Abstract
The frequent co-isolation of bacteria with Phytophthora and Pythium species suggests possible interspecies communication. Zoospore free fluids (ZFF) from bacteria-free and nutrient-depleted zoospore suspensions were examined to investigate production of autoinducer-2 (AI-2), a bacterial interspecies signal molecule, by zoosporic oomycetes. ZFF from P. nicotianae, P. sojae and Py. aphanidermatum triggered luminescence of Vibrio harveyi AI-2 reporter, indicating the presence of AI-2 in zoospore extracellular products and the potential of cross-kingdom communication between oomycetes and bacteria. Production of AI-2 by zoospores was confirmed by chemical assays. These results provide new insight into the physiology and ecology of oomycetes.
Keywords: oomycetes, AI-2, quorum sensing, cross-kingdom communication
1. Introduction
Phytophthora and Pythium in Oomycota of Stramenopila are phylogenetically related to marine algae but morphologically resemble fungi. Many species in these two genera are destructive pathogens that attack a broad range of economically important agricultural and ornamental crops as well as forest tree species. They produce asexual sporangia that release flagellate zoospores as their primary dispersal and infection agents (Deacon & Donaldson, 1993, Judelson & Blanco, 2005). Zoospores secrete a host of molecules during the homing process, however, with the exception of Ca2+ and an adhesive protein involved in aggregation, germination, and plant attachment (Deacon & Donaldson, 1993, Reid, et al., 1995, Robold & Hardham, 2005), little is known of the presence of other products and their relevance to zoospore communication.
In contrast, the identification of autoinducers or small hormone-like molecules has provided unparalleled insight into cell-to-cell communication and its role in the physiology, ecology, evolution, and pathogenesis of bacteria and a few fungal species (Winans & Bassler, 2008). The vast majority of molecules, such as acyl-homoserine lactones (AHLs) or oligopeptides from bacteria (Waters & Bassler, 2005), and small primary alcohols from fungi (Hogan, 2006), are species specific and used for intraspecific communication. One signal molecule called autoinducer-2 (AI-2) can be produced by half of the known bacterial population (Sun, et al., 2004) and by some eukaryotic plants (Gao, et al., 2003, Hauck, et al., 2003) although its production has not been reported in Fungi and Stramenopila. This molecule facilitates interspecific communication among bacteria (Xavier & Bassler, 2005). AI-2 is a collective term for a group of signal molecules derived from 4,5-dihydroxy-2,3-pentanedione (DPD) and is used interchangeably with DPD since conversion of DPD to various forms of AI-2 is a spontaneous ring closure process (Miller, et al., 2004).
The notorious presence of bacteria in Phytophthora and Pythium cultures and stimulation of Phytophthora zoospore and oospore production by bacterial metabolites (Zentmyer, 1965, Malajczuk, 1983) led us to hypothesize that zoosporic pathogens may produce AI-2 to communicate with bacteria. To test this, we analyzed zoospore free fluid (ZFF) from bacterium-free and nutrient-depleted zoospore suspensions for AI-2 activity using a AI-2 bacterial reporter strain (Bassler, et al., 1997) and chemical methods (Hauck, et al., 2003, Zhao, et al., 2003). We then discuss AI-2 production pathways and implications of AI-2 production in oomycte cross-kingdom communication.
2. Materials and methods
2.1. Phytophthora species and culture conditions
Two morphological and phylogenetically distinct Phytophthora species, and a species from the closely-related genus Pythium, were used in this study. Phytophthora nicotianae (Syn. P. parasitica) isolate 1B11, P. sojae (genotype I) isolate 23G8, and Pythium aphanidermatum isolate 18H1 were maintained in clarified 20 % vegetable juice medium supplemented with 1.5 % agar (CV8A) at 23 °C.
