Abstract
Hypertension and atherosclerosis are each important causes of morbidity and mortality in the developed world. We have investigated the interaction between these conditions by breeding mice that are atherosclerotic due to lack of apolipoprotein (apo) E with mice that are hypertensive due to lack of endothelial nitric oxide synthase (eNOS). The doubly deficient mice (nnee) have higher blood pressure (BP) and increased atherosclerotic lesion size but no change in plasma lipoprotein profiles compared with normotensive but atherosclerotic (NNee) mice. The nnee mice also develop kidney damage, evidenced by increased plasma creatinine, decreased kidney weight/body weight ratio, and glomerular lipid deposition and calcification. Enalapril treatment abolishes the deleterious effects of eNOS deficiency on BP, atherosclerosis, and kidney dysfunction in nnee mice. In striking contrast, a genetic lack of inducible NOS, which does not affect BP, has no effect on the development of atherosclerotic lesions in Apoe–/– mice. We also observed a positive relationship between BP and size of atherosclerotic lesions These results suggest that the atherogenic effects of eNOS deficiency can be partially explained by an increase in BP and reemphasize the importance of controlling hypertension in preventing atherosclerosis.
Introduction
Hypertension and atherosclerosis are major factors in the etiology of ischemic heart disease and cerebrovascular disease — the 2 leading causes of death worldwide — and are important in the development of kidney dysfunction, congestive heart failure, and angina (1, 2). Considerable epidemiological evidence suggests that high blood pressure (BP) may have a direct role in enhancing atherosclerotic lesion formation (3). Atherosclerosis is 3 times more common in patients with hypertension, and there is a positive, although not linear, correlation between BP and atherosclerosis (4). In addition, atheroma formation occurs in muscular arteries but not in lower-pressure veins, and hypertension promotes lesion formation in the presence of hypercholesterolemia (3). Clinically, many antihypertensive drugs are effective in reducing morbidity and mortality from atherosclerotically mediated cardiovascular events (5, 6). Despite this body of evidence, the effect of hypertension on atherosclerosis has been difficult to study in humans because of confounding variables and the complexity of the genetics underlying each condition. Animal studies have been equally hindered by the lack of appropriate models with simultaneous genetic predispositions for atherosclerosis and hypertension (3). This report describes such a model generated by breeding mice that spontaneously develop atherosclerosis due to apo E deficiency (Apoe –/– or ee mice) with mice that are hypertensive due to lack of the endothelial nitric oxide synthase gene (eNOS –/– or nn mice).
eNOS serves important basal regulatory functions in the vasculature. In response to stimuli such as shear stress or acetylcholine, eNOS catalyzes the production of nitric oxide (NO) from L-arginine. The NO diffuses across the endothelial cell membrane into neighboring smooth muscle cells and induces vasodilation. NO also acts locally to prevent platelet and leukocyte aggregation and inhibits vascular smooth muscle cell proliferation (7). Direct evidence that eNOS mutations can cause hypertension has been presented by Shesely et al. (8) and Huang et al. (9), who showed that mice lacking eNOS have increased BP, decreased heart rate, and increased plasma renin activity, but no atherosclerosis (8). Although linkage between genetic polymorphisms in the eNOS gene and essential hypertension has not been conclusively documented in humans (10), there is substantial evidence that NO pathways are disrupted in both hypertension and atherosclerosis (11, 12).
Apo E is an amphipathic protein that plays a pivotal role in lipoprotein trafficking by both stabilizing and solubilizing lipoprotein particles. Apo E, as a constituent of chylomicrons, VLDL, IDL, and HDL acts as a ligand for the receptor-mediated clearance of these particles (13). Apoe–/– mice are normotensive (14) but have 4–5 times normal plasma cholesterol levels and develop atherosclerotic lesions spontaneously even when fed a regular, low-cholesterol diet (15).
Here we demonstrate that mice lacking both eNOS and apo E (nnee mice) have significantly increased BP, develop larger plaques, and have more severe kidney damage than do apo E–deficient mice with intact eNOS function (NNee mice). These deleterious effects caused by the genetic loss of eNOS are partially relieved by pharmacological inhibition of NOS with L-NAME. The effects are fully ameliorated by treatment with the angiotensin-converting enzyme (ACE) inhibitor enalapril. A significant, positive relationship between lesion size and BP was observed regardless of the method used to change BP (genetic, L-NAME, or enalapril). Finally, we demonstrate that inactivating the inducible NOS (iNOS) gene, which abolishes the induction of NO synthesis in macrophages but does not alter BP, does not affect atherosclerosis in Apoe–/– mice fed a normal chow diet. These experiments demonstrate a direct relationship between the eNOS system and atherosclerosis that can be partially explained by an increase in BP.
Methods
Mouse experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee.
