Abstract
Background
After delivery, many women experience symptoms of postpartum depression (PPD), and early identification of women at risk is therefore important. The opioid peptide β-endorphin has been implicated in non-puerperal depression but its role in the development of PPD is unknown.
Methods
Three hundred and seven women with a singleton, full-term (>37.0 weeks’ GA) pregnancy were recruited early in pregnancy and followed up into the postpartum period. Blood samples were obtained at 15, 19, 25, 31 and 37 weeks’ gestational age (GA) and at 9 weeks postpartum for assessment of β-endorphin. Depressive symptoms were assessed with the Center for Epidemiological Studies – Depression scale at the last four pregnancy visits and with the Edinburgh Postnatal Depression Scale postpartum.
Results
Among women who were euthymic at 25 weeks’ GA, those who proceeded to develop PPD symptoms had higher levels of β-endorphin throughout pregnancy compared to women without PPD symptoms (all t > 2.11, p < .05). At each assessment, women above the cut-off score for β-endorphin were at more than three-fold risk for PPD symptoms (odds ratios 3.19 – 4.68) compared to women below the cut-off score.
Limitations
Self-report of depressive symptoms, no mental health history.
Conclusions
β-Endorphin may be a useful early predictor of PPD symptoms in women who do not report depressive symptoms in midpregnancy. If replicated, these findings have clinical implications for the identification and treatment of this at risk group and further suggest that some of the pathways leading to this complex disorder may be specific to subgroups of women.
Keywords: β-endorphin, postpartum depression, pregnancy
Introduction
Postpartum depression (PPD) is a common mood disorder that affects as many as 19% (7% for major PPD alone) of women within the first three months postpartum (Gavin et al., 2005). It has many of the same symptoms and consequences as non-puerperal depression. In at least two ways, however, PPD is unique: It adversely affects the cognitive, behavioral and emotional development of the newborn (Beck, 1998; Feldman et al., 2009; Grace et al., 2003), and it is preceded by physiological changes unique to pregnancy and parturition that may underlie vulnerability to postpartum mood disorders (Halbreich, 2005a; Hochberg et al., 2003; Payne et al., 2009).
Well-established risk factors for PPD include a history of stress, depression, and anxiety; a history of pre- and postnatal depression; lack of social support; and low self-esteem (Beck, 2001; O'Hara, 2009; Robertson et al., 2004). Endocrine risk factors for PPD have been identified as well, including a history of premenstrual syndrome and oral contraceptive induced mood changes (Bloch et al., 2003; Bloch et al., 2006) as well as pregnancy- and parturition-related hormonal changes (Halbreich, 2005b; Hochberg et al., 2003; Yim et al., 2009).
We recently reported that accelerated placental corticotropin-releasing hormone (pCRH) increases around 25 weeks’ gestational age (GA) are predictive of PPD symptoms (Yim et al., 2009). Postpartum depression is a multifactorial disorder, and it is unlikely that pCRH is the only marker during pregnancy that has the potential to serve as an early predictor. The opioid peptide β-endorphin, a 31 amino-acid long fragment of the pro-opiomelanocortin (POMC) molecule, is associated with the activity of the stress-sensitive hypothalamic-pituitary-adrenal (HPA) axis. After stimulation with hypothalamic CRH, it is co-released with adrenocorticotropic hormone from the anterior pituitary (Guillemin et al., 1977; Li and Chung, 1976; Li et al., 1976). In addition, CRH has been shown to modulate β-endorphin release through immunological pathways (Carr et al., 1990; Likar et al., 2007; Matejec et al., 2009). During pregnancy, β-endorphin is synthesized in the placenta (Ng et al., 1996), but its likely source in maternal peripheral blood is the pituitary (Chan and Smith, 1992).
There are at least two reasons why β-endorphin has potential as an early marker of PPD symptoms. First, β-endorphin synthesis undergoes dynamic changes throughout pregnancy with highest levels during labor, and rapid decreases after delivery (Browning et al., 1983; Chan and Smith, 1992; Chan et al., 1993; Lindow et al., 1996; McLean et al., 1994; Raisanen, 1988; Smith et al., 1990). Second, in addition to its well-characterized analgesic and euphoria-inducing properties (Amalric et al., 1987; Foley et al., 1979), β-endorphin hyperactivity has previously been associated with non-puerperal depression in some (Akil et al., 1993; Goodwin et al., 1993; Kennedy et al., 2006; Navines et al., 2008; Risch, 1982) but not all studies (Cohen et al., 1984; Djurovic et al., 1999; Gerner and Sharp, 1982). One potential explanation for these inconsistent results is that β-endorphin may be elevated in some subtypes of depression but not in others.
