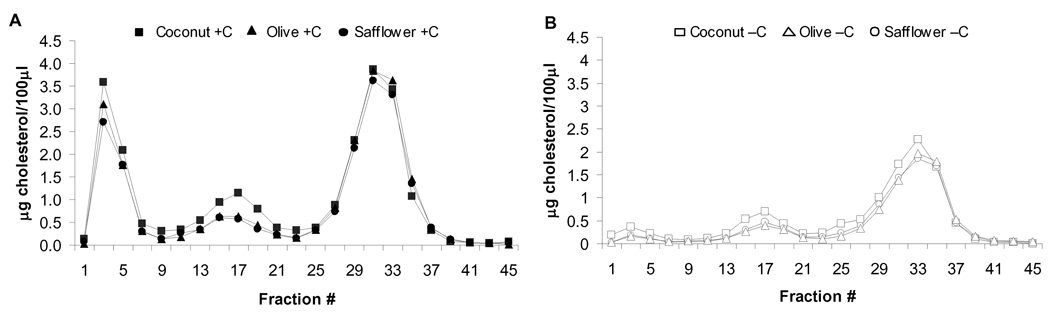
At baseline, there were no significant differences in the plasma lipid and lipoprotein concentrations among the six diet groups (Supplementary Table 1). By design, after 12 weeks of diet treatment, the cholesterol-depleted hamsters had significantly lower plasma total, non-HDL and HDL cholesterol, and triglyceride concentrations than cholesterol-supplemented hamsters, regardless of dietary fat type (Figure 1). Plasma FPLC patterns indicated that in the cholesterol-depleted hamsters the majority of cholesterol was carried on the HDL fraction, whereas, in the cholesterol-supplemented hamsters the majority of cholesterol was distributed between the VLDL and HDL fractions, again regardless of dietary fat type (Figure 2). Independent of cholesterol status, hamsters fed the coconut oil diets had significantly higher total and non-HDL cholesterol concentrations compared to hamsters fed olive and safflower oil diets.
Figure 1.
Effect of cholesterol status and dietary fat type on fasting plasma lipid and lipoprotein cholesterol concentrations. Hamsters were fed diets enriched with coconut, olive or safflower oil and 0.1% cholesterol (cholesterol-supplemented, +C) or 0.01% cholesterol for 12-weeks plus 0.15% lovastatin and 2% cholestyramine one week prior to killing (cholesterol-depleted, −C). Data represent means ± SEM, n = 15–16 animals per group. Appropriate transformations of the data (log HDL cholesterol; square root total cholesterol, non-HDL cholesterol; inverse triglyceride) were made before statistical analysis. Bars with different letters (lowercase for cholesterol-depleted, uppercase for cholesterol-supplemented) are significantly different, P≤0.05. Asterisks indicate significant differences between cholesterol-depleted and cholesterol-supplemented hamsters within a dietary fat treatment, P≤0.05.
Figure 2.
Effect of cholesterol status and dietary fat type on plasma FPLC cholesterol profiles. Hamsters were fed diets enriched with coconut, olive or safflower oil and 0.1% cholesterol (cholesterol-supplemented, +C) (A) or 0.01% cholesterol for 12-weeks plus 0.15% lovastatin and 2% cholestyramine one week prior to killing (cholesterol-depleted, −C) (B). Cholesterol concentrations were measured in odd numbered fractions using standard enzymatic reagents. Data represent the mean of 4 plasma pools of 3 hamsters per pool.
3.2. Red blood cell fatty acid profile and hepatic lipid composition
Red blood cell membrane fatty acid profiles reflected the dietary fatty type (Supplementary Table 2). Differences among individual fatty acids on the basis of cholesterol status were modest and unlikely to be of biological significance. Hepatic liver weight, total cholesterol and esterified cholesterol levels were significantly lower in cholesterol-depleted relative to cholesterol-supplemented hamsters, among all dietary fat groups (Table 4). Interestingly, hepatic triglyceride levels were 2 to 2.8-fold higher in cholesterol-depleted relative to cholesterol-supplemented hamsters. Of note, in the cholesterol-supplemented, but not cholesterol-deleted hamsters hepatic esterified cholesterol levels were highest in the olive oil, lowest in the coconut oil and intermediate in the safflower oil fed animals, with an almost 2-fold range of differences among dietary fat type groups.
Table 4.
Effects of cholesterol status and dietary fat type on liver lipid composition
| Coconut oil | Olive oil | Safflower oil | ||
|---|---|---|---|---|
| grams | ||||
| Liver weight | ||||
| −C | 5.1 ± 0.2* | 5.1 ± 0.1* | 4.9 ± 0.1* | |
| +C | 7.3 ± 0.2 | 6.7 ± 0.3 | 7.1 ± 0.2 | |
| µg/mg protein | ||||
| Total cholesterol | ||||
| −C | 23.8 ± 1.1* | 27.4 ± 1.6* | 24.3 ± 0.7* | |
| +C | 173.5 ± 10.0C | 326.3 ± 15.4A | 265.0 ± 24.4B | |
| Esterified cholesterol | ||||
| −C | 1.8 ± 0.2* | 3.7 ± 1.4* | 2.6 ± 0.3* | |
| +C | 142.4 ± 8.9C | 267.0 ± 15.8A | 221.2 ± 24.2B | |
| Free cholesterol† | ||||
| −C | 21.8 ± 1.3* | 22.8 ± 1.9* | 21.8 ± 0.6* | |
| +C | 33.3 ± 2.1B | 62.3 ± 6.5A | 43.8 ± 2.8B | |
| Triglyceride | ||||
| −C | 126.3 ± 14.3* | 140.9 ± 8.6* | 107.5 ± 7.3* | |
| +C | 45.0 ± 3.5B | 68.8 ± 6.4A | 46.1 ± 3.1B | |
Values are expressed as means ± SEM, n=15–16 animals per group.