Generation of nnee and NNee mice.
eNOS–/– (nnEE) (8) and Apoe–/– (NNee) (15) mice, backcrossed at least 6 times to C57BL6/J mice, were bred to yield mice heterozygous at both loci (NnEe). These double heterozygotes were crossed with NNee mice to yield eNOS+/–Apoe–/– mice (Nnee), which were intercrossed to yield mice that were Apoe–/– (ee) and wild type (NN), heterozygous (Nn), or homozygous (nn) at the eNOS locus. Most of the animals used in these experiments were offspring of a second intercross between NNee or nnee mice. Apoe–/– mice and iNOS–/– mice (16) were crossed in the same scheme as above to generate animals that were wild type, heterozygous, or homozygous for the lack of iNOS (IIee, Iiee, and iiee, respectively).
Apoe–/– male mice used in the L-NAME experiments were backcrossed at least 6 times to C57BL6/J mice and have since been maintained in our colony for more than 4 years.
L-NAME AND ENALAPRIL TREATMENT.
L-NAME (0.1 g/L) and enalapril (0.12 g/L; Sigma-Aldrich, St. Louis, Missouri, USA) were dissolved in water and added directly to the drinking water of the animals (which was changed at least weekly). Treatment was started at 8 weeks of age and was continued until sacrifice 8 weeks later.
Systolic BP analysis.
BP was measured noninvasively using a tail-cuff method on conscious, restrained mice as described (14), when the animals were between 3 months and 4 months of age. All BPs were calculated as the average of 5–10 sessions per day for 6 consecutive days of measurements.
Atherosclerotic lesion analysis.
All animals were sacrificed at 4 months of age, and the vascular tree was perfused with 4% paraformaldehyde under physiological pressures. Segments of the aortic sinus were embedded, sectioned, and stained as described previously (17); 4 sections chosen by strict anatomical criteria were used for morphological evaluation. The average of the lesion sizes of the 4 sections was taken as the average lesion size for each animal. Lesions were scored by a person who did not know the genotypes of the animals. Degrees of fibrous caps and microaneurysms were scored on a scale of 1 to 5 using sections of the sinus area. To evaluate plaque formation in other parts of the aorta, the aortic tree was dissected free of surrounding tissue. Plaques visible under a dissection microscope were counted in individual animals.
Blood and urine chemical analysis.
Total cholesterol, HDL, and triglyceride measurements were performed using standard colorimetric tests (Sigma-Aldrich) on plasma from animals that had been fasted for 4 hours. Plasma creatinine and urinary protein analyses were measured using a VT250 Clinical Chemical analyzer (Johnson & Johnson, Rochester, New York, USA) at the University of North Carolina Animal Clinical Core Facility. Plasma glucose was determined by a glucose Trinder test (Sigma-Aldrich). The amount of lipid peroxide–modified proteins in plasma was determined by ELISA using a polyclonal antibody that specifically recognizes lipid peroxide–modified proteins as described (18).
Histology.
The percentage of calcified glomeruli was determined by counting 100 glomeruli from sections stained with hematoxylin and eosin. Similar techniques were used on Sudan IVB–stained sections to evaluate glomerular lipid deposition.
Plasma lipoprotein analysis.
One hundred microliters of pooled plasma from either NNee or nnee (n = 10) mice was fractionated using a superose 6 HR10/30 column (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) (17). Total cholesterol and triglyceride were determined for each fraction (0.5 mL) as described for plasma.
Statistical analysis.
Data were analyzed using the JMP software package (SAS Institute Inc., Cary, North Carolina, USA). All data are presented as mean ± SE.
Results
nnee animals have higher BP and develop larger atherosclerotic lesions than do NNee mice.
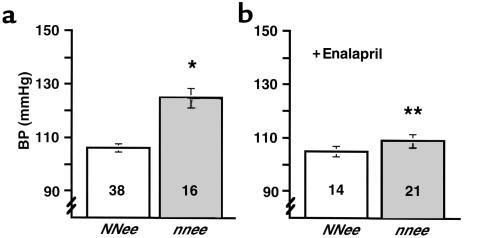
The mean systolic BP of NNee mice was not different from that of wild-type mice by tail-cuff assessment, but the nnee mice had a mean BP that was 20 mmHg higher than that of NNee mice (P < 0.0001; Figure 1a). This increase is comparable to that originally seen in nnEE mice (8). nnee mice have a 10% lower heart rate than NNee mice (614 ± 35, n = 14, vs. 683 ± 24, n = 22). There was no difference in BP or heart rate between sexes.
Figure 1.
(a) BP of NNee and nnee mice. An asterisk indicates a significant increase in the BP of nnee animals compared with NNee controls (P < 0.0001). Number inside bar is sample size. (b) BP of NNee and nnee mice treated with enalapril (0.12 g/L drinking water). Two asterisks indicate a significant decrease in BP of nnee animals treated with enalapril vs. untreated animals (P < 0.001) (but not in BP of enalapril-treated vs. untreated NNee animals); (P = 0.75). Error bars represent SE.