Few studies have empirically addressed the possibility that β-endorphin is predictive of PPD, and existing work examined the association of β-endorphin late in pregnancy with depressive symptoms in the first postnatal week, thus likely capturing symptoms of “postpartum blues”, a milder and usually transient condition. These studies yielded inconsistent results, suggesting positive (Smith et al., 1990), negative, (Newnham et al., 1984), or no associations (Brinsmead et al., 1985).
Because previous research provided some evidence that β-endorphin is associated with symptoms of non-puerperal depression and postpartum blues, the dynamic changes in β-endorphin that occur throughout pregnancy could be useful in identifying women at increased risk for the development of PPD symptoms. The aim of the present study is to explore this possibility.
Methods
Participants
Participants were selected from a larger sample of 509 women with a singleton, intrauterine pregnancy (Davis et al., 2005; Ellman et al., 2008; Glynn et al., 2007; Sandman et al., 2006; Yim et al., 2009). This sample was recruited at the University of California, Irvine Medical Center and the Cedars-Sinai Medical Center in Los Angeles, California. Women were followed starting early in pregnancy into the postnatal period with serial measures of biological and psychosocial markers. Exclusion criteria in the larger study were non-English speaking, age less than 18 years, any condition that could affect neuroendocrine function such as endocrine, hepatic and renal disorder or the use of corticosteroid medications, and drug or alcohol abuse within six months before the index pregnancy.
The present sample comprised the 307 women who delivered at term (GA > 37 weeks) and had completed the questionnaire assessing PPD symptoms. At the time of delivery, mean (SD) age was 30.3 (5.3) years. The sample included 48.9% non-Hispanic White, 20.2% Hispanic White, 10.1% African-American, 9.1% Asian, and 11.7% multi-ethnic or other. The majority of women were married (75.9%), almost all had completed high school (98%), and about half (49.5%) had a college degree. Reported annual household income ranged from less than $5,000 to more than $100,000, with the median income in the $60,000 – $70,000 range. All pregnancies resulted in live-births (49.2% female infants). Deliveries were 73.6% vaginal and 26.4% cesarean section. Mean (SD; range) birth weight was 3,526g (510; 1,790 – 5,796) and mean gestational length was 39.4 (1.2; 37.0 – 42.3) weeks. The majority of women had no previous live-born children (57.8%). Nine participants (2.9%; 3 with and 6 without PPD symptoms) reported the use of antidepressant medication during pregnancy. There was no significant association between antidepressant use and PPD symptoms (χ2=.17, n.s.) or beta-endorphin levels at any time point (all t < 1.77, n.s.), and antidepressant use is therefore not considered further.
Overall Procedure
Blood samples were obtained during pregnancy at a mean (SD; range) of 15.3 (.97; 12.3 – 17.0), 19.3 (.78; 17.0 – 21.9), 25.0 (.86; 23.1 – 28.9), 31.0 (.74; 28.9 – 33.9) and 36.7 (.70; 35.1 – 39.3) weeks’ GA and at 9.0 (3.8; 3.0 – 30.0) weeks postpartum for assessment of β-endorphin. On study days, women were instructed to refrain from caffeine intake and exercise, and no potentially stressful study procedures were scheduled before the blood draw. The blood sample was always drawn in a quiet environment. The time of day of the blood draw varied between 8:14 am and 6:30 pm. Depressive symptoms were assessed at the last four pregnancy visits and again postpartum. The first study visit at 15 weeks’ GA was optional and data is available for 147 women. At the remaining time points data is available from 303/ 297 (β-endorphin / depressive symptoms) women at 19 weeks’ GA, 301/276 women at 25 weeks’ GA, 302/300 women at 31 weeks’ GA, 274/275 women at 37 weeks’ GA and 300/ 307 women at the postpartum visit. Complete data for β-endorphin was available from 258 women; of these, 125 also participated in the optional visit at 15 weeks’ GA. Written consent was obtained from all women. This study was approved by the Institutional Review Boards of participating institutions.
