Abstract
The perceptual differentiation of odors can be measured behaviorally using generalization gradients. The steepness of these gradients defines a form of olfactory acuity for odor quality that depends on neural circuitry within the olfactory bulb and is regulated by cholinergic activity therein as well as by associative learning. Using this system as a reduced model for age-related cognitive decline, we show that aged mice, while maintaining almost the same baseline behavioral performance as younger mice, are insensitive to the effects of acutely elevated acetylcholine, which sharpens generalization gradients in young adult mice. Moreover, older mice exhibit evidence of chronically elevated acetylcholine levels in the olfactory bulb, suggesting that their insensitivity to further elevated levels of acetylcholine may arise because the maximum capacity of the system to respond to acetylcholine has already been reached. We propose a model in which an underlying, age-related, progressive deficit is mitigated by a compensatory cholinergic feedback loop that acts to retard the behavioral effects of what would otherwise be a substantial age-related decline in olfactory plasticity.
We also treated mice with ten-day regimens of olfactory environmental enrichment and/or repeated systemic injections of the acetylcholinesterase inhibitor physostigmine. Each treatment alone sharpened odor quality acuity, but administering both treatments together had no greater effect than either alone. Age was not a significant main effect in this study, suggesting that some capacity for acetylcholine-dependent plasticity is still present in aged mice despite their sharply reduced ability to respond to acute increases in acetylcholine levels.
These results suggest a dynamical framework for understanding age-related decline in neural circuit processing in which the direct effects of aging can be mitigated, at least temporarily, by systemic compensatory responses. In particular, a decline in cholinergic efficacy can precede any breakdown in cholinergic production, which may help explain the limited effectiveness of cholinergic replacement therapies in combating cognitive decline.
Keywords: Acetylcholine, age-related cognitive decline, olfactory bulb, cholinergic hypothesis, Alzheimer’s disease, basal forebrain, palliative response
1. Introduction
The olfactory bulb (OB) is a cortical structure that directly receives afferent input from primary olfactory sensory neurons. It is the final common path for olfactory afferent signals before they diverge to multiple cortical and subcortical targets, and it receives copious projections from several regions of the brain that regulate its processing of olfactory sensory information. The OB performs several computational tasks common to early sensory processing across modalities; in particular, it is thought to play a major role in odor stimulus decorrelation, a process that defines the early delineation and categorization of odors based upon stimulus features and learned contingencies (Cleland et al., 2007; Cleland and Sethupathy, 2006). This process is measured behaviorally using generalization gradients (Shepard, 1987), in which experience with one odor is generalized to a range of novel but perceptually similar odors (Cleland et al., 2002; Cleland and Narla, 2003). This gradient is steepened by increased learning (Cleland et al., 2009) and by cholinergic neuromodulation within the OB (Chaudhury et al., 2009; Mandairon et al., 2006a). Owing to the relatively well-described circuitry of the OB, the cellular and circuit mechanisms of this cortical network can be related to behavioral performance via computational modeling (Cleland et al., 2007; Mandairon et al., 2006a). As a reduced model system for integrated cortical function, the OB enables study of learning mechanisms and the convergence of bottom-up and top-down factors on sensory representations across levels of organization, from cellular physiology to functional networks to behavior.
Normal aging results in correspondingly increased rates of self-reported chronic olfactory dysfunction in humans (Hoffman et al., 1998). In particular, olfactory identification deficits, in which odors become more difficult to differentiate from one another, increase with age and are predictive of broader cognitive and learning impairments (Wilson et al., 2007) including clinical dementias (Serby et al., 1991). In rodents, aging induces deficits in olfactory perception (Nakayasu et al., 2000), including odor discrimination (Enwere et al., 2004; Prediger et al., 2005; Prediger et al., 2006) and impairments in olfactory learning and memory (Guan and Dluzen, 1994; Prediger et al., 2005; Rosenzweig and Bennett, 1996; Schoenbaum et al., 2002; Terranova et al., 1994). Notably, two mouse models of human dementias also exhibit odor habituation deficits and/or a broadening of olfactory generalization gradients, both characteristic of impaired olfactory learning (Bath et al., 2008; Guerin et al., 2007).
The olfactory system receives cholinergic input from the basal forebrain, specifically the horizontal limb of the diagonal band of Broca (Shipley and Ennis, 1996; Wenk et al., 1977). Cholinergic activity in the olfactory system influences a range of behavioral tasks reflecting learning, memory, and the regulation of perceptual differentiation, including habituation (Hunter and Murray, 1989; Mandairon et al., 2006a), short-term memory (Ravel et al., 1994; Roman et al., 1993), perceptual learning (Fletcher and Wilson, 2002), proactive interference (De Rosa and Hasselmo, 2000; De Rosa et al., 2001) and behavioral generalization (Linster and Cleland, 2002; Linster et al., 2001). Cholinergic dysfunction is widely implicated in degenerative dementias, most prominently Alzheimer’s dementia but including normal age-related cognitive decline. However, the nature of this dysfunction is unclear, as cholinergic replacement therapies have yielded somewhat disappointing results.
Environmental enrichment alters physiological responses, enhances learning and neural plasticity, and influences cholinergic neuromodulatory systems (Del Arco et al., 2007a; Del Arco et al., 2007b; Rosenzweig and Bennett, 1996; van Praag et al., 2000). In the olfactory analogue of environmental enrichment (see Methods), odor response patterns in the OB can be modified by odor exposure in both juvenile (Woo et al., 2007) and adult rodents (Buonviso and Chaput, 2000; Spors and Grinvald, 2002). At a behavioral level, olfactory enrichment can improve short-term odor memory (Rochefort et al., 2002) and improve olfactory discrimination capacities (Escanilla et al., 2008; Mandairon et al., 2006b; Mandairon et al., 2006c). Moreover, olfactory enrichment selectively affects the perception of odorants which activate at least partially overlapping regions of the OB, and manipulation of the OB network suffices to produce long-lasting perceptual changes (Mandairon et al., 2006b). While general environmental enrichment can delay or mitigate cognitive decline (Berardi et al., 2007; O’Callaghan et al., 2009), it is not yet known whether this olfactory analogue of enrichment will have a similar effect upon olfactory learning.
We sought to test whether aged wildtype mice would exhibit a broadening of associative olfactory generalization gradients (an olfactory correlate of reduced learning, hence a model for age-related cognitive decline) in comparison to young adult mice, and whether such deficits could be mitigated by acute cholinergic potentiation via acetylcholinesterase inhibition. We then assessed the capacities of two 10-day treatment regimens, olfactory enrichment and/or daily administration of an acetylcholinesterase inhibitor, to persistently sharpen generalization gradients (i.e., at least 24 hours after the final treatment) in both aged and young animals. Finally, we measured the levels of acetylcholinesterase present in the OB to assess the chronic level of acetylcholine release in that structure. We found that age sharply reduces the capacity of mice to respond to acetylcholine. Chronic treatments of enrichment or cholinergic potentiation, however, were still somewhat effective, indicating that the aged system retained some capacity for plasticity on a slower timescale. Aged mice exhibited substantially higher levels of acetylcholinesterase in the OB than did younger mice, suggesting that they maintain chronically elevated rates of acetylcholine release therein. Notably, the effect of age alone on generalization gradients in these studies was weak; specifically, it was not significant in the two behavioral experiments described above, though it was shown to be significant in an third, larger study. We suggest that aged mice are able to retain near-normal behavioral capacities in response to progressive underlying degeneration by employing a compensatory strategy of chronically elevating the level of acetylcholine released into the OB.
