Abstract
Trace-eyeblink conditioning is a forebrain-dependent learning paradigm that has assisted in our understanding of age-related hippocampal neuronal plasticity; however, the hippocampus is not believed to be the permanent site for most long-term-memory storage. Studies in adult subjects have suggested the neocortex as one such site. Whisker plucking studies have further suggested that the ability for plasticity in the neocortex declines with age. Mice were trained in trace- and delay-eyeblink conditioning with whisker or auditory stimulation as the conditioned stimulus to examine possible age-related behavioral and neocortical abnormalities. Whisker stimulation was determined to be a more effective stimulus for examining age-related behavioral abnormalities in C57 mice. Additionally, neocortical barrel expansion, observed in trace conditioned adult mice and rabbits, does not occur in mice conditioned on a delay paradigm or in old mice unable to learn the whisker trace association. Abnormalities in neocortical memory storage in the elderly could contribute to normal age-dependent declines in associative learning abilities.
Keywords: Whisker, Barrel cortex, PNBSF, Somatosensory, Hippocampus, Aging
1. Introduction
The ability to acquire many forebrain-dependent associative learning tasks is impaired with age; however, the neuronal cause for this relationship remains unknown. Trace conditioning has assisted in our understanding of age-induced learning abnormalities. In trace conditioning a neutral conditioned stimulus (CS) is paired with a salient unconditioned stimulus (US) temporally separated by a stimulus free interval (trace). After repeated CS-US pairings the subject learns that the CS predicts US onset. This form of conditioning is dependent upon neocortical, as discussed below, and cerebellar (Woodruff-Pak et al., 1985; Gruart et al., 2000; Gerwig et al., 2005; Campolattaro and Freeman, 2009; Pakaprot et al., 2009) processing. Trace conditioning is also impaired with age (Graves and Solomon, 1985; Finkbiner and Woodruff-Pak, 1991; Kishimoto et al., 2001; Knuttinen et al., 2001b; Knuttinen et al., 2001a; Villarreal et al., 2004; Woodruff-Pak et al., 2007) and is initially dependent upon hippocampal processing (Solomon et al., 1986; Moyer et al., 1990; Kim et al., 1995; McGlinchey-Berroth et al., 1997; Clark and Squire, 1998; Weiss et al., 1999; Takehara et al., 2002; Tseng et al., 2004), a structure shown to exhibit age-related functional alterations (Landfield and Pitler, 1984; Moyer et al., 1992; Oh et al., 1999; Moyer et al., 2000).
The neocortex, the most likely site of permanent storage for trace-associations (Eichenbaum et al., 1992; Christian and Thompson, 2003; Squire et al., 2004; Smith and Squire, 2009), also exhibits age-related functional alterations including a reduction in NMDAR-independent (Ohmura et al., 2003) and -dependent long-term-potentiation (LTP) (Baskys et al., 1990; Mullany and Lynch, 1997; Yoshimura et al., 2003); reduced LTP-induced protein expression (Mullany and Lynch, 1997); and an absence of layer IV metabolic barrel deprivation-induced reorganization (Skibinska et al., 2000). These studies suggest a loss in neocortical plasticity with age, indicating that neocortical memory consolidation may also be impaired with aging.
The neocortex is also essential for acquisition and retrieval of trace-associations. Neuronal activity during an auditory CS (ACS) and trace interval in medial prefrontal cortex (Baeg et al., 2001; Gilmartin and McEchron, 2005; Takehara-Nishiuchi and McNaughton, 2008), caudal anterior cingulate (Weible et al., 2003) and auditory cortex (Woody et al., 1976; Woody, 1977) are modulated during trace conditioning. Conditioning with whisker stimulation as the CS (WCS) induces an expansion of the conditioned metabolic neocortical barrel representation (Galvez et al., 2006). Furthermore, lesions of barrel somatosensory neocortex for WCS (Galvez et al., 2007) and medial prefrontal neocortex for ACS (Kronforst-Collins and Disterhoft, 1998; Weible et al., 2000; McLaughlin et al., 2002; Takehara et al., 2002) hinder both acquisition and retrieval of trace-associations. These observations along with reported age-related neocortical abnormalities, suggest that age-related trace conditioning deficits could be at least partially due to altered neocortical memory storage.
We examined abnormalities in acquisition for an associative task and subsequent conditioning-induced neocortical plasticity in aging mice. This study demonstrated 1) WCS is suitable for examining age related abnormalities in C57 mice, 2) age-related behavioral abnormalities are present in old mice, and 3) the lack of conditioning induced neocortical barrel plasticity in old mice unable to learn trace-eyeblink conditioning.
2. Materials and methods
All mice were housed on a 14-hr/10-hr light/dark cycle, and fed ad libitum. All procedures described were performed in accordance with guidelines approved by Northwestern University’s Animal Care and Use Committee.