Hormone Assessment
A 25-mL blood sample was obtained by antecubital venipuncture and drawn into EDTA-treated test tubes (Vacutainers; Becton Dickinson and Company, Sumter, South Carolina). Samples were chilled on ice and spun for 15 minutes at 2,000 g. The plasma was then decanted into polypropylene tubes containing 500 kallikrein inhibitor units/mL of aprotinin (Sigma-Aldrich Corp, St. Louis, Missouri) and stored at −70°C until assayed.
Plasma levels of β-endorphin were determined by a direct solid phase two-site immunoradiometric assay (IRMA) using antibodies against synthetic human β-endorphin (Nichols Institute Diagnostics; San Juan Capistrano, California). The antiserum has 16% cross-reactivity with beta-lipotropin at 500 pg/mL and has <0.01% cross-reactivity with related opiates at 5 µg/mL. The β-endorphin IRMA measures intact β-endorphin1–31 in a radiolabeled soluble sandwich complex bound to two antibodies with high affinity and specificity for β-endorphin coupled to a solid bead matrix. Briefly, plasma samples (200 µL) were incubated in duplicate with an antibody coated bead and 125-I-labeled antiserum (100 µL) for 20+1 hours at room temperature. The beads were then washed twice with phosphate buffered saline and aspirated to dryness. Radiolabeled antibody complex bound to the solid phase was measured using an ICN Biomedical (formerly Micromedic) Isoflex Gamma Counter. The calculated assay sensitivity was 5.7 pg/mL with intra- and inter-assay variations of 4.2% and 8.3%, respectively. All plasma β-endorphin values were interpolated from a modified standard curve enriched with two additional low level standards using a four-parameter logistics program (Rodbard and Hutt, 1974).
Assessment of Depressive Symptoms
Symptoms of depression during pregnancy were assessed with a 9-item version of the Center for Epidemiological Studies – Depression (CES-D) scale (Santor and Coyne, 1997). On a 4-point scale, participants indicated how often they experienced each symptom during the past week. Validation analyses show higher associations with the Structured Interview for DSM-III-R when items are rescored categorically (Santor and Coyne, 1997). Thus, each item was scored 0 if option 0 or 1 was endorsed, and was scored 1 if option 2 or 3 was endorsed, resulting in a possible range of scores from 0 to 9. The suggested cutoff score for at least minor depressive symptoms is 4 or more. This scale has good internal consistency (Kuder-Richardson formula 20 = 0.87), and correlates highly with the original scale (r = 0.97).
At the postpartum visit, participants completed the 10-item Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987), a scale specifically developed to assess PPD symptoms. Participants indicated how often they experienced a symptom in the past week on a 4-point scale. Total scores ranged from 0 to 30. A cutoff score of 10 or more has been suggested for studies including minor depression (Cox et al., 1987; Matthey et al., 2006). The scale has good reliability (split-half, 0.88; standardized α: 0.87).
Statistical Methods
β-Endorphin was log transformed to reduce skewness. Changes of β-endorphin and CES-D scores across study visits were tested with a series of paired samples t-tests. Pearson Product moment correlations were performed to test for associations between β-endorphin levels and depressive symptoms. Time of day of blood draw was covaried, and in no case changed the significance of the results.
Four binary logistic regressions (19, 25, 31 and 37 wks’ GA) were conducted with β-endorphin levels and CES-D scores as predictors and PPD symptoms as the outcome (1 = PPD, 0 = no PPD). In Step 2, the β-endorphin X CES-D interaction was entered into the model to test for a moderation effect of prenatal depression. The model fit (χ2), the change in model fit (χ2 change), and the strength of the association (Nagelkerke R2) are reported. Wald statistic was used to test for the significance of individual independent variables. These analyses were not possible for the 15-week time point, because no CES-D scores were available.
At gestational time points when the β-endorphin X CES-D interaction was significant, the sample was split into women with and without depressive symptoms, based on the suggested CES-D cutoff score. Binary logistic regressions were computed for both subgroups with β-endorphin as the predictor and PPD symptoms as the outcome (1 = PPD, 0 = no PPD).