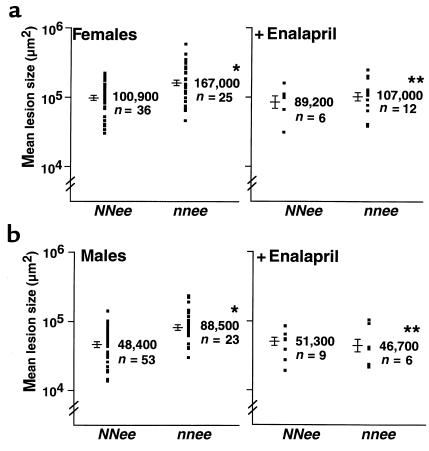
When the atherosclerotic plaques in the proximal aorta of animals were measured at 4 months of age, the lesions in the nnee mice were significantly larger than those in NNee controls (Figure 2). The mean size of lesions in the proximal aorta of nnee males was 182% of that in NNee males; the mean lesion size in nnee females was 165% of the mean lesion size in the proximal aorta of NNee females (P < 0.0001 for males and P < 0.002 for females; Figure 2, a and b). The effect of eNOS genotype (P < 0.0001) and the previously described effects of sex on atherosclerotic plaque size in Apoe–/– animals (P < 0.0001) were significant by two-way ANOVA. There was no interaction between sex and genotype (P = 0.75).
Figure 2.
(a) Left panel: Atherosclerotic lesion size expressed on a log scale in 4-month-old female NNee and nnee animals. There is an increase in the lesion size of female nnee mice compared with NNee mice. *P < 0.002. Each dot represents the mean lesion size of 4 sections from each animal. Logarithmic mean value of lesion size (in μm2) from each group is shown to the right of the dots. Data are shown as the range of logarithmic mean ± SE. Right panel: Atherosclerotic lesion size in nnee female mice is reduced by treatment with enalapril (0.12 g/L drinking water) for 2 months. **P = 0.05 compared with untreated nnee female mice. (b) Arrangement is the same as in a, except male mice are shown. *P < 0.0001 compared with NNee mice. **P < 0.02 compared with untreated nnee mice.
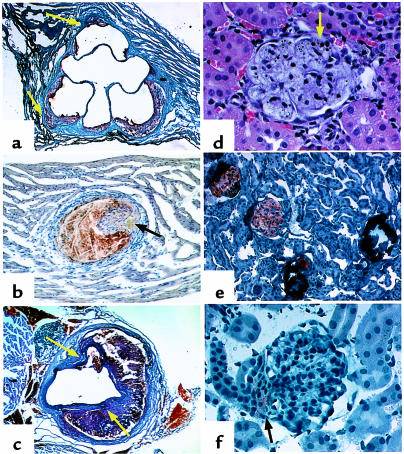
Additionally, we found that nnee mice had alterations in the distribution of atherosclerotic lesions: 90% of 4-month-old male nnee mice had 1 or more atherosclerotic plaques in the descending thoracic aorta that were readily visible under a dissection microscope, compared with 20% of NNee males (P < 0.03). Histologically, plaques in the proximal aortic area of nnee male mice were larger and more advanced; well-formed fibrous caps were twice as frequent in nnee male mice (Figure 3a) as in NNee male mice (not shown). In addition, microaneurysmal dilatations were seen in approximately 20% of the nnee mice (Figure 3a), and some of the small coronary vessels were affected (Figure 3b). Aneurysmal breaks of aortic walls were often found associated with large plaques in the descending aorta, with the plaque material contained by adventitial tissues (Figure 3c). These features are uncommon in male NNee mice of the same age (17).
Figure 3.
Representative histological sections from 4-month-old nnee animals. (a) Proximal aorta section showing atherosclerotic lesions. Sudan IVB with hematoxylin counterstain; ×40. Arrows indicate microaneurysms. (b) A plaque in a small vessel in myocardium. Sudan IVB with hematoxylin counterstain; ×200 (initial magnification). Arrow indicates lumen. (c) Aneurysms in an abdominal aortic section. Sudan IVB with hematoxylin counterstain; ×40 (original magnification). Arrows indicate sites of dissection. (d) Foam cells in glomerulus. Hematoxylin and eosin; ×165. Arrow indicates small area of calcification. (e) Four glomeruli demonstrating the transition from heavy lipid deposition (which appears orange) to dystrophic calcification (which appears dark blue). Sudan IVB with hematoxylin counterstain; ×82.5. (f) Glomerulus of enalapril-treated nnee mouse. Lipid deposition is light (arrow) and is confined to extraglomerular mesangial cells. Sudan IVB and hematoxylin; ×165. This pattern of lipid staining is similar to that seen in NNee mice.
We next examined whether the effects of eNOS deletion can be replicated by the pharmacological inhibition of NOS with L-NAME in male Nnee mice. Oral treatment of 2-month-old NNee males (n = 9) with L-NAME (0.1 g/L drinking water, equivalent to approximately 20 mg/kg per day) for 8 weeks resulted in a significant increase in BP (100 mmHg to 113 mmHg; P < 0.02). These mice also showed a trend toward increased lesion size (126% of that of nontreated mice), although this increase did not reach significance (P = 0.26; data not shown). Thus, a pharmacologically induced decrease in NO increases BP and may have an effect on the development of atherosclerosis in apo E–deficient mice.
Treatment of nnee mice with enalapril reduces both BP and lesion size.