2.1. Surgery
Fourteen Adult (3 month), 10 Middle Aged (12 month), and 8 Old (22 month) C57Bl6 mice were affixed with a headbolt for eyeblink conditioning as previously described (Tseng et al., 2004; Weiss and Disterhoft, 2008). Briefly, once anesthetized with Ketamine (6.0mg/g) and Xylazine (1.0mg/g) a connector with two Teflon-coated stainless steel wires (45μm), and one bare stainless steel wire (30 μm) was cemented to the skull with dental acrylic. The two coated wires were subcutaneously passed through the periorbital region caudal to the eye. The bare wire was secured to a stainless steel skull screw (00–90 X 1/16 in.) to serve as an electrical ground. Mice were allowed seven days to recover from surgery.
2.2. Conditioning
Each mouse was lightly anesthetized with isoflorane and all the whiskers except the C row on both hemispheres were trimmed to 0.5cm (the whisker stimulator sits approximately 1cm from the face (Galvez et al., 2008)) 24 hours prior to conditioning. Mouse whiskers grow approximately 1mm / day (Ibrahim and Wright, 1975, 1983). The mice were habituated to the training chamber 24 hours later. The mice were then WCS trained for 4 days on either a trace- or pseudo-eyeblink paradigm (Fig.1) (Galvez et al., 2008). This was then followed by ACS training for 4 days on a trace- or pseudo-eyeblink paradigm with an additional 2 days on a delay- or pseudo-eyeblink paradigm (Fig.1). A subset of the Adult (n=9) and all of the Middle Aged mice were sacrificed 24 hours following ACS-delay conditioning. The remaining Adult (n=5) and the Old mice had all the whiskers except the C row on both hemispheres trimmed to 0.5cm under light isoflorane anesthesia and then 24 hours later WCS trained for 2 days on a delay- or pseudo-eyeblink paradigm. Adult and Old mice were sacrificed for cytochrome oxidase analysis 24 hours following training (Fig. 1).
Figure 1.
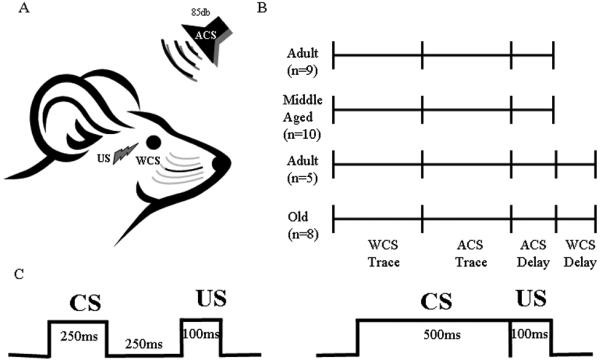
Schematic of training paradigms. A) Illustration of mouse head with the periorbital shock unconditioned stimulus (US; lightening bolt) and two conditioned stimuli, stimulation of C row whiskers (WCS; black whiskers) and auditory tone (ACS; speaker) delineated. B) Illustration of the training regimes for the three age groups. C) Illustration of the two training paradigms used. Left - Trace; Right - Delay
For trace-eyeblink conditioning, freely-moving, tethered mice were conditioned with a 250ms CS followed by a 250ms stimulus free trace interval and then a 100ms US (4mA periorbital square wave shock, 60Hz, 0.5ms pulses) (Tseng et al., 2004). For delay eyeblink conditioning the CS was extended to 500ms and the trace interval was eliminated (Fig. 1). The WCS generated a rostral-caudal 60 Hz 250μm deflection (Galvez et al., 2008). Training using WCS was conducted in the presence of 70db white noise. Activation of the whisker stimulator generates a <55db auditory stimulus that can be masked with 70db white noise (Galvez et al., 2006; Galvez et al., 2008). The ACS was an 85db white noise tone. A custom made optic sensor was used to monitor eyeblinks (Weiss and Disterhoft, 2008). Mice were given 30 trials per session with a mean inter-trial interval (ITI) of 45s (randomly varied within 30–60s) and 1 session per day. Pseudo conditioned mice randomly received 30 CSs and 30 periorbital shocks with a mean ITI of 22s (randomly varied within 15–30s) per day. A CR, closure of the eyelid, was defined as a 4 standard deviation change in voltage from an infrared reflective sensor immediately prior to US onset (Galvez et al., 2008). An alpha response was defined as a 4 standard deviation change in voltage within 35ms of CS onset. Appearance of an alpha response did not exclude the trial from further analyses. Thus a trial exhibiting an alpha response could also exhibit a CR as defined above. Since >1% of trials produced alpha responses (see Results below), this CR response definition had no substantial effect on the results.
2.3. Histology
Following conditioning the mice were given an overdose of Euthasol (390mg/ml Sodium Pentobarbital and 50 mg/ml Sodium Phenytoin) and transcardially perfused with 0.1M PBS (pH 7.4) followed by 2% paraformaldehyde. The neocortex was dissected off, flattened (Strominger and Woolsey, 1987) and post fixed overnight in 4% paraformaldehyde. Flattened neocortices were then sectioned (30 μm) and stained for cytochrome oxidase (CO; 0.05% DAB, 0.03% cytochrome C and 4% sucrose at 37°C for 4 hours) (Galvez et al., 2006).
