Abstract
Metabolic syndrome (MetS) is a cluster of risk factors, including elevated mean arterial pressure (MAP), atherogenic dyslipidemia (elevated triglycerides [TRG]), abdominal obesity (increased body mass index [BMI]), glucose intolerance (elevated glucose [GLU]), and prothrombotic/inflammatory state (increases in uric acid [UA]), that are associated with increased risk of cerebrovascular disease. We studied if an association existed between MetS components and human immunodeficiency virus (HIV)-associated cryptogenic strokes—those not caused by HIV complications, endocarditis, or stimulant abuse. We performed a retrospective case-control study. Eleven cryptogenic strokes were identified from 2346 HIV-infected (HIV+) participants. Each case was matched by age, sex, and date of stroke diagnosis to five HIV+ controls without stroke. Nonparametric stratified Wilcoxon ranked sum tests with subsequent mixed effect logistic regression determined the influence of each MetS component on HIV-associated cryptogenic stroke. Although each MetS component appeared higher for HIV+ cases with cryptogenic strokes than HIV+ controls, only MAP (odds ratio [OR] = 5.70, 95% confidence interval [CI] = 1.15–28.3) and UA (OR = 1.88, 95% CI = 1.06–3.32) were statistically different. A significantly higher percentage of HIV-associated cryptogenic stroke cases met criteria for MetS (4/11 = 36%) compared to HIV+ controls (6/55 = 11%). This observational study suggests a possible role for MetS components in HIV+ cryptogenic stroke cases. Although MetS is defined as a constellation of disorders, elevated hypertension and hyperuricemia may be involved in stroke pathogenesis. Reducing MetS component levels in HIV+ patients could therefore protect them from subsequent stroke.
Keywords: HIV, metabolic syndrome, stroke
Introduction
A causal relationship between human immunodeficiency virus (HIV) infection and stroke remains uncertain (Berger, 2004), with some studies demonstrating an increased risk compared to HIV-uninfected controls (Cole et al, 2004; Engstrom et al, 1989) and others finding no association (Mochan et al, 2003). Because many strokes are asymptomatic, the clinical observation of strokes in HIV+ patients (Rabinstein, 2003) is markedly less than at autopsy (Connor et al, 2000).
The etiology of strokes in HIV-infected (HIV+) patients can include complications due to HIV (e.g., opportunistic infections, malignancies, vasculitis, and hypercoagulable states), cerebrovascular atherosclerosis, chronic inflammation, or risky behaviors associated with HIV (e.g., stimulant drug abuse or endocarditis) (Pinto, 1996). Combination antiretroviral therapy (cART) has markedly reduced the mortality and morbidity due to HIV but has also led to an increase in metabolic and anthropomorphic side effects (Falutz, 2007; Germinario, 2003; Nolan, 2003).
Metabolic syndrome (MetS) is a constellation of disorders, including abdominal obesity, atherogenic dyslipidemia, hypertension, glucose intolerance, and a prothrombotic/inflammatory state, that increase the risk of cerebrovascular disease (Boden-Albala, 2006). Having just a single condition is not diagnostic of MetS, but does increase the overall risk of stroke (Bonora, 2006). MetS components have been defined to include (1) hypertension as defined by elevated systolic blood pressure (≥130 mm Hg), elevated diastolic blood pressure (≥85 mm Hg), or elevated mean arterial pressure (MAP) (≥100 mm Hg); (2) dyslipidemia with elevated fasting triglycerides (TRG) (≥150 mg/dl) and reduced high-density lipoprotein cholesterol (HDL-C) (<35 mg/dL [males] or <39 mg/dL [females]); (3) central obesity, with a waist:hip ratio >0.90 (males) or >0.80 (females), and/or elevated body mass index (BMI) (>30 kg/m2); and (4) elevated fasting plasma glucose (GLU) (≥110 mg/dl) (Pi-Sunyer, 2007). Controversy exists concerning the completeness and precision of this MetS definition using these cutoffs. Additional prothrombotic/inflammatory markers have been suggested for inclusion (Eckel et al, 2005; Magliano et al, 2006). Elevations in the proinflammatory marker serum uric acid (UA) (≥5 mg/dl) has been observed with MetS (Cirillo et al, 2006; Coutinho Tde et al, 2007; Hjortnaes et al, 2007; Kawamoto et al, 2006; Onat et al, 2006) and has been associated with increased risk for subclinical strokes as noted by white matter magnetic resonance imaging (MRI) hyperintensities (Schretlen et al, 2007).