2. Methods
2.1 Subjects
Three cohorts of male CD-1 mice (outbred strain; Charles River Laboratories, Wilmington, MA) served as subjects in these studies. The first cohort consisted of 16 mice – 8 young adult (5 months) and 8 aged (19 months) – and was used to investigate the effect of the acute potentiation of the cholinergic system on olfactory generalization in young and old mice (Experiment 1). A second cohort, consisting of 64 mice, including 32 young adult (2–5 months) and 32 aged (11–14 months) animals, was used to investigate the persistent effects of olfactory enrichment and/or a 10-day regimen of acetylcholinesterase inhibitors on odor quality acuity (Experiment 2). This second cohort was also subsequently used in a forced-choice discrimination control study and for AChE histology. Finally, a third cohort, consisting of 230 mice, 77 young adult (2–4 months), 73 middle-aged (7–9 months), and 80 aged (14–16 months), was used to test whether an age-related decline in behavioral performance per se could be measured behaviorally given enough statistical power (Experiment 3). All mice were group-housed (2–4 per cage) and maintained on a reversed 12:12 light cycle in an environmentally controlled room (dark period: 9:00 – 21:00 daily). All behavioral training was conducted during the afternoon (14:00 – 18:00), during the mice’s dark cycle. Water was continuously available; mice were food-deprived for 18 hours preceding each session to motivate them to obtain sucrose rewards. Mice were fed immediately following an experimental session, and were not deprived of food on two subsequent days. All procedures were performed under the auspices of a protocol approved by the Cornell University Institutional Animal Care and Use Committee in accordance with NIH guidelines.
2.2 Odor sets
Three to five odor sets were used in each experiment to enable counterbalancing among subjects and ensure that results were not specific to any given odor set (Cleland and Narla, 2003). Each odor set comprised three or four odorants: a conditioned stimulus odorant CS, one or two structurally and perceptually similar odorants S1 and S2, chosen so that S1 was more similar to the CS than was S2, and a structurally and perceptually dissimilar odorant D used as a control (Table 1). In Experiment 2, the S2 odorants were omitted. Homologous series of structurally similar aliphatic odorants such as the CS, S1, and S2 odorants in each set have been demonstrated to be perceptually similar to one another in proportion to their structural similarity (Cleland et al., 2002; Cleland and Narla, 2003). All odorants were differentially diluted in mineral oil so as to achieve similar vapor-phase partial pressures (sometimes referred to herein as vapor-phase odor concentrations). Specifically, vapor pressures of pure odorants were estimated with the Hass-Newton equation as implemented in ACD/Boiling Point & Vapor Pressure Calculator (version 4.5; Advanced Chemistry Development, Toronto, Ontario, Canada); pure stock odorants were correspondingly diluted in mineral oil to concentrations theoretically emitting vapor-phase partial pressures of 0.01 Pa (Cleland et al., 2002). The corresponding vol/vol liquid dilutions in mineral oil are listed in Table 1. Odorants were diluted several hours in advance of each experiment and agitated in order to ensure an even distribution of odorant within the mineral oil solvent.
Table 1.
Odor sets used for associative generalization task, with corresponding vol/vol dilutions in mineral oil to achieve vapor-phase partial pressures of 0.01 Pa.
| Odor Sets | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Odors | |||||
| C | hexyl acetate 22.7 × 10−6 | propanoic acid 0.3 × 10−5 | octanal 14.7 × 10−6 | butyl butyrate 1.7 × 10−5 | octanoic acid 13.7 × 10−4 |
| S1 | amyl acetate 7.2 × 10−6 | butanoic acid 1.3 × 10−5 | heptanal 7.1 × 10−6 | butyl pentanoate 5.7 × 10−5 | heptanoic acid 4.6 × 10−4 |
| S2 | butyl acetate 2.2 × 10−6 | pentanoic acid 4.5 × 10−5 | hexanal 2.2 × 10−6 | butyl hexanoate 16.3 × 10−5 | hexanoic acid 1.5 × 10−4 |
| D | anisole 5.2 × 10−6 | 5-methylfurfural 3.0 × 10−5 | 3-heptanone 6.5 × 10−6 | citronellal 16.6 × 10−5 | neryl acetate 16.4 × 10−4 |
2.3 Apparatus
All behavioral training took place in a modified mouse cage (28×17×12 cm) divided into two chambers of equal size (A and B) by a sliding, opaque Plexiglas board. Glass petri dishes (Pyrex, 60 mm diameter, 15 mm height) were used for placement of odorants and rewards. At the beginning of each training session, separate dishes were prepared for the conditioning trials using the conditioning odorant (CS) and for each of the double-blinded test odorants (the conditioning odorant CS itself, the structurally and perceptually similar odorants S1 and S2, and a structurally and perceptually dissimilar odorant D; Table 1). Each dish was filled with ~10 ml of white play sand (YardRight; Easton, PA) and inoculated with 100 μl of the appropriate diluted odorant. During conditioning trials (but not test trials), a 5 mg sucrose pellet reward (Noyes Precision Pellets; Research Diets, Inc., New Brunswick, NJ), was buried in the sand of a dish scented with the conditioning odorant CS. The sand and the odorant in each dish were replaced after every trial.
2.4 Shaping
Mice were first shaped by being taught to retrieve a reward by digging in dishes of scented sand. Mice were placed in chamber A of the modified cage apparatus, with the divider between the two chambers closed. Two sand-filled dishes were then placed in chamber B: one containing a reward and scented with the conditioning odorant CS, the other containing no reward and no odorant. Each trial began when the divider was removed, at which point the mouse entered chamber B and was allowed to dig in both dishes until it retrieved the reward (i.e., self-correction was permitted). The mouse was then returned to chamber A for the one-minute intertrial interval, during which the dishes were replaced for the next trial. To speed learning, the reward was placed on top of the sand in the scented dish during the first few trials; after the mouse reliably retrieved the reward, the reward was buried more deeply in the sand. Dishes were moved around randomly within chamber B on subsequent trials such that odor was the only reliable predictor of which dish contained a buried reward. Shaping was considered complete when a mouse would (1) reliably identify the reward-containing dish and retrieve deeply buried rewards, (2) dig in the odor-containing dish even in the absence of a reward (thus controlling for the possibility of mice directly detecting the reward), and (3) show no interest after training in digging in odors scented with dissimilar odorants, indicating that their reward associations were odor-specific. All shaping was performed using diluted odorants perceptually dissimilar to those used in the experiments.
2.5 Behavioral testing: generalization (Experiments 1–3)
We used an odor generalization paradigm to measure the degree to which mice generalized between test odorants (Cleland et al., 2002; Cleland and Narla, 2003; Cleland et al., 2009; Linster and Hasselmo, 1999). The conditioning phase followed the procedure described in the Shaping section above. Each mouse was conditioned over six conditioning trials in which it had a choice between a dish scented with the odorant CS and containing a buried reward and an unscented dish containing no reward. Subsequently, four unrewarded test trials (in which the mouse was offered a choice between a dish scented with the test odorant and an unscented dish) were conducted in a pseudorandom order; these test trials were separated by two rewarded conditioning trials to prevent extinction of the association between the odorant CS and the reward. (In Experiment 2, three unrewarded test trials were conducted). During each test trial, the total digging time in the scented dish was recorded with a stopwatch and served as the dependent variable. The duration of test trials was 1 minute, whereas conditioning trials ended after mice recovered the sucrose reward (up to a maximum of 1 minute). Intertrial intervals were 1 minute long, and test trials began directly after the completion of the conditioning trials. Each odorant was tested once per mouse, and mice were assigned to different experimental groups in subsequent odor sets according to a pseudorandomized, counterbalanced schedule (with the exception of age groups). The experimenter was blind to the identity of the test odorants during performance of these experiments.