Analysis of barrel size was conducted as previously described (Galvez et al., 2006). Briefly, the mean barrel length (the anterior-posterior extent) was calculated by summing the length of the first 3 barrels from an optical density histogram generated using NIH ImageJ Ver 1.32J and dividing by three (Fig. 3). The mean barrel width (the medial-lateral distance) was calculated by summing the width of the first 3 barrels in a row from an optical density histogram and dividing by three. Only the first 3 barrels in each row were measured to avoid animal, whisker row and sectioning variability in the number of stained barrels per row. Neocortical barrels for straddler whiskers were not measured.
Figure 3.
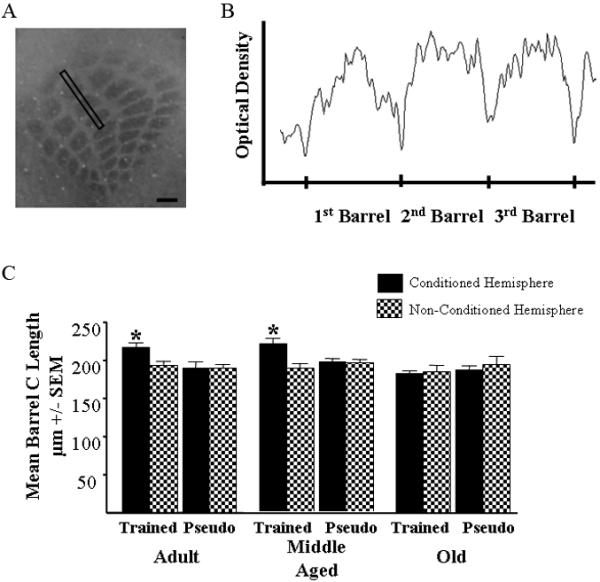
Metabolic expansion of conditioned barrels is dependent upon acquisition of the WCS-trace association. A) Illustration of a cytochrome oxidase stained cortical barrel field with a sampling area over the C row. Scale bar = 200μm. B)Illustration of an optical density histogram of the row of barrels outlined in the barrel field illustrated in A. Note tic marks on the x axis delineating the point between two barrel walls. Mean barrel width was calculated by measuring the distance from the first to the third barrel and dividing by three. C) Adult and Middle Aged mice that were trained on the WCS trace paradigm exhibited an increase in the length of the metabolic neocortical barrel representation compared to the contralateral hemisphere and pseudo conditioned mice. Old mice, unable to learn the WCS trace association did not exhibit a significant increase in the length of the metabolic neocortical barrel representation compared to the contralateral hemisphere and pseudo conditioned mice. It is important to note that the Old mice learned the WCS delay association, although this caused no change in metabolic representation in the barrel cortex. Error bars = standard error of the mean (SEM). *= p<0.05
For analysis of optical density in the barrel hollow, the mean value from 3 circular 80um diameter sampling regions randomly positioned in the hollow was acquired. For analysis of optical density in the barrel wall, the mean value from 3 circular 12um diameter sampling regions randomly positioned in the wall were acquired. All analyses were conducted blind.
2.4. Statistics
For behavioral analysis the percent conditioned response was analyzed using a mixed general-linear-model (GLM)-ANOVA with days of training as within-subject and training group and age as between-subject variables. For CR properties on the last day of training for each training paradigm, a mixed model (GLM)-ANOVA with training group and age as between-subject variables was used. For barrel length, width, and optical density, a mixed model MANOVA with row and hemisphere as within-subject and training group and age as between-subject variables was used.
3. Results
3.1. Trace and delay conditioning
Adult (6 month), Middle Aged (12 month) and Old (22 month) mice were trained on a WCS-trace paradigm (Fig. 1). The Adult and Middle Aged mice were able to acquire the association as compared to age-matched pseudo-controls while the Aged mice were not (Fig. 2; Adult - F(1,11)=18.80; p<0.05; Middle Aged - F(1,7)=11.42; p<0.05). UR properties on the last day of training for pseudo-conditioned mice revealed no significant differences among the groups, suggesting that the ability to blink was not altered with age (Table S1).
Figure 2.