To test whether treatment with an antihypertensive drug could decrease the enhanced atherosclerosis in nnee mice, 2-month-old NNee mice and nnee mice were treated with the ACE inhibitor enalapril (0.12 g/L, ∼30 mg/kg per day) in their drinking water for 8 weeks; this dose of enalapril has been shown to lower the BP of mice (14). The enalapril treatment caused a significant decrease in the BP of nnee animals (mean pressure decreased from 125 mmHg to 109 mmHg, a level similar to that in NNee animals; P < 0.001) (Figure 1b). There was no difference between sexes in the BP of mice treated with enalapril.
Enalapril treatment also caused a significant decrease in the lesion size of both male (52% decrease; P < 0.02) and female (64% decrease; P = 0.05) nnee mice to a level similar to that in NNee animals (see below) (Figure 2, a and b). ANOVA analysis in nnee animals confirmed the significant effect of sex (P < 0.0001) and of drug treatment (P < 0.005) on lesion size in Apoe–/– mice, but there was no interaction between sex and drug. The distribution and histological characteristics of plaques in nnee mice treated with enalapril were not different from those in NNee mice.
In comparable experiments with NNee mice, enalapril had no effect on either BP or atherosclerotic lesion size. ANOVA analysis showed a significant effect of sex on lesion size (P < 0.01), but the effect of drug treatment was not significant (P = 0.47; Figure 2, a and b).
Relationship between BP and lesion size.
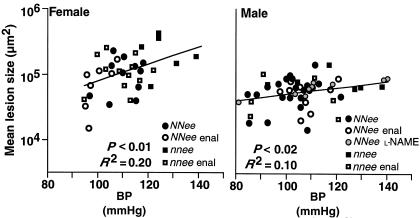
To investigate the overall relationship between BP and lesion size, we combined the data from all 4-month-old animals (n = 88) in which both BP and lesion size were measured; thus NNee and nnee animals, treated and nontreated with enalapril or with L-NAME are represented in the analysis. Figure 4 compares the resulting distribution of atherosclerotic lesion size with the distribution of BP. As shown in the figure, there is a significant positive correlation between BP and lesion size in both females (P < 0.01; n = 36) and males (P < 0.02; n = 52). When each variable (genetic, L-NAME, or enalapril) is considered separately, the correlation remains positive. Thus, there is a significant relationship between BP and lesion size that is valid regardless of which of the 3 methods was used to induce BP changes.
Figure 4.
Atherosclerotic lesion size on a log scale expressed as a function of BP in female (left) and male (right) 4-month-old mice. Genotype and drug treatments are as indicated. There is a significant relationship between BP and lesion size in both female (P < 0.01) and male (P < 0.02) mice, independent of how BP was altered (by genotype, treatment, or a combination of the two). n = 36 for females and n = 52 for males. Enal, enalapril.
Levels of plasma lipids, glucose, and oxidized plasma proteins did not differ between nnee and NNee mice.
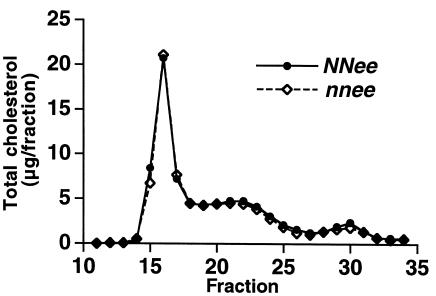
Because elevated levels of plasma lipids and glucose can cause enhanced atherosclerosis, we measured total cholesterol, HDL, triglycerides, and blood glucose, and found no differences between the NNee and nnee animals (data not shown). Fast performance liquid chromatography also revealed no differences in lipoprotein profiles of NNee and nnee animals (Figure 5). Likewise, because it has been hypothesized that NO, as a free-radical scavenger, may play a role in determining the oxidation state of the vessel wall and of plasma lipoproteins (19), we examined the levels of lipid peroxide–modified plasma proteins using ELISA (OD 405 nm). There was no detectable difference in the levels of these proteins between NNee and nnee animals (0.64 ± 0.12 OD units, n = 9 vs. 0.70 ± 0.10 ODunits, n = 9, respectively) (18). Nor did enalapril treatment affect the levels of plasma lipids or oxidized plasma proteins (data not shown).
Figure 5.
Analysis of plasma cholesterol by fast performance liquid chromatography. There were no differences in distribution of lipoproteins from NNee mice (closed circles) and nnee mice (open diamonds).
Lack of iNOS does not affect lesion development in apo E–deficient mice.
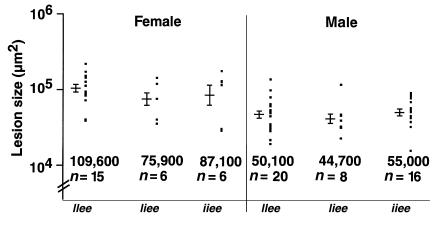
eNOS is a member of a family of enzymes that produce NO. Another member of this family, iNOS, is present mainly in macrophages and neutrophils, and produces NO in response to cytokine or endotoxin stimulation (11). Atherosclerotic lesions have been shown to contain iNOS, but its role in plaque development is unclear (20). To investigate the involvement of iNOS in atherosclerotic lesion development, we generated apo E–deficient mice that were wild type, heterozygous, or homozygous with respect to disruption of the iNOS gene (IIee, Iiee, or iiee, respectively). No effect of iNOS genotype on lesion development was seen in either female or male Apoe–/– animals when they were maintained on a normal chow diet (Figure 6). Note that mice deficient for iNOS are normotensive (21).