2.2. Preparation of zoospore-free fluid (ZFF)
ZFF was prepared from nutrient-depleted zoospore suspensions at high densities. A 5-mm2 CV8A mycelial plug was seeded in 10% CV8 in 90-mm Petri dishes. The dishes were incubated at 23°C in the dark for 3 days for P. sojae, 4 days for Py. aphanidermatum and 1–2 wks for P. nicotianae to induce sporangia. After the seed plugs and medium were removed, the mycelial mats were rinsed five times with sterile distilled water (SDW) to eliminate nutrients from the remaining medium. The drained mycelial mats were incubated for 16–18 h for P. sojae and Py. aphanidermatum, and one week for P. nicotianae under fluorescent light at 23°C. When numerous sporangia formed, the mats were rinsed an additional 5 times with SDW to remove residues from the medium. The dilution factor for the 10 % CV8 was then 1.08 × 109 as measured experimentally. To induce zoospore release, the mats were flooded with 8 ml of chilled SDW and kept under light until the desired zoospore density was reached. The density for 1B11 was up to 106 zoospores ml−1 in 1 h; for 23G8 and 18H1 was up to 5×104 and 3×104 zoospores ml−1 in 3 h, respectively. All procedures were performed under sterile conditions to prevent bacterial contamination.
To obtain ZFF, zoospore suspensions were filtered through sterile miracloth to remove mycelia, sporangia and other structures, then vortexed briefly to facilitate chemical release. The suspensions were then filtered through a 0.2 µm syringe filter to remove the cysts. ZFF was used fresh or stored at −20°C.
2.3. Detection of AI-2 in ZFF using bioluminescence assay
The bacterial AI-2 reporter Vibrio harveyi BB170 [luxN::TnS] (ATCC BAA-1117) was used to test the activity of ZFF and detect the presence of AI-2. The assay was conducted using a combined protocol based on procedures described previously (Bassler, et al., 1997, DeKeersmaecker & Vanderleyden, 2003). Briefly, BB170 was cultured overnight in MB medium then diluted 10,000× into AB medium. Aliquots of 90 µl from the resulting overnight culture were dispensed into each well of a 96-well plate, followed by the addition (10 µl per well) of test solutions. The plate was then incubated at 30°C with aeration. Light production was monitored using a CCD camera after 3 h incubation for a period of 8 h, and Integrated Optical Density (IOD) was measured using LabWorks Image Acquisition and Analysis Software (UVP, California, USA). Each assay included triplicate wells of ZFF samples with negative and positive controls. The negative controls included SDW and 10, 000 × diluted CV8. The positive controls consisted of a 10-fold dilution series from a 550 µM stock solution of enzymatically synthesized 4,5-dihydroxy-2,3-pentanedione (DPD), produced and quantified as described previously [17]. The experiment was repeated twice. For quantification, a standard curve was generated based on IOD measured at 6 h of incubation with the DPD dilution series. The standard curve was then used to plot the IOD from treatments to obtain AI-2 concentrations.
2.4. Chemical analysis of presence of AI-2 in ZFF
To confirm the presence of AI-2 (DPD) in ZFF and rule out false positives from the bioassay (DeKeersmaecker & Vanderleyden, 2003), ZFF samples were tested for DPD-derived quinoxaline generated via the chemical reaction with 1,2-diaminobenzene (Hauck, et al., 2003, Zhao, et al., 2003). Test solutions were mixed with 10 mM 1,2-diaminobenzene individually. After incubation overnight at 37 °C at pH 4.5, the resulting solution was extracted three times with an equal volume of ethyl ether. The organics were concentrated by rotary evaporation and then dissolved in methanol (500 µl).
The extracts were analyzed using liquid chromatography-mass spectrometry (LC-MS) for DPD-derived quinoxaline on a Surveyor HPLC system coupled to a Finnagan LCQ Deca XP mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Samples were loaded on a self-packed reversed-phase column (75 µm i.d. × 15 cm, Magic C18 resin, 3 µm particle size, 200 Å pore size, Michrom Bioresources, Auburn, CA). The column was equilibrated with 1% acetonitrile (solvent A) and 0.1% formic acid in water (solvent B) and eluted with the following solvent gradient starting from 1% solvent A for 10 min and increasing to 25% solvent A over 25 min, then to 50% solvent A over 5 min, and finally a constant 50% solvent A for 5 min. The flow rate was kept at a constant 160 µl min−1.
Data from LC-MS were processed using Xcalibar Data System 2.0 (Thermo Fisher Scientific, San Jose, CA). Quinoxaline was identified by extracted-ion chromatogram and fragmentation pattern analyses (Hauck, et al., 2003). Additional confirmation was made by co-elution with a DPD-derived quinoxaline standard prepared from the synthesized DPD. To quantify DPD-derived quinoxaline, the peak density at m/z 205 was plotted using a calibration curve generated from the synthetic DPD samples of known concentrations.