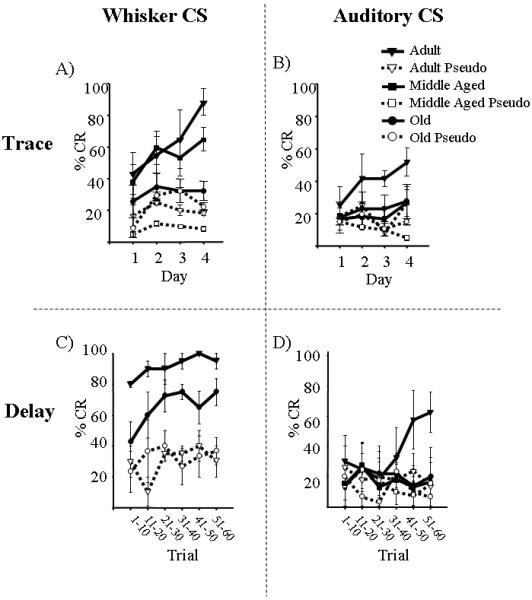
Acquisition of WCS and ACS trace and delay associations. A) WCS-Trace Conditioning. Adult and Middle Aged WCS trace conditioned mice exhibited a higher percent conditioned response (CR) compared to age matched pseudo-controls, demonstrating acquisition of the trace association. Old WCS trace conditioned mice did not perform significantly different from age matched pseudo-controls, demonstrating that they were not able to learn the whisker trace association. B) ACS-Trace Conditioning. Adult ACS mice were the only age group able to learn the trace association. C) WCS-Delay Conditioning. Old delay conditioned mice exhibited a significant increase in percent CR compared to age matched pseudo-controls when switched to WCS delay conditioning, demonstrating that they learned the association. Adult delay conditioned mice did not exhibit a significant change in percent CR; however, they had previously learned the WCS trace association and exhibited significantly better performance than age matched pseudo-controls. D) ACS-Delay Conditioning. Adult ACS mice were the only age group able to learn the delay association. Error bars = standard error of the mean (SEM).
To compare with prior findings using ACS (Woodruff-Pak, 2006) each mouse was then ACS trained on a trace- and then delay-eyeblink paradigm (Fig. 1). These analyses demonstrated that only the Adult mice were able to learn the association, as they showed significantly more CRs on the last day of training compared to age-matched pseudo-controls (Fig. 2; Trace - F(1,11)=8.33; p<0.05; Delay- F(1,10)=7.58; p<0.05). Middle Aged mice, while able to learn the WCS-trace association, were not able to learn the ACS-trace or -delay associations. The most probable explanation for the inability of Middle Aged mice to learn the ACS associations is the well characterized age-related hearing loss in C57 mice (Parham and Willott, 1988; Erway et al., 1993; Zheng et al., 1999; Johnson et al., 2000). The Old mice were unable to learn either the ACS-trace or -delay associations (Fig. 2).
To determine if the Old mice were able to learn any association, a subset of the Adult and all of the Old mice were WCS-delay conditioned (Fig. 1). Old mice exhibited a significant increase in percent CRs across delay training sessions (F(5,10)=7.80; p<0.05) and significantly more CRs on the last training session compared to age-matched pseudo-controls (F(1,6)=9.22; p<0.05), demonstrating that they learned the delay association (Fig. 2). Further analysis of performance on the last training session of WCS-delay conditioning revealed that Adult mice were blinking more (F(1,6)=7.13; p<0.05) and earlier (F(1,6)=8.01; p<0.05) compared to Old mice, suggesting that the Adult mice had acquired the association more securely compared to Old mice (Fig. 2 & Table S2).
All other CR properties on the last day of each of the four training paradigms across the 3 ages examined did not significantly differ (Table S2). Occurrence of alpha responses were extremely low for all mice (>1% of the trials) and no significant differences between age and behavioral groups were detected.
3.2. Learning induced plasticity
Analysis of barrel length (the anterior-posterior extent) for Adult and Middle Aged mice demonstrated an increase in the mean length for cytochrome oxidase (CO) stained trained barrels (C-row) compared to the non-trained hemisphere or pseudo-controls (Fig. 3, Table S3 & S4; Adult - F(3,17)=5.09; p<0.05; Middle Aged - F(3,14)=5.24; p<0.05). These analyses support prior findings from trace conditioned adult rabbits and offer additional support that the neocortex is a site of storage for an aspect of the trace association. The unconditioned whiskers (rows A, B, D, and E) of the trained hemisphere did not demonstrate a similar increase in length compared to the non-trained hemisphere or pseudo-controls, suggesting that this increase (C-row) is specific to the portion of the sensory cortex that processed the CS during training. Analysis of barrel length for the Old mice, unlike that in Adult and Middle Aged mice, did not reveal any significant differences (Fig. 3 & Table S5), suggesting that acquisition of the trace association is necessary for inducing the observed neocortical plasticity.
Note that all data for Adult mice sacrificed after training in WCS-trace and -delay conditioning were combined as there was no difference between the groups; all Old mice were trained in both WCS-trace and -delay conditioning (Fig. 1). This analysis further suggests that acquisition of the delay association, a task that all age groups were able to acquire, is not sufficient for inducing the observed neocortical plasticity (Fig. 3).