Receiver operating characteristic (ROC) curves were computed at each time point with β-endorphin as the test variable and PPD symptoms as the outcome. Areas under the ROC curve (AUCs) were calculated. The AUC values can range from 0.5 to 1.0, with 1.0 indicating a perfect test. The Youden index (sensitivity + [specificity-1]) was computed to obtain the optimal cutoff score for β-endorphin. Odds ratios were calculated to compare the likelihood to develop PPD symptoms in women whose β-endorphin levels were above versus below the cutoff score.
A series of ancillary analyses (2-tailed, independent samples t-tests, χ2-tests) revealed no significant associations between sociodemographic (ethnicity, marital status, education, household income) or pregnancy-related variables (birth weight, length of gestation, infant gender, mode of delivery, parity) and PPD symptoms (all p > 0.18), with the exception of maternal age, t(304) = 2.51, p = 0.01. However, including maternal age into the analyses did not change the significance of the results. In addition, maternal age was not associated with β-endorphin at any time point (all r = −0.01 – 0.04, p > 0.48), and results are presented without controlling for maternal age. At the postpartum visit, no association was found between PPD symptoms and the number of weeks since delivery, t(298) = 1.50, p = 0.13.
Results
As expected, β-endorphin levels increased significantly between each assessment during pregnancy, all t > 4.22, p < 0.001, and then dropped sharply after delivery, t(267) = 28.84, p < 0.001. CES-D scores did not change throughout pregnancy, except for a moderate increase between 25 and 31 weeks’ GA, t(253) = 2.65, p = 0.03. β-Endorphin was not significantly correlated with concurrently assessed depressive symptoms during pregnancy (r = −0.004 to r = 0.11, all p > 0.07) or postpartum (r = 0.02, p = 0.68) .
At 25 weeks’ GA, β-endorphin (Wald = 8.43, p = 0.004), CES-D scores (Wald = 10.73, p = 0.001), and the β-endorphin x CES-D interaction (Wald = 8.02, p = 0.005) were significant predictors of PPD symptoms (χ2 = 41.35, p < 0.001; Table 1). To explore the interaction effect, the sample was split into women with (symptomatic women: n = 56) and without symptoms of depression (euthymic women: n = 207) at 25 weeks’ GA. Twenty six (46.4%) of the symptomatic women and 35 (16.9%) of the euthymic women proceeded to develop PPD symptoms. In euthymic women, higher β-endorphin levels predicted PPD symptoms (χ2 = 7.65, p = 0.006, Nagelkerke R2 = 0.06; Wald = 7.15, p = 0.008). The association between β-endorphin levels and PPD symptoms was not significant in symptomatic women (χ2 = 2.05, p = 0.15, Nagelkerke R2 = 0.05; Wald = 1.95, p = 0.16). Of note, no overall group differences in β-endorphin levels at 25 weeks’ GA were found between symptomatic (1.50 pg/mL) and euthymic (1.52 pg/mL) women, t(257) = 0.79, p = 0.43.
Table 1.
Predictors of PPD at 25 Weeks’ Gestational Age.
| χ2 (p) | χ2 change (p) | R2 | Wald Statistic (p) | |
|---|---|---|---|---|
| Step 1 | 32.505 (<0.001) | 0.178 | ||
| β-Endorphin | 0.986 (0.321) | |||
| CES-D | 26.851 (<0.001) | |||
| Step 2 | 41.346 (<0.001) | 8.841 (0.003) | 0.223 | |
| β-Endorphin | 8.428 (0.004) | |||
| CES-D | 10.728 (0.001) | |||
| β-Endorphin X CES-D | 8.015 (0.005) | |||
Note: R2 is Nagelkerke R2
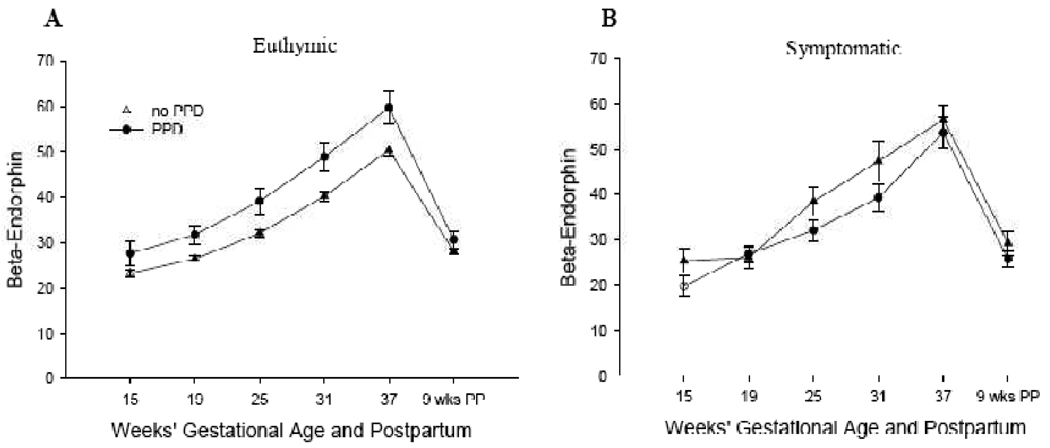