3. Results
3.1. Zoospore extracellular products affect bacterial quorum sensing of AI-2 reporter
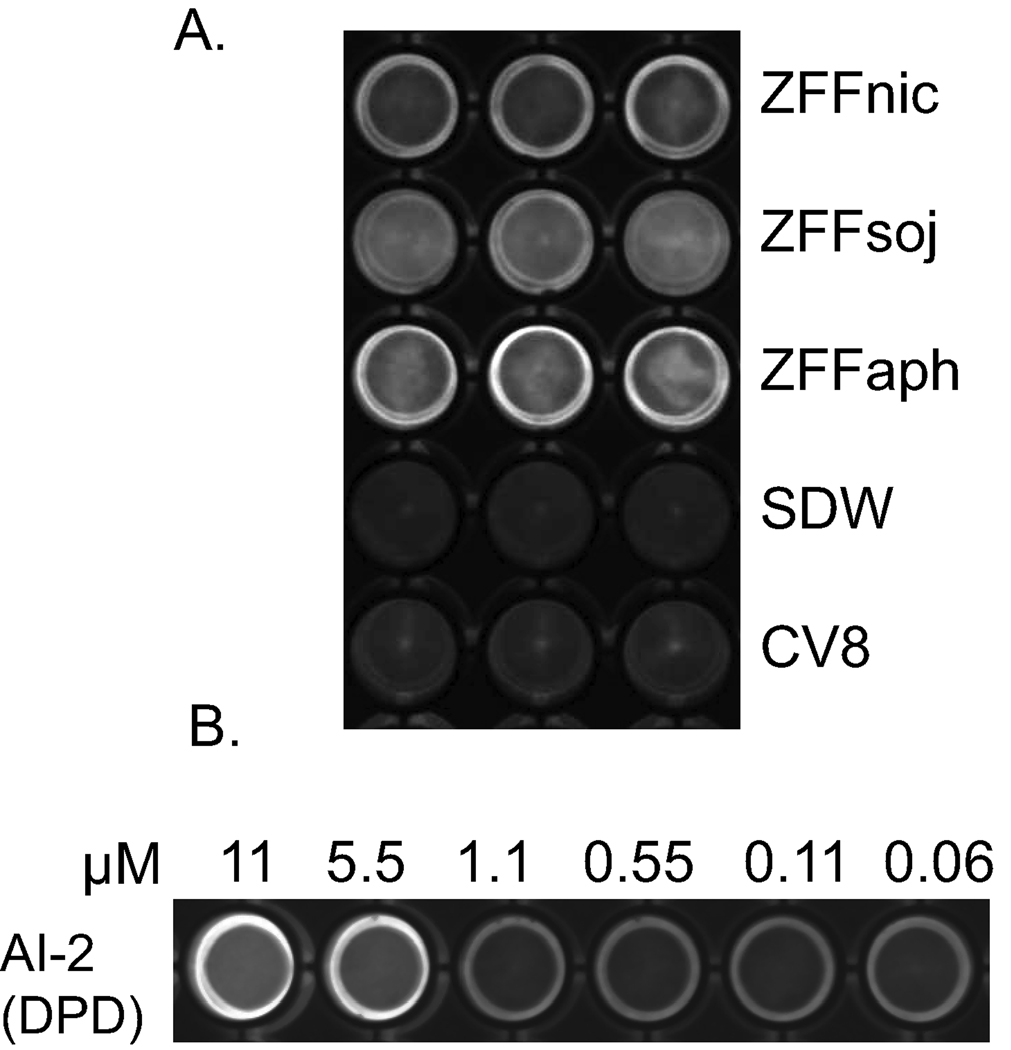
ZFF triggered luminescence production of Vibrio harveyi AI-2 reporter strain BB170. Intensive light production was observed in ZFF treated wells but not in control wells containing SDW and 104 × diluted CV8 at 6 h (Fig. 1A). Based on the light intensity induced by synthetic AI-2 (Fig. 1B), the concentration of AI-2 in ZFF samples was estimated between 1.1- 5.5 µM. Within ZFF treatments, ZFFaph displayed the highest light intensity, followed by ZFFsoj and ZFFnic. Stimulation of light production of V. harveyi indicates zoospore extracellular products regulate quorum sensing of the bacteria and contain AI-2.
Figure 1. Induced luminescence production of AI-2 reporter strain Vibrio harveyi BB170.