Analysis of barrel width (the medial-lateral distance of the barrel, perpendicular to the row) demonstrated no significant change in response to conditioning (Table S3, S4 and S5) for any of the 3 age groups. Optical density values for the barrel hollow were significantly darker than the wall in all subjects (within-subject F(1,110)=51.36; p<0.05), but neither the hollow nor wall exhibited a significant change in optical density intensity in response to conditioning or age (Table S6). These observations suggest that WCS during forebrain-dependent conditioning does not significantly increase the metabolic activity within a barrel but rather increases the size of the cortical whisker metabolic representation, as previously reported in trace conditioned rabbits (Galvez et al., 2006).
4. Discussion
The neocortex is hypothesized to be the most likely site of storage for trace associations (Eichenbaum et al., 1992; Christian and Thompson, 2003; Squire et al., 2004; Smith and Squire, 2009); yet how this process occurs and if it is altered with age remains largely unknown. We examined age-related abnormalities in acquisition of a trace association and subsequent neocortical plasticity related to behavioral learning. These analyses demonstrated that while Adult and Middle Aged mice were able to learn the trace association with whisker stimulation as the CS (WCS), only the Adult mice were able to learn the trace and delay association with an auditory CS (ACS). The Middle Aged mice were not able to learn either the forebrain-dependent (trace) or -independent (delay) version of the eyeblink task with ACS. This finding, along with published analyses of age-related hearing loss in C57 mice (Parham and Willott, 1988; Erway et al., 1993; Zheng et al., 1999; Johnson et al., 2000) suggests that the Middle Aged mice could not hear the ACS and thus could not learn the association.
It is interesting to note that WCS-trace resulted in a higher level of performance than ACS-trace or ACS-delay (Fig. 2). Furthermore, we did not observe any savings of the learned association between WCS-trace, ACS-trace and ACS-delay as has been observed between light and auditory CS (Harlow, 1949; Kehoe, 1988; Campolattaro and Freeman, 2009). Although these findings are puzzling, the rates and level of acquisition obtained in both ACS-trace and - delay eyeblink conditioning are comparable to those previously observed in our laboratory (Tseng et al., 2004). Future analyses will need to examine this difference in the level of acquisition between sensory modalities, and lack of savings when the CS modality is changed in our preparation.
Using WCS, we found that the Adult and Middle Aged mice were able to learn the trace association but Old mice were not. These findings do not indicate if this age-related learning deficit is due to a forebrain abnormality or reflects an inability to form associative memories due to brainstem-cerebellar deficits. C57 mice exhibit age-dependent loss in the number of Purkinje cells in the cerebellum (Woodruff-Pak, 2006), a brain region critical for acquisition of eyeblink conditioning (Christian and Thompson, 2003; Woodruff-Pak and Disterhoft, 2008). To determine if the observed age related trace learning impairments in Old mice were due to an inability to form eyeblink associations per se, the Old mice were then trained on a WCS-delay paradigm. Analysis of their performance revealed a significant improvement across training sessions, showing that their inability to learn the forebrain-dependent whisker trace association was not due to a complete inability to form eyeblink associations. It is interesting to note that Adult mice did perform significantly better than Old mice on the last training session of WCS-delay conditioning. This behavioral difference could be due to age-related Purkinje cell death in Old C57 mice (Woodruff-Pak, 2006) or savings from prior learned associations in Adult mice (Harlow, 1949; Kehoe, 1988; Campolattaro and Freeman, 2009). Future studies will need to determine if failure of Old mice to learn the WCS-trace association was due to age-related changes in the neocortex (Baskys et al., 1990; McGlinchey-Berroth et al., 1997; Skibinska et al., 2000; Ohmura et al., 2003; Yoshimura et al., 2003; Luebke et al., 2004), hippocampus (Landfield and Pitler, 1984; Moyer et al., 1992; Oh et al., 1999; Moyer et al., 2000), or most probably, due to a combination of age-related changes in the hippocampus, neocortex, other forebrain regions, as well as cerebellum.
Analysis of the metabolic neocortical barrel representation in Adult and Middle Aged mice that learned the WCS-trace association revealed a learning-induced expansion for the conditioned barrels; consistent with WCS trace conditioned adult rabbits (Galvez et al., 2006) and with adult rats that learn to associate whisker stimulation with a foot shock (Siucinska and Kossut, 1996) or water reward (Siucinska and Kossut, 2004.) Although both barrel width (across rows) and length (within a row) were examined, the observed expansion in mice only reached significance in the length analysis. Prior analyses of whisker trace conditioning induced neocortical barrel expansion in adult rabbits demonstrated an increase in both conditioned barrel width and length (Galvez et al., 2006) while rearing rats in a naturalistic environment induced an expansion of barrel width (across rows) and septa (the region between barrels) (Machin et al., 2004; Polley et al., 2004). Although it is not difficult to hypothesize that rearing in a naturalistic environment could result in a different neocortical barrel expansion due to differing memory and activity demands, it is not clear why acquisition of a trace associative task would result in a slightly different neocortical expansion in different species. Analysis of neuronal projections in mice within the somatosensory barrel neocortex have demonstrated that barrels within a row are more interconnected than barrels between rows (Bernardo et al., 1990; Brecht et al., 2003), suggesting that neuronal communication within a row would be dramatically different than across rows. Such an analysis has not been conducted in rabbits, making it difficult to determine the cause for this conditioning induced difference in neocortical barrel expansion. It is important to note that both species exhibited a conditioning-induced expansion of neocortical barrels that was specific to the barrels that processed the CS; the difference between the species only resides in the details of how the barrels expand (length and width vs. only length).