Both HIV and MetS could each increase the risk of stroke by common pathways (Mangili et al, 2007). Overlapping mechanisms of vasculopathy could include lipid abnormalities, elevated proinflammatory cytokines, endothelial dysfunction, and oxidative stress (Bandaru et al, 2007; Sacktor et al, 2004). Estimates of the prevalence of MetS in HIV+ patients are quite variable and range from 25% to 96% (Carr et al, 1999; Fantoni et al, 2002).
We evaluated the relationship between MetS components and HIV-associated cryptogenic strokes by taking advantage of a comprehensive database available on 2346 HIV+ persons evaluated at the University of California San Diego (UCSD) HIV Neurobehavioral Research Center (HNRC). Specifically, we compared MetS components in cases of HIV+ persons with symptomatic cryptogenic strokes to HIV+ controls without strokes matched for age, sex, and date of stroke diagnosis.
Results
Table 1 shows the demographic features of the 11 HIV+ cases with cryptogenic strokes. This group was mostly male (82%), Caucasian (64%), with an average age of 41±6 years. There was no difference in self-reported duration of infection between those with strokes (124±57 months) compared to those without strokes (118±75 months). Of the 11 HIV+ cases, 8 had carotid Doppler studies of the neck arteries, 5 had transthoracic echocardiograms (TTEs) for assessment of possible endocarditis, and 3 had coagulation panels for hypercoagulable states performed as part of their clinical evaluation. Moderate stenosis (<55% patency) of the internal carotid artery corresponding to the side of stroke was observed in 3 of the 8 cases examined (patients 1, 9, and 11). None had evidence of endocarditis or coagulation abnormalities. When available, neuroimaging results were reviewed. Radiological confirmation of stroke was confirmed in 64% of the patients. The 11 cryptogenic strokes were primarily ischemic (82%), with clinical presentation typically consisting of unilateral weakness and occasionally sensory changes (Table 1). Ten of the 11 cases were tested serologically for syphilis and all were unreactive.
Table 1.
Demographic and clinical characteristics of the 11 HIV-infected cases with cryptogenic strokes
| Patient no. | Age | Race | Sex | Stroke type | Symptoms | MAP | BMI | GLU | TRG | UA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Cauc | F | Isch | Right leg paresis | 95 | 26 | 91 | 242 | 5.9 |
| 2 | 42 | Hisp | M | Hem | Left hemiplegia | NA | 36 | 110 | 151 | 3.6 |
| 3 | 41 | Cauc | M | Isch | Right arm paresis | 102 | 42 | 130 | 735 | 5.2 |
| 4 | 35 | Cauc | M | Isch | Right arm parsesis | 101 | 22 | 91 | NA | NA |
| 5 | 53 | AA | F | Isch | Left hemiparesis | 97 | 23 | 67 | 126 | 10.9 |
| 6 | 48 | Cauc | M | Isch | Right hemiparesis | 104 | 41 | 279 | 771 | NA |
| 7 | 41 | AA | M | Hem | Coma | 77 | 27 | 78 | 129 | 6 |
| 8 | 41 | Cauc | M | Isch | Left hemiparesis | 96 | 23 | 76 | 147 | 5.1 |
| 9 | 34 | Cauc | M | Isch | Left leg paresis | 119 | 25 | 118 | 427 | 5.6 |
| 10 | 34 | Cauc | M | Isch | Left arm paresis | 111 | 30 | 138 | NA | 8 |
| 11 | 48 | Cauc | M | Isch | Right arm paresis | 103 | 31 | 91 | 238 | 5.9 |
Note. Cauc = Caucasian; Hisp = Hispanic; AA = African American; MAP = mean arterial pressure; BMI = body mass index; GLU = glucose; TRG = triglycerides; UA = serum uric acid; NA = not available; Isch = ischemia; Hem = hemorrhagic.
MetS components were compared between HIV+ cases with cryptogenic strokes and matched HIV+ controls (Table 2). On average, recorded outpatient laboratory measures were obtained at 23±3 months after the onset of stroke symptoms and ensured that acute effects due to stroke did not contribute to observed elevations in these values. Although each MetS component (MAP, random TRG, random GLU, BMI, and serum UA) appeared higher for HIV+ associated cryptogenic cases compared to the HIV+ controls, only mean MAP and serum UA were significantly different. Overall, 72% of the HIV+ associated cryptogenic cases had hypertension, whereas only 20% of the HIV+ controls met the criteria (P = .0003). The odds ratio (95% confidence interval [CI]) for each 10 mm Hg increase in MAP was 5.70 (1.15–28.3) and for each 1 mg/dl increase in serum UA was 1.88 (1.06–3.32). Although we identified a relatively small cohort, the effect size as determined by Cohen's d value for MAP (Cohen's d = 1.46) and serum UA (Cohen's d = 0.96) were large.
Table 2.