β-Endorphin levels of women who were euthymic at 25 weeks’ GA were then assessed across pregnancy. Women who proceeded to develop PPD symptoms had higher levels of β-endorphin at each time point during pregnancy compared to women who did not develop PPD symptoms, all t > 2.11, p < 0.05 (Fig 1a). This finding is of clinical significance because it suggests that among women who are euthymic at 25 weeks’ GA, β-endorphin levels at any time point in pregnancy may provide useful information about a woman’s risk of developing PPD symptoms. No differences in β-endorphin levels were found post partum, t(201) = 1.43, p = 0.16. Among women who were symptomatic at 25 weeks’ GA, no significant differences in β-endorphin levels were observed at any time point during pregnancy or postpartum, all t < 1.83, p > 0.08 (Fig 1b).
Figure 1.
β-Endorphin and Postpartum Depression in Women who are Euthymic (A) or Symptomatic (B) at 25 Weeks’ Gestational Age.
ROC analyses were computed for each time point in pregnancy including only women who were euthymic at 25 weeks’ GA (Table 2). Area under the ROC curves ranged between 0.63 and 0.65 (15 wks p = .11; all other ps = .01 – .02). Ideal cut-off scores were computed for each time point, and women with β-endorphin levels above the cut-off score were at more than three-fold risk (odds ratios 3.19 – 4.68) to develop PPD symptoms compared to women whose values fell below the cut-off score (Table 2).
Table 2.
Likelihood to Develop PPD by β-Endorphin Among Women who are Euthymic at 25 Weeks’ GA.
| GA | ROC Area Under the Curve (CI; p) |
β-Endorphin Cutoff (pg/mL) |
Women Below Cutoff % (n) |
Women Above Cutoff % (n) |
Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| PPD | no PPD | PPD | no PPD | ||||
| 15 | 0.63 (0.48 – 0.79; 0.11) | 21.90 | 6.7 (3) | 93.3 (42) | 19.3 (11) | 80.7 (46) | 3.37 |
| 19 | 0.64 (0.53 – 0.74; 0.05) | 24.86 | 8.9 (8) | 91.1 (82) | 23.9 (27) | 76.1 (86) | 3.19 |
| 25 | 0.63 (0.51 – 0.74; 0.02) | 41.80 | 12.0 (20) | 88.0 (147) | 38.9 (14) | 61.1 (22) | 4.68 |
| 31 | 0.65 (0.54 – 0.76; 0.01) | 43.77 | 10.3 (14) | 89.7 (122) | 27.9 (19) | 72.1 (49) | 3.37 |
| 37 | 0.63 (0.52 – 0.75; 0.02) | 62.13 | 11.9 (17) | 88.1 (126) | 34.1 (15) | 65.9 (29) | 3.83 |
Note: Odds Ratio computed as: odds for PPD in women below cutoff divided by odds for PPD in women above cutoff score
Binary logistic regression analyses were also conducted for the assessments at 19, 31 and 37 weeks’ GA. However, at these time points only CES-D scores emerged as significant predictors of PPD symptoms (all Wald = 18.84 – 23.09, all ps < 0.001). No significant main effects of β-endorphin (all Wald = 0.03 – 3.50, p > 0.06) were observed, and inclusion of the β-endorphin X CES-D interaction did not improve the models (all χ2 change < 1.38, p > 0.24).
Discussion
Our study suggests that among women who are euthymic at 25 weeks’ GA, increased β-endorphin levels at any time point during pregnancy are associated with a more than three-fold increase in the risk of developing PPD symptoms. Thus, there is an at-risk group of women who show no overt symptoms of depression in mid-pregnancy (and thus cannot be identified with self-report questionnaires), but display indications of altered HPA axis regulation, as evidenced by a more pronounced pregnancy-induced increase in β-endorphin levels.