2.6 Acute physostigmine injection (Experiment 1)
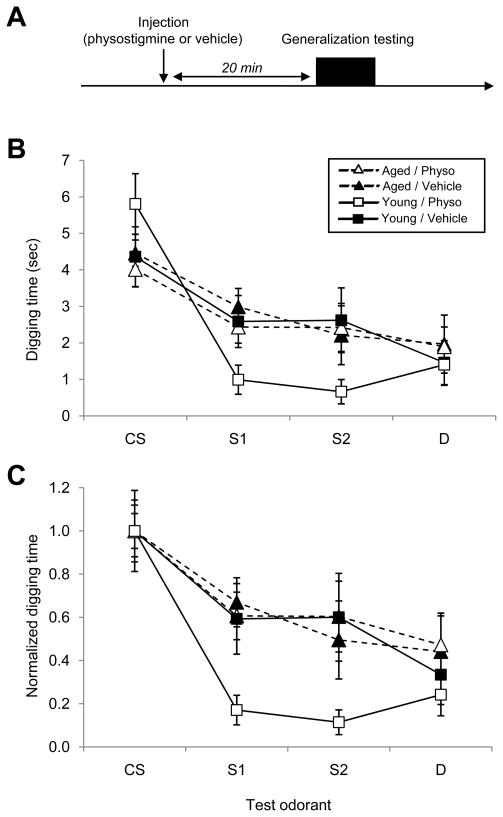
A cohort of mice was used in a 2×2 matrix paradigm to test the effect of acute physostigmine administration on odor generalization in young adult and aged mice (Figure 1A). Physostigmine is an inhibitor of acetylcholinesterase (AChE), the enzyme that degrades secreted acetylcholine; hence, physostigmine serves to potentiate endogenous cholinergic effects by impairing the degradation of normally released acetylcholine. All mice were trained and tested 20 min after intraperitoneal injection of either physostigmine (0.05 mg/kg; (Doty et al., 1999)) or 0.9% physiological saline vehicle (control) to evaluate the effects and interaction of age and acute cholinergic potentiation on olfactory generalization gradients.
Figure 1.
Acute injections of the acetylcholinesterase inhibitory physostigmine sharpen olfactory generalization gradients in young adult but not aged mice. A. Protocol. Either 0.05 mg/kg physostigmine or a like volume of plain 0.9% saline vehicle were injected intraperitoneally 20 minutes before the start of a ~20 minute training/testing regimen measuring odor generalization. Specifically, when trained to associate a conditioned odorant stimulus (CS) with reward, mice will dig in a dish scented with that CS odor as well as in dishes scented with structurally and perceptually similar odorants (S1, S2). In the training protocol used herein, broader generalization gradients are associated with impaired olfactory learning. Each mouse underwent the training and testing series no more than once per day. B. Generalization gradients measured in young adult (5 months old) and aged (19 months old) mice, after either physostigmine or vehicle injection. The injection of physostigmine significantly sharpened the generalization gradients of young mice, whereas it had no significant effect on the gradients of aged mice (see Results). C. Data from B normalized so that the mean response to the CS in each age group is unity. Error bars denote the standard error of the mean.
2.7 Olfactory enrichment and repeated cholinergic potentiation regimens (Experiment 2)
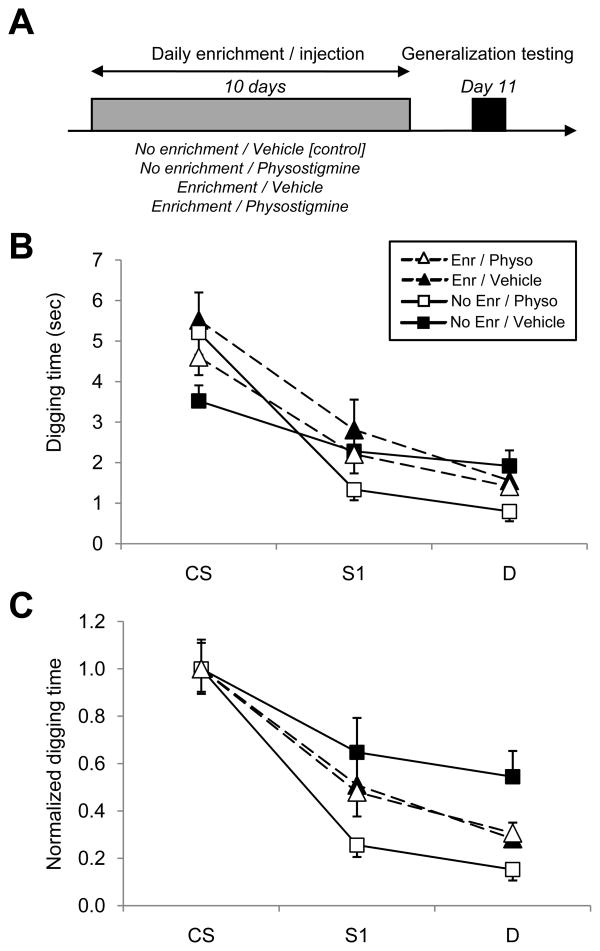
Two new cohorts of mice, one young adult (2–5 months) and one aged (11–14 months), were divided into four groups each: non-enriched/vehicle (controls), non-enriched/physostigmine, enriched/vehicle, and enriched/physostigmine (n=8 per group per age, total n=64; Table 2). The enrichment process consisted of housing animals in an odor-enriched environment for one hour daily over 10 days (Figure 2A). Specifically, two swabs, each containing 100 μl of a subtly different pure odorant ((−)-limonene and (+)-limonene), were placed inside two tea balls which were hung inside the home cage for a one-hour period each day. These odorants were selected because they are highly perceptually similar yet distinguishable by rodents (Linster et al., 2002), and this procedure is known to increase the behavioral differentiation of similar odorants (Mandairon et al., 2006c). Control mice were treated identically except that the two tea balls were left empty. The administration of physostigmine followed the same time schedule as olfactory enrichment; specifically, each mouse received an intraperitoneal injection of either physostigmine (0.05 mg/kg; Sigma) or 0.9% physiological saline vehicle (control) 20 minutes before the daily enrichment or faux enrichment period began. After 10 days of enrichment and/or drug treatment, mice underwent behavioral testing to measure their gradients of generalization among series of similar odorants. Neither enrichment nor drug treatments were administered on the day of training and testing (day 11).
Table 2.
Enrichment odors and drug regimens for the 8 experimental groups of Experiment 2.
| Group number | Enrichment odors | Drug regimen | Age (months) |
|---|---|---|---|
| 1 | (+)-limonene, (−)-limonene | Saline vehicle | 2–5 |
| 2 | (+)-limonene, (−)-limonene | Physostigmine | 2–5 |
| 3 | No odor | Saline vehicle | 2–5 |
| 4 | No odor | Physostigmine | 2–5 |
| 5 | (+)-limonene, (−)-limonene | Saline vehicle | 11–14 |
| 6 | (+)-limonene, (−)-limonene | Physostigmine | 11–14 |
| 7 | No odor | Saline vehicle | 11–14 |
| 8 | No odor | Physostigmine | 11–14 |
Figure 2.
Ten-day regimens of either daily odor enrichment (see Methods) or daily physostigmine injections sharpen generalization gradients 24 hours after the discontinuation of treatment. A. Protocol. Four groups of mice were studied in a 2×2 experimental design. The first group was injected with 0.05 mg/kg physostigmine and exposed to enriching odor stimuli for an hour each day. The second group was injected with a like volume of saline vehicle but received the same enriching odor treatment. The third group was not given odor enrichment, but was injected with physostigmine daily, whereas the fourth group was not enriched and was injected only with vehicle. On the eleventh day, on which no enrichment or injections were performed, mice were trained and tested normally and their olfactory generalization gradients measured. B. Generalization gradients measured in the presence and absence of enrichment or physostigmine regimens. Both the drug treatment and the olfactory enrichment regimens significantly sharpened odor generalization gradients; however, mice given both physostigmine and enrichment did not exhibit gradients sharper than those receiving only one of these treatments (see Results). C. Data from B normalized so that the mean response to the CS in each age group is unity. Error bars denote the standard error of the mean.