Metabolic syndrome components in HIV+ cases with cryptogenic strokes and HIV+ control subjects
| HIV + cryptogenic stroke cases (n = 11) | HIV + control subjects (n = 55) | P value | Difference (95% confidence interval) | Odds ratio (95% confidence interval) | Cohen's d effect size | |
|---|---|---|---|---|---|---|
| Mean arterial blood pressure (MAP) (mm Hg) | 101±11 | 86±10 | .003* | 14.8 (8.4, 21.3) | 5.85 (1.15, 29.7) | 1.46 |
| Random triglycerides (TRG) (mg/dL) | 327±260 | 217±117 | .46 | 60.0 (−20.8, 177.5) | 1.61 (0.76, 3.40) | 0.48 |
| Body mass index (BMI) (kg/m2) | 28.6±7.4 | 24.7±4.1 | .10 | 3.4 (0.3, 7.0) | 2.00 (1.01, 3.96) | 0.73 |
| Random glucose (GLU) (mg/dL) | 115±59 | 95±19 | .44 | 12.4 (−2.8, 30.2) | 1.60 (0.83, 3.08) | 0.53 |
| Serum uric acid (UA) (mg/dL) | 6.3±2.2 | 4.8±1.2 | .02* | 1.38 (0.27, 2.73) | 2.67 (1.06, 6.73) | 0.96 |
Note. Data are mean±standard deviation (SD).
P < .05. The odds ratio is for stroke, per 10 mm Hg MAP increase, per 1 mg/dL, or kg/m2.
For this table the “Difference” column contains the difference in the means (and 95% CI) between the stroke and control groups, controlling for matching. These differences were computed using linear mixed-effects models, with a random effect for the matching groups. A log transformation was used for glucose, uric acid, triglycerides, and BMI. The differences (and 95% CI) on the log-scale were then back-transformed to the natural scale. The odds ratio is the odds ratio of stroke (and 95% CI) per 1 SD increase in the covariate.
Because hemorrhagic strokes are more common in hypertensive cases, we hypothesized that our two cases of hemorrhagic stroke might have influenced observed relationships. However, even after excluding two cases with hemorrhage, MAP remained a risk factor in the remaining nine patients with ischemic stroke. These cases remained included in the analysis as we were unsure if the initial event was ischemic with hemorrhagic conversion.
Because MetS reflects a syndromic combination of multiple components, we assessed for differences in prevalence of zero, one, two, or there or more of these factors between groups. There was no significant difference between HIV+ associated cryptogenic stroke cases and HIV+ controls in regards to number of MetS components present (data not included). Although none of the HIV+ cases with cryptogenic strokes were clinically diagnosed with MetS, 4 of 11 cases (36%) met criteria the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) criteria (Grundy et al, 2005). This prevalence of retrospectively diagnosed MetS was greater than in HIV+ controls (6/55 = 11%; P = .031).
We also assessed the possible impact of other laboratory and clinical variables on HIV-associated cryptogenic strokes. Neither clinical (duration of infection, duration of antiretroviral use, history of substance abuse, history of stimulant abuse, or Centers for Disease Control and Prevention [CDC] classification) nor laboratory (hemoglobin, hematocrit, platelet count, total serum cholesterol, CD4 lymphocyte counts, nadir CD4, plasma HIV RNA levels) variables significantly differed between the HIV+ cryptogenic cases and HIV+ controls (Table 3). HIV+ cases were also classified at the outpatient visit according to their cART regimen (either protease inhibitor [PI] or not PI based). Although a higher percentage of HIV+ cases with cryptogenic strokes were taking PIs (55%) than HIV+ control (33%), this difference was not statistically significant (P = .34; 95% CI: −0.12, 0.43) (Table 3).
Table 3.