Means in a row without common letters (lowercase for −C [cholesterol-depleted], uppercase for +C [cholesterol-supplemented) are significantly different, P≤0.05. Asterisks indicate significant differences between −C and +C hamsters within a dietary fat group, P≤0.05.
Data were log-transformed prior to statistical analysis.
3.3. Hepatic and intestinal mRNA levels
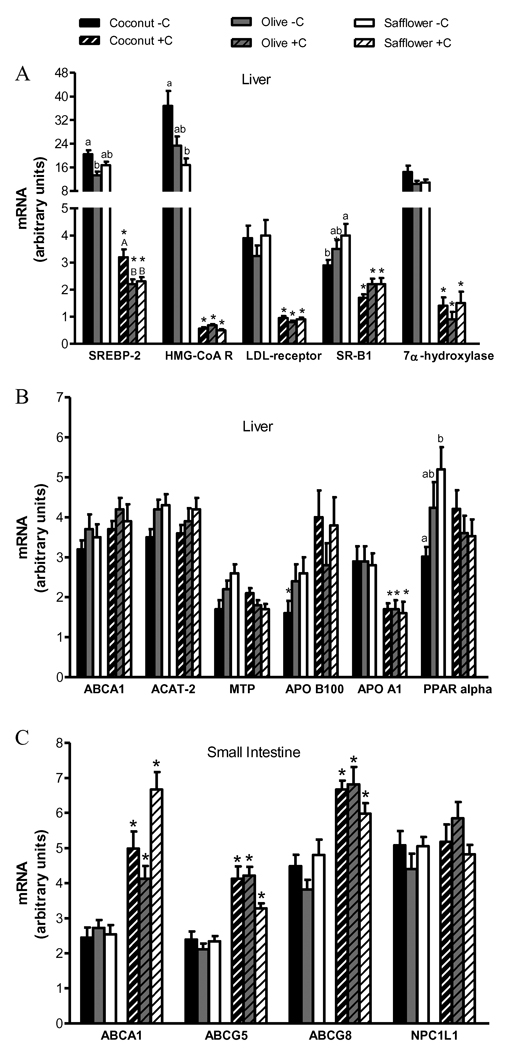
Regardless of dietary fat type, relative to cholesterol-supplemented hamsters, acute whole body cholesterol-depletion resulted in an up-regulation of hepatic mRNA levels of genes involved in cholesterol biosynthesis (HMG-CoA reductase, 34 to 65-fold), uptake (LDL receptor, 4.1 to 4.4-fold; SR-B1, 1.6 to 1.8-fold), metabolism (7α-hydroxylase, 7.3 to11.6-fold), and plasma transport (apo A-I, 1.7-fold) (Figure 3A, B). Hepatic SREBP-2 mRNA levels were significantly higher (6 to 7.3-fold) in cholesterol-depleted relative to cholesterol-supplemented hamsters, consistent with its role in transcriptional regulation of the HMG-CoA reductase and LDL receptor genes. Cholesterol-depleted hamsters had modestly lower intestinal mRNA levels of the sterol transporters ABCA1 (1.5 to 2.6-fold), ABCG5 (1.4 to 2-fold) and ABCG8 (1.3 to 1.8-fold) relative to cholesterol-supplemented hamsters for all dietary fat types (Figure 3C).
Figure 3.
Hepatic and small intestinal gene expression in response to alterations in cholesterol homeostasis and dietary fat type. Real time PCR was used to measure gene expression in the liver (A and B) and small intestine (C). Values are expressed as mean ± SEM, n=14–16 animals per group. Appropriate transformations of the data (log SREBP-2, CYP7A1, MTP, apo B-100, ABCA1, HMG-CoA R [HMG-CoA reductase]; square root SR-B1, SREBP-1c, apo A-I) were made before statistical analysis. Bars with different letters (lowercase for cholesterol-depleted, −C; uppercase for cholesterol-supplemented, +C) are significantly different, P≤0.05. Asterisks indicate significant differences between +C and −C hamsters within a dietary fat treatment, P≤0.05.