2.8 Behavioral testing: forced-choice discrimination
Forced-choice simultaneous discrimination tasks differ from generalization tasks in that they reward animals for successfully distinguishing between two stimuli presented together (Cleland et al., 2002; Linster et al., 2002). We used this protocol to rule out potential confounding effects of age, specifically ensuring that aged mice were still as capable of detecting and selecting rewarded odors at 0.01 Pa in the digging task as were the younger mice. Each training set comprised 20 consecutive discrimination trials using the same odorant paired with an unscented dish. Behavioral training took place in the same divided chamber used for generalization studies. Mice were placed in chamber A with the divider closed. Each trial began when the divider was removed, enabling the mouse to enter chamber B which contained two sand-filled dishes, one of which was scented with the CS odorant and contained a sucrose reward and the other of which was unscented and contained no reward. Mice were permitted to dig in only one of the two dishes and to retrieve only one of the two rewards each trial; i.e., self-correction was not permitted. The spatial locations of the dishes within chamber B were varied randomly among trials. The dish in which a mouse dug first in each trial served as the dependent variable. Trials were terminated after 1 min and scored as failures if the mouse did not dig at all. To control for the possibility of mice smelling the sucrose reward directly rather than by learning the association with the odorant, every fifth trial the reward was not placed in the dish; during these trials, after the mouse registered a preference by digging in the scented pot, the reward was dropped onto the scented bedding to maintain the association of the odorant with the reward. As only 20 trials were performed in each discrimination task, these data strongly reflect learning rates (as well as any steady-state error probabilities). This experiment was performed using the mice from Experiment 2, and was repeated five times such that each mouse was tested with each odorant CS from Table 1.
2.9 Acetylcholinesterase histochemistry and analysis
Four mice were pseudorandomly selected from each of the 8 experimental groups in Experiment 2 two days after the last day of training and sacrificed under deep anesthesia (urethane, 2g/kg i.p.) by intracardiac perfusion of 50 ml fixative (4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4). The brains were removed and cryoprotected by immersion in 20% sucrose in 0.1M phosphate buffer at 4°C for 4 days, and then frozen at −45°C. Serial frontal sections (15 μm) were cut on a cryostat and mounted onto poly-L-lysine-coated slides (0.05%, Sigma, St Louis, MO). The mounted sections were incubated at 4° C overnight in an incubation medium made up of 0.68% sodium acetate, 0.1% copper sulfate, 0.12% glycine, and 0.15% acetylthiocholine iodide, and including 0.03% ethopropazine (Sigma) as a nonspecific esterase inhibitor. Acetylcholinesterase (AChE) activity was then rendered visible by treatment with 1% sodium sulfide for 10 minutes. Finally, the mounted sections were dehydrated through an ethanol series, cleared with xylene, and coverslipped with Entellan embedding agent.
Omission of the AChE substrate acetylthiocholine iodide was carried out as a control for AChE histochemistry; no residual activity was observed in these controls. Photomicrographs were taken on a Zeiss microscope with a 5× objective and analyzed with image analysis software (Morpho Pro, La Rochelle, France).
2.10 Behavioral data analysis
For generalization tasks, the dependent variable was digging time during test trials. Only mice that dug for at least 1 second in the dish scented with the odor CS during unrewarded test trials were included in the analysis, in order to exclude mice that might have learned to detect the reward directly. Repeated-measures analyses of variance were performed with odorant (i.e., conditioned odorant CS, similar odorants S1/S2, and dissimilar control odorant D) as the within-subjects factor and other experimental variables as between-subjects factors (see Results). Interactions between odorant and one or more of the between-subjects factors indicated that the effect of the latter depended on the similarity of the odorant to the CS – i.e., that the trajectory of the generalization gradient was affected, typically yielding an overall sharpening or broadening effect; these were the main results of interest (Cleland et al., 2009). Subsequent simple effects analyses were also performed within-subjects using Greenhouse-Geisser corrections. Statistical analyses were performed with SPSS software (SPSS, Chicago, IL); the threshold for assessing significance was α = 0.05.
For the forced-choice discrimination task, the number of trials in which a mouse dug in the scented dish first (“correct” responses) served as the dependent variable. ANOVA testing for a main effect of age was performed to identify any age-dependent differences in olfactory discrimination capabilities.
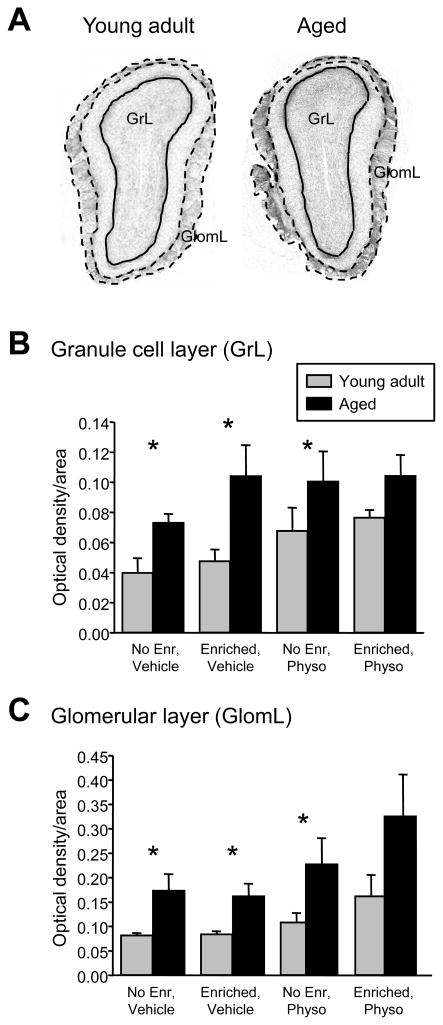
Acetylcholinesterase histological results from the OB were analyzed as follows. Two regions of interest (ROI) in each OB slice image were manually delimited: the granule cell layer (Figure 5A, GrL), which also included the internal plexiform and mitral cell layers, and the glomerular layer (Figure 5A, GlomL), which included only the glomerular layer proper. The external plexiform layer was omitted from analysis. The mean optical densities of each ROI were then calculated with Morpho Pro, and the expression levels of AChE in each ROI were compared among the different experimental groups. Specifically the optical density was measured in the granule cell and glomerular layer ROIs in each animal from four frontal sections spaced at 300 μm intervals, each normalized to the staining level of the olfactory nerve layer. Measurements of optical density per unit area were then averaged across the animals within each group; between-groups statistical comparisons were performed by ANOVA using SPSS software. All AChE quantifications were performed while blind to the experimental group.
Figure 5.
Aged mice express significantly higher levels of acetylcholinesterase (AChE) in the olfactory bulb than do young adult mice. A. Micrographs of olfactory bulb cross-sections; darker staining indicates greater AChE activity. GrL: granule cell layer; GlomL: glomerular layer. B. AChE expression levels in the granule cell layer (GrL; including the internal plexiform and mitral cell layers). Statistical analyses of main effects are presented in the Results; differences among young adult and aged mice within each individual experimental group are as follows: naïve, saline regimen, F(1,11) = 12.463, p < 0.01; enriched, saline regimen, F(1,26) = 7.816, p < 0.01; naïve, physostigmine regimen, F(1,23) = 5.681, p < 0.05; enriched, physostigmine regimen, F(1,15) = 1.594, p > 0.05. Error bars denote the standard error of the mean. C. AChE expression levels in the glomerular layer (GlomL). Statistical analyses of main effects are presented in the Results; differences among young adult and aged mice within each individual experimental group are as follows: naïve, saline regimen, F(1,20) = 10.591, p < 0.01; enriched, saline regimen, F(1,27) = 16.098, p < 0.001; naïve, physostigmine regimen, F(1,25) = 16.789, p < 0.001; enriched, physostigmine regimen, F(1,19) = 2.858, p > 0.05). Error bars denote the standard error of the mean.