Light production was photographed using a CCD camera at 6 h at 30°C after addition of (A) ZFFnic from a zoospore suspension of Phytophthora nicotianae at 5×105 ml−1, ZFFsoj from a suspension of P. sojae at 104 ml−1, ZFFaph from a suspension of Py. aphanidermatum at 3×104 ml−1, SDW and 104 × diluted CV8 controls; (B) a dilution series of synthetic AI-2. Each assay contained 3 wells and was repeated twice.
Quantification of AI-2 in ZFF using the DPD standard curve (AI-2=0.376*IOD-28.93) indicated that the amount of AI-2 in ZFF varied with species. ZFFaph contained the highest concentration of AI-2 (1.66 ±0.25 µM) among the three species tested followed by ZFFsoj (1.40 ±0.18 µM), both from zoospores at 104 ml−1 levels, while ZFFnic from zoospores at 5×105 ml−1 contained the least AI-2 (0.66 ±0.13 µM). Negative values were obtained for the SDW and CV8 controls, indicating that zoospores produced AI-2, and the AI-2 activity was not from residual CV8 broth.
3.2. Confirmation of AI-2 production by chemical method
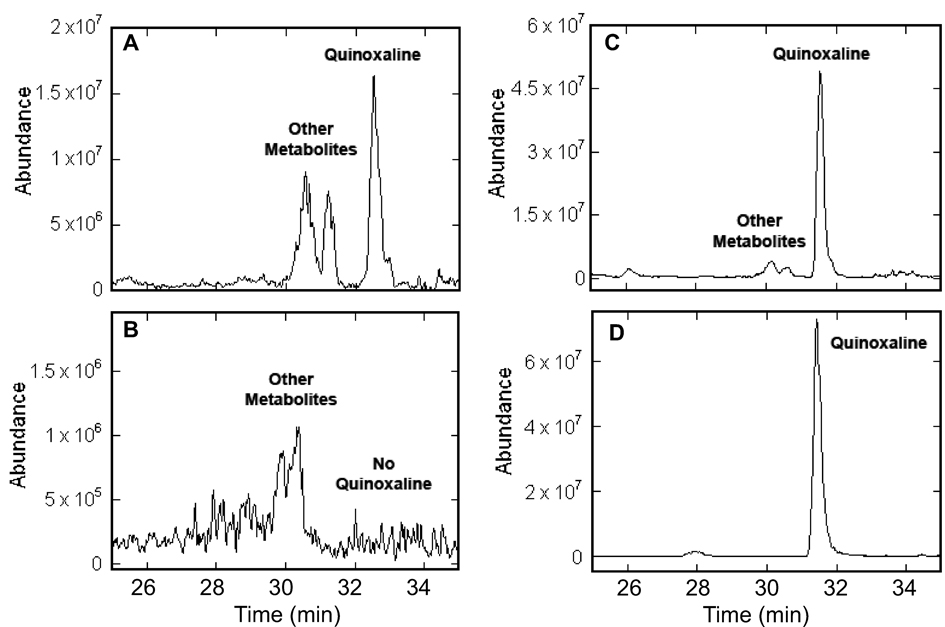
To confirm the results from the luminescence assay, ZFFnic and ZFFsoj were analyzed using chemical methods. The formation of quinoxaline derived from DPD, specifically 2-(1,2-dihydroxyethyl)-3-methylquinoxaline, was detected by liquid chromatography-mass spectrometry (LC-MS). First, a peak identical to quinoxaline from synthetic DPD (Fig. 2D) was identified in the extracted ion chromatograms (EIC) at m/z 205 from ZFFsoj (Fig. 2A) and ZFFnic (Fig. 4A middle). Second, quinoxaline products from ZFF coeluted with the quinoxaline standard (Fig. 2C, Fig. 4 top). Lastly, the fragmentation patterns of the quinoxaline derived from ZFF samples were identical to those from synthetic DPD (Fig. 3A, B; Fig. 4. B, C). Furthermore, the quinoxaline peak was not detected in the negative control, a 103 × dilution of the CV8 broth equivalent to 106 times what was left in ZFFs (Fig. 2B Fig. 4A bottom). These cumulative results indicate AI-2 originated from zoospores.
Figure 2. DPD-derived quinoxaline from zoospore-free fluid (ZFF) of Phytophthora sojae detected by LC-MS.