Additional HIV-related clinical and laboratory factors in stroke cases and HIV-infected controls
| HIV-related cryptogenic stroke (n = 11) | HIV-infected controls (n = 55) | P value | Difference in proportion (95% confidence interval) | Odds ratio (95% confidence interval) | Cohen's d effect size | |
|---|---|---|---|---|---|---|
| Center for Disease Control (CDC) classification (% AIDS) | 73% | 67% | .89 | 8% (−16%, 43%) | 1.45 (0.33, 6.44) | |
| Duration of known HIV infection in months | 139±57 | 118±75 | 0.42 | 22 (−24, 68) | 1.35 (0.59, 3.11) | 0.30 |
| % taking combination antiretroviral therapy | 82% | 70% | 0.68 | 12% (−10%, 49%) | 1.89 (0.35, 10.37) | |
| % taking protease inhibitors (PIs) | 55% | 33% | 0.34 | 22% (−12%, 43%) | 2.40 (0.61, 9.38) | |
| Duration of combination antiretroviral therapy (cART) in months | 25±28 | 19±19 | 0.77 | 6 (−14, 25) | 1.30 (0.52, 3.25) | 0.28 |
| % history of previous substance abuse | 64% | 71% | 0.29 | −7% (−27%, 25%) | 0.72 (0.18, 2.94) | |
| % history of previous stimulant abuse | 45% | 44% | 0.70 | 1% (−29%, 28%) | 1.08 (0.28, 4.14) | |
| Laboratory values | ||||||
| Hemoglobin (g/dL) | 14.4±1.6 | 13.6±1.7 | 0.12 | 0.7 (−0.2, 1.7) | 1.64 (0.78, 3.46) | 0.46 |
| Hematocrit (%) | 43±2 | 40±5.0 | 0.086 | 2.4 (−0.4, 5.3) | 1.81 (0.82, .4.01) | 0.52 |
| Platelets (103/μl) | 262±113 | 232±74 | 0.47 | 35 (−16, 86) | 1.53 (0.77, 3.06) | 0.46 |
| Cholesterol (mg/dL) | 192±47 | 174±39 | 0.60 | 18 (−12, 47) | 1.55 (0.73, 3.28) | 0.44 |
| CD4 count (cells/μL) | 441±383 | 373±276 | 0.78 | −1.9 (−8.2, 4.5) (on square root scale) |
0.81 (0.38, 1.69) | 0.19 |
| CD4 nadir (cells/μL) | 219±319 | 210±240 | 0.61 | −0.7 (−5.9, 46) (on square root scale) |
0.92 (0.46, 1.85) | 0.08 |
| plasma HIV-1 RNA viral load (% detectable) | 50% | 49% | 0.84 | −1% (−33%, 32%) | 0.96 (0.20, 4.57) |
Note. Data are mean±SD.
Discussion
In this case-control study we found a 3-fold higher prevalence of retrospectively diagnosed MetS components in the HIV-associated cryptogenic stroke patients than in HIV+ controls without strokes. Although the mean values of MetS components appeared to be higher in the 11 HIV-related cryptogenic stroke cases than in the 55 HIV+ controls matched for age, sex, and date of stoke diagnosis, only mean arterial pressure (MAP) and serum uric acid (UA) were statistically different between the two groups.
This study, like earlier analyses, has shown that cerebral infarction and not hemorrhage is the predominant form of stroke in HIV+ patients (Cole et al, 2004; Engstrom et al, 1989; Ortiz et al, 2007; Qureshi et al, 1997). This finding and the normal platelet counts in our patients suggests that immune-mediated thrombocytopenia, a common HIV-associated complication, does not frequently contribute to HIV-associated cryptogenic strokes (Pinto, 1996).
Neither HIV replication rates, as assessed by plasma HIV levels, nor degree of immunosuppression (current or nadir CD4 lymphocyte cell counts) contributed to risk of stroke (Ortiz et al, 2007). This study does not address the role of HIV-associated immunosuppression in risk of stroke-like symptoms from central nervous system (CNS) opportunistic infections and malignancies, as such cases were excluded by design. Risk of such strokes is likely to be increased with advanced immunosuppression.
Both MAP and UA, a proinflammatory marker, were significantly greater for HIV+ cases with cryptogenic strokes than HIV+ controls. In regards to serum UA, normal elderly adults with higher UA values have a 4- to 5-fold increase in risk of cerebral ischemic disease, as assessed by hyperintensities in white matter by MRI (Schretlen et al, 2007). Although the mechanism remains unknown, our findings support a link between elevations in serum UA and cerebrovascular disease. UA can damage the vascular endothelium (Reynolds, 2007) causing accelerated atherosclerosis (Kanellis and Kang, 2005). UA can also produce superoxide anions and lead to increases in oxidative stress. A similar mechanism has been proposed for vascular damage caused directly by HIV infection (Sacktor et al, 2004).
The use of combined antiretroviral therapy (cART), in particular PIs, has been associated with the development of MetS (Wang et al, 2007). However, we found no correlation between cART and stroke, even after controlling for PI use. Our results are similar to those of a recent study that demonstrated no relationship between PI use and MetS (Mangili et al, 2007). In addition, the self-reported duration of exposure to cART was similar for both groups, suggesting that the observed increases in MAP and serum UA may act through other mechanisms. There was no significant difference in the frequency of PI-based regimens between HIV+ stroke patients and HIV+ controls (55% versus 33%). A possible contribution of PIs to risk of cryptogenic stroke was assessed by post hoc power calculations (Hoenig and Heisey, 2001). For example, we have calculated that the calculated differences in PI use between these two groups could in fact be as small as −12% or as large as 43% due to the limited sample size (Table 3). Larger powered studies are required.