3. Results
3.1 Age-dependent effects of acute cholinergic potentiation
Associative learning narrows olfactory generalization gradients, progressively increasing odor quality acuity (Cleland et al., 2009) as measured in a standard associative olfactory generalization paradigm (Cleland et al., 2002). We hypothesized that age-related cortical learning deficits would impair this plasticity, thereby reducing olfactory quality acuity in affected animals. Moreover, reductions in cholinergic activity – whether systemic (Linster and Cleland, 2002; Linster et al., 2001; Wilson, 2001) or specifically targeted to the OB (Chaudhury et al., 2009; Mandairon et al., 2006a) – are also known to reduce olfactory quality acuity. Using the progressive sharpening of olfactory generalization gradients as a model for cortical learning, we asked whether administration of acetylcholinesterase inhibitors could mitigate any age-related behavioral impairment observed by this measure. Specifically, we tested whether acute intraperitoneal administration of the acetylcholinesterase inhibitor physostigmine could sharpen olfactory generalization gradients in young adult (5 months, n=8) and aged (19 months, n=8) mice (Figure 1). A repeated-measures ANOVA was used to assess the effect of age and drug on generalization gradients with odorant as the within-subjects factor. The ability of cholinergic potentiation to sharpen generalization gradients depended significantly on age (Wilks’ lambda; interaction of drug, age, and odorant, p < 0.05), although neither age nor physostigmine exerted significant effects alone (Wilks’ lambda; interaction of age and odorant, p = 0.053; interaction of drug and odorant, p = 0.052). Specifically, whereas the olfactory quality acuity of young adult mice was significantly improved by physostigmine (simple effects analysis, (F(2,29) = 3.999; p < 0.05), the drug had no significant effect in aged mice (F(3,41) = 0.203; p > 0.05). That is, aged mice were resistant to the effects of acute cholinergic potentiation.
3.2 Effects of odor enrichment and chronic cholinergic potentiation
In order to help distinguish the acute effect of acetylcholine on olfactory generalization gradients from its role in potentiating the effects of learning (Wilson et al., 2004), we then tested whether ten-day treatment regimens of olfactory environmental enrichment (see Methods) and/or intraperitoneal physostigmine injections could sharpen olfactory quality acuity using the same associative olfactory generalization paradigm (Figure 2). Two new cohorts of mice, one young adult (2–5 months) and one aged (11–14 months), were divided into four groups each: non-enriched/vehicle (controls), non-enriched/physostigmine, enriched/vehicle, and enriched/physostigmine (n=16 per group). Neither enrichment nor physostigmine administration was performed on the day of generalization testing (day 11); hence the results do not reflect any acute effects of these manipulations. The effects of age, enrichment, and drug regimen on generalization gradients were assessed using a repeated-measures ANOVA with odorant as the within-subjects factor. In agreement with the previous study, age alone did not exert a significant effect on generalization gradients (Wilks’ lambda; interaction of age and odorant, p = 0.073). However, enrichment and chronic physostigmine treatments both significantly sharpened odor generalization gradients (Wilks’ lambda; interaction of enrichment, drug regimen, and odorant, p < 0.05; Figure 2B,C). Specifically, the effect of the physostigmine regimen was highly significant among non-enriched animals (simple effects analysis, F(2,139) = 6.105, p < 0.005) but nonsignificant among enriched animals (F(2,202) = 0.481, p > 0.05). Similarly, the effect of enrichment was significant in mice treated only with vehicle (F(2,164) = 3.677, p < 0.05), but was nonsignificant among physostigmine-treated animals (F(2,165) = 2.013, p > 0.05). That is, both the physostigmine and olfactory enrichment regimens sharpened olfactory generalization gradients compared to non-enriched, vehicle-injected controls; however, mice receiving both treatments did not exhibit sharper gradients than did mice receiving only one of the treatments. These results suggest a ceiling effect, or potentially a genuine interaction whereby the process of enrichment utilizes the cholinergic feedback regulatory processes of the olfactory system (Linster and Cleland, 2002; Linster and Hasselmo, 2000), thereby adapting to or simply superseding the effects of periodic physostigmine administration.
3.3 Modest age-dependent decline in olfactory quality acuity
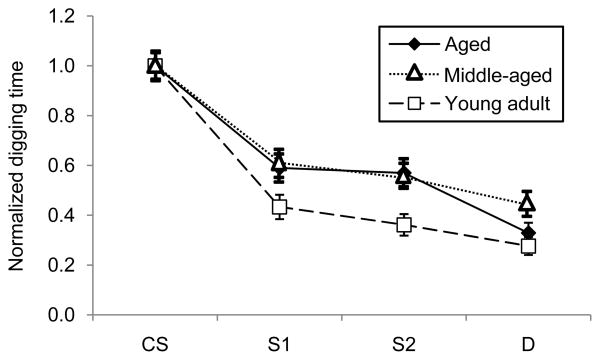
The marginal nonsignificance of age alone as a main effect in the above two studies (acute study: p = 0.053; enrichment study: p = 0.073) suggested that a small age-related effect might exist at the ages studied that could be revealed by a larger, simpler study. We tested this hypothesis by training larger groups of young adult (2–4 months; n =77), middle-aged (7–9 months; n=73) and aged (14–16 months; n =80) mice using the same associative olfactory generalization paradigm and asking whether age was a significant determinant of the shape of olfactory generalization gradients. A repeated-measures ANOVA with odorant as the within-subjects factor and age as the between-subjects factor showed that aging had a modest but significant effect on generalization gradients (Wilks’ lambda; interaction of age and odorant, F(6,450) = 2.843, p < 0.01). Specifically, given the same associative conditioning regimen, older mice exhibited broader generalization gradients than did younger mice (Figure 3). As generalization gradients progressively narrow with increased numbers of conditioning trials in the paradigm used, broader gradients are indicative of reduced learning (Cleland et al., 2009). These results indicate that olfactory quality acuity can measure an effect of age-related decline in cortical learning capacities, though the behavioral impairment is modest at the ages tested.
Figure 3.
A modest effect of age alone on olfactory generalization gradients is measurable in a sufficiently large study. Middle-aged and aged mice exhibited broader generalization gradients than did young adult mice. Young adult mice: 2–4 months old; middle-aged mice: 7–9 months old; aged mice: 14–16 months old. Digging times are scaled so that the mean response to the CS in each age group is unity. S1: odorant highly similar to the CS; S2: odorant moderately similar to the CS; D: an odorant structurally and perceptually dissimilar to the CS (Table 1). Error bars denote the standard error of the mean.
3.4 Confirmation of ability to detect test odorants
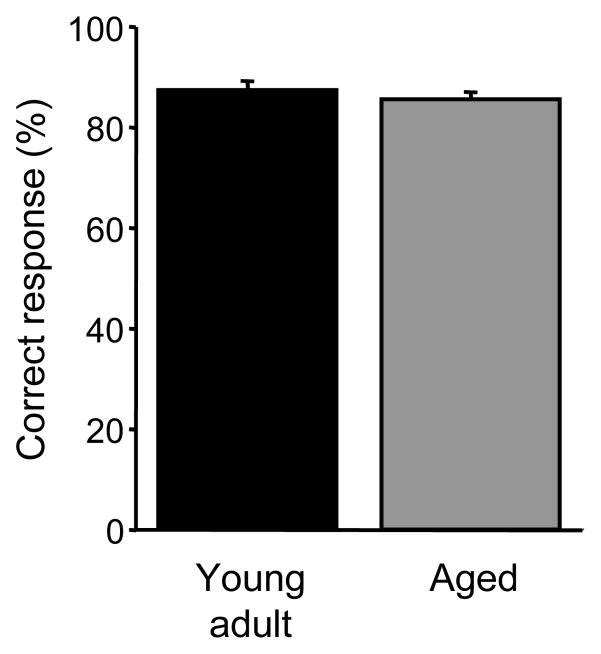
We performed an olfactory discrimination test between a scented and a nominally unscented dish to ensure that the aged mice were still as capable as the younger mice of detecting test odorants at a vapor-phase odorant concentration of 0.01 Pa. One-way analysis of variance on the overall number of correct responses (see Methods) yielded no significant effect of age (F(1,77) = 0.792, p > 0.05), indicating that the aged mice in this study were no less capable of detecting test odors under the present conditions than were the young adult mice (Figure 4).