Unlike Adult and Middle Aged mice, Old mice did not exhibit a conditioning induced expansion for the conditioned barrels. The Old mice were unable to learn the WCS-trace association, but did learn the WCS-delay association. In addition, there were no differences in the metabolic data sets for the subgroups of Adult mice that learned only WCS trace-conditioning as compared to the mice that learned both WCS trace- and delay-conditioning. These observations suggest that acquisition of a forebrain-independent association (delay-conditioning) is not sufficient for induction of neocortical metabolic barrel expansion. However, given that WCS-delay conditioned Adult mice performed significantly better than Old mice on the last day of training, induction of forebrain-independent neocortical plasticity may require a certain level of proficiency not reached by the Old mice. Furthermore, forebrain-independent training has been shown to induce other forms of neocortical plasticity. For example, neocortical receptive field size for the preferred frequency in primary auditory cortex is altered with forebrain-independent frequency discrimination training (Disterhoft and Stuart, 1976; Kitzes et al., 1978; Diamond and Weinberger, 1986, 1989; Edeline et al., 1993; Recanzone et al., 1993; Chowdhury and Suga, 2000) and auditory fear conditioning (Ji and Suga, 2003). Furthermore, training that preferentially favors increased sensitivity to a specific sensory modality for an extended period of time not only results in enhanced sensitivity for that specific sensory modality, but also induces behaviorally relevant alterations in cortical primary map representations (Jenkins et al., 1990; Recanzone et al., 1990; Recanzone et al., 1992a; Recanzone et al., 1992b; Recanzone et al., 1993; Nudo et al., 1996; Xerri et al., 1996). These studies suggest that some forebrain-independent conditioning tasks induce neocortical plasticity with acquisition. Finally, we should note that whisker pairing studies, a paradigm where all the whiskers are removed except two, have been shown to modulate neocortical barrel receptive field size (Diamond et al., 1993; Armstrong-James et al., 1994; Diamond et al., 1994). However, these studies do not typically control for the amount or timing between stimulation of the two whiskers, making any direct comparisons between these findings and the conditioning studies difficult.
Prior studies have demonstrated that lesions of primary somatosensory barrel cortex impair both acquisition and retention of WCS-trace-eyeblink associations (Galvez et al., 2006). Further analyses have demonstrated that WCS-trace-eyeblink conditioning also results in an expansion of the metabolic neocortical representation for the conditioned whiskers (Galvez et al., 2007). These analyses suggest that primary somatosensory neocortex is one site of storage for trace associations. We should note that an alternative hypothesis could be that layer IV of the barrel cortex is acting as a site of enhanced processing that facilitates memory storage in some other brain region such as higher neocortical regions or deep cerebellar nuclei. However, the fact that barrel cortical lesions impair performance for already learned trace associations (Galvez et al., 2007), along with findings from the current study demonstrating no change in the metabolic neocortical representation of Old mice that only learned the delay association, argue against this hypothesis and in favor of primary somatosensory barrel cortex acting as a site of storage for the trace association.
Prior studies have also demonstrated that acquisition of forebrain dependent associative tasks, like trace-eyeblink conditioning are dependent upon the age of the subject. The older the subject the more difficult it is to acquire trace associations (Graves and Solomon, 1985; Finkbiner and Woodruff-Pak, 1991; Kishimoto et al., 2001; Knuttinen et al., 2001b; Knuttinen et al., 2001a; Villarreal et al., 2004; Woodruff-Pak et al., 2007). We have expanded upon these findings and demonstrated that not only is whisker stimulation ideally suited for examination of age-related behavioral changes in C57 mice, but that conditioning induced neocortical plasticity is dependent upon acquisition of the trace association and related to the age of the subject. Further analyses will be needed to determine if age-related abnormalities in neocortical plasticity alone hindered acquisition of the trace association in the Old mice, or if the age-related abnormalities in learning were dependent upon changes in other brain regions such as the hippocampus, that in turn depressed the neocortical plasticity.
Supplementary Material
5. Acknowledgements
This work was supported by Mechanisms of Aging and Dementia training grant T32 AG20506, and R37 AG008796.