Figure 6.
Effect of iNOS deficiency on the size of atherosclerotic plaques. There is no significant difference in the atherosclerotic lesion size between iNOS+/+Apoe–/– (IIee) mice and iNOS–/–Apoe–/– (iiee) mice on normal chow. Error bars represent SE.
nnee animals have decreased kidney weight/body weight ratios, impaired renal function, increased glomerular lipid deposition, and dystrophic calcification.
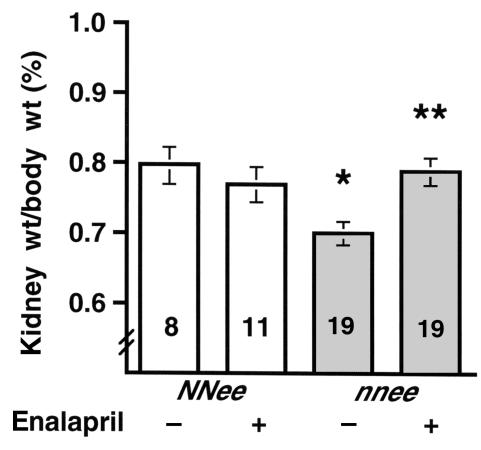
Both hypertension and atherosclerosis often contribute to kidney damage. We found that the ratio of kidney weight to body weight in nnee mice is 15% lower than that of NNee mice (0.70 ± 0.02, n = 19 vs. 0.80 ± 0.03, n = 8; P < 0.005) (Figure 7). The plasma creatinine levels of the double-knockout mice were 66% higher than in NNee mice (0.25 ± 0.03 mg/dL, n = 23 vs. 0.15 ± 0.01 mg/dL, n = 21; P < 0.002), but there was no difference in the level of total urinary protein between the groups. Histologically, about 5% of the glomeruli in NNee kidneys had small lipid deposits, mostly limited to extraglomerular mesangial cells. In contrast, about 3 times more glomeruli (15%) in the nnee kidneys contained large lipid deposits in foam cells that filled the glomeruli, rather than being limited to mesangial cells (Figure 3d). In the nnee mice, the atherosclerotic glomerular injury progressed from fatty deposits to dystrophic calcification (3%), with eventual glomerular loss (Figure 3e). No such glomerular alterations were seen in kidneys of the NNee mice. Although the smaller kidney weight suggests the possibility of ischemia, no ischemic changes were seen in nnee mice, and no occlusion or narrowing of blood vessels was evident in either nnee or NNee kidneys.
Figure 7.
Ratios of kidney weight to body weight in 4-month-old NNee mice and nnee mice treated (+) and untreated (–) with enalapril in drinking water (0.12 g/L) for 2 months. The nnee mice show a significant decrease in the kidney weight/body weight ratio compared with NNee control mice (*P < 0.01). This decrease is abolished by enalapril treatment (**P < 0.002, nnee untreated compared with nnee treated). Error bars represent SE.
Chronic inhibition of NOS in NNee animals with L-NAME treatment also produced signs of kidney dysfunction. L-NAME treatment increased plasma creatinine (0.25 ± 0.03 mg/dL, n = 9 vs. 0.15 ± 0.01 mg/dL, n = 21; P < 0.001), although it did not lead to glomerular calcification, and there was no difference in lipid-containing glomeruli.
Enalapril treatment abrogates kidney damage in nnee mice.
Enalapril treatment reduced the kidney damage in nnee animals. The decreased kidney weight/body weight ratios seen in the nnee animals were restored to normal levels (0.70 ± 0.02, n = 19, untreated vs. 0.79 ± 0.02, n = 19, treated; P < 0.005) (Figure 7). Histologically, the enalapril-treated nnee mice had half as many lipid-containing glomeruli (8% vs. 15%) (Figure 3f) and two-thirds less glomerular calcification (1% vs. 3%) compared with untreated nnee mice. Plasma creatinine levels (0.11 ± 0.01 mg/dL, n = 11) were less than half of those seen in untreated nnee mice (0.25 ± 0.03 mg/dL, n = 23; P < 0.0001), and were similar to those of NNee control mice. The kidney weight/body weight ratios of NNee animals were unchanged after enalapril treatment (0.77 ± 0.03, n = 11).
Kidneys from mice lacking eNOS but wild type for apo E (nnEE) had no evidence of glomerular calcification or lipid-containing glomeruli. The level of plasma creatinine in the nnEE mice was similar to that in NNee mice (0.10 ± 0.00 mg/dL, n = 4 vs. 0.15 ± 0.01 mg/dL, n = 21), even though the nnEE mice had elevated BP similar to nnee mice. Thus, both elevated BP and a disturbance in lipid metabolism are necessary for the development of the observed kidney abnormalities.