Figure 4.
Young adult and aged mice exhibit the same capacity to detect test odors in a rewarded odor discrimination task. Mice underwent 20 sequential trials in which a sucrose reward was buried in a scented dish in the presence of a nominally unscented distractor dish. Digging in the scented dish first in each trial was scored as a correct response. The aged mice exhibited no deficit either in detecting the scented dish or learning to associate it with the reward. Error bars denote the standard error of the mean.
3.5 Acetylcholinesterase expression in the olfactory bulb
The release of acetylcholine within the OB has been repeatedly associated with a sharpening of olfactory quality acuity in both associative and nonassociative generalization paradigms (Chaudhury et al., 2009; Linster and Cleland, 2002; Linster et al., 2001; Mandairon et al., 2006a). We therefore measured the levels of acetylcholinesterase (AChE) expressed in the OBs of each of the eight experimental groups as an indicator of the average chronic levels of acetylcholine endogenously released into the OB. Specifically, AChE expression was compared among the OBs of the eight different experimental groups of mice depicted in Figure 2 (i.e., two ages × two enrichment histories × two drug regimens) using measurements of optical density taken from serial frontal sections of the OB granule cell (GrL, including the internal plexiform and mitral cell layers) and glomerular (GlomL) layers (Figure 5A; see Methods). A three-way between-subjects ANOVA with age, enrichment, and drug regimen as main effects showed that age had a highly significant effect on AChE levels in GrL (F(1,74) = 14.839; p < 0.001, Figure 5B). The physostigmine regimen also exerted a significant effect on chronic AChE levels in GrL (F(1,74) = 5.672; p < 0.05), whereas enrichment was not a significant factor (F(1,74) = 1.323; p > 0.05). AChE levels in GlomL exhibited the same pattern; the effects of age and drug regimen were both highly significant whereas olfactory enrichment was not a significant factor affecting AChE expression (age: F(1,92) = 22.708, p < 0.001; enrichment: F(1,92) = 2.065, p > 0.05; drug regimen: F(1,92) = 14.289, p < 0.001; Figure 5C).
Simple effects analyses in GrL further showed that age had a significant effect on AChE levels irrespective of the other factors (enriched, F(1,41) = 14.0, p < 0.001; non-enriched: F(1,45) = 10.80, p < 0.01; physostigmine regimen, F(1,39) = 7.0, p < 0.05; vehicle regimen, F(1,39) = 20.0, p < 0.001). In GlomL, simple effects analyses again showed the same pattern; age had a significant effect across all other between-subjects factors (enriched, F(1,47) = 13.0, p < 0.01; non-enriched: F(1,33) = 8.0, p < 0.01; physostigmine regimen, F(1,47) = 20.1, p < 0.01; vehicle regimen, F(1,49) = 6.2, p < 0.05). The significance of the effect of age on AChE expression levels within each individual experimental group is illustrated in Figure 5B,C. In sum, older mice expressed significantly higher levels of AChE than did young adult mice in both layers of the OB and irrespective of other experimental factors.
4. Discussion
4.1 Overview
Olfactory generalization gradients are learning-dependent measures of perceptual differentiation that rely substantially upon the neural circuitry of the OB. Steeper gradients correspond to greater olfactory quality acuity, and are associated with increased learning in multiple testing paradigms (Cleland et al., 2009), whereas shallower gradients can be indicative of learning impairments. We here show that a modest deficit in quality acuity incurred by aging is likely to conceal a more substantial deficit that appears to be compensated in large part by chronically increased cholinergic release into the OB. The efficacy of this compensation also may decline with age. Some lasting improvement in olfactory acuity can be derived from 10-day treatment regimens of olfactory enrichment or repeated administration of the acetylcholinesterase inhibitor physostigmine; in mice given either of these treatments, generalization gradients were still sharpened 24 hours after the end of treatment.
Broader olfactory generalization gradients after training are potentially indicative of learning impairments; if correlated with age, such effects are likely to reflect a form of age-related cognitive decline. However, the effect of age alone on olfactory generalization gradients was modest, requiring an unusually large study to establish its significance (Figure 3); age alone was not a significant main effect in smaller studies (Figures 1, 2). In contrast, the interaction of age with cholinergic potentiation was robust: administration of an AChE inhibitor significantly sharpened olfactory generalization gradients in young adult mice but had no effect on aged mice (Figure 1). This intervening effect of age on the efficacy of cholinergic potentiation directly reflects non-olfactory studies of age-related changes in cortical cholinergic function and associated cognitive/behavioral capacities. Specifically, whereas age as a main effect in such studies is often subtle or nonsignificant, it can interact strongly with other experimental manipulations, particularly those related to the vulnerability or reduced compensatory capacity of the cholinergic system (Sarter and Bruno, 1998).
The identification of age as an intervening variable (as opposed to a main effect) in studies of acetylcholine-dependent cognitive capacities generally has been interpreted to mean that aging progressively damages the basal forebrain cholinergic system (Sarter and Bruno, 1998), such that any effects of aging on behavioral performance will only be observed when the capacities of the cholinergic system are taxed. Indeed, populations of basal forebrain neurons are significantly reduced in postmortem studies of advanced-stage dementia patients. In rats, the population of basal forebrain cholinergic neurons declines with advanced age, although this decline is not significant until roughly 24 months of age (Sarter and Bruno, 1998). In CD-1 mice, this decline is not significant even at 24 months of age (Mesulam et al., 1987). While these data reflect cholinergic cell counts in the nucleus of Meynert rather than in the horizontal limb of the diagonal band of Broca (HDB), from which cholinergic fibers project to the OB, they nevertheless suggest that the cholinergic basal forebrain in general is relatively intact in the 14-month old aged CD-1 mice used in the present study, a result corroborated by the high bulbar AChE levels expressed by aged mice (Figure 5). Indeed, the generally disappointing efficacy of cholinergic replacement therapies for age-related and Alzheimer’s dementias clearly indicates that the underlying problem is not a simple lack of capacity to deliver acetylcholine to cortical structures.
The density of muscarinic cholinergic receptors in the rat OB declines substantially and significantly with age (Gurwitz et al., 1987), as it also does in the cortices of elderly humans and dementia patients (Dewey et al., 1990; Nordberg et al., 1992; Suhara et al., 1993). Reductions in receptor density reduce the absolute number of activated, ligand-bound receptor complexes for any given nonsaturating concentration of agonist, thereby increasing the agonist concentration required to achieve a given level of postsynaptic effectiveness. Indeed, in the present study, aged mice expressed significantly higher levels of AChE in the OB than did young adult mice, irrespective of other experimental variables (Figure 5), indicating that older mice are releasing chronically elevated levels of acetylcholine into the OBs compared to their younger counterparts. As the OB does not contain cholinergic cell somata, these levels reflect ACh released from basal forebrain projection fibers (Durand et al., 1998). Thus, it appears that as the density of OB muscarinic receptors declines with age, perhaps along with other related mechanisms (e.g., (Warpman et al., 1993), older mice secrete correspondingly higher levels of acetylcholine into the OB so as to maintain a level of perceptual/behavioral performance – as measured by the ability to regulate generalization gradients – comparable to that of younger mice. That is, in the early stages of progressive age-related decline in this model system, the basal forebrain cholinergic system serves as a compensatory mechanism, and is likely to not be the location of original dysfunction. A similar model has been proposed in human studies, in which older adults appear to use selective attention to compensate for difficulties in visual processing (McCalley et al., 1995). Putatively compensatory anticorrelations between choline acetyltransferase activity and muscarinic receptor density also have been observed in the hippocampi of patients with Alzheimer’s dementia (Nordberg et al., 1983).