Consistent with this modest response of plasma lipid and lipoprotein concentrations to dietary fat type, there were only modest effects on message levels for the genes of interest. Cholesterol-supplemented hamsters fed coconut oil had significantly higher hepatic mRNA levels of hepatic SREBP-2 than hamsters fed olive or safflower oil (1.5 and 1.2-fold, respectively) (Figure 3A). In contrast, cholesterol-depleted hamsters fed coconut oil had significantly higher hepatic mRNA levels of HMG-CoA reductase (2.2-fold) and lower levels of SR-B1 (1.4-fold) and ACAT-2 (1.3-fold) than hamsters fed safflower oil (Figure 3A, B). Cholesterol-depleted hamsters fed coconut oil had modestly, albeit, significantly higher levels of SREBP-2 (1.4-fold) than both cholesterol-depleted hamsters fed olive and safflower oil. Additionally, cholesterol-depleted hamsters fed safflower oil had modest but significantly higher levels of PPAR alpha (1.7-fold) than cholesterol-depleted hamsters fed safflower oil. Intestinal NPC1L1 mRNA levels were similar on the basis of either cholesterol status or dietary fat type. Dietary fat type had no significant effect on intestinal mRNA levels of ABCA1, ABCG5 or ABCG8 (Figure 3C).
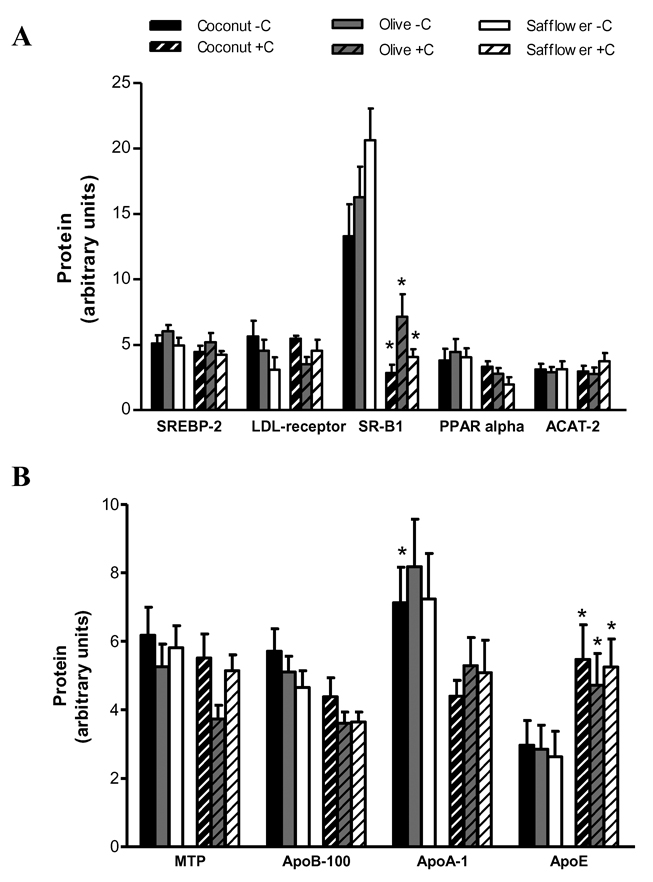
3.4. Hepatic protein levels
Similar to the pattern observed for mRNA expression, differences in protein expression were primarily observed in response to cholesterol status rather than dietary fat type. Relative to cholesterol-supplemented hamsters, cholesterol-depleted hamsters had significantly higher hepatic levels of SR-B1 (2 to 5-fold) and lower levels of apo E (1.7 to 2-fold) (Figure 4A, B and Supplementary Figure 1). Cholesterol status had no significant effect on hepatic SREBP-2, PPAR alpha, MTP, ACAT-2, apo B-100 or LDL receptor protein levels. Despite modest differences in both plasma lipid and lipoprotein concentrations, and hepatic lipid levels, in the animal model used for this work there was no significant effect of dietary fat type on hepatic SREBP-2, PPAR alpha, LDL receptor, SR-B1, apo A-I, apo B-100, apo E, MTP or ACAT-2 protein levels (Figure 4A, B).
Figure 4.
Hepatic protein expression in response to alterations in cholesterol homeostasis and dietary fat type. LDL receptor was detected in the membrane fraction, SREBP-2 was detected in the nuclear fraction, and SRB1, ACAT-2, MTP, apo B-100, apo A-I, and apo E were detected in the cell lysate. Values are expressed as mean ± SEM, n = 14–16 animals per group. Appropriate transformations of the data (log apo A-I, apo E, LDL receptor, SREBP-1, SR-B1; square root apo B-100) were made before statistical analysis. Bars with different letters (lowercase for cholesterol-depleted, −C; uppercase for cholesterol-supplemented, +C) are significantly different, P≤0.05. Asterisks indicate significant differences between −C and +C hamsters within a dietary fat group, P≤0.05.
Discussion
This work was designed to develop a novel experimental animal model for use to determine in multiple tissue systems, simultaneously, the mechanism(s) regulating circulating lipid and lipoprotein concentrations in response to changes in dietary fat type within the context of altered cholesterol status. The results indicate that FIB hamsters fed semi-purified diets enriched with fats high in saturated, monounsaturated and polyunsaturated fatty acids were only modestly responsive to dietary fat type, despite dramatic differences in cholesterol status induced by a high cholesterol diet or low cholesterol diet plus acute treatment with cholesterol lowering drugs. Nonetheless, a number of findings shed light on the original intent of the work.