Discussion
We used a genetic approach to study the interaction between hypertension and atherosclerosis. Lack of eNOS increases BP, enhances atherosclerotic lesion size, and causes greater renal damage in Apoe–/– mice than in eNOS+/+Apoe–/– mice. These deleterious effects are completely abolished by treatment with an ACE inhibitor, enalapril. There is a direct and significant positive correlation between BP and the severity of atherosclerosis in animals; the relationship is seen regardless of the method used to change BP (genetic, L-NAME, or enalapril).
Previous studies in mice have also shown correlations between BP and atherosclerosis (22, 23). Rheologic mechanisms can explain the observed relationship between BP and lesion size. For example, increases in BP lead to increased turbulence and stagnation of lipoprotein particles, and increased arterial wall stretch (24, 25), both of which are major determinants of the localization of atherosclerotic lesions (26–28). Additionally, the decreased heart rate of nnee mice predisposes them to a considerable increase in pulse pressure, which can be expected to increase turbulent flow and stretch, thereby acting as an atherogenic stimulus.
Besides the effects on BP and heart rate, the eNOS system is potentially antiatherogenic through many other mechanisms. For example, a direct role for NO in decreasing vascular smooth muscle cell proliferation after a remodeling stimulus has been described in eNOS–/– mice (29). In addition, NO inhibition of platelet aggregation and monocyte adherence to the vessel wall are potentially antiatherogenic (7, 11), and antioxidant functions of NO may prevent proatherogenic changes in lipoproteins (19). Many of the local functions of NO counterbalance proatherogenic effects of angiotensin II, which mediates vasoconstriction, smooth muscle cell migration and proliferation, and monocyte adhesion. It is important to note that eNOS-deficient mice have increased plasma renin activity (8), and probably also have increased production of angiotensin II.
Although we and others saw no statistically significant decrease in either the BP or lesion size of NNee mice treated with enalapril (20 mg/kg per day) or with losartan (30 mg/kg per day; ref. 23), others have reported that 3 months of treatment with captopril (50 mg/kg per day) or losartan (25 mg/kg per day) decreases the atherosclerotic lesion size of Apoe–/– mice by 70% and 80%, respectively (30, 31); BP was not measured in these experiments. A reduction of in vitro oxidizability of lipoproteins in animals treated with captopril and losartan has been described (31, 32).
Clinical benefits of ACE inhibitors on atherosclerosis seen in humans (6, 33, 34) or in animal models (35) do not always correlate with BP decreases (36). These observations have led to the hypothesis that ACE inhibition protects the vasculature from atheroma development by locally decreasing tissue production of angiotensin II (34), thereby enhancing NO bioavailability (34, 37). Our present results showing that ACE inhibition is effective in the absence of NO production by endothelial cells suggest that the observed beneficial effect of enalapril does not depend on NO. The direct correlation between BP and the severity of atherosclerosis that we see when BP is altered by 3 different means, combined with the fact that enalapril did not significantly decrease the BP or lesion size of normotensive NNee animals in our experiments, suggests that at least part of the beneficial effect of enalapril is directly due to decreased BP. Furthermore, other antihypertensive drugs, including β-adrenergic blockers and calcium antagonists that do not act through the renin-angiotensin system, are also antiatherogenic in both humans and cholesterol-fed animal models (6, 38–40). Testing these drugs on nnee mice will be informative in distinguishing whether the mechanism of NO protection against atherosclerosis is due to its local effects or to its systemic effects on BP.
The atherosclerotic effects of the genetic lack of eNOS were only partly mimicked by inhibition of NOS with L-NAME, in agreement with results reported previously (41). L-NAME at the dose we used (0.1 g/L) might have abolished the production of NO only partially, because the BP increase in our L-NAME–treated NNee mice was about 65% of the difference between untreated nnee mice and untreated NNee mice. Nevertheless, because L-NAME inhibits all 3 forms of NOS, inhibition of NO production by the other synthases must be considered. For example, the observation of increased iNOS levels in atherosclerotic plaques (42) has led to the hypothesis that NO produced by iNOS in macrophages and neutrophils may contribute to plaque development; any proatherogenic effects of L-NAME through eNOS inhibition could therefore be masked by concomitant antiatherogenic effects through iNOS inhibition. However, our genetic experiment, in which absence of iNOS had no effect on lesion size, makes it unlikely that iNOS plays any significant role in the atherogenesis of Apoe–/– mice.
Both hypertension and hyperlipidemia contribute to renal dysfunction (43). Chronic NOS inhibition with L-NAME in rats has been shown to cause both hypertension and kidney damage (44, 45), both preventable by simultaneous treatment with losartan or nifedipine — drugs with very different modes of action (45, 46). This suggests that the damage is mediated by increased BP. Atherosclerosis can also lead to renal dysfunction (47) and glomerular lipid deposition (48). However, neither hypertension nor atherosclerosis alone can account for the phenotype we see in the nnee mice. In hypertensive, but not atherosclerotic nnEE mice, we saw no glomerular lipid deposition, no glomerular calcification, and no increase in plasma creatinine. Untreated atherosclerotic (but normotensive) NNee mice likewise have normal plasma creatinine and very little kidney pathology. Yet when the 2 genetic defects are combined in the nnee mice, the kidney damage is severe, suggesting that eNOS deficiency and atherosclerosis mutually enhance end-organ damage.