The extracted ion chromatograms (m/z 205) for the organic extract of the ZFFsoj (prepared from a zoospore suspension at 5×104 ml−1 (A) and a 1,000-fold dilution of CV8 (B) treated with 1,2-diaminobenzene. The treated ZFF mixed with an equivalent amount of the quinoxaline standard derived from synthetic DPD (C) displayed identical peak as quinoxaline standard (D).
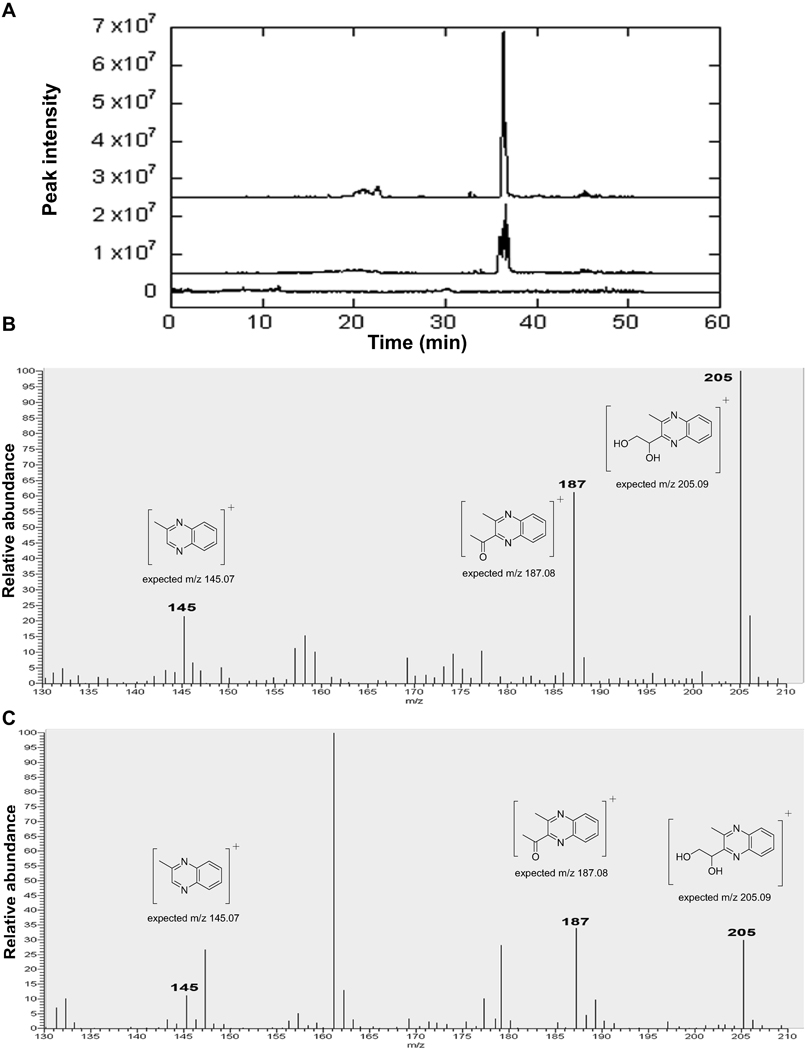
Figure 4. Detection of AI-2 in zoospore-free fluid (ZFF) of Phytophthora nicotianae using the chemical method.
(A). The extracted ion chromatograms (m/z 205) for the organic extract of the ZFFnic from a zoospore suspension at 106 ml−1 treated with 1,2-diaminobenzene (middle trace), the same sample mixed with an equivalent amount of the quinoxaline standard (top trace), and the organic extract of a 1,000 × dilution of CV8 treated with 1,2-diaminobenzene (bottom trace). Mass spectra of the DPD-derived quinoxaline in the extract of ZFFnic from a zoospore suspension at 106 ml−1 presenting the characteristic parent ion of m/z 205 and fragment ions at m/z 187 and 145 (C) to those in authentic DPD samples (B).
Figure 3. Detection of AI-2 in zoospore-free fluid (ZFF) of Phytophthora sojae using electrospray ionisation-mass spectrometry (ESI-MS).
Mass spectra of the DPD-derived quinoxaline in the extract of synthetic DPD (A), ZFFsoj from a zoospore suspension at 5×104 ml−1 (B), and a 1,000 × dilution of CV8 (C). DPD-derived quinoxaline was detected in the extract of ZFFsoj, but not in that of CV8 negative control, as indicated by presence of the characteristic parent ion of m/z 205 and fragment ions at m/z 187 and 145 as those in authentic DPD samples above the noise level between 30 to 35 min (the quinoxaline standard eluted from the column at ~32 min).