4.2 Model of cholinergic signaling with aging: compensation and decline
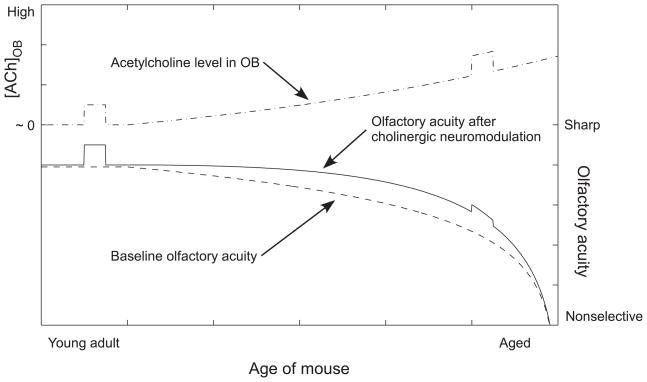
A model of the progressive age-related decline of learning performance in this model system is illustrated in Figure 6. With respect to a baseline performance level in young adult mice, aged mice exhibit progressive reductions in the intrinsic capacity for cortical plasticity in the OB, among which factors is the decline of muscarinic acetylcholine receptor expression (Gurwitz et al., 1987). At first, the potentially deleterious effects of this decline are readily compensated by increasing the levels of acetylcholine secreted into the OB; hence, reasonably aged mice show only minor deficits. However, the underlying pathology can be observed by measuring the effects of cholinergic potentiation at different ages. In young mice, a moderate dosage of an AChE inhibitor exerts a substantial effect upon odor quality acuity (Figure 6; height of short pulse in solid line). However, in aged mice, the same drug dosage exerts a minimal effect upon odor quality acuity, both because the ability of the system to respond to the drug is reduced and because the ligand is already being endogenously released at near-saturating levels. These aged mice consequently exhibit elevated AChE levels in the OB compared to younger mice (Figure 5), and also derive little or no improvement in olfactory acuity from cholinergic potentiation (Figure 1). Owing to this compensation, the effect of age alone on behavioral performance is predicted to be marginal during this period (Figure 1, 2), though potentially still measurable (Figure 3).
Figure 6.
Illustrative model of decline and compensation during aging. An underlying progressive decline in olfactory bulb-dependent olfactory quality acuity (Baseline olfactory acuity, reflecting a diminishing intrinsic learning capacity) is mitigated by gradually increasing chronic levels of acetylcholine secretion into the olfactory bulb (Acetylcholine level in OB). Because of this increased secretion of acetylcholine, aged mice persistently exhibit a level of performance comparable to that of younger mice until a relatively advanced age (Olfactory acuity after cholinergic neuromodulation); however, eventually the efficacy of this neuromodulation declines even as the absolute levels of cholinergic secretion continue to rise. Moreover, the effects of acutely elevated acetylcholine levels (identical square pulses in top curve), such as would be generated by administration of acetylcholinesterase inhibitors, on performance are substantial in young animals but ineffective in aged animals (dissimilar responses in middle curve to these pulses) because of the animals’ progressive inability to respond to the neuromodulator. The middle curve, Olfactory acuity after cholinergic neuromodulation, is a function of the other two; that is, the same putatively bulbar mechanisms of decline that reduce baseline olfactory acuity also reduce its capacity to respond to cholinergic compensatory neuromodulation, requiring progressively higher levels of secretion in order to have an effect. This alternative strategy eventually breaks down, in part directly because of this loss of sensitivity to acetylcholine but also potentially owing to the eventual breakdown of overworked basal forebrain cholinergic neurons. See text for details.
Such chronically elevated levels of acetylcholine production and release are metabolically expensive and may tax the basal forebrain cholinergic system, perhaps contributing to its eventual decline via oxidative stress (Mattson and Pedersen, 1998; Toliver-Kinsky et al., 1997). Certainly a substantial decline in cholinergic production capacity is likely to underlie a variety of cognitive deficits, as has been repeatedly observed in late-stage dementia patients and in animal models. However, this is unlikely to be the problem in the present model system, and indeed may not be the problem during the onset phase of human dementias. Studies of early-stage symptoms in Alzheimer’s dementia and normal aging have shown increases in rostral basal forebrain cholinergic activity (Ishunina and Swaab, 2003); it is in more advanced stages that breakdown of the cholinergic system is observed, and even then this breakdown includes deficiencies of cortical origin or of axonal transport in addition to cell loss in the basal forebrain (Etienne et al., 1986), suggesting that cholinergic system dysfunction can originate in the recipient cortical tissues. Reductions in cholinergic efficacy – whether due to its unavailability or an inability to respond to it – are theoretical risk factors for runaway synaptic modification in associative memory networks, a current hypothesis for the initiation and progression of Alzheimer’s dementia (Hasselmo, 1997).
The development of the olfactory bulb as a reduced cortical model system provides a computationally tractable microcosm of the interacting physiological and behavioral effects of cortical function and dysfunction, including the degenerative effects of aging and of heritable dementias (Bath et al., 2008). The need for such simplified yet relevant systems for study is increasingly apparent as the complexities and heterogeneity of aging and dementia effects resist clear attribution to specific genetic polymorphisms or other factors (Lindenberger et al., 2008). Selected perceptual/behavioral capacities dependent on olfactory bulb circuitry can be examined and quantitatively modeled (e.g., (Cleland et al., 2007; Mandairon et al., 2006a) in order to study the function and failures of cortical networks with full respect for the layers of compensatory responses that may conceal accumulating deficits until the overall dynamic range of the complex system is finally exceeded.
Acknowledgments
Supported by Marie Curie Foundation MOIF-CT-2005-51474 to NM and NIDCD R01 DC009948 to TAC. We are grateful to Mia Castro, Patrick Drummond, Olga Escanilla, Erin Johnson, Kathy Ko, Alan Leung, Max Hao Liu, Varun Ponmudi, Thalia Segal, Julia Tian, and Stephanie Zimmerman for help in behavioral experiments.