The pathologic transition from lipid deposition to dystrophic calcification observed in the glomeruli of the nnee mice has not been described previously to our knowledge, and provides direct evidence that hyperlipidemia induces glomerular injury. In humans, glomerular foam cells are very uncommon (48, 49). Interestingly, in a case report of a hypertensive woman with apo E deficiency, the kidneys were atrophic and contained lipid-filled mesangial foam cells in the glomeruli (49). Conceivably, the decreased kidney weight and the increase in plasma creatinine in untreated nnee mice could result from generalized ischemia (50), but no such changes were histologically evident.
The fact that enalapril abrogates the kidney damage seen in nnee animals is important but not surprising (51, 52). It is possible that renal damage contributes to enhancement of aortic atherosclerosis in nnee mice, and that some of the beneficial effects of enalapril on atheroma development are the result of improved renal function.
In conclusion, our genetically defined mouse model lends insight into the complex relationship between the eNOS system and atherosclerosis, and reaffirms the “response to injury” hypothesis of atherogenesis described by Ross (53). In our model, eNOS deficiency and plasma lipid dysfunction caused by lack of apo E generate an environment particularly favorable for atheroma formation and end-organ damage (3, 53). Lack of iNOS does not affect lesion size, but treatment with enalapril interrupts the cycle of atheroma formation and kidney damage. Our demonstration of a linear relationship between lesion size and BP reemphasizes the importance of controlling hypertension in preventing atherosclerotic disease.
Acknowledgments
The authors would like to thank Sampath Parthasarathy and Nailini Santanam (Emory University, Atlanta, Georgia, USA) for analysis of lipid peroxide–modified proteins. We also thank Hyung-Suk Kim, Shinja Kim, Elena Avdievich, Jennifer Wilder, Peter Heeringa, Allyson Ricks, Myron Hinsdale, David Carraway, and John Hagaman for experimental assistance, and Howard Rockman, Michael Goy, Michael Knowles, Nobuyuki Takahashi, Tracey Dawson, and Eric Meissner for helpful comments. This work was supported by National Institutes of Health grants HL-42630 (N. Maeda), HL-62845 (N. Maeda), and HL-49277 (O. Smithies).
Footnotes
Edward G. Shesely’s current address is: Hypertension and Vascular Research Division, Henry Ford Hospital, Detroit, Michigan 48202, USA.
References
- 1.World Health Organization. 1998. The world health report, 1998. World Health Organization. Geneva, Switzerland. 125–126.
- 2.Braunwald E. Shattuck lecture — cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Alexander RW. Exacerbation of atherosclerosis by hypertension. Potential mechanisms and clinical implications. Arch Intern Med. 1996;156:1952–1956. [PubMed] [Google Scholar]
- 4.Doyle, A.E. 1990. Does hypertension predispose to coronary artery disease? In Hypertension: pathophysiology, diagnosis and management. J.H. Laragh and B.M. Brenner, editors. Raven Press Ltd. New York, NY. 119–125.
- 5.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 6.Collins R, et al. Blood pressure, stroke, and coronary heart disease. II. Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 8.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang PL, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 10.Bonnardeaux A, et al. Lack of evidence for linkage of the endothelial cell nitric oxide synthase gene to essential hypertension. Circulation. 1995;91:96–102. doi: 10.1161/01.cir.91.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- 12.Cox DA, et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 13.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 14.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 15.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 16.Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang SH, Reddick RL, Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest. 1994;94:937–945. doi: 10.1172/JCI117460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JG, et al. Generation of a polyclonal antibody against lipid peroxide-modified proteins. Free Radic Biol Med. 1997;23:251–259. doi: 10.1016/s0891-5849(96)00615-6. [DOI] [PubMed] [Google Scholar]
- 19.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 20.Esaki T, et al. Expression of inducible nitric oxide synthase in T lymphocytes and macrophages of cholesterol-fed rabbits. Atherosclerosis. 1997;128:39–46. doi: 10.1016/s0021-9150(96)05976-x. [DOI] [PubMed] [Google Scholar]
- 21.MacMicking JD, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase [erratum 1995, 81:1170] Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama F, et al. Acceleration of atherosclerotic lesions in transgenic mice with hypertension by the activated renin-angiotensin system. Lab Invest. 1997;76:835–842. [PubMed] [Google Scholar]
- 23.Makaritsis KP, Gavras H, Du Y, Chobanian AV, Brecher P. Alpha1-adrenergic plus angiotensin receptor blockade reduces atherosclerosis in apolipoprotein E-deficient mice. Hypertension. 1998;32:1044–1048. doi: 10.1161/01.hyp.32.6.1044. [DOI] [PubMed] [Google Scholar]