This study has multiple limitations. First, although over 2000 cases were examined, the sample size was small, reflecting the relatively low rate of cryptogenic strokes. It is possible that our observed values actually underestimate the prevalence of HIV-associated strokes due to the loss of patients who died, who may have dropped out because of disabilities associated with their strokes, or who failed to recall or recognize subtle clinical symptoms (Wright et al, 2008). Second, only random outpatient blood GLU and TRG levels were available, introducing variation that may have obscured relationships between stroke and both these variables and MetS, of which they are components. However, random and fasting levels have been found to correlate in patients with MetS, suggesting that the former may be used as a surrogate for the latter (Ahmadani et al, 2008). Third, because only BMI, but not waist - circumference or anterior-posterior waist diameter, was available, we used BMI to assess central obesity. Fortunately, the relationship between BMI and waist circumference is linear for BMI values between 20 and 30 kg/m2 (Booth et al, 2000). Fourth, we were unable to exclude with certainty HIV complications and endocarditis as mechanisms of strokes because only 73% had carotid imaging to detect large vessel atheromas, 45% had TTE for evaluation of endocarditis, and 27% had other tests for cerebral vasculopathy and hypercoagulation (Ortiz et al, 2007). Previous studies have demonstrated that these conditions could increase the risk of both ischemic and hemorrhagic stroke with HIV infection (Berger, 2004). Finally, MetS risk factors were assessed an average of nearly 2 years after diagnosis of stroke, allowing for greater variation compared to the extended period before the stroke when they may have operated to increase risk. Although we were surprised that laboratory values for some MetS risk factors were elevated at these later time points, we believe that the observed effects may be even greater for longitudinal studies that assessed cases earlier after their event. Future larger prospective studies to assess these metabolic risk factors are needed to confirm these findings.
This is first study to examine the relationship of MetS and its components to HIV-related stroke in the cART era and in comparison to matched HIV-infected patients without stroke (Ortiz et al, 2007). Comparisons in the pre-cART era could not examine stroke risk associated with the antiretroviral protease inhibitors or non-nucleoside reverse transcriptase inhibitors that contribute to MetS. The fact that that the effect sizes were quite robust for hypertension and hyperuricemia (Cohen's d = 1.46 for MAP and for 0.96 for serum UA) suggests the importance of examining these potential predictors in larger cohorts.
We conclude that detecting elevations in MetS components, especially hypertension and hyperuricemia, and reducing them by lifestyle modifications and/or pharmacological therapy may benefit the increasingly aging HIV+ population of most developed countries by preventing strokes.
Materials and methods
A retrospective chart and database analysis was performed of 2346 participants followed at the HIV Neurobehavioral Research Center (HNRC) of the University of California San Diego (UCSD) in multiple longitudinal studies. All participants consented to participation in the parent studies, to the use of their available clinical data, and to post hoc analyses of their data. The Institutional Review Board (IRB) at UCSD approved all HNRC studies. From this group of systematically surveyed volunteers, cases of stroke-like illnesses were selected based on reported International Classification of Disease, 9th revision (ICD-9) codes (Reker et al, 2002). Codes included the following potential stroke classifications: 342: Hemiplegia and Hemiparesis; 430: Subarachnoid hemorrhage; 431: Intracerebral hemorrhage; 432: Other and unspecified intracranial hemorrhage; 433: Occlusion and stenosis of precerebral arteries; 434: Occlusion of cerebral arteries; 435: Transient cerebral ischemia; 436: Acute, but ill-defined cerebrovascular disease; 437: Other and ill-defined cerebrovascular disease; 438: Late effects of cerebrovascular disease. Stroke was defined according to World Health Organization (WHO) criteria (Bonita et al, 2004) as a condition characterized by rapidly developing symptoms and signs of a focal brain lesion, with symptoms lasting for more than 24 hours or leading to death, with no apparent cause other than that of vascular origin. All strokes were classified as either ischemic or hemorrhagic using National Institute of Neurological Disorders and Stroke criteria (Ballard et al, 2004).
From these coding procedures, 21 cases were initially identified. Charts were subsequently reviewed, with strokes classified by likely etiology based on existing additional evidence of confounding factors, such as intracerebral HIV-related pathologies (opportunistic infections, primary central nervous system lymphoma, metastatic malignancy, meningoencephalitis, progressive multifocal leukoencephalopathy, cerebral abscesses, and tuberculosis), endocarditis, or stimulant abuse (according to the Diagnostic and Statistical Manual of Mental Disorders- Fourth Edition [DSM-4] criteria). Of these 21 HIV+ cases, 10 had stroke-like illnesses most likely due to either endocarditis (n = 2), primary central nervous system lymphoma (n = 2), metastatic Kaposi sarcoma (n = 1), progressive multifocal leukoencephalopathy (n = 3), or cerebral abscess (n = 2). The remaining 11 HIV+ cases were classified as “cryptogenic” strokes (i.e., not attributable to central nervous system complications of HIV infections, endocarditis, or stimulant drug abuse) (Figure 1). For each cryptogenic stroke case, five HIV+ control participants without stroke were identified from the same HNRC cohort using the following matching criteria: age (±4 years), sex, and outpatient visit date (±4 years).