Quantification of DPD-derived quinoxaline in ZFF samples also showed variation in AI-2 concentrations with species. P. sojae produced a higher amount of AI-2 than P. nicotianae. Based on the standard curve generated from synthetic DPD, AI-2 concentration in ZFFnic from a suspension of 106 zoospores ml−1 was 1.1±0.1 µM while in ZFFsoj from a suspension of 5 × 104 zoospores ml−1 was 10.1±2.0 µM (Fig. 4), similar to what was observed in the bio-luminescence assay.
Discussion
AI-2 represents an interspecies signaling molecule and is involved in regulation of luminescence, virulence factor secretion and biofilm formation in bacteria (Vendeville, et al., 2005, Xavier & Bassler, 2005). Here, we demonstrate for the first time the production of AI-2 by P. nicotianae, P. sojae and Py. aphanidermatum, members of Pythiaceae in the eukaryotic Stramenopila kingdom, which will provide insight into the physiology and ecology of zoosporic pathogens.
Detection of AI-2 in zoospore free fluid (Fig. 1–4) raises a question regarding the AI-2 production pathway in oomycete species. Currently, there are three known pathways for AI-2 (DPD) production (Winzer, et al., 2002, Hauck, et al., 2003, Nichols, et al., 2009). Within these pathways, the pentose-phosphate pathway used by some plant and bacterial species (Hauck, et al., 2003, Tavender, et al., 2008) is most likely to be adopted by oomycetes. The reasons are three fold. First, the LuxS-dependent pathway is only available in bacterial species (Sun, et al., 2004). Oomycetes, like other eukaryotes, lack LuxS homology in their genome (genome.jgi-psf.org, broad.mit.edu, vmd.vbi.vt.edu). Second, the noncanonical abiotic/biotic reaction pathway reported in thermal tolerant bacteria requires hydrothermal environments to form DPD (Nichols, et al., 2009). Such conditions are not encountered by these oomycete “water molds”. Lastly, in the pentose-phosphate pathway, DPD is formed spontaneously by converting pentose-phosphates to D-ribulose-5-phosphate using isomerases (RPI). In searching oomycete genome databases we found that pentose phosphates are common metabolic products, and all four published genome sequences of Phytophthora species contain conserved sequences for RPI, suggesting zoosporic oomycetes may form DPD through the central intermediate ribose-5-phosphate. Silencing the RPI gene and testing mutant AI-2 production may provide direct evidence to test this presumption. However, it is possible that other unknown pathways are responsible for the production of AI-2.
Although it is not clear whether oomycetes use AI-2 to encode information for communication within the population to coordinate behaviors such as aggregation and plant infection, AI-2 production by Pythiaceae species raises the possibility that zoosporic pathogens may use AI-2 as a common signal to communicate with bacteria. Communication with bacteria may be beneficial to these pathogens as shown by their ability to survive in soil with a wide range of bacteria and their tolerance to frequent culture contamination by bacteria. It will be interesting to know if this cross-kingdom relationship is bridged by AI-2. In fact, triggering luminescence of Vibrio harveyi by ZFF (Fig. 1) has verified that oomycetes can communicate with bacteria and affect their quorum sensing through this molecule. This process may give oomycetes an advantage in fitness and possibly virulence. Bacteria and bacterial metabolites have been shown to stimulate Phytophthora reproduction (Zentmyer, 1965) and contribute to Phytophthora colonization on plants (Yang, et al., 2001).
Acknowledgements
We gratefully acknowledge supplies of isolates of Phytophthora and Pythium from Drs. Brett Tyler, Michael Benson and Gary Moorman; expression strains for AI-2 production from Drs. Kenneth Cornell, Michael Riscoe, Mark Hilgers and Martha Ludwig. We thank Dr. Brett Tyler for assistance with oomycete bioinformatics; and Patricia Richardson for reading this manuscript. This work is supported in part by grants to C. Hong from USDA-CSREES (2005-51101-02337) and to Z. S. Zhou from NIAID/NIH (1R01AI058146). This is publication number 939 from the Barnett Institute.