Part of the approach used to deplete whole body cholesterol, acute treatment with cholestyramine, increased hepatic cholesterol demand for use in de novo bile acid synthesis. This was coupled with a drug that inhibited endogenous cholesterol synthesis. Elevated SR-B1 mRNA and protein levels facilitate the hepatic uptake of cholesterol from HDL, the preferred cholesterol source for bile acid biosynthesis, [49, 50]. Consistent with this sequence, we observed higher 7α-hydroxylase mRNA levels and lower circulating HDL cholesterol concentrations in the cholesterol-depleted hamsters. Both SR-B1 and 7α-hydroxylase are regulated by bile acids via pathways involving FXR, providing additional support for the coordinate regulation of SR-B1 activity and bile acid metabolism [51, 52]. In contrast, hepatic MTP and ABCA1, also important regulators of hepatic intracellular cholesterol and lipoprotein metabolism, remained unaffected by cholesterol status of the hamsters.
Acute cholesterol depletion, compared to cholesterol supplementation, was also associated with higher hepatic apo A-I mRNA levels, yet lower plasma HDL cholesterol concentrations. Consistent with higher SR-B1 expression in the cholesterol-depleted hamsters, these data suggest an up-regulation of reverse cholesterol transport. Two-fold higher levels of hepatic apo A-I mRNA induced by simvastatin or cholestryamine has been reported in rats without a concomitant increase in plasma apo A-I concentrations [53]. Feeding bile acids to human apo A-I transgenic mice has also been shown to inhibit apo A-I expression via a pathway involving FXR [54]. Previous studies in F1B hamsters have shown an increase in HMG-CoA reductase activity in response to bile acid sequestrants and this was associated with a decrease in hepatic cholesterol concentrations and increase in fecal bile acid excretion [55,56]. Additionally, treatment with lovastatin has been shown to result in an up-regulation of HMG-CoA reductase mRNA levels in the F1B hamster [57].
Intestinal cholesterol absorption is one factor that modulates plasma cholesterol concentrations and this mechanism has been exploited as a therapeutic target [33]. Intestinal ABCA1, ABCG5 and ABCG8 mRNA levels were significantly higher in cholesterol-supplemented compared to cholesterol-depleted hamsters, whereas NPC1L1 was unaffected. These data are consistent with that observed in other hamster studies [47]. ABCA1, ABCG5 and ABCG8 are regulated by the nuclear receptor LXR for which oxysterols are ligands [47, 58–60]. Our work suggests that in vivo changes in hepatic cholesterol synthesis, uptake and secretion induced by cholesterol status were more important modifiers of lipoprotein metabolism than changes in expression of intestinal cholesterol transporters.
Hepatic cholesterol levels are tightly regulated by the transcription factor SREBP-2. Consistent with this role, cholesterol-depletion resulted in higher SREBP-2 mRNA levels relative to cholesterol-supplementation. This in turn was associated with higher mRNA levels of two genes regulated by SREBP-2, HMG Co-A reductase and the LDL receptor [1]. A similar response has been reported in hamsters fed lovastatin and cholestryamine, and is consistent with the cholesterol-depleted model used in our study [15, 39]. Nonetheless, there was no significant effect of whole body cholesterol status on nuclear SREBP-2 protein levels. This discrepancy may be attributable to the prandial state of the hamsters [61, 62]. The ratio of nuclear to membrane SREBP-2 is indicative of proteolytic regulation [39]. However, because we were unable to detect membrane SREBP-2, we were unable to address this issue further.
Secondary to statin treatment higher hepatic HMG-CoA reductase mRNA levels were observed in the cholesterol-depleted, relative to cholesterol-supplemented hamsters, as has been reported [63, 64]. This is consistent with the transcriptional regulation of HMG-CoA reductase observed in hamsters in response to alterations in cholesterol homeostasis [65]. The modestly higher HMG-CoA reductase mRNA levels in cholesterol-depleted hamsters fed coconut oil, relative to olive or safflower oil is likely the result of increased SREBP-2 transcription. These results suggest that in this animal model regulation of cholesterol synthesis is primarily at the level of transcription and enzyme activity [66]. Nonetheless, we cannot rule out the possibility that other pleiotropic effects on statin treatment influenced the outcomes observed.
The LDL receptor is the main route of LDL cholesterol uptake in both hamsters and humans, thus, is a major determinant of plasma non-HDL cholesterol concentrations [6]. A 4-fold up-regulation of LDL receptor mRNA levels, but not protein levels, was observed in cholesterol-depleted, relative to the cholesterol-supplemented hamsters. These differences were accompanied by lower plasma non-HDL cholesterol concentrations. A similar observation of increased LDL receptor mRNA levels and no difference in protein levels in response to cholesterol-depletion has been reported in rats [63, 65]. These data suggest an increased rate of receptor recycling [65].
The hamsters responded to different dietary fat types with modest alterations in circulating lipid and lipoprotein concentrations, and gene expression. Regardless of cholesterol status, coconut oil fed hamsters had higher SREBP-2 mRNA levels than olive or safflower oil fed hamsters. Intracellular sterol levels regulate SREBP-2 expression and activity. Lower hepatic free cholesterol levels in hamsters fed coconut oil, relative to olive or safflower oil, may have been a reflection of these higher SREBP-2 mRNA levels [1]. This observation is consistent with the proposed mechanism by which saturated fatty acids increases SREBP-2 expression [19]. PPAR gamma coactivator-1β, an activator of the SREBP family of transcription factors, is stimulated by saturated fatty acids and may have contributed to higher SREBP-2 mRNA levels observed in the coconut oil fed hamsters [67].