Increases in β-endorphin can be the result of an impairment of the negative feedback system controlling HPA axis activity (Akil et al., 1993). Impaired HPA feedback regulation has previously been described in healthy individuals at high risk for affective disorders (Holsboer et al., 1995), suggesting that HPA dysregulation may precede the onset of affective disorders. The subgroup of women described here may be particularly vulnerable to HPA-induced mood changes, and the substantial decrease of β-endorphin levels after delivery may put these women at risk. This may particularly implicate β-endorphin and not other HPA axis hormones because it is an opioid peptide with euphorigenic properties. A similar effect was not found for women who were symptomatic at 25 weeks’ GA. The importance of identifying endophenotypes that underlie pathophysiological processes specific to subtypes of PPD has previously been emphasized (Nemeroff, 2008). In line with this idea, our findings suggest that β-endorphin plays an important role in the development of PPD symptoms only among women who are euthymic in mid-pregnancy.
Importantly, depressive symptoms had to be assessed at 25 weeks’ GA to detect this effect, adding to our previous reports that have identified this time in mid-gestation as a possible window of vulnerability for adverse postnatal maternal and infant outcomes (Davis et al., 2005; Yim et al., 2009). Future studies should systematically investigate the antecedents and consequences of mid-pregnancy alterations in hormones of the HPA-placental axis.
Previous studies on β-endorphin as a predictor of “postpartum blues” in the first few days after delivery were inconclusive (Brinsmead et al., 1985; Newnham et al., 1984; Smith et al., 1990). Our study is different from these reports because we assessed (i) the trajectory of β-endorphin throughout gestation, and (ii) PPD symptoms nine weeks after delivery and not postpartum blues within the first postnatal week. To our knowledge, only one other study aimed to relate β-endorphin to PPD symptoms at eight weeks postpartum, and that study suggests a lack of association (Brinsmead et al., 1985). However, that study was based on 19 participants, and only considered the change in β-endorphin from 38 weeks’ GA until the fourth postnatal day.
A notable limitation to this study is that we assessed depressive symptoms by self-report questionnaire. Therefore, our findings should be replicated using further diagnostic instruments, discriminating between subtypes of depression and considering the role of anxiety, as these distinctions have been shown to be of importance with regard to HPA axis function in perinatal depression (e.g., Heron et al., 2004; Kammerer et al., 2006). In addition, although we did control for depressive symptoms in the pregnancy, we have no information about life-time history of depression. While it is likely that the effects of current depressive symptoms are stronger than any additional variance explained by a history of depression, the importance of this variable as a predictor of PPD symptoms is evident (Beck, 2001; Robertson et al., 2004), and should be included in future studies. Finally, it should be noted that β-endorphin was measured in maternal plasma, and is not necessarily reflective of central opioid activity (Baker et al., 1997).
The present study in a large cohort of women has important implications. First, we found that the opioid peptide β-endorphin predicted PPD symptoms in women who are euthymic at 25 weeks’ GA. Thus, β-endorphin may play an important role in the pathophysiology of PPD symptoms in this subgroup of women. Moreover, this finding, if replicable, indicates that among the clinically interesting group of women who are euthymic and are therefore not easily diagnosed as at risk, a blood draw at any time during pregnancy may assist in identifying women at risk of developing PPD symptoms.
Acknowledgements
Dr Hobel holds the Miriam Jacobs Chair in the Division of Maternal-Fetal Medicine at Cedars-Sinai Medical Center.
Role of the Funding Source:
This research was supported by US PHS (NIH) research awards from the National Institute of Child Health and Human Development (HD28413 and HD51852 to Dr Sandman). This funding source had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Yim conducted the statistical analyses and took the lead in writing the manuscript. All other authors contributed to and have approved the final manuscript. Sandman obtained funding for the study. Glynn, Dunkel Schetter, Hobel and Sandman designed the study. Chicz-DeMet oversaw the bioassays.
Conflict of Interest:
All authors declare that they have no conflict of interest.