Footnotes
6. Disclosure Statement
Publishing these findings impose no actual or potential conflicts of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Armstrong-James M, Diamond ME, Ebner FF. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Baskys A, Reynolds JN, Carlen PL. NMDA depolarizations and long-term potentiation are reduced in the aged rat neocortex. Brain Res. 1990;530:142–146. doi: 10.1016/0006-8993(90)90671-w. [DOI] [PubMed] [Google Scholar]
- Bernardo KL, McCasland JS, Woolsey TA, Strominger RN. Local intra- and interlaminar connections in mouse barrel cortex. J Comp Neurol. 1990;291:231–255. doi: 10.1002/cne.902910207. [DOI] [PubMed] [Google Scholar]
- Brecht M, Roth A, Sakmann B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. The Journal of physiology. 2003;553:243–265. doi: 10.1113/jphysiol.2003.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Cerebellar inactivation impairs cross modal savings of eyeblink conditioning. Behav Neurosci. 2009;123:292–302. doi: 10.1037/a0014483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SA, Suga N. Reorganization of the frequency map of the auditory cortex evoked by cortical electrical stimulation in the big brown bat. J Neurophysiol. 2000;83:1856–1863. doi: 10.1152/jn.2000.83.4.1856. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–360. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Role of context in the expression of learning-induced plasticity of single neurons in auditory cortex. Behav Neurosci. 1989;103:471–494. doi: 10.1037//0735-7044.103.3.471. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci U S A. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Stuart DK. Trial sequence of changed unit activity in auditory system of alert rat during conditioned response acquisition and extinction. J Neurophysiol. 1976;39:266–281. doi: 10.1152/jn.1976.39.2.266. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus--what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison D. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hearing Research. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging. 1991;6:109–117. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- Galvez R, Weible AP, Disterhoft JF. Cortical barrel lesions impair whisker-CS trace eyeblink conditioning. Learn Mem. 2007;14:94–100. doi: 10.1101/lm.418407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF. Vibrissa-signaled eyeblink conditioning induces somatosensory cortical plasticity. J Neurosci. 2006;26:6062–6068. doi: 10.1523/JNEUROSCI.5582-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Cua S, Disterhoft J. A novel method for precisely timed stimulation of mouse whiskers in a freely moving preparation: Application for delivery of the conditioned stimulus in trace eyeblink conditioning. J Neurosci Methods. 2008;177:434–439. doi: 10.1016/j.jneumeth.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Haerter K, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Thilmann AF, Gizewski ER, Timmann D. Trace eyeblink conditioning in human subjects with cerebellar lesions. Exp Brain Res. 2005:1–15. doi: 10.1007/s00221-005-0171-2. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Graves CA, Solomon PR. Age-related disruption of trace but not delay classical conditioning of the rabbit’s nictitating membrane response. Behav Neurosci. 1985;99:88–96. doi: 10.1037//0735-7044.99.1.88. [DOI] [PubMed] [Google Scholar]
- Gruart A, Guillazo-Blanch G, Fernandez-Mas R, Jimenez-Diaz L, Delgado-Garcia JM. Cerebellar posterior interpositus nucleus as an enhancer of classically conditioned eyelid responses in alert cats. J Neurophysiol. 2000;84:2680–2690. doi: 10.1152/jn.2000.84.5.2680. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychol Rev. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Wright EA. The growth of rats and mice vibrissae under normal and some abnormal conditions. Journal of embryology and experimental morphology. 1975;33:831–844. [PubMed] [Google Scholar]
- Ibrahim L, Wright EA. Effect of castration and ttestosterone propionate on mouse vibrissae. Journal of embryology and experimental morphology. 1983;108:321–326. doi: 10.1111/j.1365-2133.1983.tb03971.x. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. Journal of neurophysiology. 2003;90:1904–1909. doi: 10.1152/jn.00363.2003. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A Major Gene Affecting Age-Related Hearing Loss Is Common to at Least Ten Inbred Strains of Mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ. A layered network model of associative learning: learning to learn and configuration. Psychol Rev. 1988;95:411–433. doi: 10.1037/0033-295x.95.4.411. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001;12:3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Farley GR, Starr A. Modulation of auditory cortex unit activity during the performance of a conditioned response. Exp Neurol. 1978;62:678–697. doi: 10.1016/0014-4886(78)90277-7. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Power JM, Preston AR, Disterhoft JF. Awareness in classical differential eyeblink conditioning in young and aging humans. Behav Neurosci. 2001a;115:747–757. doi: 10.1037//0735-7044.115.4.747. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects on eyeblink conditioning in the F344 x BN F1 hybrid rat. Neurobiol Aging. 2001b;22:1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the Caudal Area of Rabbit Medial Prefrontal Cortex Impair Trace Eyeblink Conditioning. NEUROBIOLOGY OF LEARNING AND MEMORY. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+−dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Machin R, Blasco B, Bjugn R, Avendano C. The size of the whisker barrel field in adult rats: minimal nondirectional asymmetry and limited modifiability by chronic changes of the sensory input. Brain Res. 2004;1025:130–138. doi: 10.1016/j.brainres.2004.07.077. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Carrillo MC, Gabrieli JD, Brawn CM, Disterhoft JF. Impaired trace eyeblink conditioning in bilateral, medial-temporal lobe amnesia. Behav Neurosci. 1997;111:873–882. doi: 10.1037//0735-7044.111.5.873. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial Prefrontal Cortex and Pavlovian Conditioning: Trace Versus Delay Conditioning. Behavioral Neuroscience. 2002;116:37–47. [PubMed] [Google Scholar]