Interestingly, cholesterol-supplemented hamsters fed the olive oil enriched diet had the highest concentrations of hepatic esterified cholesterol levels. ACAT-2 mRNA and protein levels were not altered by dietary fat type or cholesterol status. The preference of ACAT-2 for oleoyl CoA may account for this observation [16, 68–70]. These data are particularly striking in light of higher levels of hepatic esterified cholesterol, mainly cholesteryl oleate, that have been observed in African green monkeys and apoB-100 transgenic, LDLr−/− mice fed monounsaturated fatty acid enriched, relative to saturated fatty acids or n-6 polyunsaturated fatty acid enriched diets [70, 71] and confirm the unanticipated effect of a dietary fat high in monounsaturated fatty acids.
Hepatic triglyceride levels were markedly higher and plasma triglyceride concentrations were lower in the cholesterol-depleted hamsters compared to cholesterol-supplemented hamsters. This unique finding in this animal model may be secondary due to insufficient hepatic cholesterol, apo E or apo B-100 levels to support VLDL formation and secretion [26, 28, 72–75].
Coconut oil was used as the experimental saturated fat, rather than butter, a more common fat the diet of humans, because it has been used extensively in prior hamster studies [76, 77]. In the hamster, coconut oil has been reported to predominantly increase non-HDL cholesterol concentration, as was observed in the current study, and also has been demonstrated to induce a cytokine response [78–81]. Nonetheless, we observed only a modest response to changing the major dietary fat type with respect to plasma lipid and lipoprotein concentrations, less than anticipated [66, 82–85].
In conclusion, the experimental hamster model system developed as part of this study to assess simultaneously in multiple tissue systems the mechanism(s) for differences in response to dietary fat type on circulating lipid and lipoprotein concentrations was not ideal because the hamsters responded only modestly to dietary fat type regardless of dramatic differences in cholesterol status. Nonetheless, this work demonstrated that lower plasma non-HDL cholesterol concentrations in cholesterol-depleted, relative to cholesterol-supplemented hamsters was in part accounted for by increased expression of genes associated with hepatic cholesterol uptake (LDL receptor), metabolism (7α-hydroxylase) and reverse cholesterol transport (SR-B1 and apo A-I). Lower hepatic apo E protein and cholesterol levels in cholesterol-depleted hamsters was associated with higher hepatic triglyceride levels, and lower plasma non-HDL cholesterol and triglyceride concentrations suggesting lower rates of de novo VLDL synthesis. Nonetheless, on the basis of the response to dietary fat type the experimental animal model developed, in general, has limited value to study diet induced atherosclerosis.
Supplementary Material
Acknowledgements
We gratefully acknowledge Debra Cromly for her technical assistance with the FPLC assay, Dr. Donald Smith and Andrea Pinella for their invaluable help with the animals, and Dr. Suzanne Dorfman for her guidance and expertise in establishing the animal model. We thank Drs. Margaret Brousseau and Alice Dillard for their helpful comments during manuscript preparation. Finally, we would like to thank Merck & Co. Inc. for their generous donation of lovastatin.
Supported by NIH/NHLBI 5T32HL069772 (JLL) and USDA agreement 588-1950-9-001. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the USDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest with this paper.
References
- 1.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 2.Soccio R, Breslow J. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 3.Jump DB. Dietary polyunsaturated fatty acids and regulation of gene expression. Curr Opin Lipidol. 2002:155–164. doi: 10.1097/00041433-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kritchevsky D. Diet and atherosclerosis. J Nutr Health Aging. 2001;5:155–159. [PubMed] [Google Scholar]
- 5.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb Vasc Biol. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Song W, Redinger RN. Effects of dietary cholesterol on hepatic production of lipids and lipoproteins in isolated hamster liver. Hepatology. 1996;24:423–434. doi: 10.1002/hep.510240222. [DOI] [PubMed] [Google Scholar]
- 7.Nistor A, Bulla A, Filip DA, Radu A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis. 1987;68:159–173. doi: 10.1016/0021-9150(87)90106-7. [DOI] [PubMed] [Google Scholar]
- 8.Reaves S, Wu J, Wu Y, Franco J, Wang Y, Lei P, Lei K. Regulation of intestinal apolipoprotein B mRNA editing levels by a zinc deficient diet and cDNA cloning of editing protein in hamsters. J Nutr. 2000;130:2166–2173. doi: 10.1093/jn/130.9.2166. [DOI] [PubMed] [Google Scholar]
- 9.Remillard P, Shen G, Milne R, Maheux P. Induction of cholesteryl ester transfer protein in adipose tissue and plasma of the fructose fed hamster. Life Science. 2001;69:677–687. doi: 10.1016/s0024-3205(01)01168-7. [DOI] [PubMed] [Google Scholar]