References
- Akil H, Haskett RF, Young EA, Grunhaus L, Kotun J, Weinberg V, Greden J, Watson SJ. Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry. 1993;33:73–85. doi: 10.1016/0006-3223(93)90305-w. [DOI] [PubMed] [Google Scholar]
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Orth DN, Hill KK, Nicholson WE, Ekhator NN, Bruce AB, Wortman MD, Keck PE, Jr, Geracioti TD., Jr Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinology. 1997;22:517–529. doi: 10.1016/s0306-4530(97)00053-x. [DOI] [PubMed] [Google Scholar]
- Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rotenberg N, Koren D, Klein E. Risk factors for early postpartum depressive symptoms. Gen Hosp Psychiatry. 2006;28:3–8. doi: 10.1016/j.genhosppsych.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Brinsmead M, Smith R, Singh B, Lewin T, Owens P. Peripartum concentrations of beta endorphin and cortisol and maternal mood states. Aust N Z J Obstet Gynaecol. 1985;25:194–197. doi: 10.1111/j.1479-828x.1985.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Browning AJ, Butt WR, Lynch SS, Shakespear RA. Maternal plasma concentrations of beta-lipotrophin, beta-endorphin and gamma-lipotrophin throughout pregnancy. Br J Obstet Gynaecol. 1983;90:1147–1151. doi: 10.1111/j.1471-0528.1983.tb06462.x. [DOI] [PubMed] [Google Scholar]
- Carr DJ, DeCosta BR, Jacobson AE, Rice KC, Blalock JE. Corticotropin-releasing hormone augments natural killer cell activity through a naloxone-sensitive pathway. J Neuroimmunol. 1990;28:53–61. doi: 10.1016/0165-5728(90)90040-t. [DOI] [PubMed] [Google Scholar]
- Chan EC, Smith R. Beta-endrophin immunoreactivity during human pregnancy. J Clin Endocrinol Metab. 1992;75:1453–1458. doi: 10.1210/jcem.75.6.1464647. [DOI] [PubMed] [Google Scholar]
- Chan EC, Smith R, Lewin T, Brinsmead MW, Zhang HP, Cubis J, Thornton K, Hurt D. Plasma corticotropin-releasing hormone, beta-endorphin and cortisol inter-relationships during human pregnancy. Acta Endocrinol (Copenh) 1993;128:339–344. doi: 10.1530/acta.0.1280339. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Pickar D, Extein I, Gold MS, Sweeney DR. Plasma cortisol and beta-endorphin immunoreactivity in nonmajor and major depression. Am J Psychiatry. 1984;141:628–632. doi: 10.1176/ajp.141.5.628. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- Djurovic D, Milic-Askrabic J, Majkic-Singh N. Serum beta-endorphin level in patients with depression on fluvoxamine. Farmaco. 1999;54:130–133. doi: 10.1016/s0014-827x(99)00005-1. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Dunkel Schetter C, Hobel CJ, Czics-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Foley KM, Kourides IA, Inturrisi CE, Kaiko RF, Zaroulis CG, Posner JB, Houde RW, Li CH. beta-Endorphin: analgesic and hormonal effects in humans. Proc Natl Acad Sci U S A. 1979;76:5377–5381. doi: 10.1073/pnas.76.10.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Sharp B. CSF beta-endorphin-immunoreactivity in normal, schizophrenic, depressed, manic and anorexic subjects. Brain Res. 1982;237:244–247. doi: 10.1016/0006-8993(82)90574-1. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28:1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Austin MP, Curran SM, Ross M, Murray C, Prentice N, Ebmeier KP, Bennie J, Carroll S, Dick H, et al. The elevation of plasma beta-endorphin levels in major depression. J Affect Disord. 1993;29:281–289. doi: 10.1016/0165-0327(93)90018-f. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F. beta-Endorphin and adrenocorticotropin are secreted concomitantly by the pituitary gland. Science. 1977;197:1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The association between pregnancy processes, preterm delivery, low birth weight, and postpartum depressions--the need for interdisciplinary integration. Am J Obstet Gynecol. 2005a;193:1312–1322. doi: 10.1016/j.ajog.2005.02.103. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Postpartum disorders: multiple interacting underlying mechanisms and risk factors. J Affect Disord. 2005b;88:1–7. doi: 10.1016/j.jad.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Heron J, O'Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80:65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24:523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health. 2006;9:187–196. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Li CH, Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976;73:1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Chung D, Doneen BA. Isolation, characterization and opiate activity of beta-endorphin from human pituitary glands. Biochem Biophys Res Commun. 1976;72:1542–1547. doi: 10.1016/s0006-291x(76)80189-1. [DOI] [PubMed] [Google Scholar]