Figure 1.
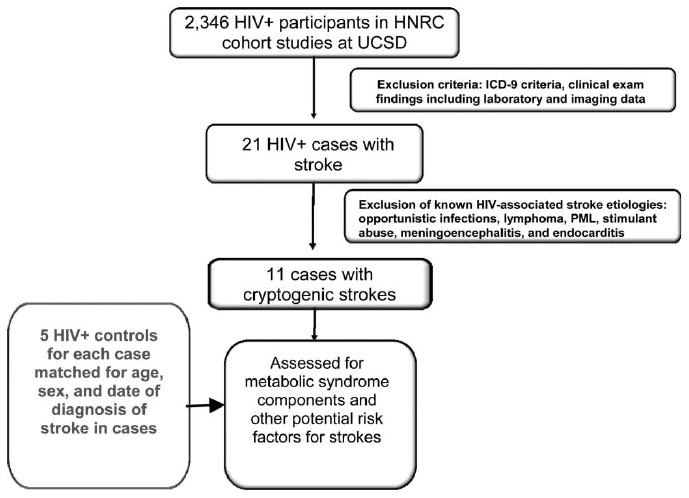
Selection criteria for identifying cases with cryptogenic strokes not due to HIV complications (n = 11). HNRC = HIV Neurobehavioral Research Center; UCSD = University of California San Diego; ICD9 = International Classification of Disease, 9th revision; PML = progressive multifocal leukoencephalopathy.
For these 66 participants (11 HIV+ cryptogenic stroke and 55 HIV+ controls), MetS components (MAP, TRG, BMI, GLU, and UA), clinical, and additional laboratory data were assessed. All BP measurements were obtained at the HNRC using a calibrated mercury sphygmomanometer and appropriate cuff size. Both systolic and diastolic BP measures were obtained from a subject in the sitting position to ensure standardized conditions (Banegas et al, 2007; Pickering et al, 2005). For all participants, MetS laboratory component values were extracted from outpatient research records closest to the reported stroke. Only random and not-fasting TRG and GLU were available. Additional variables surveyed included the self-reported duration of infection, history of previous substance abuse (cocaine, amphetamines, alcohol, cannabis, opioids, and hallucinogens), medication history, cholesterol, hemoglobin, hematocrit, platelets, current and lowest (nadir) reported blood CD4+ cell count, and plasma viral load. Participants were also classified as having acquired immunodeficiency syndrome (AIDS) according to the Centers for Disease Control and Prevention (CDC) classification (i.e., CD4 count less than 200 or an AIDS-defining illness) (Buehler and Ward, 1993).
To assess for possible differences in MetS components between HIV+ cases with cryptogenic strokes and HIV+ controls, we performed nonparametric Wilcoxon ranked sum tests controlled for matching. A logarithmic transformation was used for random TRG, random GLU, UA, and BMI due to their skewness and unequal variances (homoscedasticity). The difference in the means (and 95% CI) was determined between HIV+ cases with cryptogenic strokes and HIV+ controls. These differences were computed using linear mixed-effects models, with a random effect for the matching groups. A log transformation was used for random TRG, random GLU, UA, and BMI with differences (and 95% CI) back-transformed to the natural scale (Breslow and Clayton, 1993). To assess for possible differences in clinical and laboratory values between HIV+ cases with cryptogenic strokes and HIV+ controls, we performed either a nonparametric stratified Wilcoxon ranked sum test (for plasma viral load) or a stratified Gehan-Wilcoxon rank test, which accounted for values censored below the limit of detection of the assay. To assess effects sizes, a Cohen's d value was determined for each MetS component. To delineate if MetS components were associated with protease inhibitor (PI) exposure or duration, for each individual we classified cART regimens as either PI containing or not PI containing and used Cochran-Mantel-Haenszel tests to compare HIV+ cases with cryptogenic strokes to HIV+ controls.
Acknowledgments
This research was supported by the Foundation for AIDS Research (amFAR) Fellowship (106729-40-RFRL) (B.A.), Dana Foundation Brain-Immuno Imaging Award (DF 3857-41880) (B.A.), NIH grants 1K23MH081786 (B.A.), and P30 MH62512 (HNRC Group). The HNRC is supported by Center Award MH 62512 from the National Institute of Mental Health (NIMH).