- 10.Kowala MC, Nunnari JJ, Durham SK, Nicolosi RJ. Doxazosin and cholestyramine similarly decrease fatty streak formation in the aortic arch of hyperlipidemic hamsters. Atherosclerosis. 1991;91:35–49. doi: 10.1016/0021-9150(91)90185-6. [DOI] [PubMed] [Google Scholar]
- 11.McAteer MA, Grimsditch DC, Vidgeon-Hart M, Benson GM, Salter AM. Dietary cholesterol reduces lipoprotein lipase activity in the atherosclerosis susceptible Bio-F(1)B hamster. Br J Nutr. 2003;89:341–350. doi: 10.1079/BJN2002802. [DOI] [PubMed] [Google Scholar]
- 12.Field FJ, Born E, Murthy S, Mathur SN. Regulation of sterol regulatory element-binding proteins in hamster intestine by changes in cholesterol flux. J Biol Chem. 2001;276:17576–17583. doi: 10.1074/jbc.M010917200. [DOI] [PubMed] [Google Scholar]
- 13.Tyburczy C, Major C, Lock AL, Destaillats F, Lawrence P, Brenna JT, Salter AM, Bauman DE. Individual trans octadecenoic acids and partially hyrdogenated vegetable oil differentially affect hepatic lipid and lipoprotein metabolism in golden Syrian hamsters. J Nutr. 2009;139:257–263. doi: 10.3945/jn.108.098004. [DOI] [PubMed] [Google Scholar]
- 14.Weinhofer I, Kunze M, Rampler H, Bookout A, Forss-Petter S, Berger J. LXRalpha intereferes with SREBP-1c mediated Abcd2 expression: novel cross-talk in gene regulation. J Biol Chem. 2005;280:41243–41251. doi: 10.1074/jbc.M509450200. [DOI] [PubMed] [Google Scholar]
- 15.Sheng Z, Otani H, Brown MS, Goldstein JL. Independent regulation of sterol regulatory element-binding proteins 1 and 2 in hamster liver. Proc Natl Acad Sci USA. 1995;92:935–938. doi: 10.1073/pnas.92.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman DS, Deykin D, Shiratori T. The formation of cholesterol esters with rat liver enzymes. J Biol Chem. 1964;239:1335–1345. [PubMed] [Google Scholar]
- 17.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 18.Shimano H. Sterol regulatory element binding proteins (SREBP's): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40:439–452. doi: 10.1016/s0163-7827(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 19.Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol. 2001;12:129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue specific induction of intestinal ABCA1 expression with liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- 23.Ragozin S, Niemeier A, Laatsch A, Loeffler B, Merkel M, Beisiegel U, Heeren J. Knockdown of hepatic ABCA1 by RNA interference decreases plasma HDL cholesterol levels and influences postprandial lipemia in mice. Arterioscler Thromb Vasc Biol. 2005:1433–1438. doi: 10.1161/01.ATV.0000166616.86723.d0. [DOI] [PubMed] [Google Scholar]
- 24.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-B1 as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 25.Fungwe TV, Cagen LM, Wilcox HG, Heimberg M. Effects of dietary cholesterol on hepatic metabolism of free fatty acid and secretion of VLDL in the hamster. Biochem Biophys Res Commun. 1994;200:1505–1511. doi: 10.1006/bbrc.1994.1621. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Cianflone K, Sniderman AD. Role of cholesterol ester mass in regulation of secretion of ApoB100 lipoprotein particles by hamster hepatocytes and effects of statins on that relationship. Arterioscler Thromb Vasc Biol. 1999;19:743–752. doi: 10.1161/01.atv.19.3.743. [DOI] [PubMed] [Google Scholar]
- 27.Shelness GS, Ingram MF, Huang XF, DeLozier JA. Apolipoprotein B in the rough endoplasmic reticulum: translation, translocation and the initiation of lipoprotein assembly. J Nutr. 1999;129:456S–462S. doi: 10.1093/jn/129.2.456S. [DOI] [PubMed] [Google Scholar]
- 28.Kuipers F, Jong MC, Lin Y, Eck M, Havinga R, Bloks V, Verkade HJ, Hofker MH, Moshage H, et al. Impaired secretion of very low density lipoprotein-triglycerides by apolipoprotein E- deficient mouse hepatocytes. J Clinic Invest. 1997;100:2915–2922. doi: 10.1172/JCI119841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 30.White DA, Bennett AJ, Billett MA, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br J Nutr. 1998;80:219–229. [PubMed] [Google Scholar]
- 31.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: The assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 32.Davis HR, Jr., Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 33.Sudhop T, Lutjohann D, von Bergmann K. Sterol transporters: targets of natural sterols and new lipid lowering drugs. Pharmacol Ther. 2005;105:333–341. doi: 10.1016/j.pharmthera.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Mulligan JD, Flowers MT, Tebon A, Bitgood JJ, Wellington C, Hayden MR, Attie AD. ABCA1 is essential for efficient basolateral cholesterol efflux during the absorption of dietary cholesterol in chickens. J Biol Chem. 2003;278:13356–13366. doi: 10.1074/jbc.M212377200. [DOI] [PubMed] [Google Scholar]