- Moyer JR, Jr., Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr., Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany P, Lynch MA. Changes in protein synthesis and synthesis of the synaptic vesicle protein, synaptophysin, in entorhinal cortex following induction of long-term potentiation in dentate gyrus: an age-related study in the rat. Neuropharmacology. 1997;36:973–980. doi: 10.1016/s0028-3908(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Power JM, Thompson LT, Moriearty PL, Disterhoft JF. Metrifonate increases neuronal excitability in CA1 pyramidal neurons from both young and aging rabbit hippocampus. J Neurosci. 1999;19:1814–1823. doi: 10.1523/JNEUROSCI.19-05-01814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura T, Ming R, Yoshimura Y, Komatsu Y. Age and experience dependence of N-methyl-D-aspartate receptor-independent long-term potentiation in rat visual cortex. Neurosci Lett. 2003;341:95–98. doi: 10.1016/s0304-3940(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Pakaprot N, Kim S, Thompson RF. The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav Neurosci. 2009;123:54–61. doi: 10.1037/a0014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham K, Willott JF. Acoustic Startle Response in Young and Aging C57BL/6J and CBA/J Mice. Behavioral Neuroscience. 1988;102:881–886. doi: 10.1037//0735-7044.102.6.881. [DOI] [PubMed] [Google Scholar]
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Dinse HR. Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical microstimulation. Cereb Cortex. 1992a;2:181–196. doi: 10.1093/cercor/2.3.181. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Allard TT, Jenkins WM, Merzenich MM. Receptive-field changes induced by peripheral nerve stimulation in SI of adult cats. J Neurophysiol. 1990;63:1213–1225. doi: 10.1152/jn.1990.63.5.1213. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992b;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex--a 2DG study. Cereb Cortex. 1996;6:506–513. doi: 10.1093/cercor/6.3.506. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Experience-dependent changes in cortical whisker representation in the adult mouse: a 2-deoxyglucose study. Neuroscience. 2004;127:961–971. doi: 10.1016/j.neuroscience.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Skibinska A, Glazewski S, Fox K, Kossut M. Age-dependent response of the mouse barrel cortex to sensory deprivation: a 2-deoxyglucose study. Exp Brain Res. 2000;132:134–138. doi: 10.1007/s002210000341. [DOI] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Medial temporal lobe activity during retrieval of semantic memory is related to the age of the memory. J Neurosci. 2009;29:930–938. doi: 10.1523/JNEUROSCI.4545-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Strominger RN, Woolsey TA. Templates for locating the whisker area in fresh flattened mouse and rat cortex. J Neurosci Methods. 1987;22:113–118. doi: 10.1016/0165-0270(87)90004-5. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322:960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Takatsuki K, Kirino Y. Time-limited role of the hippocampus in the memory for trace eyeblink conditioning in mice. Brain Res. 2002;951:183–190. doi: 10.1016/s0006-8993(02)03159-1. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Villarreal JS, Dykes JR, Barea-Rodriguez EJ. Fischer 344 rats display age-related memory deficits in trace fear conditioning. Behav Neurosci. 2004;118:1166–1175. doi: 10.1037/0735-7044.118.6.1166. [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. J Neurophysiol. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft J. Evoking blinks with natural stimulation and detecting then with a noninvasive optical device: a simple, inexpensive method for use with freely moving animals. J neurosci methods. 2008;173:108–113. doi: 10.1016/j.jneumeth.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Stereological estimation of Purkinje neuron number in C57BL/6 mice and its relation to associative learning. Neuroscience. 2006;141:233–243. doi: 10.1016/j.neuroscience.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends in neurosciences. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Seta SE, Roker LA, Lehr MA. Effects of paradigm and inter-stimulus interval on age differences in eyeblink classical conditioning in rabbits. Learn Mem. 2007;14:287–294. doi: 10.1101/lm.504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CD. Changes in activity and excitability of cortical auditory receptive units of the cat as a function of different behavioral states. Ann N Y Acad Sci. 1977;290:180–199. doi: 10.1111/j.1749-6632.1977.tb39726.x. [DOI] [PubMed] [Google Scholar]
- Woody CD, Knispel JD, Crow TJ, Black-Cleworth PA. Activity and excitability to electrical current of cortical auditory receptive neurons of awake cats as affected by stimulus association. J Neurophysiol. 1976;39:1045–1061. doi: 10.1152/jn.1976.39.5.1045. [DOI] [PubMed] [Google Scholar]
- Xerri C, Coq JO, Merzenich MM, Jenkins WM. Experience-induced plasticity of cutaneous maps in the primary somatosensory cortex of adult monkeys and rats. J Physiol Paris. 1996;90:277–287. doi: 10.1016/s0928-4257(97)81438-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Research. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