- Likar R, Mousa SA, Steinkellner H, Koppert W, Philippitsch G, Stein C, Schafer M. Involvement of intra-articular corticotropin-releasing hormone in postoperative pain modulation. Clin J Pain. 2007;23:136–142. doi: 10.1097/01.ajp.0000210954.93878.0d. [DOI] [PubMed] [Google Scholar]
- Lindow SW, Newham A, Hendricks MS, Thompson JW, van der Spuy ZM. The 24-hour rhythm of oxytocin and beta-endorphin secretion in human pregnancy. Clin Endocrinol (Oxf) 1996;45:443–446. doi: 10.1046/j.1365-2265.1996.8290840.x. [DOI] [PubMed] [Google Scholar]
- Matejec R, Locke G, Muhling J, Harbach HW, Langefeld TW, Bodeker RH, Hempelmann G. Release of melanotroph- and corticotroph-type proopiomelanocortin derivatives into blood after administration of corticotropin-releasing hormone in patients with septic shock without adrenocortical insufficiency. Shock. 2009;31:553–560. doi: 10.1097/SHK.0b013e318188dfb8. [DOI] [PubMed] [Google Scholar]
- Matthey S, Henshaw C, Elliott S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale - implications for clinical and research practice. Arch Womens Ment Health. 2006;9:309–315. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- McLean M, Thompson D, Zhang HP, Brinsmead M, Smith R. Corticotrophin-releasing hormone and beta-endorphin in labour. Eur J Endocrinol. 1994;131:167–172. doi: 10.1530/eje.0.1310167. [DOI] [PubMed] [Google Scholar]
- Navines R, Martin-Santos R, Gomez-Gil E, Martinez de Osaba MJ, Gasto C. Interaction between serotonin 5-HT1A receptors and beta-endorphins modulates antidepressant response. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1804–1809. doi: 10.1016/j.pnpbp.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Understanding the pathophysiology of postpartum depression: implications for the development of novel treatments. Neuron. 2008;59:185–186. doi: 10.1016/j.neuron.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Newnham JP, Dennett PM, Ferron SA, Tomlin S, Legg C, Bourne GL, Rees LH. A study of the relationship between circulating beta-endorphin-like immunoreactivity and post partum 'blues'. Clin Endocrinol (Oxf) 1984;20:169–177. doi: 10.1111/j.1365-2265.1984.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Ng ML, Healy DL, Rajna A, Fullerton M, O'Grady C, Funder JW. Presence of pro-opiomelanocortin peptides and corticotropin-releasing factor in human placenta. Malays J Pathol. 1996;18:59–63. [PubMed] [Google Scholar]
- O'Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65:1258–1269. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harv Rev Psychiatry. 2009;17:72–86. doi: 10.1080/10673220902899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisanen I. Plasma levels and diurnal variation of beta-endorphin, beta-lipotropin and corticotropin during pregnancy and early puerperium. Eur J Obstet Gynecol Reprod Biol. 1988;27:13–20. doi: 10.1016/s0028-2243(88)80005-4. [DOI] [PubMed] [Google Scholar]
- Risch SC. beta-Endorphin hypersecretion in depression: possible cholinergic mechanisms. Biol Psychiatry. 1982;17:1071–1079. [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Hutt D. Statistical analysis of radioimmunoassays and immunoradiometric (labeled antibody) assays. In: Rodbard D, Hutt D, editors. Proceedings, Symosium on Radioimmunoassays and Related Procedures in Medicine. Vienna: International Atomic Energy Agency; 1974. pp. 165–192. [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9:233–243. [Google Scholar]
- Smith R, Cubis J, Brinsmead M, Lewin T, Singh B, Owens P, Chan EC, Hall C, Adler R, Lovelock M, et al. Mood changes, obstetric experience and alterations in plasma cortisol, beta-endorphin and corticotrophin releasing hormone during pregnancy and the puerperium. J Psychosom Res. 1990;34:53–69. doi: 10.1016/0022-3999(90)90008-r. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel Schetter C, Hobel CJ, Chicz-Demet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]