- 35.Altmann SW, Davis HR, Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 37.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 38.Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molec Endocrin. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 39.Shimomura I, Bashmakov Y, Shimano H, Horton JD, Goldstein JL, Brown MS. Cholesterol feeding reduces nuclear forms of sterol regulatory element binding proteins in hamster liver. Proc Natl Acad Sci USA. 1997;94:12354–12359. doi: 10.1073/pnas.94.23.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Cho H, O'Malley S, Park JH, Clarke SD. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and -2 in three distinct stages and by different mechanisms. J Nutr. 2002;132:3333–3339. doi: 10.1093/jn/132.11.3333. [DOI] [PubMed] [Google Scholar]
- 41.Tsukamoto K, Smith P, Glick JM, Rader DJ. Liver-directed gene transfer and prolonged expression of three major human ApoE isoforms in ApoE-deficient mice. J Clin Invest. 1997;100:107–114. doi: 10.1172/JCI119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millar JS, Cromley DA, McCoy M, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46:2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 44.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84:497–504. doi: 10.1093/ajcn/84.3.497. [DOI] [PubMed] [Google Scholar]
- 46.Matthan NR, Raeini-Sarjaz M, Lichtenstein AH, Ausman LM, Jones PJ. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 2000;35:1037–1044. doi: 10.1007/s11745-000-0616-9. [DOI] [PubMed] [Google Scholar]
- 47.Field FJ, Born E, Mathur SN. Stanol esters decrease plasma cholesterol independently of intestinal ABC sterol transporters and Niemann-Pick C1-like 1 protein gene expression. J Lipid Res. 2004;45:2252–2259. doi: 10.1194/jlr.M400208-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Dorfman SE, Wang S, Vega-Lopez S, Jauhiainen M, Lichtenstein AH. Dietary fatty acids and cholesterol differentially modulate HDL cholesterol metabolism in Golden-Syrian hamsters. J Nutr. 2005;135 doi: 10.1093/jn/135.3.492. [DOI] [PubMed] [Google Scholar]
- 49.Pieters MN, Schouten D, Van Berkel TJC. In vitro and in vivo evidence for the role of HDL in reverse cholesterol transport. Biochim Biophys Acta. 1993;1125:125–134. doi: 10.1016/0925-4439(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz CC, Halloran LG, Vlahcevic ZR, Gregory DH, Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978;200:62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- 51.Malerod L, Juvet LK, Hanssen-Bauer A, Eskild W, Berg T. Oxysterol-activated LXRalpha/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem Biophys Res Commun. 2002;299:916–923. doi: 10.1016/s0006-291x(02)02760-2. [DOI] [PubMed] [Google Scholar]
- 52.Malerod L, Sporstol M, Juvet LK, Mousavi SA, Gjoen T, Berg T, Roos N, Eskild W. Bile acids reduce SR-B1 expression in hepatocytes by a pathway involving FXR/RXR, SHP, and LRH-1. Biochem Biophys Res Commun. 2005;336:1096–1105. doi: 10.1016/j.bbrc.2005.08.237. [DOI] [PubMed] [Google Scholar]
- 53.Staels B, Van Tol A, Fruchart J, Auwerx J. Effects of hypolipidemic drugs on the expression of genes involved in high density lipoprotein metabolism in the rat. Israel J Med Sci. 1996;32:490–498. [PubMed] [Google Scholar]
- 54.Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, Fruchart J, Dallongeville J, Hum DW, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-1 transcription via a negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suckling K, Benson G, Bond B, Gee A, Glen A, Haynes C, Jackson B. Cholesterol lowering and bile acid excretion in the hamster with cholestyramine treatment. Atherosclerosis. 1991;89:183–190. doi: 10.1016/0021-9150(91)90059-c. [DOI] [PubMed] [Google Scholar]
- 56.Wilson T, Nicolosi R, Rogers E, Sacchiero R, Goldberg D. Studies of cholesterol and bile acid metabolism, and early atherogenesis in hamsters fed GT16-239, a novel bile acid sequestrant (BAS) Atherosclerosis. 1998;140:315–324. doi: 10.1016/s0021-9150(98)00135-x. [DOI] [PubMed] [Google Scholar]
- 57.Pitman W, Osgood D, Smith D, Schaefer EJ, Ordovas J. The effects of diet and lovastatin on regression of fatty streak lesions and on hepatic and intestinal mRNA levels for the LDL receptor and HMG CoA reductase in F1B hamsters. Atherosclerosis. 1998;138:43–52. doi: 10.1016/s0021-9150(97)00302-x. [DOI] [PubMed] [Google Scholar]
- 58.Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002;43:2–12. [PubMed] [Google Scholar]
- 59.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 60.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver × receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 61.Bennett MK, Seo YK, Datta S, Shin DJ, Osborne TF. Selective binding of SREBP isoforms and co-regulatory proteins to promoters for lipid metabolic genes in liver. J Biol Chem. 2008;283:15628–15637. doi: 10.1074/jbc.M800391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and re-fed mice. Proc Natl Acad Sci USA. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ness GC, Chambers CM, Lopez D. Atorvastatin action involves diminished recovery of hepatic HMG-CoA reductase activity. J Lipid Res. 1998;39:75–84. [PubMed] [Google Scholar]
- 64.Bergstrom JD, Bostedor RG, Rew DJ, Geissler WM, Wright SD, Chao YS. Hepatic responses to inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase: a comparison of atorvastatin and simvastatin. Biochim Biophys Acta. 1998;1389:213–221. doi: 10.1016/s0005-2760(97)00182-3. [DOI] [PubMed] [Google Scholar]