Footnotes
5.1 Disclosure Statement
None of the authors have actual or potential conflicts of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28 (10):2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Braschi C, Capsoni S, Cattaneo A, Maffei L. Environmental enrichment delays the onset of memory deficits and reduces neuropathological hallmarks in a mouse model of Alzheimer-like neurodegeneration. J Alzheimers Dis. 2007;11 (3):359–370. doi: 10.3233/jad-2007-11312. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Chaput M. Olfactory experience decreases responsiveness of the olfactory bulb in the adult rat. Neuroscience. 2000;95 (2):325–332. doi: 10.1016/s0306-4522(99)00450-9. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci. 2009;29 (1):52–60. doi: 10.1523/JNEUROSCI.4036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Johnson BA, Leon M, Linster C. Relational representation in the olfactory system. Proc Natl Acad Sci U S A. 2007;104 (6):1953–1958. doi: 10.1073/pnas.0608564104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116 (2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Narla VA. Intensity modulation of olfactory acuity. Behav Neurosci. 2003;117 (6):1434–1440. doi: 10.1037/0735-7044.117.6.1434. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Narla VA, Boudadi K. Multiple learning parameters differentially regulate olfactory generalization. Behav Neurosci. 2009;123(1) doi: 10.1037/a0013991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 2006;7:7. doi: 10.1186/1471-2202-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Hasselmo ME. Muscarinic cholinergic neuromodulation reduces proactive interference between stored odor memories during associative learning in rats. Behav Neurosci. 2000;114 (1):32–41. [PubMed] [Google Scholar]
- De Rosa E, Hasselmo ME, Baxter MG. Contribution of the cholinergic basal forebrain to proactive interference from stored odor memories during associative learning in rats. Behav Neurosci. 2001;115 (2):314–327. [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007a;114 (1):43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007b;176 (2):267–273. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Volkow ND, Logan J, MacGregor RR, Fowler JS, Schlyer DJ, Bendriem B. Age-related decreases in muscarinic cholinergic receptor binding in the human brain measured with positron emission tomography (PET) J Neurosci Res. 1990;27 (4):569–575. doi: 10.1002/jnr.490270418. [DOI] [PubMed] [Google Scholar]
- Doty RL, Bagla R, Kim N. Physostigmine enhances performance on an odor mixture discrimination test. Physiol Behav. 1999;65 (4–5):801–804. doi: 10.1016/s0031-9384(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Durand M, Coronas V, Jourdan F, Quirion R. Developmental and aging aspects of the cholinergic innervation of the olfactory bulb. Int J Dev Neurosci. 1998;16 (7–8):777–785. doi: 10.1016/s0736-5748(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24 (38):8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanilla O, Mandairon N, Linster C. Odor-reward learning and enrichment have similar effects on odor perception. Physiol Behav. 2008;94 (4):621–626. doi: 10.1016/j.physbeh.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Etienne P, Robitaille Y, Wood P, Gauthier S, Nair NP, Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer’s disease. Neuroscience. 1986;19 (4):1279–1291. doi: 10.1016/0306-4522(86)90142-9. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22(2):RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav Brain Res. 1994;61 (1):87–90. doi: 10.1016/0166-4328(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Guerin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Gurwitz D, Egozi Y, Henis YI, Kloog Y, Sokolovsky M. Agonist and antagonist binding to rat brain muscarinic receptors: influence of aging. Neurobiol Aging. 1987;8 (2):115–122. doi: 10.1016/0197-4580(87)90020-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. A computational model of the progression of Alzheimer’s disease. MD Comput. 1997;14 (3):181–191. [PubMed] [Google Scholar]
- Hoffman HJ, Ishii EK, MacTurk RH. Age-related changes in the prevalence of smell/taste problems among the United States adult population. Results of the 1994 disability supplement to the National Health Interview Survey (NHIS) Ann N Y Acad Sci. 1998;855:716–722. doi: 10.1111/j.1749-6632.1998.tb10650.x. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Murray TK. Cholinergic mechanisms in a simple test of olfactory learning in the rat. Psychopharmacology (Berl) 1989;99 (2):270–275. doi: 10.1007/BF00442821. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Increased neuronal metabolic activity and estrogen receptors in the vertical limb of the diagonal band of Broca in Alzheimer’s disease: relation to sex and aging. Exp Neurol. 2003;183 (1):159–172. doi: 10.1016/s0014-4886(03)00138-9. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2 (2):234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw. 2002;15 (4–6):709–717. doi: 10.1016/s0893-6080(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001;115 (4):826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 1999;66 (3):497–502. doi: 10.1016/s0031-9384(98)00324-2. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Neural activity in the horizontal limb of the diagonal band of broca can be modulated by electrical stimulation of the olfactory bulb and cortex in rats. Neurosci Lett. 2000;282 (3):157–160. doi: 10.1016/s0304-3940(00)00885-5. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22 (16):6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006a;24 (11):3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci U S A. 2006b;103 (36):13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Enrichment to odors improves olfactory discrimination in adult rats. Behav Neurosci. 2006c;120 (1):173–179. doi: 10.1037/0735-7044.120.1.173. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA. Effects of amyloid precursor protein derivatives and oxidative stress on basal forebrain cholinergic systems in Alzheimer’s disease. Int J Dev Neurosci. 1998;16 (7–8):737–753. doi: 10.1016/s0736-5748(98)00082-3. [DOI] [PubMed] [Google Scholar]
- McCalley LT, Bouwhuis DG, Juola JF. Age changes in the distribution of visual attention. J Gerontol B Psychol Sci Soc Sci. 1995;50 (6):P316–331. doi: 10.1093/geronb/50b.6.p316. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Rogers J. Age-related shrinkage of cortically projecting cholinergic neurons: a selective effect. Ann Neurol. 1987;22 (1):31–36. doi: 10.1002/ana.410220109. [DOI] [PubMed] [Google Scholar]
- Nakayasu C, Kanemura F, Hirano Y, Shimizu Y, Tonosaki K. Sensitivity of the olfactory sense declines with the aging in senescence-accelerated mouse (SAM-P1) Physiol Behav. 2000;70 (1–2):135–139. doi: 10.1016/s0031-9384(00)00234-1. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J Neurosci Res. 1992;31 (1):103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Larsson C, Adolfsson R, Alafuzoff I, Winblad B. Muscarinic receptor compensation in hippocampus of Alzheimer patients. J Neural Transm. 1983;56 (1):13–19. doi: 10.1007/BF01243370. [DOI] [PubMed] [Google Scholar]
- O’Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19 (10):1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26 (6):957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Prediger RD, De-Mello N, Takahashi RN. Pilocarpine improves olfactory discrimination and social recognition memory deficits in 24 month-old rats. Eur J Pharmacol. 2006;531 (1–3):176–182. doi: 10.1016/j.ejphar.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Ravel N, Elaagouby A, Gervais R. Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behav Neurosci. 1994;108 (2):317–324. doi: 10.1037//0735-7044.108.2.317. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22 (7):2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman FS, Simonetto I, Soumireu-Mourat B. Learning and memory of odor-reward association: selective impairment following horizontal diagonal band lesions. Behav Neurosci. 1993;107 (1):72–81. doi: 10.1037//0735-7044.107.1.72. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78 (1):57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Age-related changes in rodent cortical acetylcholine and cognition: main effects of age versus age as an intervening variable. Brain Res Brain Res Rev. 1998;27 (2):143–156. doi: 10.1016/s0165-0173(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002;23 (4):555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer’s disease. Am J Psychiatry. 1991;148 (3):357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Shepard RN. Toward a universal law of generalization for psychological science. Science. 1987;237 (4820):1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30 (1):123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34 (2):301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Suhara T, Inoue O, Kobayashi K, Suzuki K, Tateno Y. Age-related changes in human muscarinic acetylcholine receptors measured by positron emission tomography. Neurosci Lett. 1993;149 (2):225–228. doi: 10.1016/0304-3940(93)90777-i. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Perio A, Worms P, Le Fur G, Soubrie P. Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav Pharmacol. 1994;5 (1):90–98. [PubMed] [Google Scholar]
- Toliver-Kinsky T, Papaconstantinou J, Perez-Polo JR. Age-associated alterations in hippocampal and basal forebrain nuclear factor kappa B activity. J Neurosci Res. 1997;48 (6):580–587. doi: 10.1002/(sici)1097-4547(19970615)48:6<580::aid-jnr11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1 (3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Warpman U, Alafuzoff I, Nordberg A. Coupling of muscarinic receptors to GTP proteins in postmortem human brain--alterations in Alzheimer’s disease. Neurosci Lett. 1993;150 (1):39–43. doi: 10.1016/0304-3940(93)90103-r. [DOI] [PubMed] [Google Scholar]
- Wenk H, Meyer U, Bigl V. Centrifugal cholinergic connections in the olfactory system of rats. Neuroscience. 1977;2 (5):797–800. doi: 10.1016/0306-4522(77)90033-1. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8 (5):279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Fletcher ML, Sullivan RM. Acetylcholine and olfactory perceptual learning. Learn Mem. 2004;11 (1):28–34. doi: 10.1101/lm.66404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64 (7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Woo CC, Hingco EE, Johnson BA, Leon M. Broad activation of the glomerular layer enhances subsequent olfactory responses. Chem Senses. 2007;32 (1):51–55. doi: 10.1093/chemse/bjl035. [DOI] [PMC free article] [PubMed] [Google Scholar]