- 24.Gimbrone MA., Jr Vascular endothelium, hemodynamic forces, and atherogenesis. Am J Pathol. 1999;155:1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann Thorac Surg. 1995;59:1594–1603. doi: 10.1016/0003-4975(94)01037-d. [DOI] [PubMed] [Google Scholar]
- 26.Zand T, et al. Lipid deposition in rat aortas with intraluminal hemispherical plug stenosis. A morphological and biophysical study. Am J Pathol. 1999;155:85–92. doi: 10.1016/S0002-9440(10)65103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giddens DP, Zarins CK, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 28.Meyer G, Merval R, Tedgui A. Effects of pressure-induced stretch and convection on low-density lipoprotein and albumin uptake in the rabbit aortic wall. Circ Res. 1996;79:532–540. doi: 10.1161/01.res.79.3.532. [DOI] [PubMed] [Google Scholar]
- 29.Rudic RD, et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayek T, Attias J, Smith J, Breslow JL, Keidar S. Antiatherosclerotic and antioxidative effects of captopril in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 1998;31:540–544. doi: 10.1097/00005344-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Keidar S, Attias J, Smith J, Breslow JL, Hayek T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 1997;236:622–625. doi: 10.1006/bbrc.1997.6844. [DOI] [PubMed] [Google Scholar]
- 32.Benzie IF, Tomlinson B. Antioxidant power of angiotensin-converting enzyme inhibitors in vitro. Br J Clin Pharmacol. 1998;45:168–169. doi: 10.1046/j.1365-2125.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancini GB, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) [erratum 1996, 94:1490] Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 34.Dzau VJ. Mechanism of protective effects of ACE inhibition on coronary artery disease. Eur Heart J. 1998;19(Suppl.):J2–J6. [PubMed] [Google Scholar]
- 35.Chobanian AV, Haudenschild CC, Nickerson C, Drago R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1990;15:327–331. doi: 10.1161/01.hyp.15.3.327. [DOI] [PubMed] [Google Scholar]
- 36.Kowala MC, Grove RI, Aberg G. Inhibitors of angiotensin converting enzyme decrease early atherosclerosis in hyperlipidemic hamsters. Fosinopril reduces plasma cholesterol and captopril inhibits macrophage-foam cell accumulation independently of blood pressure and plasma lipids. Atherosclerosis. 1994;108:61–72. doi: 10.1016/0021-9150(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 37.Gibbons GH. Vasculoprotective and cardioprotective mechanisms of angiotensin-converting enzyme inhibition: the homeostatic balance between angiotensin II and nitric oxide. Clin Cardiol. 1997;20(Suppl. 2):II-18–25. [PubMed] [Google Scholar]
- 38.Chobanian AV, Brecher P, Chan C. Effects of propranolol on atherogenesis in the cholesterol-fed rabbit. Circ Res. 1985;56:755–762. doi: 10.1161/01.res.56.5.755. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–1372. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- 40.Henry PD, Bentley KI. Suppression of atherogenesis in cholesterol-fed rabbit treated with nifedipine. J Clin Invest. 1981;68:1366–1369. doi: 10.1172/JCI110384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elhage R, et al. Prevention of fatty streak formation of 17beta-estradiol is not mediated by the production of nitric oxide in apolipoprotein E-deficient mice. Circulation. 1997;96:3048–3052. doi: 10.1161/01.cir.96.9.3048. [DOI] [PubMed] [Google Scholar]
- 42.Behr D, Rupin A, Fabiani JN, Verbeuren TJ. Distribution and prevalence of inducible nitric oxide synthase in atherosclerotic vessels of long-term cholesterol-fed rabbits. Atherosclerosis. 1999;142:335–344. doi: 10.1016/s0021-9150(98)00254-8. [DOI] [PubMed] [Google Scholar]
- 43.Manttari M, Tiula E, Alikoski T, Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995;26:670–675. doi: 10.1161/01.hyp.26.4.670. [DOI] [PubMed] [Google Scholar]
- 44.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro MO, Antunes E, Muscara MN, De Nucci G, Zatz R. Nifedipine prevents renal injury in rats with chronic nitric oxide inhibition. Hypertension. 1995;26:150–155. doi: 10.1161/01.hyp.26.1.150. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20:298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- 47.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 48.Magil AB. Interstitial foam cells and oxidized lipoprotein in human glomerular disease. Mod Pathol. 1999;12:33–40. [PubMed] [Google Scholar]
- 49.Amatruda JM, Margolis S, Hutchins GM. Type 3 hyperlipoproteinemia with mesangial foam cells in renal glomeruli. Arch Pathol Lab Med. 1974;98:51–54. [PubMed] [Google Scholar]
- 50.Meyrier A, Hill GS, Simon P. Ischemic renal diseases: new insights into old entities. Kidney Int. 1998;54:2–13. doi: 10.1046/j.1523-1755.1998.00968.x. [DOI] [PubMed] [Google Scholar]
- 51.Hollenberg NK, Raij L. Angiotensin-converting enzyme inhibition and renal protection. An assessment of implications for therapy. Arch Intern Med. 1993;153:2426–2435. [PubMed] [Google Scholar]
- 52.Maschio G, et al. Angiotensin-converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. J Cardiovasc Pharmacol. 1999;33(Suppl. 1):S16–S20; discussion S41–43. doi: 10.1097/00005344-199900001-00004. [DOI] [PubMed] [Google Scholar]
- 53.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]