- 65.Ness GC, Zhao Z, Lopez D. Inhibitors of cholesterol biosynthesis increase hepatic low-density lipoprotein receptor protein degradation. Arch Biochem Biophys. 1996;325:242–248. doi: 10.1006/abbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 66.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 68.Woollett LA, Daumerie CM, Dietschy JM. Trans-9-octadecenoic acid is biologically neutral and does not regulate the low density lipoprotein receptor as the cis isomer does in the hamster. J Lipid Res. 1994;35:1661–1673. [PubMed] [Google Scholar]
- 69.Daumerie CM, Woollett LA, Dietschy JM. Fatty acids regulate hepatic low density lipoprotein receptor activity through redistribution of intracellular cholesterol pools. Proc Natl Acad Sci USA. 1992;89:10797–10801. doi: 10.1073/pnas.89.22.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudel LL, Haines J, Sawyer JK, Shah R, Wilson MS, Carr TP. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clinc Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell TA, 3rd, Wilson MD, Kelley K, Sawyer JK, Rudel LL. Monounsaturated fatty acyl-coenzyme A is predictive of atherosclerosis in human apoB-100 transgenic, LDLr−/− mice. J Lipid Res. 2007;48:1122–1131. doi: 10.1194/jlr.M600526-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Bocan TM, Mueller SB, Brown EQ, Lee P, Bocan MJ, Rea T, Pape ME. HMG-CoA reductase and ACAT inhibitors act synergistically to lower plasma cholesterol and limit atherosclerotic lesion development in the cholesterol-fed rabbit. Atherosclerosis. 1998;139:21–30. doi: 10.1016/s0021-9150(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 73.Krause BR, Newton RS. Lipid-lowering activity of atorvastatin and lovastatin in rodent species: triglyceride-lowering in rats correlates with efficacy in LDL animal models. Atherosclerosis. 1995;117:237–244. doi: 10.1016/0021-9150(95)05576-i. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell A, Fidge N, Griffiths P. The effect of the HMG-CoA reductase inhibitor simvastatin and of cholestyramine on hepatic apolipoprotein mRNA levels in the rat. Biochim Biophys Acta. 1993;1167:9–14. doi: 10.1016/0005-2760(93)90210-z. [DOI] [PubMed] [Google Scholar]
- 75.Maugeais C, Tietge UJ, Tsukamoto K, Glick JM, Rader DJ. Hepatic apolipoprotein E expression promotes very low density lipoprotein-apolipoprotein B production in vivo in mice. J Lipid Res. 2000;41:1673–1679. [PubMed] [Google Scholar]
- 76.Dietschy JM. Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations. J Nutr. 1998;128:444S–448S. doi: 10.1093/jn/128.2.444S. [DOI] [PubMed] [Google Scholar]
- 77.Kurushima H, Hayashi K, Shingu T, Kuga Y, Ohtani H, Okura Y, Tanaka K, Yasunobu Y, Nomura K, Kajiyama G. Opposite effects on cholesterol metabolism and their mechanisms induced by dietary oleic acid and palmitic acid in hamsters. Biochim Biophys Acta. 1995;1258:251–256. doi: 10.1016/0005-2760(95)00122-s. [DOI] [PubMed] [Google Scholar]
- 78.Alexaki A, Wilson TA, Atallah MT, Handelman G, Nicolosi RJ. Hamsters fed diets high in saturated fat have increased cholesterol accumulation and cytokine production in the aortic arch compared with cholesterol-fed hamsters with moderately elevated plasma non-HDL cholesterol concentrations. J Nutr. 2004;134:410–415. doi: 10.1093/jn/134.2.410. [DOI] [PubMed] [Google Scholar]
- 79.Wilson T, Nicolosi R, Kotyla T, Sundram K, Kritchevsky D. Different palm oil preparations reduce plasma cholesterol concentrations and aortic cholesterol accumulation compared to coconut oil in hypercholesterolemic hamsters. J Nutr Biochem. 2005;16:633–640. doi: 10.1016/j.jnutbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Tzang B, Yang S, Fu S, Yang H, Sun H, Chen Y. Effects of dietary flaxseed oil on cholesterol metabolism in hamsters. Food Chem. 2009;114:1450–1455. [Google Scholar]
- 81.Mangiapane E, McAteer M, Benson G, White DS,AM. Modulation of the regression of atherosclerosis in the hamster by dietary lipids: comparison of coconut oil and olive oil. Br J Nutr. 1999;82:401–409. doi: 10.1017/s0007114599001646. [DOI] [PubMed] [Google Scholar]
- 82.Jones PJH, Ridgen JE, Benson AP. Influence of dietary fatty acid composition on cholesterol synthesis and esterification in hamsters. Lipids. 1990;25:815–819. doi: 10.1007/BF02535903. [DOI] [PubMed] [Google Scholar]
- 83.Horton JD, Cuthbert JA, Spady D. Dietary fatty acids regulate hepatic low density lipoprotein (LDL) transport by altering LDL receptor protein and mRNA levels. J Clin Invest. 1993;92:743–749. doi: 10.1172/JCI116645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sessions VA, Salter AM. The effects of different dietary fats and cholesterol on serum lipoprotein concentrations in hamsters. Biochem Biophys Acta. 1994;1211:207–214. doi: 10.1016/0005-2760(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 85.Woollett LA, Spady DK, Dietschy JM. Saturated and unsaturated fatty acids independently regulate low density lipoprotein receptor activity and production rate. J Lipid Res. 1992;33:77–88. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.