References
- Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. of Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon JW, Donaldson SP. Molecular recognition in the homing responses of zoosporic fungi, with special reference to Pythium and Phytophthora. Mycol. Res. 1993;97:1153–1171. [Google Scholar]
- DeKeersmaecker SCJ, Vanderleyden J. Constraints on detection of autoinducer-2 (Al-2) signalling molecules using Vibrio harveyi as a reporter. Microbiology-Sgm. 2003;149:1953–1956. doi: 10.1099/mic.0.C0117-0. [DOI] [PubMed] [Google Scholar]
- Gao M, Teplitski M, Robinson JB, Bauer WD. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Molecular Plant-Microbe Interactions. 2003;16:827–834. doi: 10.1094/MPMI.2003.16.9.827. [DOI] [PubMed] [Google Scholar]
- Hauck T, Hubner Y, Bruhlmann F, Schwab W. Alternative pathway for the formation of 4,5-dihydroxy-2,3-pentanedione, the proposed precursor of 4-hydroxy-5-methyl-3(2H)-furanone as well as autoinducer-2, and its detection as natural constituent of tomato fruit. Biochimica Et Biophysica Acta-General Subjects. 2003;1623:109–119. doi: 10.1016/j.bbagen.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hogan DA. Talking to themselves: autoregulation and quorum sensing in Fungi. Eukaryotic Cell. 2006;5:613–619. doi: 10.1128/EC.5.4.613-619.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judelson HS, Blanco FA. The spores of Phytophthora: Weapons of the plant destroyer. Nature Reviews Microbiology. 2005;3:47–58. doi: 10.1038/nrmicro1064. [DOI] [PubMed] [Google Scholar]
- Malajczuk N. Microbial antagonism to Phytophthora. In: Erwin DC, Bartnicki-Garcia S, Tsao PH, editors. Phytophthora: Its Biology, Taxonomy, Ecology, and Pathology. St. Paul, Minnesota: APS Press; 1983. pp. 197–218. [Google Scholar]
- Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorumsensing signal Al-2. Molecular Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Nichols JD, Johnson MR, Chou C-J, Kelly RM. Temperature, not LuxS, mediates AI-2 formation in hydrothermal habitats. Fems Microbiology Ecology. 2009;68:173–181. doi: 10.1111/j.1574-6941.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Reid B, Morris BM, Gow NAR. Calcium-dependent, genus-specific, autoaggregation of zoospores of phytopathogenic fungi. Exp. Mycol. 1995;19:202–213. [Google Scholar]
- Robold AV, Hardham AR. During attachment Phytophthora spores secrete proteins containing thrombospondin type 1 repeats. Current Genetics. 2005;47:307–315. doi: 10.1007/s00294-004-0559-8. [DOI] [PubMed] [Google Scholar]
- Sun JB, Daniel R, Wagner-Dobler I, Zeng AP. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways? BMC Evol. Biol. 2004;4 doi: 10.1186/1471-2148-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavender T, Halliday N, Hardie K, Winzer K. LuxS-independent formation of AI-2 from ribulose-5-phosphate. BMC Microbiology. 2008;8:98. doi: 10.1186/1471-2180-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making 'sense' of metabolism: Autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 2005;3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell. Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Winans SC, Bassler BL. Chemical Communication Among Bacteria. Washington, DC: ASM Press; 2008. [Google Scholar]
- Winzer K, Hardie KR, Burgess N, et al. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology-Sgm. 2002;148:909–922. doi: 10.1099/00221287-148-4-909. [DOI] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. Interference with Al-2-mediated bacterial cell-cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Crowley DE, Menge JA. 16S rDNA fingerprinting of rhizosphere bacterial communities associated with healthy and Phytophthora infected avocado roots. Fems Microbiology Ecology. 2001;35:129–136. doi: 10.1111/j.1574-6941.2001.tb00796.x. [DOI] [PubMed] [Google Scholar]
- Zentmyer GA. Bacterial stimulation of sporangium production in Phytophthora cinnamomi. Science. 1965;150:1178–1179. doi: 10.1126/science.150.3700.1178. [DOI] [PubMed] [Google Scholar]
- Zhao G, Wan W, Mansouri S, Alfaro JF, Bassler BL, Cornell KA, Zhou ZHS. Chemical synthesis of S-ribosyl-L-homocysteine and activity assay as a LuxS substrate. Bioorganic & Medicinal Chemistry Letters. 2003;13:3897–3900. doi: 10.1016/j.bmcl.2003.09.015. [DOI] [PubMed] [Google Scholar]