The San Diego HIV Neurobehavioral Research Center (HNRC) group includes Director: Igor Grant, MD; Co-Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and J. Allen McCutchan, MD; Center Manager: Thomas D. Marcotte, PhD; Jennifer Marquie Beck; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, MD, PhD (P.I.), J. Allen McCutchan, MD, Scott Letendre, MD, Edmund Capparelli, PharmD, Rachel Schrier, PhD, Terry Alexander, RN; Neurobehavioral Component: Robert K. Heaton, PhD (P.I.), Mariana Cherner, PhD, Steven Paul Woods, PsyD, David J. Moore, PhD, Matthew Dawson; Neuroimaging Component: Terry Jernigan, PhD (P.I.), Christine Fennema-Notestine, PhD, Sarah L., Archibald, MA, John Hesselink, MD, Jacopo Annese, PhD, Michael J. Taylor, PhD; Neurobiology Component: Eliezer Masliah, MD (P.I.), Ian Everall, FRCPsych, FRCPath, PhD, Cristian Achim, MD, PhD; Neurovirology Component: Douglas Richman, MD, (P.I.), David M. Smith, MD; International Component: J. Allen McCutchan, MD, (P.I.); Developmental Component: Ian Everall, FRCPsych, FRCPath, PhD (P.I.), Stuart Lipton, MD, PhD; Clinical Trials Component: J. Allen McCutchan, MD, J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, Scott Letendre, MD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (P.I.), Rodney von Jaeger, MPH; Data Management Unit: Anthony C. Gamst, PhD (P.I.), Clint Cushman, BA (Data Systems Manager), Daniel R. Masys, MD (Senior Consultant); Statistics Unit: Ian Abramson, PhD (P.I.), Christopher Ake, PhD, Florin Vaida PhD.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmadani MY, Hakeem R, Fawwad A, Basit A, Shera AS. Determination of reference values for elevated fasting and random insulin levels and their associations with metabolic risk factors among rural Pakistanis from Sindh Province. Metab Syndr Relat Disord. 2008;6:143–148. doi: 10.1089/met.2007.0031. [DOI] [PubMed] [Google Scholar]
- Ballard CG, Burton EJ, Barber R, Stephens S, Kenny RA, Kalaria RN, O'Brien JT. NINDS AIREN neuroimaging criteria do not distinguish stroke patients with and without dementia. Neurology. 2004;63:983–988. doi: 10.1212/01.wnl.0000138435.19761.93. [DOI] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banegas JR, Segura J, Sobrino J, Rodriguez-Artalejo F, de la Sierra A, de la Cruz JJ, Gorostidi M, Sarria A, Ruilope LM. Effectiveness of blood pressure control outside the medical setting. Hypertension. 2007;49:62–68. doi: 10.1161/01.HYP.0000250557.63490.55. [DOI] [PubMed] [Google Scholar]
- Berger JR. AIDS and stroke risk. Lancet Neurol. 2004;3:206–207. doi: 10.1016/S1474-4422(04)00704-5. [DOI] [PubMed] [Google Scholar]
- Boden-Albala B. Current understanding of multiple risk factors as the metabolic syndrome: distillation or deconstruction. Semin Neurol. 2006;26:108–116. doi: 10.1055/s-2006-933314. [DOI] [PubMed] [Google Scholar]
- Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3:391–393. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80. doi: 10.1080/07853890500401234. [DOI] [PubMed] [Google Scholar]
- Booth ML, Hunter C, Gore CJ, Bauman A, Owen N. The relationship between body mass index and waist circumference: implications for estimates of the population prevalence of overweight. Int J Obes Relat Metab Disord. 2000;24:1058–1061. doi: 10.1038/sj.ijo.0801359. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Clayton DG. Statistic in epidemiology: the case control study. J Am Stat Assoc. 1993;88:9–25. doi: 10.1080/01621459.1996.10476660. [DOI] [PubMed] [Google Scholar]
- Buehler JW, Ward JW. A new definition for AIDS surveillance. Ann Intern Med. 1993;118:390–392. doi: 10.7326/0003-4819-118-5-199303010-00012. [DOI] [PubMed] [Google Scholar]
- Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, Nakagawa T, Johnson RJ. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006;17:S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- Cole JW, Pinto AN, Hebel JR, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Price TR, Sloan MA, Stern BJ, Wityk RJ, Wozniak MA, Kittner SJ. Acquired immunodeficiency syndrome and the risk of stroke. Stroke. 2004;35:51–56. doi: 10.1161/01.STR.0000105393.57853.11. [DOI] [PubMed] [Google Scholar]
- Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD. Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV Autopsy Cohort. Stroke. 2000;31:2117–2126. doi: 10.1161/01.str.31.9.2117. [DOI] [PubMed] [Google Scholar]
- Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy PF, 2nd, Kullo IJ. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens. 2007;20:83–89. doi: 10.1016/j.amjhyper.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Engstrom JW, Lowenstein DH, Bredesen DE. Cerebral infarctions and transient neurologic deficits associated with acquired immunodeficiency syndrome. Am J Med. 1989;86:528–532. doi: 10.1016/0002-9343(89)90379-3. [DOI] [PubMed] [Google Scholar]
- Falutz J. Therapy insight: body-shape changes and metabolic complications associated with HIV and highly active antiretroviral therapy. Nat Clin Pract Endocrinol Metab. 2007;3:651–661. doi: 10.1038/ncpendmet0587. [DOI] [PubMed] [Google Scholar]
- Fantoni M, Del Borgo C, Autore C, Barbaro G. Metabolic disorders and cardiovascular risk in HIV-infected patients treated with antiretroviral agents. Ital Heart J. 2002;3:294–299. [PubMed] [Google Scholar]
- Germinario RJ. Anti-retroviral protease inhibitors—‘a two edged sword?’. IUBMB Life. 2003;55:67–70. doi: 10.1002/tbmb.718540874. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Hjortnaes J, Algra A, Olijhoek J, Huisman M, Jacobs J, van der Graaf Y, Visseren F. Serum uric Acid levels and risk for vascular diseases in patients with metabolic syndrome. J Rheumatol. 2007;34:1882–1887. [PubMed] [Google Scholar]
- Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat. 2001;55:19–24. [Google Scholar]
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Kawamoto R, Tomita H, Oka Y, Ohtsuka N. Relationship between serum uric acid concentration, metabolic syndrome and carotid atherosclerosis. Intern Med. 2006;45:605–614. doi: 10.2169/internalmedicine.45.1661. [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Shaw JE, Zimmet PZ. How to best define the metabolic syndrome. Ann Med. 2006;38:34–41. doi: 10.1080/07853890500300311. [DOI] [PubMed] [Google Scholar]
- Mangili A, Jacobson DL, Gerrior J, Polak JF, Gorbach SL, Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis. 2007;44:1368–1374. doi: 10.1086/516616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke. 2003;34:10–15. doi: 10.1161/01.str.0000043821.35051.fa. [DOI] [PubMed] [Google Scholar]
- Nolan D. Metabolic complications associated with HIV protease inhibitor therapy. Drugs. 2003;63:2555–2574. doi: 10.2165/00003495-200363230-00001. [DOI] [PubMed] [Google Scholar]
- Onat A, Uyarel H, Hergenc G, Karabulut A, Albayrak S, Sari I, Yazici M, Keles I. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am J Hypertens. 2006;19:1055–1062. doi: 10.1016/j.amjhyper.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–1261. doi: 10.1212/01.wnl.0000259515.45579.1e. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer X. The metabolic syndrome: how to approach differing definitions. Med Clin North Am. 2007;91:1025–1040. vii. doi: 10.1016/j.mcna.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- Pinto AN. AIDS and cerebrovascular disease. Stroke. 1996;27:538–543. doi: 10.1161/01.str.27.3.538. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Janssen RS, Karon JM, Weissman JP, Akbar MS, Safdar K, Frankel MR. Human immunodeficiency virus infection and stroke in young patients. Arch Neurol. 1997;54:1150–1153. doi: 10.1001/archneur.1997.00550210078016. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA. Stroke in HIV-infected patients: a clinical perspective. Cerebrovasc Dis. 2003;15:37–44. doi: 10.1159/000067120. [DOI] [PubMed] [Google Scholar]
- Reker DM, Rosen AK, Hoenig H, Berlowitz DR, Laughlin J, Anderson L, Marshall CR, Rittman M. The hazards of stroke case selection using administrative data. Med Care. 2002;40:96–104. doi: 10.1097/00005650-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Reynolds T. Serum uric acid, the endothelium and hypertension: an association revisited. J Hum Hypertens. 2007;21:591–593. doi: 10.1038/sj.jhh.1002239. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Inscore AB, Vannorsdall TD, Kraut M, Pearlson GD, Gordon B, Jinnah HA. Serum uric acid and brain ischemia in normal elderly adults. Neurology. 2007;69:1418–1423. doi: 10.1212/01.wnl.0000277468.10236.f1. [DOI] [PubMed] [Google Scholar]
- Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, Decarli C, Sacco R, Stern Y. White matter hyperintensities and subclinical infarction. Associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]


